Abstract
Copper-nickel bimetallic nanoparticles (Cu-Ni BNPs) were fabricated using an eco-friendly green method of synthesis. An extract of synthesized Gazania rigens was used for the synthesis of BNPs followed by characterization employing different techniques including UV/Vis spectrophotometer, FTIR, XRD, and SEM. Spectrophotometric studies (UV-Vis and FTIR) confirmed the formation of bimetallic nanoparticles. The SEM studies indicated that the particle size ranged from 50 to 100 nm. Analysis of the BNPs by the XRD technique confirmed the presence of both Cu and Ni crystal structure. The synthesized nanoparticles were then tested for their catalytic potential for photoreduction of methylene blue dye in an aqueous medium and DPPH radical scavenging in a methanol medium. The BNPs were found to be efficient in the reduction of methylene blue dye as well as the scavenging of DPPH free radicals such that the MB dye was completely degraded in just 17 min at the maximum absorption of 660 nm. Therefore, it is concluded that Cu-Ni BNPs can be successfully synthesized using Gazania rigens extract with suitable size and potent catalytic and radical scavenging activities.
1. Introduction
Nanotechnology has introduced many advantages to the fields of physics [1], chemistry [2,3,4], and the medical sciences [5]. In the late 20th century, the synthesis, application, and commercialization of nanomaterials in different fields have been investigated by many researchers and scientists [6]. Metals have been extensively used over the decades in various fields of chemical science [7,8], biomedical science [9], engineering [10], photonics [11], and electronics [12]. The extensive use of metal-based products in routine life has compelled scientists to find new ways of synthesizing different products. Rather than producing products incorporating metals as macroparticles, nowadays, micro- and nano-particle-based metal oxides such as CuO and CdO are being produced worldwide [13,14,15,16]. Bimetallic nanoparticles have attracted much attention due to their fascinating properties, as the combination of two metals not only creates synergistic effects but also increases their spectrum of application, such as with respect to their catalytic and antioxidant potential [17,18,19]. Bimetallic nanoparticle synthesis has played a vibrant role in the development of new materials, especially the composites of different metals. A variety of different bimetallic materials have been reported in which composites of gold, palladium, platinum, and silver [20] have been synthesized, mostly due to their noble surface plasmon resonance properties. Other metals used in bimetallic composites include the metals Fe, Cu, Co, Mo, Sn, Ni, and Zn [21,22,23].
During recent decades, water contamination has been a major problem in many developing countries around the world [24,25]. We can protect water by its treatment with many different techniques—and nanotechnology is one of them. Recently, different scientists have been searching for solutions to this world-wide problem and have highlighted the importance of nanocatalysts in the field of water treatment, especially for the removal of organic pollutants from water [26]. To address this, a number of different types of novel photocatalytic materials have been proposed for the photocatalytic reduction of organic pollutants and dyes. Cu-Ni BNPs and several novel BNPs produced by the hydrothermal method have been reported for methylene blue reduction by the photocatalytic process [27]. Copper composites with Ni, Fe, Mn, and Co have been developed for the reduction of organic pollutants and dyes [28,29].
Reactive oxygen species (ROS) and free radicals are produced in the human body as a result of many metabolic processes [7]. Free radicals and ROS are now well known to instigate various chronic and degenerative diseases [9] by causing oxidative damage to cellular molecules [10]. The excessive generation of ROS in the human body may result in oxidative stress. To overcome this state of stress and to counter the effects of damaging radicals and reactive oxygen species, antioxidant supplements from external sources are required [11,30,31,32]. Scientists and researchers are now searching for novel molecules that have antioxidant properties, employing DPPH for scavenging studies [33]. Hence, the current study focuses on the green synthesis of antioxidant-functionalized metal nanoparticles.
Metal nanoparticles belonging to d-block elements in the periodic table include Ag, Au, Pt, and Pd; these are considered noble nanoparticles due to their fascinating properties, but have low cost-effectiveness. In contrast, Cu, Co, Ni, Fe, and Mo metal nanoparticles are cheap, while exhibiting comparable properties to those of noble nanoparticles. Therefore, the current study was designed to synthesize Cu-Ni BNPs using an ecofriendly method of synthesis. In addition, the reduction of methylene blue and scavenging of DPPH radicals using Cu-Ni BNPs have been achieved successfully. Informed by our review of the literature, the research project investigated the application of novel bimetallic nanoparticles for the photocatalysis of organic dye (i.e., methylene blue) reduction.
2. Materials and Methods
2.1. Chemicals and Reagents
Methylene blue (99.99%) was purchased from Fisher Scientific UK. Sodium borohydride (99.9%), DPPH (99.95%), NiSO4 (99.98%), and CuSO4 (99.99%) were acquired from Sigma Aldrich Germany. Methanol, used as a solvent, was purchased from Sigma Aldrich Germany
2.2. Preparation of Plant Extract
Gazania rigens plants were collected from different locations in Lahore, Punjab, Pakistan. After washing, plants were dried under shade and then their constant weight was attained by placing the plants in a hot air oven for 3 h at 60 °C. The plants were then cut and ground to powder. G. rigens extract was prepared in methanol using an orbital shaker for 3 h at 150 rpm. The filtrate obtained was dried using a rotary evaporator and stored at −4 °C prior to further use [6].
