Biochar and Zeolite as Alternative Biofilter Media for Denitrification of Aquaculture Effluents
Abstract
:1. Introduction
2. Materials and Methods
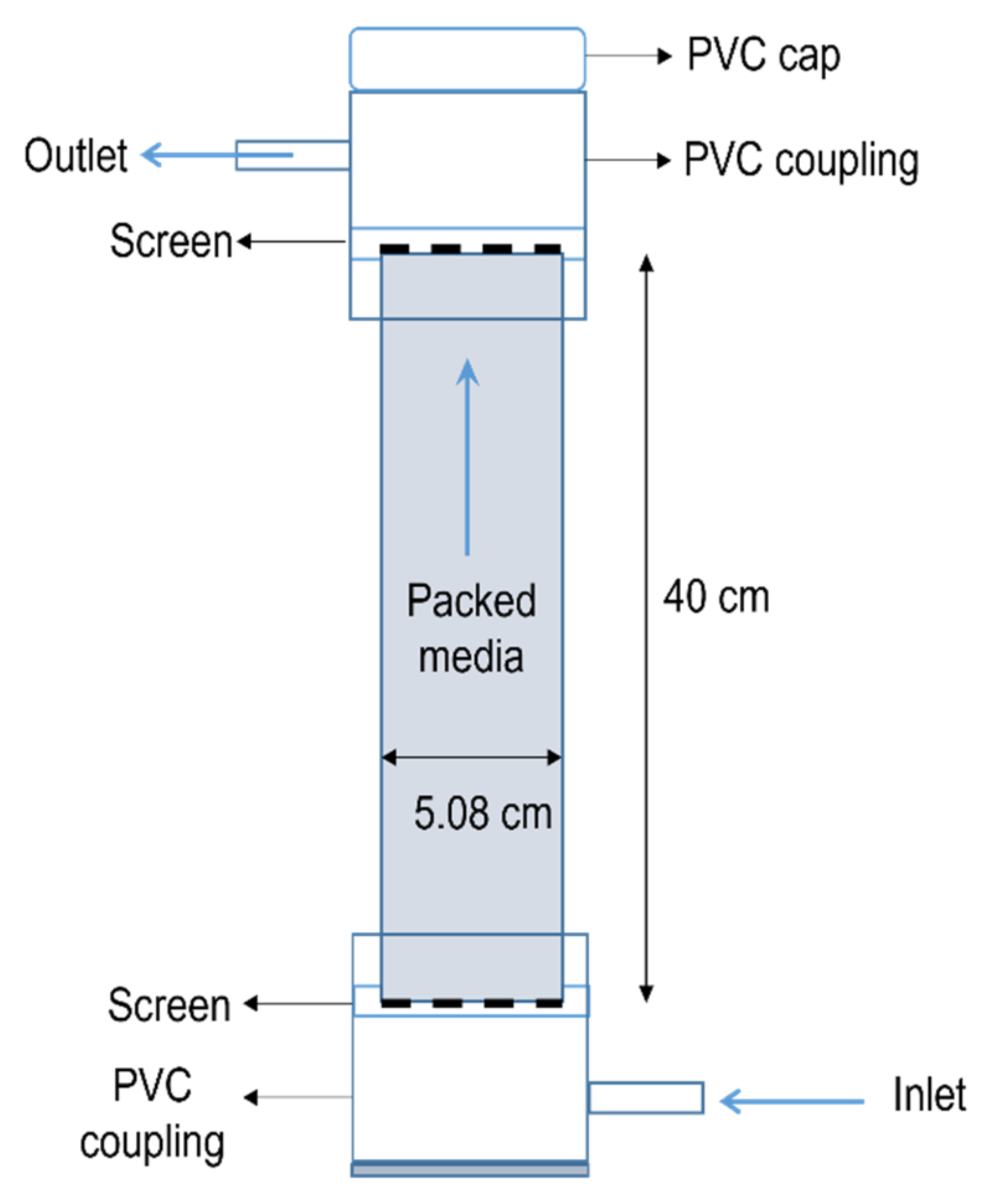
2.1. Experimental Design
2.2. Water Quality Analysis
2.3. Characterization of Biofilter Media
2.4. Statistical Analysis
3. Results
3.1. Performance of Bioreactors
3.2. Quality of the Effluents
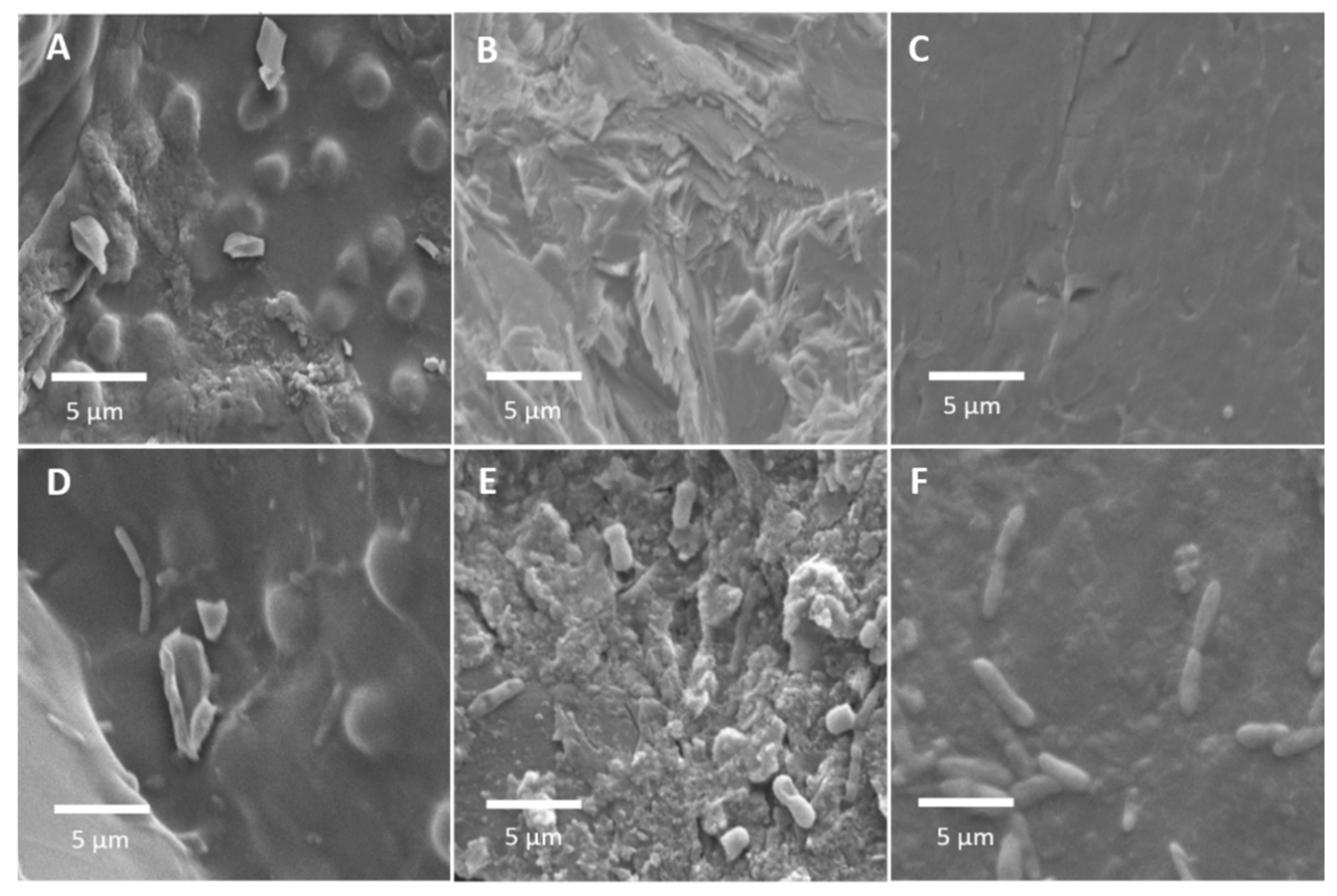
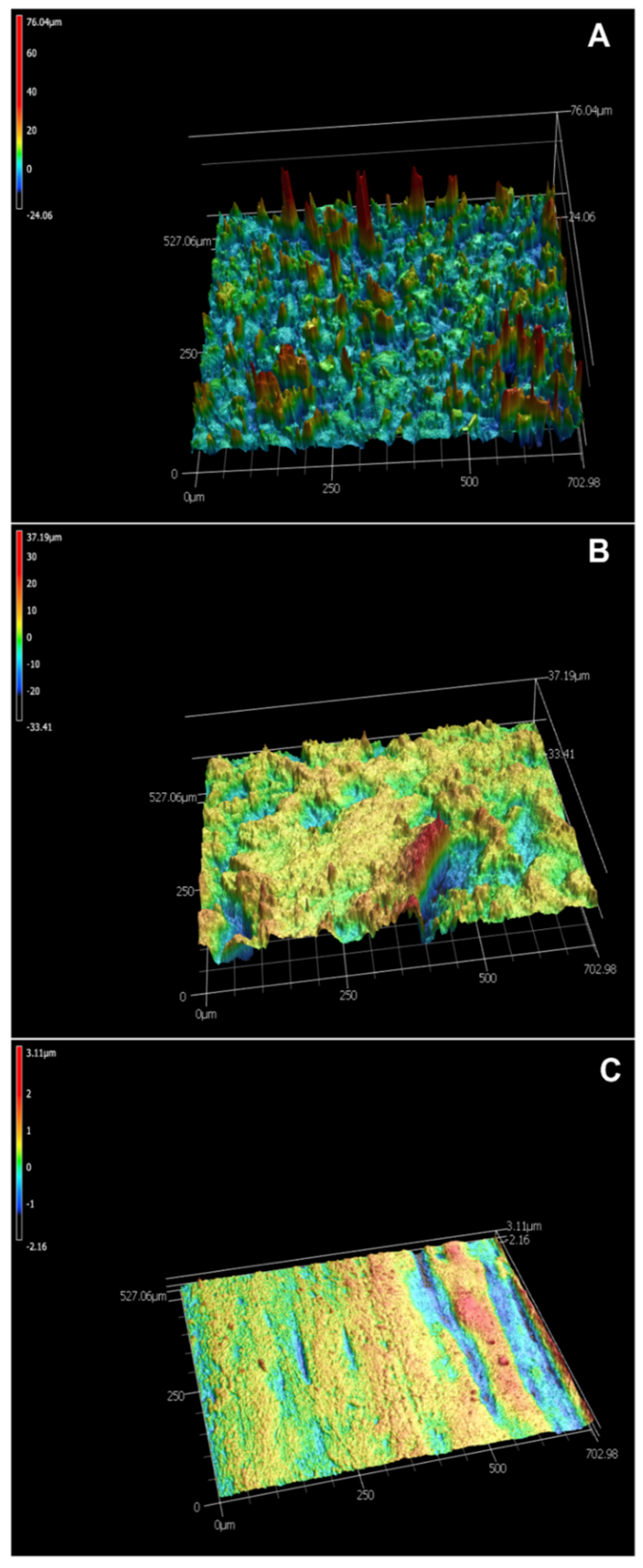
3.3. Characteristics of Biofilter Media
3.4. Cost of Media
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hall, S.G.; Campbell, M.; Geddie, A.; Thomas, M.; Paul, D.; Wilcox, D.; Smith, R.; Eddy, N.; Frinsko, M.; Wilder, S.; et al. Engineering challenges in marine aquaculture. In Proceedings of the 2018 ASABE Annual International Meeting, Detroit, MI, USA, 29 July–1 August 2018; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2018. [Google Scholar] [CrossRef]
- Paul, D. Biological and Adsorptive Removal of Nitrogenous Species from Aquaculture Wastewater. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 2021. Available online: https://www.lib.ncsu.edu/resolver/1840.20/39013 (accessed on 27 September 2021).
- FAO. The State of World Fisheries and Aquaculture 2016; Contributing to Food Security and Nutrition for all; FAO: Rome, Italy, 2016. [Google Scholar]
- Banerjee, A. Performance and flow analysis of an elliptic bladed Savonius-style wind turbine. J. Renew. Sustain. Energy 2019, 11, 033307. [Google Scholar] [CrossRef]
- Banerjee, A. High Temperature Heat Extraction from Heat Recirculating Porous Burner. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 2019. Available online: http://www.lib.ncsu.edu/resolver/1840.20/36624 (accessed on 27 September 2021).
