Release of Antibiotic-Resistance Genes from Hospitals and a Wastewater Treatment Plant in the Kathmandu Valley, Nepal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Water Samples
2.2. Quantification of Total Coliforms (TC) and Escherichia coli in Water Samples
2.3. Extraction of Bacterial DNA
2.4. Quantitative PCR (qPCR) Assays
2.5. Statistical Analysis
3. Results and Discussion
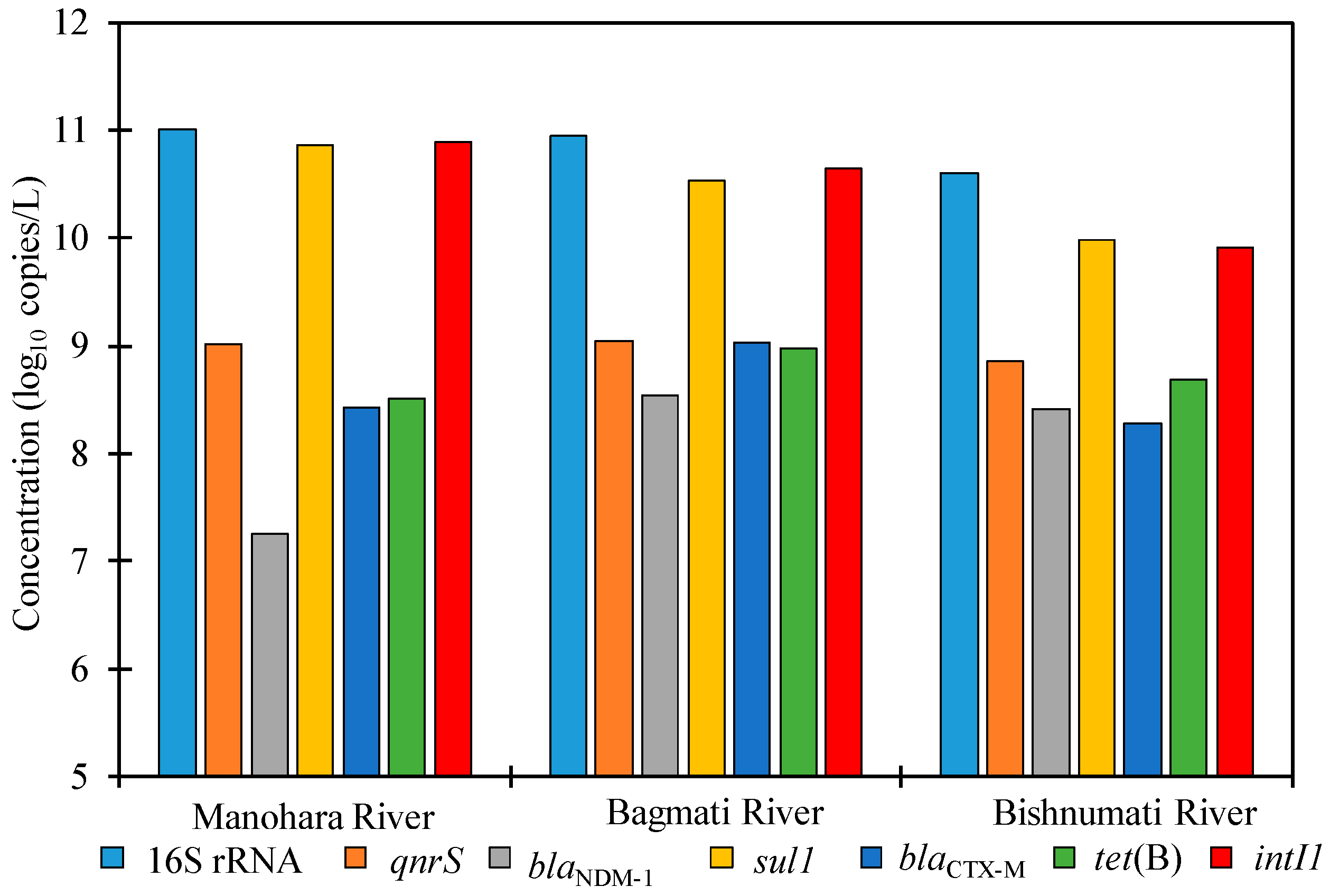
3.1. ARGs in River Water
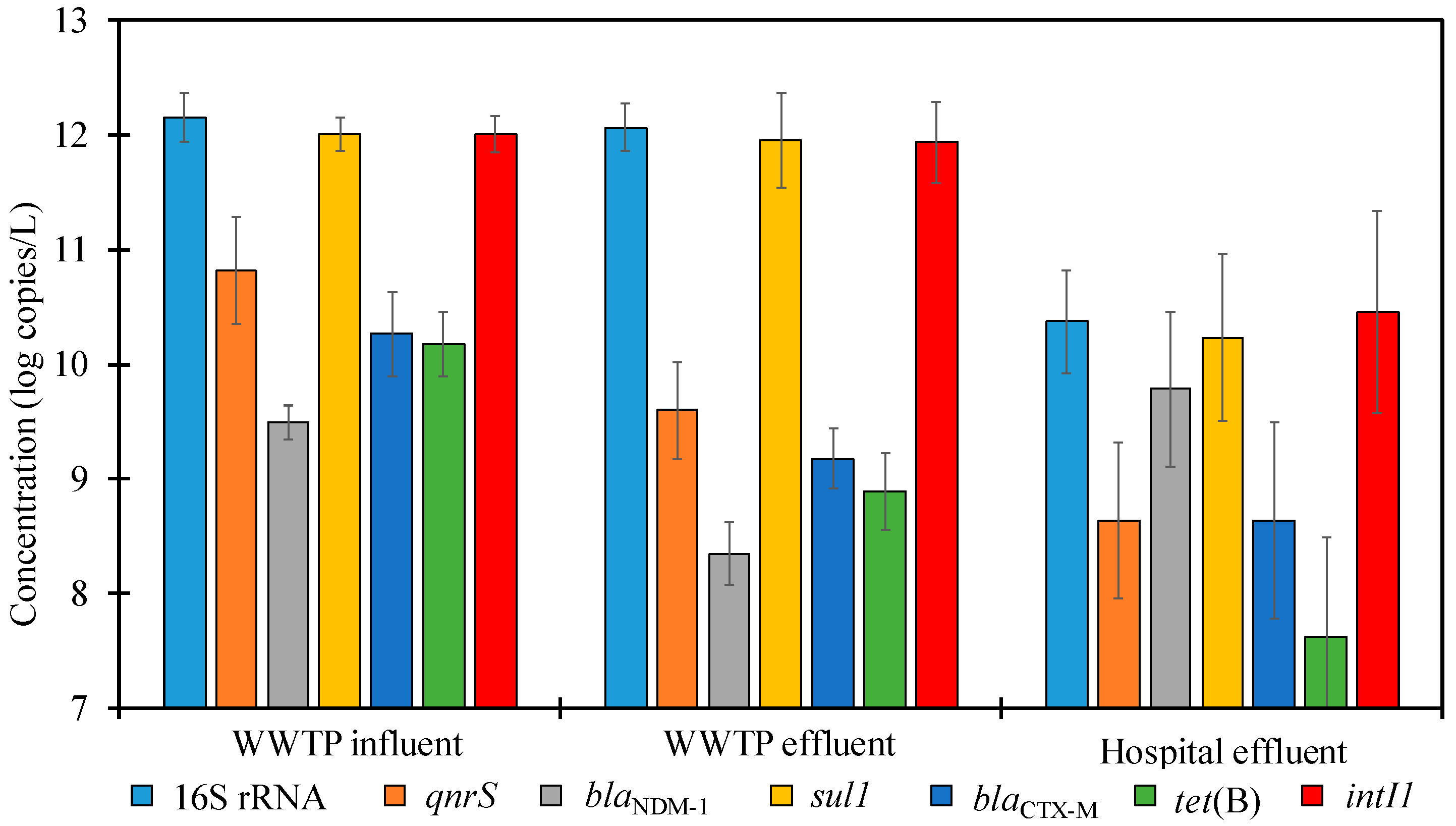
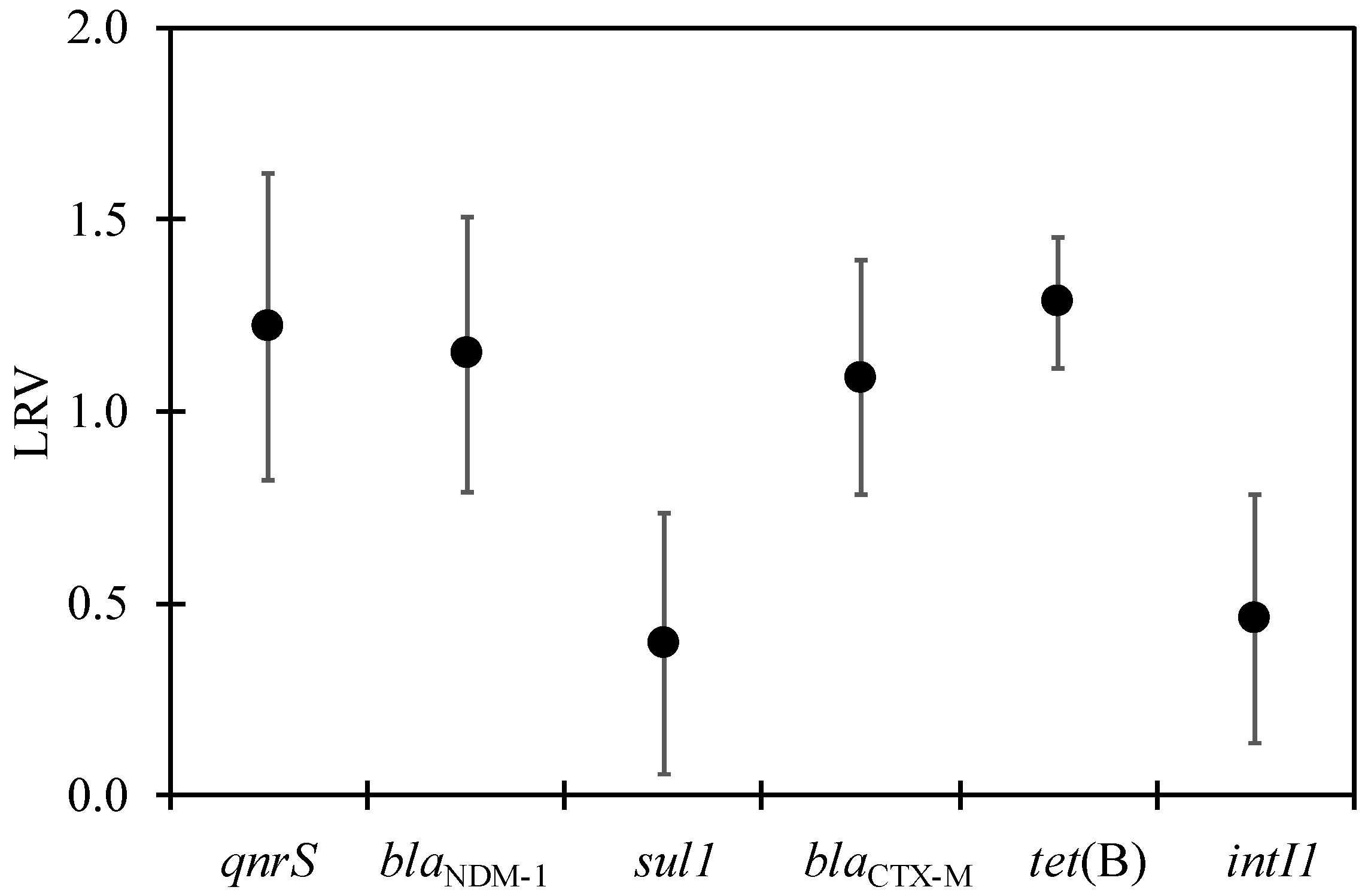
3.2. ARGs in WWTP
3.3. ARGs in the Hospital Wastewater
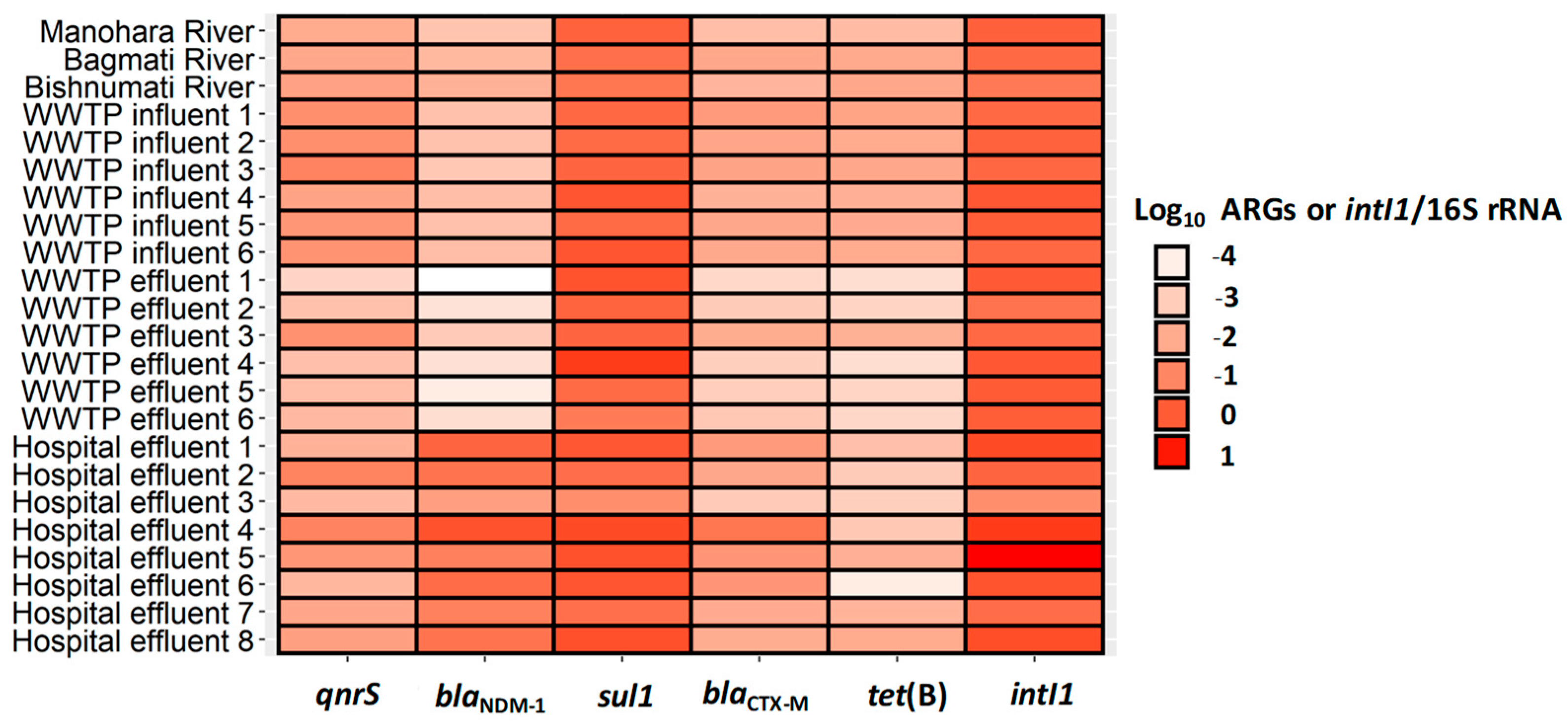