2.3. Synthesis of Cu-Ni Nanoparticles
A salt solution was prepared by combining NiSO4 and CuSO4 in 25 mL of solvent. The extract was dissolved in methanol to prepare a 1000 ppm solution. Both solutions were then mixed at varying concentrations to determine the optimal salt solution concentration to synthesize bimetallic nanoparticles; the optimal concentration was found to be 200 ppm on the basis of spectrophotometric analysis. The nanoparticles were synthesized by mixing the selected concentrations of the salt and extract solutions. This mixture was then centrifuged and dried [34].
2.4. Instrumentation
The photocatalytic potential of the synthesized nanoparticles was measured by recording the lambda max using a CECIL-7400ce UV-VIS-spectrophotometer under sunlight and by inspecting the FTIR spectra produced by an FTIR spectrophotometer. The X-ray diffraction (XRD) studies were carried out at a scanning rate of 0.05 min−1 using a Bruker D8 Advanced diffractometer, equipped with a scintillation counter using Cu Kα radiation (k = 1.5405 Å, nickel filter) at an acceleration voltage of 30 KV. A NOVA SEM 450 was utilized to obtain SEM images of synthesized BNPs with nanographs obtained for 3 different ranges [35].
2.5. Photocatalytic Reduction of Methylene Blue
The catalytic activity for Cu-Ni BNPs was evaluated following an already published method [36,37]. Solutions of methylene blue (0.086 mM), NaBH4 (26 mM), and Cu-Ni BNPs at a concentration of 100 ppm were prepared. Using a UV-VIS-spectrophotometer cuvette, 3 mL of methylene blue (acting as a substrate), and 0.4 mL of 26 mM sodium borohydride (acting as a reducing agent), were added. To this solution, containing the substrate and the reducing agent, 0.5 mL Cu-Ni BNP’s solution was added, which acted as a catalyst. All the observations were recorded at 665 nm, the maximum absorbance (λmax) for methylene blue [20].
2.6. Radical Scavenging Potential
The antioxidant capacity of the BNPs was studied in terms of their free radical scavenging potential using a 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay [38,39]. Ten milliliters of DPPH (200, 400, and 600 ppm) were taken in three separate flasks and 90 mL of distilled water was added. The mixture was thoroughly mixed and kept in the dark for 30 min. The absorbance was measured later, at 515 nm, against a blank of methanol without DPPH. The results were calculated as the percentage inhibition of the DPPH according to the following equation: % inhibition of DPPH = [(Abs control-Abs sample)/Abs control] × 100, where Abs control is the absorbance of DPPH solution without extracts [40].
3. Results and Discussion
3.1. UV-Visible Analysis of BNPs
Preliminary analysis of the Cu-Ni BNPs was carried out using a UV-visible spectrophotometer to confirm the formation of nanoparticles by the G. rigens extract (Figure 1). According to the literature, the absorbance range for Ni nanoparticles is 280–320 nm [41,42]. Therefore, spectra having maximum absorption peaks at 293.5 nm indicated the formation of Cu-Ni BNPs using the G. rigens extract.
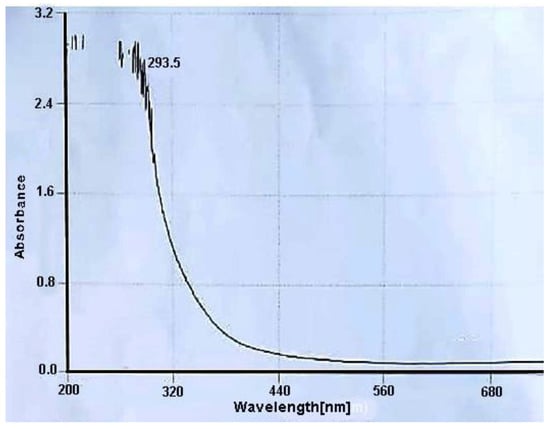
Figure 1.
The UV-Visible spectrum of Cu-Ni BNPs in the absence of dye.
3.2. FTIR Analysis of Cu-Ni BNPs
The FTIR analysis (Figure 2) of Cu-Ni BNPs was carried out to evaluate the structural bond formations in the BNPs. The spectra were recorded using a Nicolet 5PC, Nicolet Analytical Instrument (Protea, Cambridgeshire, UK) that works in the frequency range of 4000–400 cm−1. The transmittance at 3095.27 cm−1 appeared to be due to the presence of the hydroxyl group and C-H group stretching present in beta-amyrin and cholesterol in the gazania extract [43]; the value at 1662.63 cm−1 is due to N-H bending by alkaloids containing amino groups also present in the gazania extract. Additionally, carboxylic acid (-COOH), existing on the boundaries of Cu-Ni BNPs, displays a peak at 859.15 cm−1; the peak at 1054.51 cm−1 indicates the C–O–C attached to BNPs [39], while the peak appearing at 602.95 cm−1 is possibly associated with metal–carbon linkage [44].
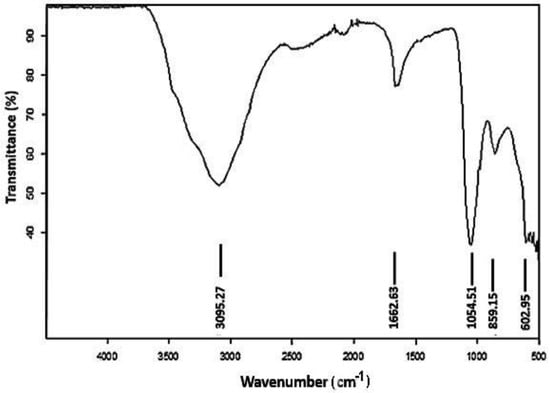
Figure 2.
The FTIR spectrum of copper-nickel bimetallic nanoparticles.
