Numerical Investigation of Degasification in an Electrocoagulation Reactor
Abstract
:1. Introduction
2. Materials and Methods
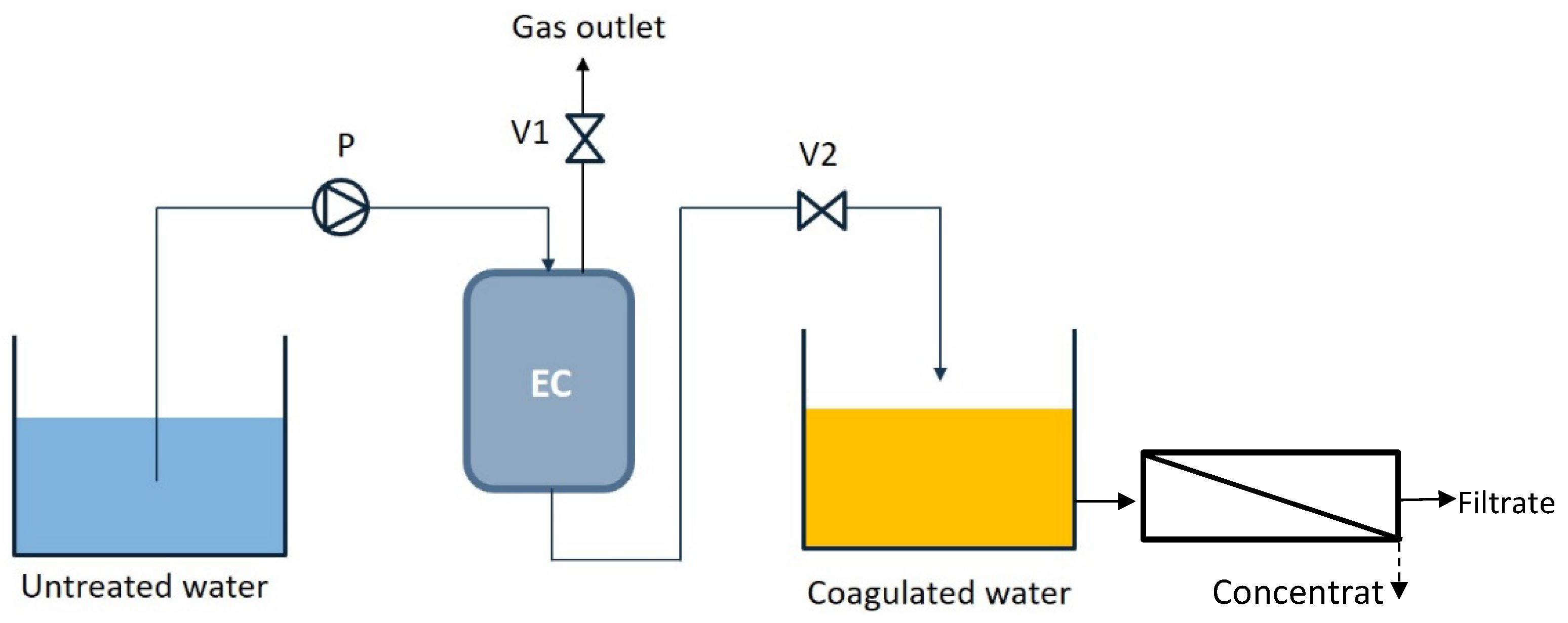
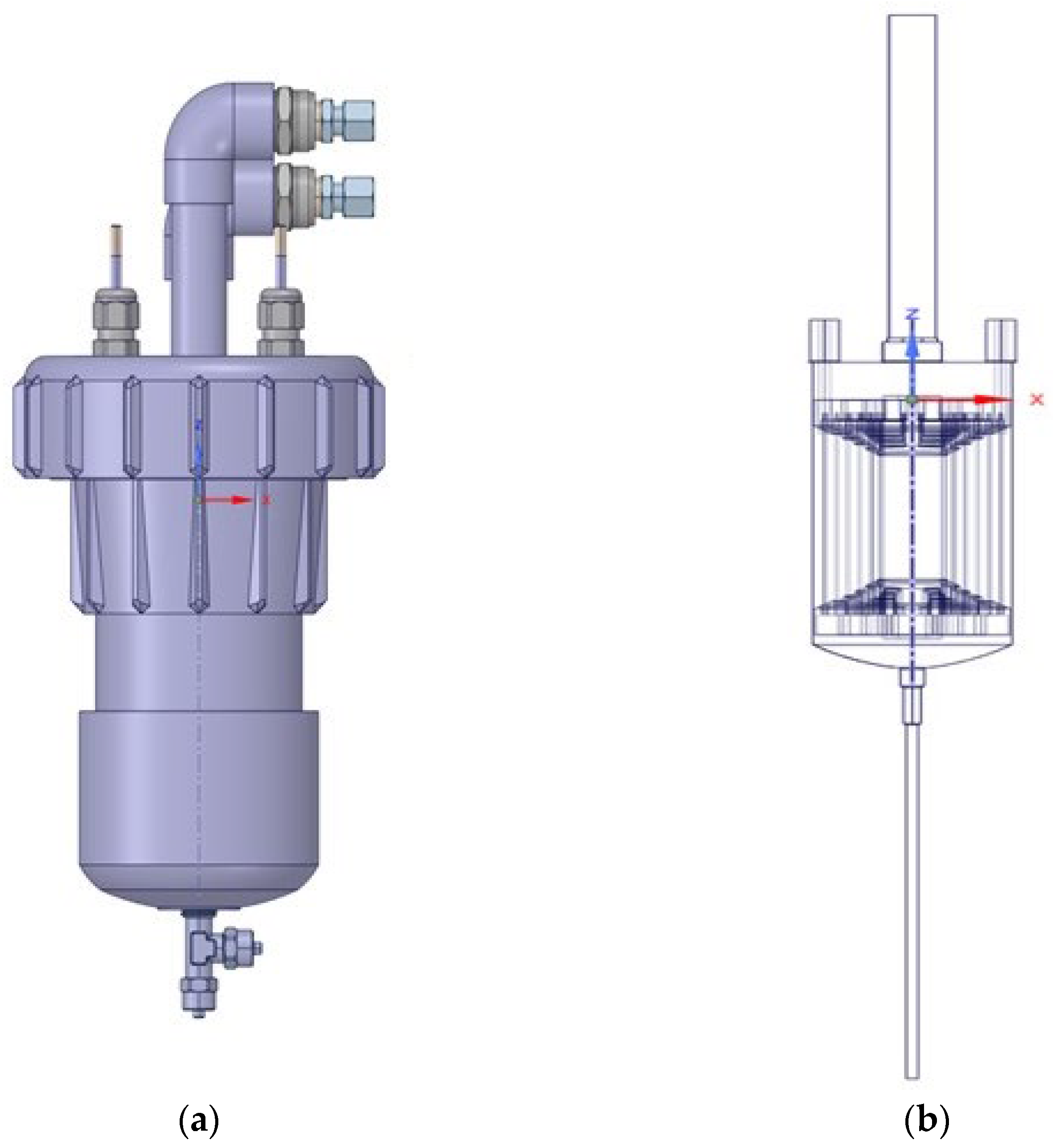
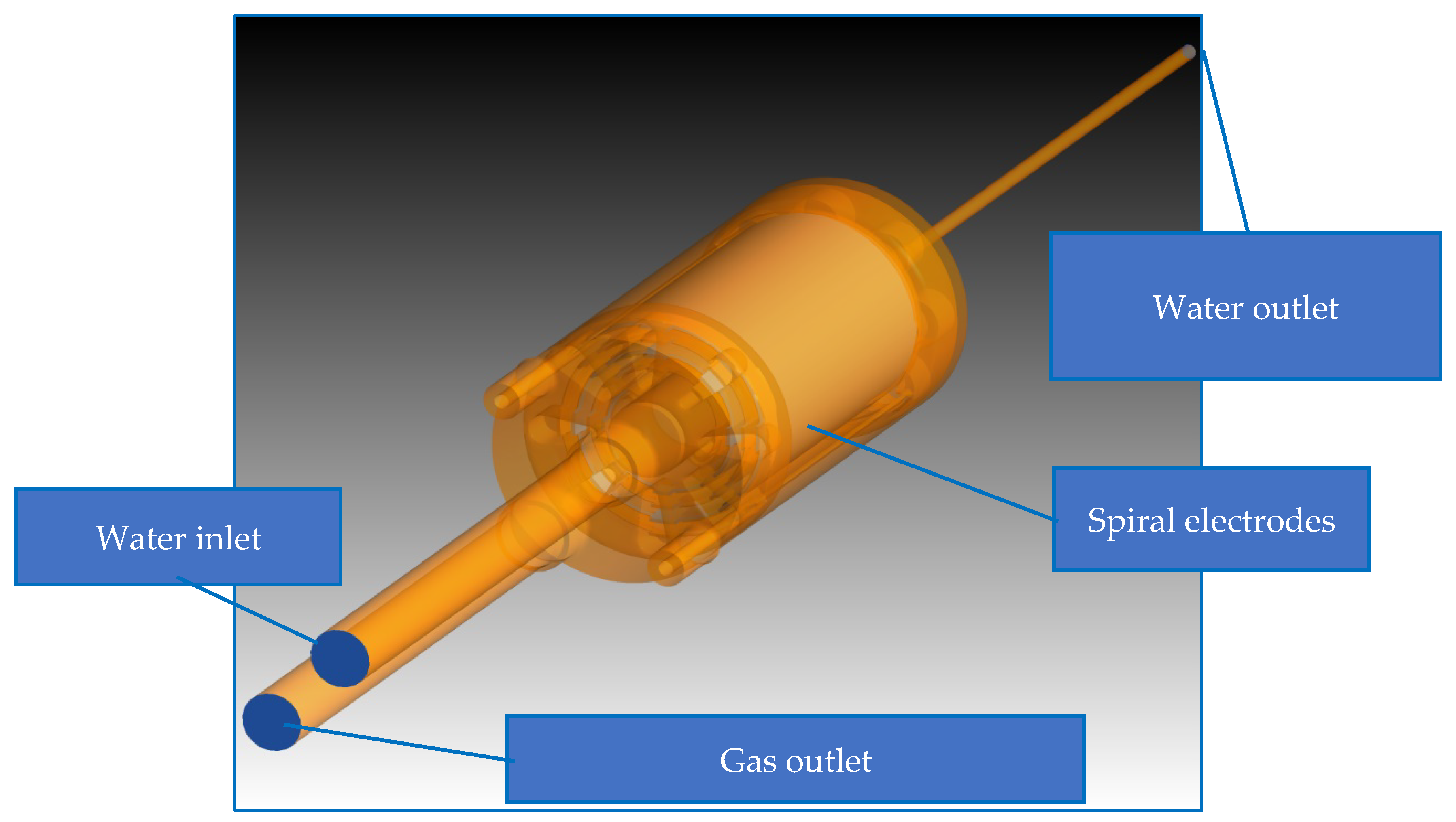
2.1. Experimental Setup
2.2. Numerical Simulation Setup
2.2.1. Geometry and Grid Generation
2.2.2. Solver Setup
2.2.3. Interphase Transfer Model
2.2.4. Turbulence Model
3. Results and Discussion
3.1. Verification and Validation
3.2. Hydraulic Retention Time
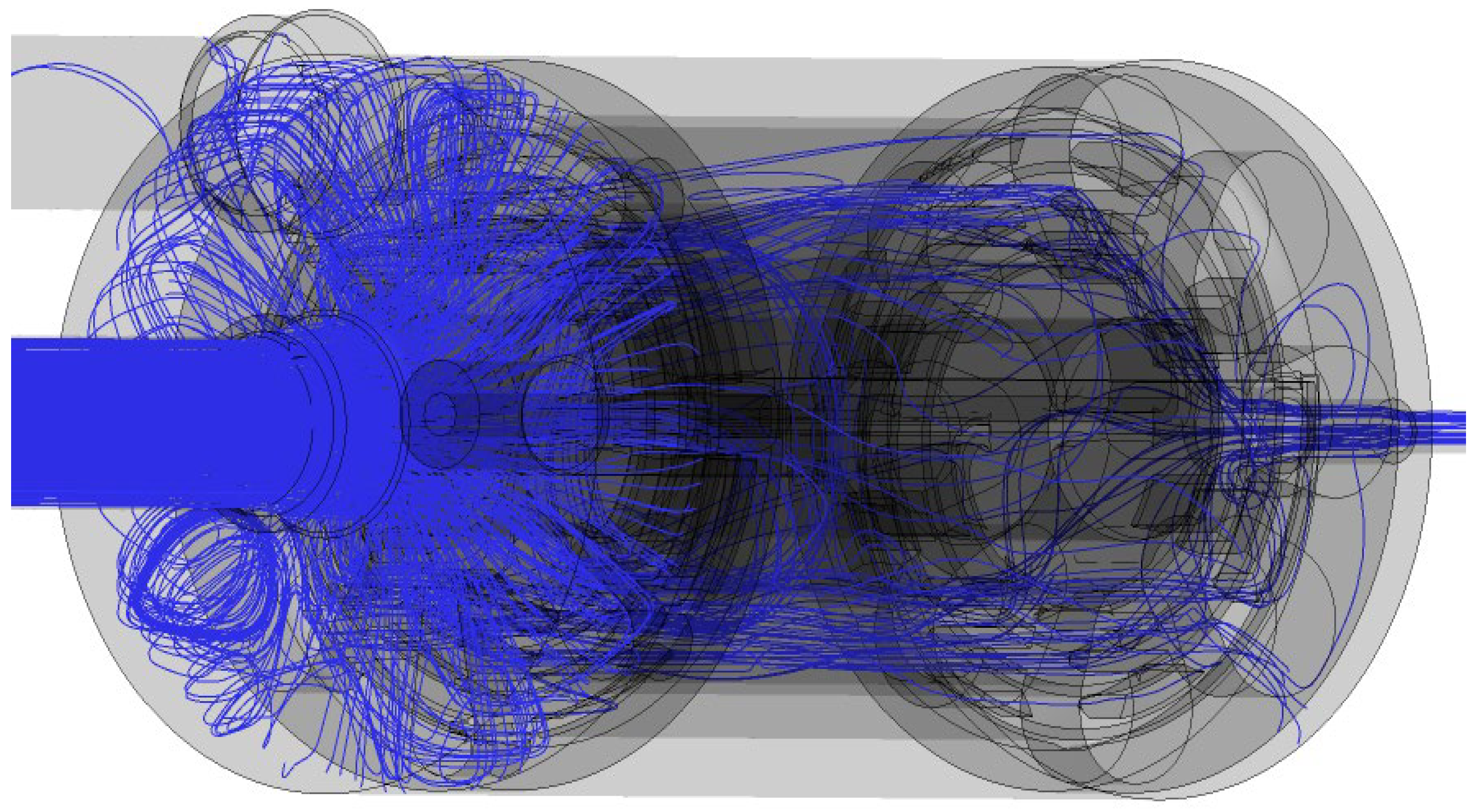
3.3. Velocity Vectors
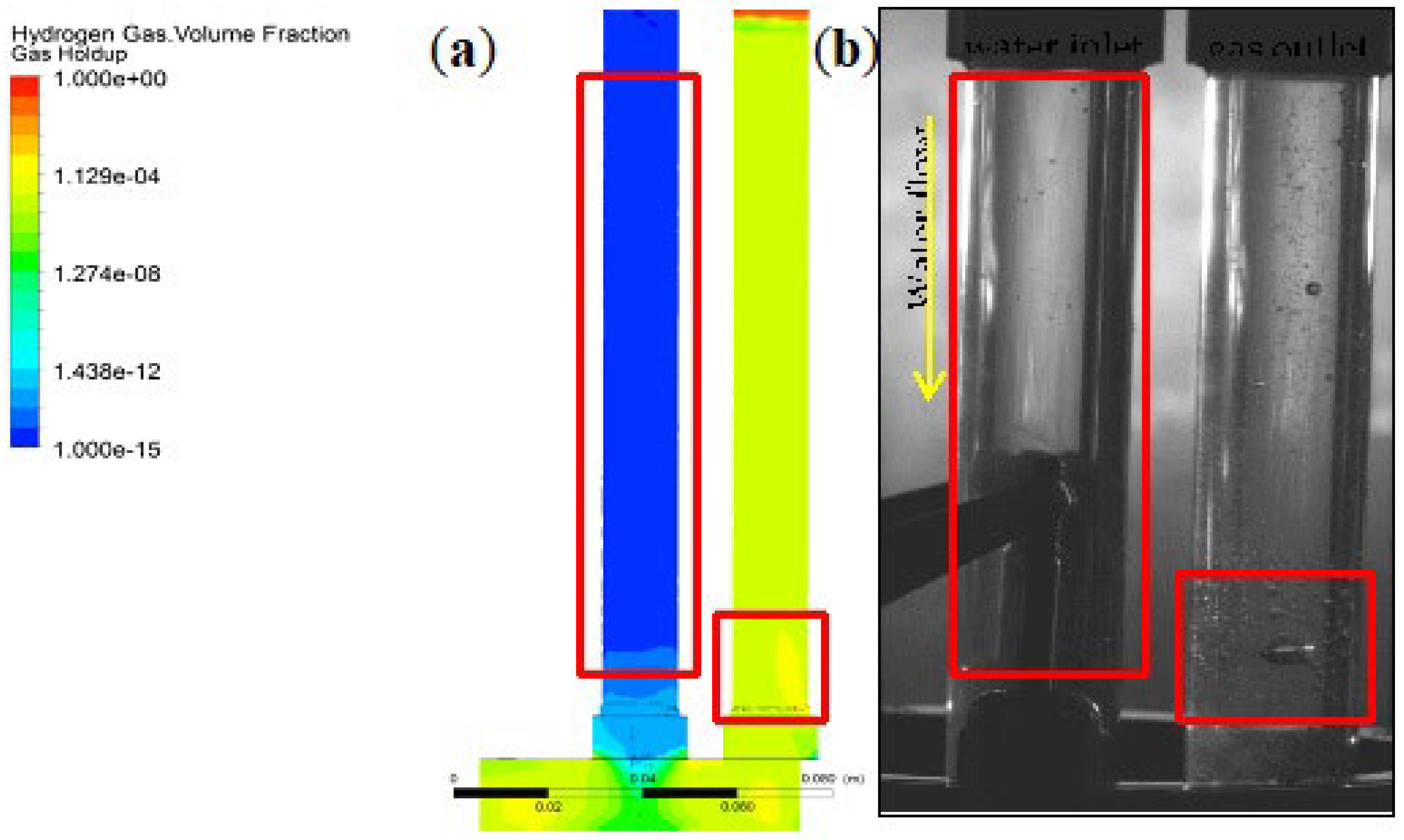
3.4. Gas Holdup
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| A | Area |
| Aαβ | Interfacial area density |
| c | Continuous phase |
| CD | Drag coefficient |
| Ci | Ion concentration |
| CL | Lift coefficient |