3.4. Correlation between Individual ARGs, intI1, and Fecal Indicator Bacteria
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toranzos, G.; Steyn, M.; Santiago-Rodriguez, T.; Sano, D. Editorial: Bacterial antibiotic resistance in the water environment. J. Water Health 2020, 18, 855–857. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B.A. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K.; Al-Ahmad, A.; Mersch-Sundermann, V. Biodegradability of some antibiotics, elimination of the genotoxicity and affection of wastewater bacteria in a simple test. Chemosphere 2000, 40, 701–710. [Google Scholar] [CrossRef]
- Kümmerer, K.; Henninger, A. Promoting resistance by the emission of antibiotics from hospitals and households into effluent. Clin. Microbiol. Infect. 2003, 9, 1203–1214. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acharya, K.P.; Wilson, R.T. Antimicrobial Resistance in Nepal. Front. Med. 2019, 6, 105. [Google Scholar] [CrossRef] [Green Version]
- Dahal, R.H.; Chaudhary, D.K. Microbial Infections and Antimicrobial Resistance in Nepal: Current Trends and Recommendations. Open Microbiol. J. 2018, 12, 230–242. [Google Scholar] [CrossRef] [Green Version]
- Joshi, H.D.; Acharya, T.; Ayer, R.; Dhakal, P.; Karki, K.B.; Dhimal, M. Health Care Waste Management Practice in Health Care Institutions of Nepal. J. Nepal Health Res. Counc. 2017, 15, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Thakali, O.; Tandukar, S.; Brooks, J.P.; Sherchan, S.P.; Sherchand, J.B.; Haramoto, E. The Occurrence of Antibiotic Resistance Genes in an Urban River in Nepal. Water 2020, 12, 450. [Google Scholar] [CrossRef] [Green Version]
- Hyoju, R.K.; Shrestha, R. Efficiency of General Hospitals in Kathmandu Valley. 2014. Available online: http://conference.ioe.edu.np/ioegc2014/papers/IOE-CONF-2014-39.pdf (accessed on 11 May 2021).