3.3. X-ray Diffraction Analysis
The X-ray diffraction patterns of Cu-Ni, as synthesized nanoparticles, were recorded in 2θ values ranging between 20° and 80° at a scan rate of 0.4° per minute via continuous scan type. According to Figure 3, the sharpness of peaks corresponds to the phase purity and crystal structure for the particular catalytic sample. The peaks appearing at 29° (110), 43° (200), 46° (100), 52° (210), 69° (111), and 74.4° (211) correspond to copper [4,45]. The peaks observed at 52° (200), 46° (100), 52° (100), 57° (210), and 61° (210) correspond to face-centered cubic Ni nanoparticles [41,46,47]. Mixing of Cu and Ni nanoparticles in a single compound displayed a single-phase system but both Cu and Ni maintained their crystallinity. However, appearance of new major peaks at 33° and 62° at 200 and 210 planes illustrates the influence of both Cu and Ni elemental composition in the crystal lattice.
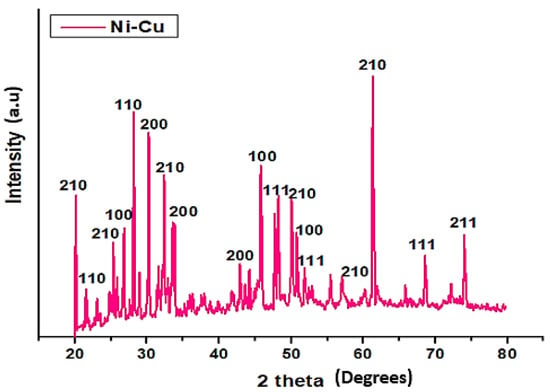
Figure 3.
The XRD diffraction pattern of Cu-Ni BNPs.
Additionally, the crystallite size “D” of the as-synthesized nanoparticles was estimated from XRD analysis by examining major peaks using the Debye–Scherrer equation given in Equation (1).
where k represents the constant shape factor equal to 0.9, λ is the X-ray wavelength, β indicates the full width half maximum (FWHM), and θ represents the Bragg’s angle. The crystallite size was found to be 47 nm.
3.4. Morphological Analysis
The SEM images for Cu-Ni BNPs presented in Figure 4a–c were obtained in three different magnifications (30 nm, 50 nm, and 100 nm). These images provide data regarding the morphology of the nanoparticles. The images appear as a heterogeneous mixture. The particle sizes vary due to the clumping of nanoparticles. The surface of the particles has not remained smooth, which may be due to their interaction with the extract [48]. However, according to the SEM results, the size of nanoparticles predicted ranged from 50 nm to 100 nm.

Figure 4.
SEM nanographs of Cu-Ni BNPs with scale bars of (a) 30 nm, (b) 50 nm, and (c) 100 nm.
3.5. Photocatalytic Reduction of Methylene Blue in Aqueous Medium
Contamination of water by organic pollutants results in adverse effects not only for aquatic life but also for humans. Organic pollutants include pharmaceuticals, detergents, pesticides, industrial waste, and dyes [26]. Dyes may cause carcinogenic effects having benzene rings in their structure. Therefore, their removal from the aqueous medium is essential, and photocatalytic reduction is widely performed to achieve this. In the reduction of methylene blue (MB) dye, the reducing agent NaBH4 has a primary role, but it requires the support of a catalyst to complete the reduction [49,50].
In the current study, MB reduction was observed in the presence of NaBH4 without a catalyst and showed no reduction (Figure 5). This may be due to no transference occurring of electron pairs from BH4− to the azo group of the MB. Correspondingly, as illustrated in Figure 5b, there is also no reduction in the absence of a reducing agent, because no electron pair is present. Both of these reactions were monitored under controlled conditions. These exemplary reactions suggest that the reduction may only be possible in the presence of a catalyst.
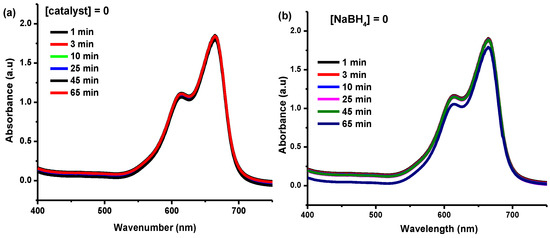
Figure 5.
(a) 0.086 mM MB, 13 mM NaBH4, and no Cu-Ni bimetallic nanocatalyst; (b) 0.086 mM MB, 100 ppm Cu-Ni bimetallic nanocatalyst, and 0 mM NaBH4.
Nanoparticles act as catalysts due to their high surface-to-volume ratio and smaller sizes are helpful in the reduction of dyes in water [51]. The reduction of MB was achieved using Cu-Ni BNPs in the presence of NaBH4. The reduction reaction was completed in 17 min. No reduction phenomenon was observed in the first 5 min while the catalyst stabilized/adjusted with the dye molecules until the generation of reactive oxidation species (ROS) involved in the photocatalytic reduction (Figure 6), which reflects the excellent capability of the Cu-Ni nanocatalyst [52]. This is referred to as the induction period where the reduction phenomenon is slow, accelerating afterwards [53,54,55]. Here, the catalyst acts as a carrier which transfers an electron pair from borohydride to the substrate and thus enables the leuco form of methylene blue to be formed. The leucomethylene blue is not harmful.