- Banerjee, A.; Saveliev, A.V. Emission characteristics of heat recirculating porous burners with high temperature energy extraction. Front. Chem. 2020, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, A.; Saveliev, A.V. Effect of heat extraction on flame position in counterflow porous burner. In ASTFE Digital Library; Begell House Inc.: Danbury, CT, USA, 2019; pp. 113–122. [Google Scholar]
- Banerjee, A.; Saveliev, A.V. High temperature heat extraction from counterflow porous burner. Int. J. Heat Mass Transf. 2018, 127, 436–443. [Google Scholar] [CrossRef]
- Chen, S. Recirculating systems effluents, and treatment. In Aquaculture and the Environment in the United States; World Aquaculture Society: Sorrento, LA, USA, 2002; pp. 119–140. [Google Scholar]
- Paul, D.; Noori, M.T.; Rajesh, P.P.; Ghangrekar, M.M.; Mitra, A. Modification of carbon felt anode with graphene oxide-zeolite composite for enhancing the performance of microbial fuel cell. Sustain. Energy Technol. Assess. 2018, 26, 77–82. [Google Scholar] [CrossRef]
- Noori, M.T.; Paul, D.; Ghangrekar, M.M.; Mukherjee, C.K. Enhancing the performance of sediment microbial fuel cell using graphene oxide–zeolite modified anode and V2O5 catalyzed cathode. J. Clean Energy Technol. 2018, 6, 150–154. [Google Scholar] [CrossRef]
- Song, X.; Yang, X.; Hallerman, E.; Jiang, Y.; Huang, Z. Effects of hydraulic retention time and influent nitrate-n concentration on nitrogen removal and the microbial community of an aerobic denitrification reactor treating recirculating marine aquaculture system effluent. Water 2020, 12, 650. [Google Scholar] [CrossRef] [Green Version]
- Gong, W.; Gao, S.; Zhu, Y.; Wang, G.; Zhang, K.; Li, Z.; Yu, E.; Tian, J.; Xia, Y.; Xie, J.; et al. Effect of the aerobic denitrifying bacterium Pseudomonas furukawaii ZS1 on microbiota compositions in grass carp culture water. Water 2021, 13, 1329. [Google Scholar] [CrossRef]
- Xu, W.; Xu, Y.; Su, H.; Xu, H.; Yang, K.; Wen, G.; Cao, Y. Characteristics of ammonia removal and nitrifying microbial communities in a hybrid biofloc-ras for intensive Litopenaeus vannamei culture: A pilot-scale study. Water 2020, 12, 3000. [Google Scholar] [CrossRef]
- Cheng, X.; Zhu, D.; Wang, X.; Yu, D.; Xie, J. Effects of nonaerated circulation water velocity on nutrient release from aquaculture pond sediments. Water 2017, 9, 6. [Google Scholar] [CrossRef] [Green Version]
- You, G.; Xu, B.; Su, H.; Zhang, S.; Pan, J.; Hou, X.; Li, J.; Ding, R. Evaluation of aquaculture water quality based on improved fuzzy comprehensive evaluation method. Water 2021, 13, 1019. [Google Scholar] [CrossRef]
- Sang, C.G.; Fu, Y.W.; Guo, S.Q.; Luo, J.J.; Zhang, Q.Z. Isolation and characterization of an aerobic denitrifier Bacillus sp. SC16 from an intensive aquaculture pond. Water 2020, 12, 3559. [Google Scholar] [CrossRef]
- Gichana, Z.; Meulenbroek, P.; Ogello, E.; Drexler, S.; Zollitsch, W.; Liti, D.; Akoll, P.; Waidbacher, H. Growth and nutrient removal efficiency of sweet wormwood (Artemisia annua) in a recirculating aquaculture system for Nile tilapia (Oreochromis niloticus). Water 2019, 11, 923. [Google Scholar] [CrossRef] [Green Version]
- Gołaś, I.; Szmyt, M.; Potorski, J.; Łopata, M.; Gotkowska-Płachta, A.; Glinska-Lewczuk, K. Distribution of Pseudomonas fluorescens and Aeromonas hydrophila bacteria in a recirculating aquaculture system during farming of European grayling (Thymallus thymallus L.) broodstock. Water 2019, 11, 376. [Google Scholar] [CrossRef] [Green Version]
- Delwiche, C. Denitrification, nitrification and atmospheric nitrous oxide. In The Nitrogen Cycle and Nitrous Oxide; Wiley & Sons: Hoboken, NJ, USA, 1981. [Google Scholar]