- Thakali, O.; Brooks, J.P.; Shahin, S.; Sherchan, S.P.; Haramoto, E. Removal of Antibiotic Resistance Genes at Two Conventional Wastewater Treatment Plants of Louisiana, USA. Water 2020, 12, 1729. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Chamorro, S.; Marti, E.; Huerta, B.; Gros, M.; Sànchez-Melsió, A.; Borrego, C.M.; Barceló, D.; Balcázar, J.L. Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river. Water Res. 2015, 69, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I.; Roberts, M. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poirel, L.; Cattoir, V.; Nordmann, P. Plasmid-Mediated Quinolone Resistance; Interactions between Human, Animal, and Environmental Ecologies. Front. Microbiol. 2012, 3, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherchan, J.B.; Hayakawa, K.; Miyoshi-Akiyama, T.; Ohmagari, N.; Kirikae, T.; Nagamatsu, M.; Tojo, M.; Ohara, H.; Sherchand, J.B.; Tandukar, S. Clinical epidemiology and molecular analysis of extended-spectrum-β-lactamase-producing Escherichia coli in Nepal: Characteristics of sequence types 131 and 648. Antimicrob. Agents Chemother. 2015, 59, 3424–3432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pokhrel, R.; Thapa, B.; Kafle, R.; Shah, P.; Tribuddharat, C. Co-existence of beta-lactamases in clinical isolates of Escherichia coli from Kathmandu, Nepal. BMC Res. Notes 2014, 7, 694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yong, D.; Toleman, M.A.; Giske, C.G.; Cho, H.S.; Sundman, K.; Lee, K.; Walsh, T.R. Characterization of a new metallo-β-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 2009, 53, 5046–5054. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, B.; Tada, T.; Shimada, K.; Shrestha, S.; Ohara, H.; Pokhrel, B.M.; Sherchand, J.B.; Kirikae, T. Emergence of Various NDM-Type-Metallo-β-Lactamase-Producing Escherichia coli Clinical Isolates in Nepal. Antimicrob. Agents Chemother. 2017, 61, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Rowe-Magnus, D.A.; Mazel, D. The role of integrons in antibiotic resistance gene capture. Int. J. Med. Microbiol. 2002, 292, 115–125. [Google Scholar] [CrossRef]
- Zhang, K.; Xin, R.; Zhao, Z.; Ma, Y.; Zhang, Y.; Niu, Z. Antibiotic Resistance Genes in drinking water of China: Occurrence, distribution and influencing factors. Ecotoxicol. Environ. Saf. 2020, 188, 109837. [Google Scholar] [CrossRef]
- Narciso-da-Rocha, C.; Varela, A.R.; Schwartz, T.; Nunes, O.C.; Manaia, C.M. blaTEM and vanA as indicator genes of antibiotic resistance contamination in a hospital–urban wastewater treatment plant system. J. Glob. Antimicrob. Resist. 2014, 2, 309–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. WHO Model List of Essential Medicines, 21st List. 2019. Available online: https://www.who.int/publications/i/item/WHOMVPEMPIAU2019.06 (accessed on 5 May 2021).