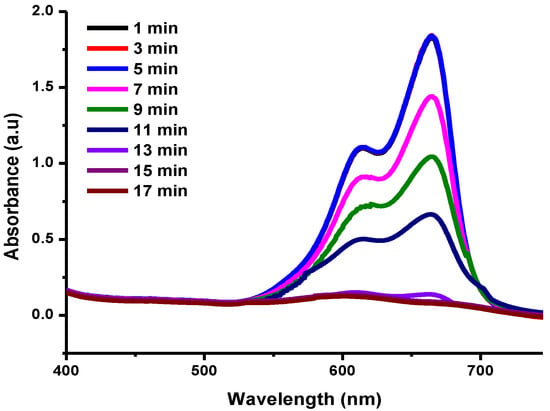
Figure 6.
UV-Vis spectra for the MB photocatalytic reduction using 100 ppm Cu-Ni bimetallic photocatalyst, 13 mM NaBH4, and 0.086 mM MB.
A kinetic study was also performed for the model-controlled reaction of MB reduction on the surface of Cu-Ni BNPs. As the reaction proceeds, absorbance at 665 nm decreases due to the charge transfer from the reductant to the reactant (MB). The reaction follows the Langmuir–Hinshelwood mechanism having pseudo first order reaction kinetics because concentration of sodium borohydride was 150 times greater than that of the methylene blue, [NaBH4]>>>[MB]. The graph presented in Figure 6 provides information about the reaction from the first stage (adsorption of the reactant to the nanocatalyst), the intermediate stage (interaction between the reactant and the reductant, i.e., the reaction time) to the end stage (reaction completion and desorption of the reactant). The slope gives the value of the experimental rate constant (kexp) 0.2505 min−1 with a half-life of 2.7664 min (Figure 7).
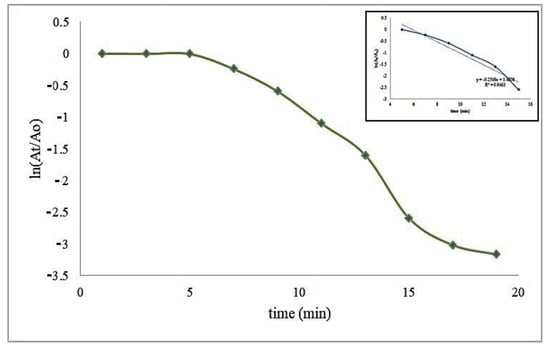
Figure 7.
A kinetic model of the photocatalytic reduction at fixed concentrations with concentrations of MB = 0.086 mM, NaBH4 = 13 mM, and 100 ppm of Cu-Ni bimetallic nanocatalyst.
3.6. Radical Scavenging Potential
Free radicals are unstable and tend to form stable bonds by accepting or donating available electrons. Nanoparticles, being zero valent and capable of donating electrons to free radicals, inhibit the initiation step of oxidative chain reactions and the formation of stable free radicals. The DPPH is a stable, nitrogen-centered free radical which has a very strong absorption band at 517 nm. The antioxidant activity of any species can be determined using spectrophotometry to assess the change in color of DPPH from violet to yellow. The change in color is attributed to the reduction of DPPH either by hydrogen or electron donation [56,57]. The DPPH radical inhibition potential of varying BNP concentration is presented in Figure 8a–c. The radical scavenging potential of BNPs is reflected in increased reaction time, as evidenced in the figures, with maximum scavenging observed after 30 min of reaction. Radical scavenging can be enhanced by increasing the concentration of BNPs [58].
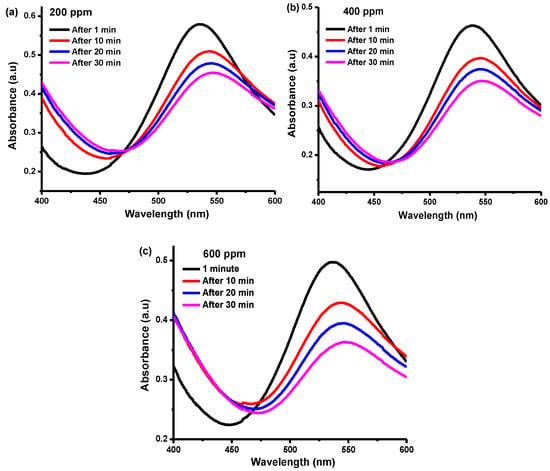
Figure 8.
Antioxidant activity of Cu-Ni BNPs at (a) 200, (b) 400, and (c) 600 ppm of DPPH.
Moreover, the position for the UV-Vis absorption maximum varies with different factors such as the nature and concentration of the solvent/s, the nature of the radical, the intensity of light, etc. [59,60]. Consequently, the position for the absorption maximum moved from 540 to 550 nm with an increase in the DPPH concentration from 200 to 600 ppm. Incident light was adsorbed for one minute before undergoing the absorption process, which produced the absorption signal at a lower wavelength, which might be attributed to a less stable form of DPPH radical [61,62]. Once the DPPH is stabilized by the antioxidant, it absorbs at a longer wavelength; i.e., a red-shift occurs [63].
The reaction between DPPH and Cu-Ni BNPs (antioxidant) (Figure 9) follows second order kinetics, as the concentrations of both the reactants are in the same proportions. All the reactions were noted at around 550 nm after an interval of 10 minutes. The concentration of DPPH used ranged from 200 to 600 ppm. In Figure 9, ln [DPPH]t/[DPPH]o is presented as a function of time that provides a value of the experimental rate constant and the half-life, as indicated in Table 1. The comparison of different reported photocatalysts using our synthesized nanoparticles for dye reduction has been represented in Table 2.