| CTD | Coefficient of turbulence dispersion force |
| CVM | Coefficient of virtual mass force |
| cμ | Viscosity coefficient |
| D | Drag force |
| d | Dispersed phase |
| db | Bubble diameter |
| dH | Horizontal bubble dimension |
| Eo | Eotvos number |
| Eo’ | Modified Eotvos number |
| F | Faraday’s constant |
| f | Volume fraction |
| Fb | Buoyancy force |
| FL | Lift force |
| Fs | Surface tension force |
| FTD | Turbulence dispersion force |
| FVM | Virtual mass force |
| g | Gravity acceleration |
| Ie | Electrical current |
| ke | Specific electrical conductivity |
| kαβ | Normal surface curvature |
| Le | Electrical charge loading |
| Mα | Interphase momentum transfer |
| nb | Bubble number |
| P | Pressure |
| Pabs | Absolute pressure |
| Pref | Reference pressure |
| Prt | Turbulent Prandtl number |
| Pstat | Static pressure |
| Ptot | Total pressure |
| Q | Volumetric flow rate |
| r | Area density |
| rc | Capillary radius |
| Re | Electrical resistance |
| S | Source term |
| t | Time |
| T | Tensor of mean motion |
| U | Mean velocity |
| u | Fluid velocity |
| u’ | Fluctuating velocity |
| V | Volume |
| x, y, z | Cartesian’s spatial direction |
| Z | Charge |
| α | Phase α (Water) |
| β | Phase β (Hydrogen) |
| δ | Kronecker delta |
| ε | Eddy |
| κ | Turbulent kinetic energy |
| μ | Dynamic viscosity |
| μt | Dynamic turbulence (eddy) viscosity |
| µtp | Particle induced eddy viscosity |
| μts | Shear-induced eddy viscosity |
| ρ | Density |
| σs | Surface tension coefficient |
| σtc | Turbulence Schmidt number for the continuous phase |
| τ | Reynolds stress tensor |
| ταβ | Interphase mass transfer |
| ζ | Zeta potential |
| ν | Kinematic viscosity |
| νt | Kinematic turbulence (eddy) viscosity |