- Malla, B.; Ghaju Shrestha, R.; Tandukar, S.; Bhandari, D.; Thakali, O.; Sherchand, J.B.; Haramoto, E. Detection of Pathogenic Viruses, Pathogen Indicators, and Fecal-Source Markers within Tanker Water and Their Sources in the Kathmandu Valley, Nepal. Pathogens 2019, 8, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, M.T.; Taylor, L.T.; DeLong, E.F. Quantitative Analysis of Small-Subunit rRNA Genes in Mixed Microbial Populations via 5′-Nuclease Assays. Appl. Environ. Microbiol. 2000, 66, 4605–4614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; Mao, D.; Rysz, M.; Zhou, Q.; Zhang, H.; Xu, L.; Alvarez, P.J.J. Trends in Antibiotic Resistance Genes Occurrence in the Haihe River, China. Environ. Sci. Technol. 2010, 44, 7220–7225. [Google Scholar] [CrossRef]
- Rutgersson, C.; Fick, J.; Marathe, N.; Kristiansson, E.; Janzon, A.; Angelin, M.; Johansson, A.; Shouche, Y.; Flach, C.-F.; Larsson, D.G.J. Fluoroquinolones and qnr Genes in Sediment, Water, Soil, and Human Fecal Flora in an Environment Polluted by Manufacturing Discharges. Environ. Sci. Technol. 2014, 48, 7825–7832. [Google Scholar] [CrossRef] [PubMed]
- Colomer-Lluch, M.; Jofre, J.; Muniesa, M. Antibiotic resistance genes in the bacteriophage DNA fraction of environmental samples. PLoS ONE 2011, 6, e17549. [Google Scholar] [CrossRef] [Green Version]
- Naas, T.; Ergani, A.; Carrër, A.; Nordmann, P. Real-Time PCR for Detection of NDM-1 Carbapenemase Genes from Spiked Stool Samples. Antimicrob. Antimicrob. Agents Chemother. 2011, 55, 4038–4043. [Google Scholar] [CrossRef] [Green Version]
- Czekalski, N.; Berthold, T.; Caucci, S.; Egli, A.; Bürgmann, H. Increased levels of multiresistant bacteria and resistance genes after wastewater treatment and their dissemination into Lake Geneva, Switzerland. Front. Microbiol. 2012, 3, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Barraud, O.; Baclet, M.C.; Denis, F.; Ploy, M.C. Quantitative multiplex real-time PCR for detecting class 1, 2 and 3 integrons. J. Antimicrob. Chemother. 2010, 65, 1642–1645. [Google Scholar] [CrossRef] [Green Version]
- Baran, W.; Adamek, E.; Ziemiańska, J.; Sobczak, A. Effects of the presence of sulfonamides in the environment and their influence on human health. J. Hazard. Mater. 2011, 196, 1–15. [Google Scholar] [CrossRef]
- Shrestha, S.; Shrestha, S.; Shindo, J.; Sherchand, J.B.; Haramoto, E. Virological quality of irrigation water sources and pepper mild mottle virus and tobacco mosaic virus as index of pathogenic virus contamination level. Food Environ. Virol. 2018, 10, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.G.; Tandukar, S.; Bhandari, D.; Sherchan, S.P.; Tanaka, Y.; Sherchand, J.B.; Haramoto, E. Prevalence of Arcobacter and Other Pathogenic Bacteria in River Water in Nepal. Water 2019, 11, 1416. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.-L.; Zhang, M.; He, L.-X.; Zou, H.-Y.; Liu, Y.-S.; Li, B.-B.; Yang, Y.-Y.; Liu, C.; He, L.-Y.; Ying, G.-G. Contamination profile of antibiotic resistance genes in ground water in comparison with surface water. Sci. Total Environ. 2020, 715, 136975. [Google Scholar] [CrossRef] [PubMed]
- Parnanen, K.M.M.; Narciso-da-Rocha, C.; Kneis, D.; Berendonk, T.U.; Cacace, D.; Do, T.T.; Elpers, C.; Fatta-Kassinos, D.; Henriques, I.; Jaeger, T.; et al. Antibiotic resistance in European wastewater treatment plants mirrors the pattern of clinical antibiotic resistance prevalence. Sci. Adv. 2019, 5, eaau9124. [Google Scholar] [CrossRef] [Green Version]