- Soares MInês, M.; Abeliovich, A. Wheat straw as substrate for water denitrification. Water Res. 1998, 32, 3790–3794. [Google Scholar] [CrossRef]
- Hunho, K.; Seagren, E.A.; Davis, A.P. Engineered bioretention for removal of nitrate from stormwater runoff. Water Environ. Res. 2003, 75, 355–367. [Google Scholar] [CrossRef]
- Robertson, W.D.; Ford, G.I.; Lombardo, P.S. Wood–based filter for nitrate removal in septic systems. Trans. ASAE 2005, 48, 121–128. [Google Scholar] [CrossRef]
- Knapik, E.; Stopa, J. Fibrous deep-bed filtration for oil/water separation using sunflower pith as filter media. Ecol. Eng. 2018, 121, 44–52. [Google Scholar] [CrossRef]
- Saliling, W.J.B.; Westerman, P.W.; Losordo, T.M. Wood chips and wheat straw as alternative biofilter media for denitrification reactors treating aquaculture and other wastewaters with high nitrate concentrations. Aquac. Eng. 2007, 37, 222–233. [Google Scholar] [CrossRef]
- Bock, E.M.; Coleman, B.; Easton, Z.M. Effect of biochar on nitrate removal in a pilot-scale denitrifying bioreactor. J. Environ. Qual. 2016, 45, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Bock, E.; Smith, N.; Rogers, M.; Coleman, B.; Reiter, M.; Benham, B.; Easton, Z.M. Enhanced nitrate and phosphate removal in a denitrifying bioreactor with biochar. J. Environ. Qual. 2015, 44, 605–613. [Google Scholar] [CrossRef]
- Reddy, K.R.; Xie, T.; Dastgheibi, S. Evaluation of biochar as a potential filter media for the removal of mixed contaminants from urban storm water runoff. J. Environ. Eng. 2014, 140, 04014043. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Loyola-Licea, C.; Crowley, D.E.; Ahmad, Z. Performance of a two-phase biotrickling filter packed with biochar chips for treatment of wastewater containing high niutrogen and phosphorous concentrations. Process Saf. Environ. Prot. 2016, 102, 150–158. [Google Scholar] [CrossRef]
- Sidibe, M. Comparative Study of Bark, Bio-Char, Activated Charcoal Filters for Upgrading Greywater: From a Hygiene Aspect. Master’s Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2014. Available online: https://core.ac.uk/download/pdf/42951469.pdf (accessed on 27 September 2021).
- Foglar, L.; Gašparac, D. Continuous-flow biological denitrification with zeolite as support for bacterial growth. Desalin. Water Treat. 2013, 51, 37–39. [Google Scholar] [CrossRef]
- Chang, W.C.; Hong, S.W.; Park, J. Effect of zeolite media for the treatment of textile wastewater in biological aerated filter. Process Biochem. 2002, 37, 693–698. [Google Scholar] [CrossRef]
- Chung, Y.C.; Son, D.H.; Ahn, D.H. Nitrogen and organics removal from industrial wastewater using natural zeolite media. Water Sci. Technol. 2000, 42, 127–134. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, C.G.; Yoon, T.I.; Kim, D.W. Evaluation of increased denitrification in an anoxic activated sludge using zeolite. Korean J. Chem. Eng. 2003, 20, 492–495. [Google Scholar] [CrossRef]
- Banerjee, A.; Roy, S.; Mukherjee, P.; Saha, U.K. Unsteady flow analysis around an elliptic-bladed savonius-style wind turbine. In Proceedings of the Gas Turbine India Conference, New Delhi, India, 15–17 December 2014; American Society of Mechanical Engineers: New York, NY, USA, 2014; Volume 49644I, p. V001T05A001. [Google Scholar] [CrossRef]
- Banerjee, A.; Paul, D. Developments and applications of porous medium combustion: A recent review. Energy 2021, 119868. [Google Scholar] [CrossRef]