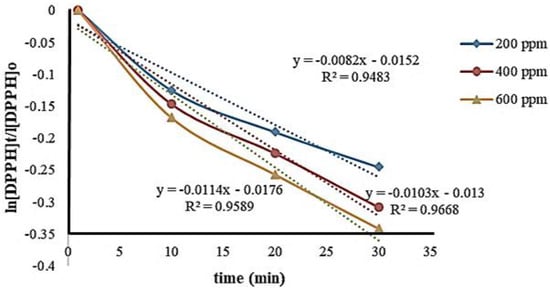
Figure 9.
Correlation between concentration and time at different ppm values.

Table 1.
Estimation of kinetic parameters of BNPs radical scavenging potential.

Table 2.
Comparison of various photocatalysts for dye reduction.
4. Conclusions
Nickel–copper bimetallic nanoparticles, prepared via a green synthetic route using Gazania rigens extract, were found to be effective in biological and photocatalytic activities. Sharp peaks in XRD pattern indicated the crystalline morphology of the Cu-Ni BNPs. The percentage transmittance in the FTIR analysis indicated the presence of carbonyl groups along with metal bond vibrations. SEM micrographs confirmed the formation of a heterogeneous surface that facilitated the catalytic process. In addition, the particle size estimated from the SEM images was found to be in the range of 50 to 100 nm. The photocatalytic potential of Cu-Ni BNPs were investigated by studying the photocatalytic reduction of methylene blue under visible light irradiation. The BNPs were found to be highly efficient photocatalysts degrading the MB in only 19 min with a rate constant of 0.2505 min−1. This implies that they are superior to several chemical nanoparticles, as well as having a greener process of synthesis. In addition, the synthesized nanoparticles were investigated for their scavenging potential for DPPH radicals and were found to be applicable to the radical scavenging purpose. Therefore, the Cu-Ni BNPs synthesized from greener routes have been found to exhibit excellent photocatalytic potential. Such BNPs, being more cost effective, simple, and ecofriendly, could be employed as photocatalysts for the reduction of several organic dyes in industrial wastewater bodies.
Author Contributions
The research scheme was designed by U.Y. and F.A. The synthesis and photocatalytic part was performed by A.G. and Z.A. Z.S. and S.K. performed the characterization analysis. S.K. and F.A. wrote the manuscript. M.P. edited the manuscript. Data Analysis done by A.A.A., and Conceptualization by M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Researchers Supporting Project Number (RSP-2021/243), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare that there is no conflict of interest related to this research article.
References
- Yan, Y.; Gong, J.; Chen, J.; Zeng, Z.; Huang, W.; Pu, K.; Liu, J.; Chen, P. Recent advances on graphene quantum dots: From chemistry and physics to applications. Adv. Mater. 2019, 31, 1808283. [Google Scholar] [CrossRef]
- Whitesides, G.M. Nanoscience, nanotechnology, and chemistry. Small 2005, 1, 172–179. [Google Scholar] [CrossRef]
- Zuorro, A.; Iannone, A.; Lavecchia, R. Water–organic solvent extraction of phenolic antioxidants from brewers’ spent grain. Processes 2019, 7, 126. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Shah, S.S.; Anjum, M.A.R.; Khan, M.R.; Janjua, N.K. Electro-oxidation of ammonia over copper oxide impregnated γ-Al2O3 nanocatalysts. Coatings 2021, 11, 313. [Google Scholar] [CrossRef]
- Rosenthal, S.J. Nanotechnology in neuroscience reveals membrane mobility matters. ACS Chem. Neurosci. 2018, 10, 30–32. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Votruba, A.R.; Farokhzad, O.C.; Langer, R. Nanotechnology in drug delivery and tissue engineering: From discovery to applications. Nano Lett. 2010, 10, 3223–3230. [Google Scholar] [CrossRef] [Green Version]
- Jaffee, R.I.; Promisel, N.E. (Eds.) The Science, Technology and Application of Titanium, Proceedings of the International Conference Organized by the Institute of Metals, the Metallurgical Society of Aime, and the American Society for Metals in Association with the Japan Institute of Metals and the Academy of Sciences, USSR, and Held at the Royal Festival Hall, Lodon, UK, 21–24, May 1968; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Khan, M.; Janjua, N.K.; Khan, S.; Qazi, I.; Ali, S.; Algarni, T.S. Electro-oxidation of ammonia at novel Ag2O− PrO2/γ-Al2O3 catalysts. Coatings 2021, 11, 257. [Google Scholar] [CrossRef]
- Hermawan, H. Updates on the research and development of absorbable metals for biomedical applications. Prog. Biomater. 2018, 7, 93–110. [Google Scholar] [CrossRef] [Green Version]
- Madej, L. Digital/virtual microstructures in application to metals engineering–A review. Arch. Civ. Mech. Eng. 2017, 17, 839–854. [Google Scholar] [CrossRef]
- Kuznetsova, R.T.; Aksenova, I.V.; Bashkirtsev, D.E.; Prokopenko, A.A.; Pomogaev, V.A.; Antina, E.V.; Berezin, M.B.; Bumagina, N.A. Photonics of coordination complexes of dipyrrins with p-and d-block elements for application in optical devices. J. Photochem. Photobiol. A Chem. 2018, 354, 147–154. [Google Scholar] [CrossRef]