- Bairán, G.; Rebollar-Pérez, G.; Chávez-Bravo, E.; Torres, E. Treatment processes for microbial resistance mitigation: The technological contribution to tackle the problem of antibiotic resistance. Int. J. Environ. Res. Public Health 2020, 17, 8866. [Google Scholar] [CrossRef]
- Amarasiri, M.; Sano, D.; Suzuki, S. Understanding human health risks caused by antibiotic resistant bacteria (ARB) and antibiotic resistance genes (ARG) in water environments: Current knowledge and questions to be answered. Crit. Rev. Environ. Sci. Technol. 2019, 50, 2016–2059. [Google Scholar] [CrossRef]
- Su, H.; Liu, S.; Hu, X.; Xu, X.; Xu, W.; Xu, Y.; Li, Z.; Wen, G.; Liu, Y.; Cao, Y. Occurrence and temporal variation of antibiotic resistance genes (ARGs) in shrimp aquaculture: ARGs dissemination from farming source to reared organisms. Sci. Total Environ. 2017, 607–608, 357–366. [Google Scholar] [CrossRef]
- Gillings, M.R.; Gaze, W.H.; Pruden, A.; Smalla, K.; Tiedje, J.M.; Zhu, Y.-G. Using the class 1 integron-integrase gene as a proxy for anthropogenic pollution. ISME J. 2015, 9, 1269–1279. [Google Scholar] [CrossRef]
- Sherchan, J.B.; Tada, T.; Shrestha, S.; Uchida, H.; Hishinuma, T.; Morioka, S.; Shahi, R.; Twi, R.T.; Kirikae, T.; Sherchand, J.B. Emergence of clinical isolates of highly carbapenem-resistant Klebsiella pneumonia co-harboring blaNDM-5 and blaOXA-181 or -232 in Nepal. Int. J. Infect. Dis. 2020, 92, 247–252. [Google Scholar] [CrossRef] [Green Version]
- Nordmann, P.; Poirel, L.; Walsh, T.R.; Livermore, D.M. The emerging NDM carbapenemases. Trends Microbiol. 2011, 19, 588–595. [Google Scholar] [CrossRef]
- Lamba, M.; Graham, D.W.; Ahammad, S.Z. Hospital Wastewater Releases of Carbapenem-Resistance Pathogens and Genes in Urban India. Environ. Sci. Technol. 2017, 51, 13906–13912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.A.; Islam, M.; Hasan, R.; Hossain, M.I.; Nabi, A.; Rahman, M.; Goessens, W.H.F.; Endtz, H.P.; Boehm, A.B.; Faruque, S.M. Environmental Spread of New Delhi Metallo-β-Lactamase-1-Producing Multidrug-Resistant Bacteria in Dhaka, Bangladesh. Appl. Environ. Microbiol. 2017, 83, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamba, M.; Gupta, S.; Shukla, R.; Graham, D.W.; Sreekrishnan, T.R.; Ahammad, S.Z. Carbapenem resistance exposures via wastewaters across New Delhi. Environ. Int. 2018, 119, 302–308. [Google Scholar] [CrossRef]
- Walsh, T.R.; Weeks, J.; Livermore, D.M.; Toleman, M.A. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: An environmental point prevalence study. Lancet Infect. Dis. 2011, 11, 355–362. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, P.; Yang, Q. Occurrence and diversity of antibiotic resistance in untreated hospital wastewater. Sci. Total Environ. 2018, 621, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Cerdeira, L.; Fernandes, M.R.; Ienne, S.; Souza, T.A.; Garcia, D.d.O.; Lincopan, N. Draft genome sequence of an environmental multidrug-resistant Klebsiella pneumoniae ST340/CC258 harbouring blaCTX-M-15 and blaKPC-2 genes. J. Glob. Antimicrob. Resist. 2017, 8, 108–109. [Google Scholar] [CrossRef]
- Zhang, C.; Qiu, S.; Wang, Y.; Qi, L.; Hao, R.; Liu, X.; Shi, Y.; Hu, X.; An, D.; Li, Z.; et al. Higher Isolation of NDM-1 Producing Acinetobacter baumannii from the Sewage of the Hospitals in Beijing. PLoS ONE 2013, 8, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Karkman, A.; Do, T.T.; Walsh, F.; Virta, M.P.J. Antibiotic-Resistance Genes in Waste Water. Trends Microbiol. 2018, 26, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Marathe, N.P.; Berglund, F.; Razavi, M.; Pal, C.; Dröge, J.; Samant, S.; Kristiansson, E.; Joakim Larsson, D.G. Sewage effluent from an Indian hospital harbors novel carbapenemases and integron-borne antibiotic resistance genes. Microbiome 2019, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Seruga Music, M.; Hrenovic, J.; Goic-Barisic, I.; Hunjak, B.; Skoric, D.; Ivankovic, T. Emission of extensively-drug-resistant Acinetobacter baumannii from hospital settings to the natural environment. J. Hosp. Infect. 2017, 96, 323–327. [Google Scholar] [CrossRef]