- Banerjee, A.; Kundu, P.; Gnatenko, V.; Zelepouga, S.; Wagner, J.; Chudnovsky, Y.; Saveliev, A. NOx minimization in staged combustion using rich premixed flame in porous media. Combust. Sci. Technol. 2019, 19. [Google Scholar] [CrossRef]
- Paul, D.; Kasera, N.; Kolar, P.; Hall, S.G. Physicochemical characterization data of pine-derived biochar and natural zeolite as precursors to catalysts. Chem. Data Collect. 2020, 30, 100573. [Google Scholar] [CrossRef]
- Kolar, P.; Jin, H. Baseline characterization data for raw rice husk. Data Brief 2019, 25, 104219. [Google Scholar] [CrossRef]
- Greensword, M.A. Rice Hull Bioreactor for Recirculating Aquaculture. Ph.D. Thesis, Lousiana State University, Baton Rouge, LA, USA, 2017. [Google Scholar]
- Volokita, M.; Belkin, S.; Abeliovich, A.; Soares, M.I.M. Biological denitrification of drinking water using newspaper. Water Res. 1996, 30, 965–971. [Google Scholar] [CrossRef]
- Mohseni-Bandpi, A.; Elliott, D.J.; Zazouli, M.A. Biological nitrate removal processes from drinking water supply-a review. J. Environ. Health Sci. Eng. 2013, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wei, W.; Xu, J.; Chen, X.; Liu, Y.; Peng, L.; Wang, D.; Ni, B.J. Denitrifying biofilm processes for wastewater treatment: Developments and perspectives. Environ. Sci. Water Res. Technol. 2021, 7, 40–67. [Google Scholar] [CrossRef]
- Chen, R.; Deng, M.; He, X.; Hou, J. Enhancing nitrate removal from freshwater pond by regulating carbon/nitrogen ratio. Front. Microbiol. 2017, 8, 1712. [Google Scholar] [CrossRef] [Green Version]
- Kamp, A.; Høgslund, S.; Risgaard-Petersen, N.; Stief, P. Nitrate storage and dissimilatory nitrate reduction by eukaryotic microbes. Front. Microbiol. 2015, 6, 1492. [Google Scholar] [CrossRef] [Green Version]
- Jones, Z.L.; Jasper, J.T.; Sedlak, D.L.; Sharp, J.O. Sulfide-induced dissimilatory nitrate reduction to ammonium supports anaerobic ammonium oxidation (anammox) in an open-water unit process wetland. Appl. Environ. Microbiol. 2017, 83, e00782-17. [Google Scholar] [CrossRef] [Green Version]
- Carlson, H.K.; Lui, L.M.; Price, M.N.; Kazakov, A.E.; Carr, A.V.; Kuehl, J.V.; Owens, T.K.; Nielsen, T.; Arkin, A.P.; Deutschbauer, A.M. Selective carbon sources influence the end products of microbial nitrate respiration. ISME J. 2020, 14, 2034–2045. [Google Scholar] [CrossRef]
- Li, B.; Irvin, S. The comparison of alkalinity and ORP as indicators for nitrification and denitrification in a sequencing batch reactor (SBR). Biochem. Eng. J. 2007, 34, 248–255. [Google Scholar] [CrossRef]
- Lepine, C.; Christianson, L.; Sharrer, K.; Summerfelt, S. Optimizing hydraulic retention times in denitrifying woodchip bioreactors treating recirculating aquaculture system wastewater. J. Environ. Qual. 2016, 45, 813–821. [Google Scholar] [CrossRef]
- Peng, Y.; Hou, H.; Wang, S.; Cui, Y.; Yuan, Z. Nitrogen and phosphorus removal in pilot-scale anaerobic-anoxic oxidation ditch system. J. Environ. Sci. 2008, 20, 398–403. [Google Scholar] [CrossRef]
- Zhang, T.C.; Bishop, P.L. Density, porosity, and pore structure of biofilms. Water Res. 1994, 28, 2267–2277. [Google Scholar] [CrossRef]
- Lazarova, V.; Manem, J. Biofilm characterization and activity analysis in water and wastewater treatment. Water Res. 1995, 25, 2227–2245. [Google Scholar] [CrossRef]
- Arnáiz Franco, C.; Gutierrez, J.C.; Lebrato Martínez, J. Support material selection for anaerobic fluidized bed reactors by phospholipid analysis. Biochem. Eng. J. 2006, 27, 240–245. [Google Scholar] [CrossRef] [Green Version]
- Pentair. Sweetwater Bio-Media. Available online: https://pentairaes.com/sweetwater-swx-bio-media.html (accessed on 9 August 2021).