- Raut, N.C.; Al-Shamery, K. Inkjet printing metals on flexible materials for plastic and paper electronics. J. Mater. Chem. C 2018, 6, 1618–1641. [Google Scholar] [CrossRef]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Kinetic modeling of azo dye adsorption on non-living cells of Nannochloropsis oceanica. J. Environ. Chem. Eng. 2017, 5, 4121–4127. [Google Scholar] [CrossRef]
- Zuorro, A.; Iannone, A.; Natali, S.; Lavecchia, R. Green synthesis of silver nanoparticles using bilberry and red currant waste extracts. Processes 2019, 7, 193. [Google Scholar] [CrossRef] [Green Version]
- Kannan, K.; Radhika, D.; Nesaraj, A.; Sadasivuni, K.K.; Reddy, K.R.; Kasai, D.; Raghu, A.V. Photocatalytic, antibacterial and electrochemical properties of novel rare earth metal oxides-based nanohybrids. Mater. Sci. Energy Technol. 2020, 3, 853–861. [Google Scholar] [CrossRef]
- Kannan, K.; Radhika, D.; Nikolova, M.P.; Andal, V.; Sadasivuni, K.K.; Krishna, L.S. Facile microwave-assisted synthesis of metal oxide CdO-CuO nanocomposite: Photocatalytic and antimicrobial enhancing properties. Optik 2020, 218, 165112. [Google Scholar] [CrossRef]
- Shen, L.; Zhou, X.; Zhang, C.; Yin, H.; Wang, A.; Wang, C. Functional characterization of bimetallic CuPdx nanoparticles in hydrothermal conversion of glycerol to lactic acid. J. Food Biochem. 2019, 43, e12931. [Google Scholar] [CrossRef]
- Panusa, A.; Petrucci, R.; Lavecchia, R.; Zuorro, A. UHPLC-PDA-ESI-TOF/MS metabolic profiling and antioxidant capacity of arabica and robusta coffee silverskin: Antioxidants vs phytotoxins. Food Res. Int. 2017, 99, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Kharisov, B.I.; Kharissova, O.V.; Yacaman, M.J. State of the art of the bi-and trimetallic nanoparticles on the basis of gold and iron. Recent Pat. Nanotechnol. 2009, 3, 81–98. [Google Scholar] [CrossRef]
- Loza, K.; Heggen, M.; Epple, M. Synthesis, structure, properties, and applications of bimetallic nanoparticles of noble metals. Adv. Funct. Mater. 2020, 30, 1909260. [Google Scholar] [CrossRef] [Green Version]
- He, P.; Wang, X.; Liu, Y.; Liu, X.; Yi, L. Comparison of electrocatalytic activity of carbon-supported Au–M (M = Fe, Co, Ni, Cu and Zn) bimetallic nanoparticles for direct borohydride fuel cells. Int. J. Hydrogen Energy 2012, 37, 11984–11993. [Google Scholar] [CrossRef]
- Ammam, M.; Easton, E.B. Oxygen reduction activity of binary PtMn/C, ternary PtMnX/C (X = Fe, Co, Ni, Cu, Mo and, Sn) and quaternary PtMnCuX/C (X = Fe, Co, Ni, and Sn) and PtMnMoX/C (X = Fe, Co, Ni, Cu and Sn) alloy catalysts. J. Power Sources 2013, 236, 311–320. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, D.; Kumar, A.; Ala’a, H.; Pathania, D.; Naushad, M.; Mola, G.T. Revolution from monometallic to trimetallic nanoparticle composites, various synthesis methods and their applications: A review. Mater. Sci. Eng. C 2017, 71, 1216–1230. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xiang, Y.; Zhou, Y.; Yang, Y.; Zhang, J.; Huang, H.; Shang, C.; Luo, L.; Gao, J.; Tang, L. Selenium contamination, consequences and remediation techniques in water and soils: A review. Environ. Res. 2018, 164, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Kumari, B.; Sinam, G.; Kumar, N.; Mallick, S. Fluoride distribution and contamination in the water, soil and plants continuum and its remedial technologies, an Indian perspective–a review. Environ. Pollut. 2018, 239, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Astruc, D. Nanocatalysts and other nanomaterials for water remediation from organic pollutants. Coord. Chem. Rev. 2020, 408, 213180. [Google Scholar] [CrossRef]
- Priya, A.S.; Geetha, D.; Karthik, K.; Rajamoorthy, M. Investigations on the enhanced photocatalytic activity of (Ag, La) substituted nickel cobaltite spinels. Solid State Sci. 2019, 98, 105992. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, Y.; Xing, S. Comparative study of Cu-based bimetallic oxides for Fenton-like degradation of organic pollutants. Chemosphere 2018, 203, 450–456. [Google Scholar] [CrossRef]
- Surendran, P.; Lakshmanan, A.; Priya, S.S.; Geetha, P.; Rameshkumar, P.; Kannan, K.; Hegde, T.A.; Vinitha, G. Fluorescent carbon quantum dots from Ananas comosus waste peels: A promising material for NLO behaviour, antibacterial, and antioxidant activities. Inorg. Chem. Commun. 2021, 124, 108397. [Google Scholar] [CrossRef]
- Rangayasami, A.; Kannan, K.; Joshi, S.; Subban, M. Bioengineered silver nanoparticles using Elytraria acaulis (Lf) Lindau leaf extract and its biological applications. Biocatal. Agric. Biotechnol. 2020, 27, 101690. [Google Scholar] [CrossRef]