- Shrestha, S.; Tada, T.; Miyoshi-Akiyama, T.; Ohara, H.; Shimada, K.; Satou, K.; Teruya, K.; Nakano, K.; Shiroma, A.; Sherchand, J.B.; et al. Molecular epidemiology of multidrug-resistant Acinetobacter baumannii isolates in a university hospital in Nepal reveals the emergence of a novel epidemic clonal lineage. Int. J. Antimicrob. Agents 2015, 46, 526–531. [Google Scholar] [CrossRef] [PubMed]
| Gene | Class of Antibiotics/Importance |
|---|---|
| 16SrRNA | Proxy for total bacterial load |
| tet(B) | Tetracycline b |
| qnrS | Fluoroquinolones a |
| blaCTX-M | Cephalosporins a,b, Penicillin, Monobactams b |
| blaNDM-1 | Carbapenems b, Cephalosporins a,b, Penicillin |
| sul1 | Sulfonamides |
| intI1 | Proxy for the spread of antibiotic-resistance genes [22] |
| Assay | Function | Sequence (5′–3′) | Size (bp) | Reference |
|---|---|---|---|---|
| 16S rRNA | Forward primer | CGGTGAATACGTTCYCGG | 142 | [25] |
| Reverse primer | GGWTACCTTGTTACGACTT | |||
| TaqMan probe | FAM-CTTGTACAC/ZEN/ACCGCCCGTC-IBFQ | |||
| tet(B) | Forward primer | CGAAGTAGGGGTTGAGACGC | 192 | [26] |
| Reverse primer | AGACCAAGACCCGCTAATGAA | |||
| qnrS | Forward primer | GATATCGAAGGCTGCCACTT | 115 | [27] |
| Reverse primer | CACGGAACTCTATACCGTAGCA | |||
| blaCTX-M | Forward primer | ACCAACGATATCGCGGTGAT | 101 | [28] |
| Reverse primer | ACATCGCGACGGCTTTCT | |||
| TaqMan probe | FAM-TCGTGCGCCGCTG-BHQ1 | |||
| blaNDM-1 | Forward primer | ATTAGCCGCTGCATTGAT | 154 | [29] |
| Reverse primer | CATGTCGAGATAGGAAGTG | |||
| TaqMan probe | FAM-CTGCCAGACATTCGGTGC-TAMRA | |||
| sul1 | Forward primer | CCGTTGGCCTTCCTGTAAAG | 67 | [30] |
| Reverse primer | TTGCCGATCGCGTGAAGT | |||
| TaqMan probe | FAM-CAGCGAGCCTTGCGGCGG-BHQ1 | |||
| intI1 | Forward primer | GCCTTGATGTTACCCGAGAG | 196 | [31] |
| Reverse primer | GATCGGTCGAATGCGTGT | |||
| TaqMan probe | FAM-ATTCCTGGCCGTGGTTCTGGGTTTT- BHQ1 |
| Indicator | sul1 | tet(B) | qnrS | blaCTX-M | blaNDM-1 |
|---|---|---|---|---|---|
| intI1 | 0.96 * | 0.73 * | 0.71 * | 0.80 * | −0.04 |
| TC | −0.16 | 0.16 | 0.22 | 0.18 | 0.33 |
| E. coli | 0.58 * | 0.74 * | 0.61 * | 0.60 * | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thakali, O.; Malla, B.; Tandukar, S.; Sthapit, N.; Raya, S.; Furukawa, T.; Sei, K.; Sherchand, J.B.; Haramoto, E. Release of Antibiotic-Resistance Genes from Hospitals and a Wastewater Treatment Plant in the Kathmandu Valley, Nepal. Water 2021, 13, 2733. https://doi.org/10.3390/w13192733
Thakali O, Malla B, Tandukar S, Sthapit N, Raya S, Furukawa T, Sei K, Sherchand JB, Haramoto E. Release of Antibiotic-Resistance Genes from Hospitals and a Wastewater Treatment Plant in the Kathmandu Valley, Nepal. Water. 2021; 13(19):2733. https://doi.org/10.3390/w13192733
Chicago/Turabian StyleThakali, Ocean, Bikash Malla, Sarmila Tandukar, Niva Sthapit, Sunayana Raya, Takashi Furukawa, Kazunari Sei, Jeevan B. Sherchand, and Eiji Haramoto. 2021. "Release of Antibiotic-Resistance Genes from Hospitals and a Wastewater Treatment Plant in the Kathmandu Valley, Nepal" Water 13, no. 19: 2733. https://doi.org/10.3390/w13192733