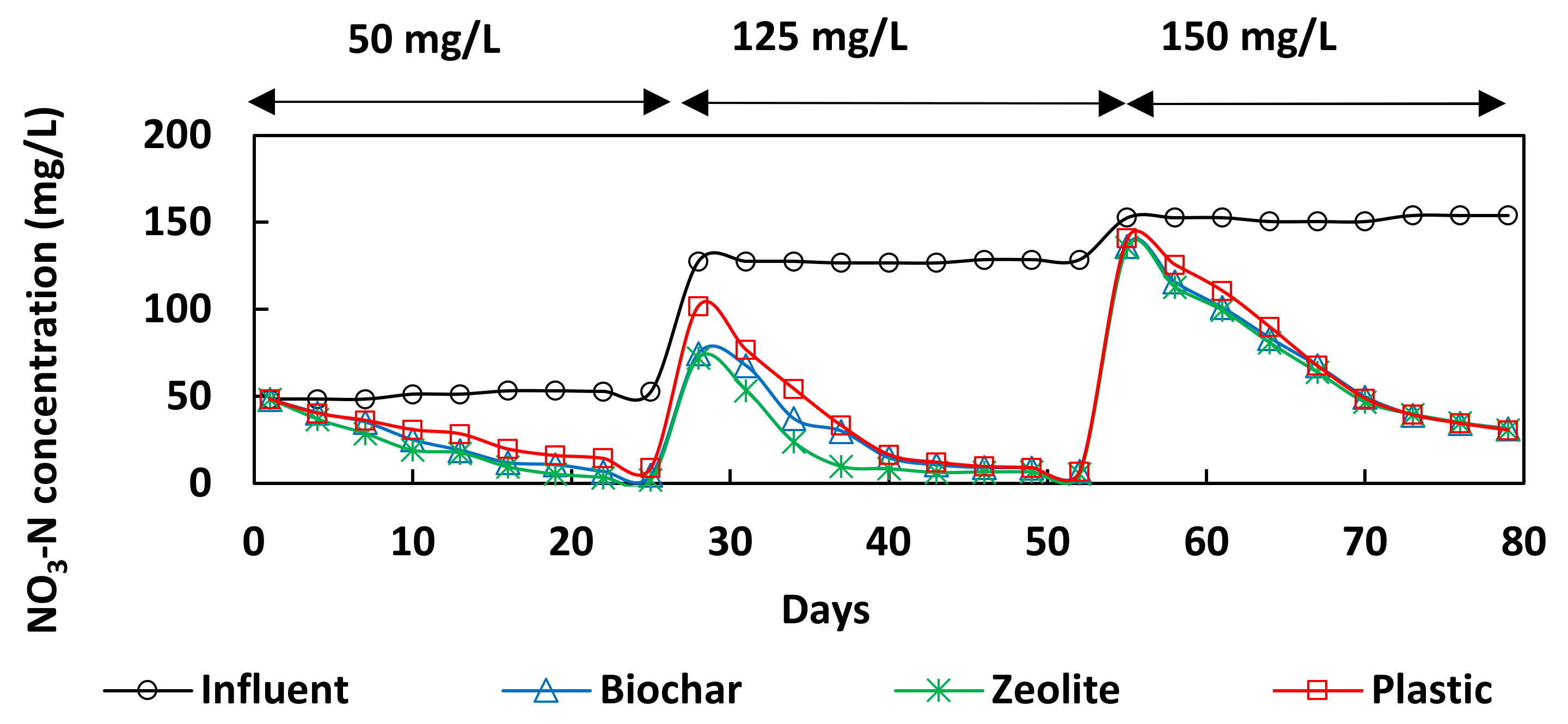

| Influent NO3-N Concentration (mg/L) | Media | Effluent NO3-N Concentration (mg/L) | % NO3-N Removal | DNR (g-N/m3/d) |
|---|---|---|---|---|
| 51.21 ± 2.11 | Biochar | 8.72 ± 3.37 | 83.59 ± 0.06 | 638.98 ± 45.29 |
| Zeolite | 5.24 ± 3.16 | 90.15 ± 0.06 | 689.11 ± 42.75 | |
| Plastic | 14.97 ± 4.43 | 71.82 ± 0.08 | 548.98 ± 61.17 | |
| 127.64 ± 0.78 | Biochar | 9.07 ± 1.59 | 92.91 ± 0.01 | 1714.20 ± 33.84 |
| Zeolite | 6.37 ± 0.62 | 95.02 ± 0.01 | 1753.00 ± 16.59 | |
| Plastic | 9.52 ± 2.24 | 92.57 ± 0.02 | 1707.85 ± 43.35 | |
| 152.47 ± 1.56 | Biochar | 35.31 ± 3.96 | 77.09 ± 0.03 | 1710.80 ± 56.98 |
| Zeolite | 35.31 ± 4.38 | 77.09 ± 0.03 | 1710.70 ± 63.04 | |
| Plastic | 35.89 ± 5.00 | 77.28 ± 0.03 | 1714.98 ± 64.07 |
| Media | DNR (g-N/m3/d) | References |
|---|---|---|
| Biochar | 1714.20 | This study |
| Zeolite | 1753.00 | This study |
| Plastic | 1714.98 | This study |
| Rice hull | 1025.00 | [40] |
| Wheat straw | 1360 | [25] |
| Wood chips | 1360 | [25] |
| Kaldness media | 1330 | [25] |
| Wheat straw | 53.00 | [21] |
| Newspaper | 22.58 | [41] |
| Parameter | Effluent Concentration | ||
|---|---|---|---|
| pH | 7.4 to 8.9 | ||
| DO | <2 mg/L | ||
| NH3-N | 0.02 ± 0.03 mg/L to 0.05 ± 0.07 mg/L | ||
| NO2-N | 0.62 ± 0.31 mg/L to 1.02 ± 0.02 mg/L | ||
| NO3-N levels | |||
| 50 mg/L | 125 mg/L | 150 mg/L | |