- Intaphong, P.; Phuruangrat, A.; Karthik, K.; Dumrongrojthanath, P.; Thongtem, T.; Thongtem, S. Effect of pH on phase, morphology and photocatalytic properties of BiOBr synthesized by hydrothermal method. J. Inorg. Organomet. Polym. Mater. 2020, 30, 714–721. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Keereesaensuk, P.-O.; Karthik, K.; Dumrongrojthanath, P.; Ekthammathat, N.; Thongtem, S.; Thongtem, T. Synthesis and characterization Ag nanoparticles supported on Bi2WO6 nanoplates for enhanced visible-light-driven photocatalytic degradation of rhodamine B. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1033–1040. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Keereesaensuk, P.-O.; Karthik, K.; Dumrongrojthanath, P.; Ekthammathat, N.; Thongtem, S.; Thongtem, T. Synthesis of Ag/Bi2MoO6 nanocomposites using NaBH4 as reducing agent for enhanced visible-light-driven photocatalysis of rhodamine B. J. Inorg. Organomet. Polym. Mater. 2020, 30, 322–329. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Bundschuh, M.; Filser, J.; Lüderwald, S.; McKee, M.S.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Wagner, S. Nanoparticles in the environment: Where do we come from, where do we go to? Environ. Sci. Eur. 2018, 30, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Galagan, Y.; Su, W.-F. Reversible photoreduction of methylene blue in acrylate media containing benzyl dimethyl ketal. J. Photochem. Photobiol. A Chem. 2008, 195, 378–383. [Google Scholar] [CrossRef]
- Zhang, J.; Lee, K.-H.; Cui, L.; Jeong, T.-s. Degradation of methylene blue in aqueous solution by ozone-based processes. J. Ind. Eng. Chem. 2009, 15, 185–189. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Dwivedi, R.P.; ALOthman, Z.A.; Mola, G.T. Novel development of nanoparticles to bimetallic nanoparticles and their composites: A review. J. King Saud Univ.-Sci. 2019, 31, 257–269. [Google Scholar] [CrossRef]
- Younas, U.; Hassan, S.T.; Ali, F.; Hassan, F.; Saeed, Z.; Pervaiz, M.; Khan, S.; Jannat, F.T.; Bibi, S.; Sadiqa, A.; et al. Radical Scavenging and Catalytic Activity of Fe-Cu Bimetallic Nanoparticles Synthesized from Ixora finlaysoniana Extract. Coatings 2021, 11, 813. [Google Scholar] [CrossRef]
- Bibi, S.; Ahmad, A.; Anjum, M.A.R.; Haleem, A.; Siddiq, M.; Shah, S.S.; al Kahtani, A. Photocatalytic degradation of malachite green and methylene blue over reduced graphene oxide (rGO) based metal oxides (rGO-Fe3O4/TiO2) nanocomposite under UV-visible light irradiation. J. Environ. Chem. Eng. 2021, 9, 105580. [Google Scholar] [CrossRef]
- Mohanty, P.; Rath, C.; Mallick, P.; Biswal, R.; Mishra, N. UV–visible studies of nickel oxide thin film grown by thermal oxidation of nickel. Phys. B Condens. Matter 2010, 405, 2711–2714. [Google Scholar] [CrossRef]
- Fu, J.; Liang, H.; Zhang, J.; Wang, Y.; Liu, Y.; Zhang, Z.; Lin, X. Enhanced optical absorbance and fabrication of periodic arrays on nickel surface using nanosecond laser. Opt. Commun. 2017, 389, 170–175. [Google Scholar] [CrossRef]
- Thou, C.Z.; Khan, F.S.A.; Mubarak, N.; Ahmad, A.; Khalid, M.; Jagadish, P.; Walvekar, R.; Abdullah, E.; Khan, S.; Khan, M.; et al. Surface charge on chitosan/cellulose nanowhiskers composite via functionalized and untreated carbon nanotube. Arab. J. Chem. 2021, 14, 103022. [Google Scholar] [CrossRef]
- Lamayi, W.; Shehu, Z.; Mai, A.J.; Magaji, B.; Adam, M.; Bunu, M.A. Green synthesis, characterization and larvicidal activity of Cu/Ni bimetallic nanoparticles using fruit extract of Palmyra palm. Int. J. Chem. Mater. Res. 2020, 8, 20–25. [Google Scholar]
- Mujtaba, A.; Janjua, N.K. Fabrication and electrocatalytic application of CuO@Al2O3 hybrids. J. Electrochem. Soc. 2015, 162, H328. [Google Scholar] [CrossRef]
- Xiaodan, L.; Min, G.; Zhang, M.; Xidong, W.; Xiao, G.; Kuochih, C. Effects of PVP on the preparation and growth mechanism of monodispersed Ni nanoparticles. Rare Met. 2008, 27, 642–647. [Google Scholar]
- Wei, Z.; Yan, P.; Feng, W.; Dai, J.; Wang, Q.; Xia, T. Microstructural characterization of Ni nanoparticles prepared by anodic arc plasma. Mater. Charact. 2006, 57, 176–181. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, R.; Chai, Y.; Hu, F. Electrochemical sensing of hydrogen peroxide using metal nanoparticles: A review. Microchim. Acta 2013, 180, 15–32. [Google Scholar] [CrossRef]
- Shahid, M.; Farooqi, Z.H.; Begum, R.; Arif, M.; Irfan, A.; Azam, M. Extraction of cobalt ions from aqueous solution by microgels for in-situ fabrication of cobalt nanoparticles to degrade toxic dyes: A two fold-environmental application. Chem. Phys. Lett. 2020, 754, 137645. [Google Scholar] [CrossRef]