| Residual COD | 34.02 ± 0.5 mg/L to 35.08 ± 0.68 mg/L | 40.36 ± 0.82 mg/L to 41.92 ± 0.26 mg/L | 49.16 ± 0.85 mg/L to 50.22 ± 1.13 mg/L |
| Alkalinity as CaCO3 | 186.99 ± 2.21 mg/L | 441.63 ± 4.14 mg/L | 514.69 ± 4.89 mg/L |
| Values of Surface Roughness | Biochar (µm) | Zeolite (µm) | Plastic (µm) |
|---|---|---|---|
| Average Sa | 0.36 | 0.89 | 0.13 |
| Average Sz | 2.22 | 6.52 | 0.67 |
| Average Sq | 0.44 | 1.17 | 0.16 |
| Media | Cost/m3 | Total Cost/m3 for a Period of 10 Years |
|---|---|---|
| Plastic | $1450 | $1450 (no reloading) |
| Biochar | $72.5 | $725 (reloaded once a year) |
| Zeolite | $300 to $600 | $700 to $1200 (reloaded every 5 years) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul, D.; Hall, S.G. Biochar and Zeolite as Alternative Biofilter Media for Denitrification of Aquaculture Effluents. Water 2021, 13, 2703. https://doi.org/10.3390/w13192703
Paul D, Hall SG. Biochar and Zeolite as Alternative Biofilter Media for Denitrification of Aquaculture Effluents. Water. 2021; 13(19):2703. https://doi.org/10.3390/w13192703
Chicago/Turabian StylePaul, Diplina, and Steven G. Hall. 2021. "Biochar and Zeolite as Alternative Biofilter Media for Denitrification of Aquaculture Effluents" Water 13, no. 19: 2703. https://doi.org/10.3390/w13192703
APA StylePaul, D., & Hall, S. G. (2021). Biochar and Zeolite as Alternative Biofilter Media for Denitrification of Aquaculture Effluents. Water, 13(19), 2703. https://doi.org/10.3390/w13192703