- Demirci, S.; Sunol, A.K.; Sahiner, N. Catalytic activity of amine functionalized titanium dioxide nanoparticles in methanolysis of sodium borohydride for hydrogen generation. Appl. Catal. B Environ. 2020, 261, 118242. [Google Scholar] [CrossRef]
- Tang, L.; Wang, J.-j.; Wang, L.; Jia, C.-t.; Lv, G.-x.; Liu, N.; Wu, M.-h. Facile synthesis of silver bromide-based nanomaterials and their efficient and rapid selective adsorption mechanisms toward anionic dyes. ACS Sustain. Chem. Eng. 2016, 4, 4617–4625. [Google Scholar] [CrossRef]
- Di Credico, B.; Bellobono, I.; D’Arienzo, M.; Fumagalli, D.; Redaelli, M.; Scotti, R.; Morazzoni, F. Efficacy of the reactive oxygen species generated by immobilized TiO2 in the photocatalytic degradation of diclofenac. Int. J. Photoenergy 2015. [Google Scholar] [CrossRef] [Green Version]
- Lei, M.; Wang, N.; Zhu, L.; Tang, H. Peculiar and rapid photocatalytic degradation of tetrabromodiphenyl ethers over Ag/TiO2 induced by interaction between silver nanoparticles and bromine atoms in the target. Chemosphere 2016, 150, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chang, W.; Wang, Y.; Ji, H.; Chen, C.; Ma, W.; Zhao, J. Rapid, photocatalytic, and deep debromination of polybrominated diphenyl ethers on Pd–TiO2: Intermediates and pathways. Chem.—A Eur. J. 2014, 20, 11163–11170. [Google Scholar] [CrossRef]
- Lei, M.; Wang, N.; Zhu, L.; Zhou, Q.; Nie, G.; Tang, H. Photocatalytic reductive degradation of polybrominated diphenyl ethers on CuO/TiO2 nanocomposites: A mechanism based on the switching of photocatalytic reduction potential being controlled by the valence state of copper. Appl. Catal. B Environ. 2016, 182, 414–423. [Google Scholar] [CrossRef]
- Chandraker, K.; Vaishanav, S.K.; Nagwanshi, R.; Satnami, M.L. Radical scavenging efficacy of thiol capped silver nanoparticles. J. Chem. Sci. 2015, 127, 2183–2191. [Google Scholar] [CrossRef]
- Shanmugam, S.; Xu, J.; Boyer, C. Aqueous RAFT photopolymerization with oxygen tolerance. Macromolecules 2016, 49, 9345–9357. [Google Scholar] [CrossRef]
- Sudha, A.; Jeyakanthan, J.; Srinivasan, P. Green synthesis of silver nanoparticles using Lippia nodiflora aerial extract and evaluation of their antioxidant, antibacterial and cytotoxic effects. Resour.-Effic. Technol. 2017, 3, 506–515. [Google Scholar] [CrossRef]
- Morgareidge, K. Influence of Solvent on Ultraviolet Absorption Maximum of Vitamin A. Ind. Eng. Chem. Anal. Ed. 1942, 14, 700–702. [Google Scholar] [CrossRef]
- Sanna, D.; Delogu, G.; Mulas, M.; Schirra, M.; Fadda, A. Determination of free radical scavenging activity of plant extracts through DPPH assay: An EPR and UV–Vis study. Food Anal. Methods 2012, 5, 759–766. [Google Scholar] [CrossRef]
- Zhang, T.; Oyama, T.; Aoshima, A.; Hidaka, H.; Zhao, J.; Serpone, N. Photooxidative N-demethylation of methylene blue in aqueous TiO2 dispersions under UV irradiation. J. Photochem. Photobiol. A Chem. 2001, 140, 163–172. [Google Scholar] [CrossRef]
- Savory, D.M.; McQuillan, A.J. Influence of formate adsorption and protons on shallow trap infrared absorption (STIRA) of anatase TiO2 during photocatalysis. J. Phys. Chem. C 2013, 117, 23645–23656. [Google Scholar] [CrossRef]
- Liu, Z.; Xiao, J.; Fu, Q.; Feng, H.; Zhang, X.; Ren, T.; Wang, S.; Ma, D.; Wang, X.; Chen, H. Synthesis and physical properties of the conjugated dendrons bearing twisted acenes used in solution processing of organic light-emitting diodes. ACS Appl. Mater. Interfaces 2013, 5, 11136–11141. [Google Scholar] [CrossRef]
- Aravind, M.; Ahmad, A.; Ahmad, I.; Amalanathan, M.; Naseem, K.; Mary, S.M.M.; Parvathiraja, C.; Hussain, S.; Algarni, T.S.; Pervaiz, M.; et al. Critical green routing synthesis of silver NPs using jasmine flower extract for biological activities and photocatalytical degradation of methylene blue. J. Environ. Chem. Eng. 2021, 9, 104877. [Google Scholar] [CrossRef]
- Bhatti, M.A.; Tahira, A.; Chandio, A.d.; Almani, K.F.; Bhatti, A.L.; Waryani, B.; Nafady, A.; Ibupoto, Z.H. Enzymes and phytochemicals from neem extract robustly tuned the photocatalytic activity of ZnO for the degradation of malachite green (MG) in aqueous media. Res. Chem. Intermed. 2021, 47, 1581–1599. [Google Scholar] [CrossRef]
- Mark, J.A.M.; Venkatachalam, A.; Pramothkumar, A.; Senthilkumar, N.; Jothivenkatachalam, K.; Jesuraj, J.P. Investigation on structural, optical and photocatalytic activity of CoMn2O4 nanoparticles prepared via simple co-precipitation method. Phys. B Condens. Matter 2021, 601, 412349. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).