| χi | Ionic conductivity |
| ω | Turbulent frequency term |
| ωc | Angular velocity |
References
- Worthington, A.C.; Hoffman, A.M. A State of the Art Review of Residential Water Demand Modelling. In School of Accounting & Finance, University of Wollongong, Working Paper 6; 2007; Available online: https://ro.uow.edu.au/accfinwp/6/ (accessed on 12 February 2021).
- Ross, N. World Water Quality Facts and Statistics—World Water Day 2010’; Pacific Institute: Oakland, CA, USA, 2010; Available online: https://pacinst.org/publication/world-water-quality-facts-and-statistics-world-water-day-2010/ (accessed on 12 February 2021).
- UNESCO. World Water Assessment Programme. 14 September 2020. Available online: https://en.unesco.org/wwap (accessed on 3 June 2021).
- Tambo, N.; Ogasawara, K. Physical and Chemical Separation in Water and Wastewater Treatment; IWA Publishing: London, UK, 2020. [Google Scholar]
- Keucken, A.; Heinicke, G.; Persson, K.M.; Köhler, S.J. Combined Coagulation and Ultrafiltration Process to Counteract Increasing NOM in Brown Surface Water. Water 2017, 9, 697. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Liu, M.; Zhang, X.; Graham, N.; Qu, J. Effect of pre-coagulation using different aluminium species on crystallization of cake layer and membrane fouling. NPJ Clean Water 2019, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Lerch, A.; Panglisch, S.; Buchta, P.; Tomita, Y.; Yonekawa, H.; Hattori, K. Direct river water treatment using coagulation/ceramic membrane microfiltration. Desalination 2005, 179, 41–50. [Google Scholar] [CrossRef]
- Aly, S.A.; Anderson, W.B.; Huck, P.M. In-line coagulation assessment for ultrafiltration fouling reduction to treat secondary effluent for water reuse. Water Sci. Technol. 2020, 83, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.S.; Ma, Z.; Qin, J.; Sim, S.H.; Toh, C.-S. Inline coagulation–ultrafiltration as the pretreatment for reverse osmosis brine treatment and recovery. Desalination 2015, 365, 242–249. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X. Ultrafiltration with in-line coagulation for the removal of natural humic acid and membrane fouling mechanism. J. Environ. Sci. 2006, 18, 880–884. [Google Scholar] [CrossRef]
- Arkhangelsky, E.; Lerch, A.; Uhl, W.; Gitis, V. Organic fouling and floc transport in capillaries. Sep. Purif. Technol. 2011, 80, 482–489. [Google Scholar] [CrossRef]
- Lerch, A. Fouling layer formation by flocs in inside-out driven, horizontal aligned capillary ultrafiltration membranes. Desalination 2011, 283, 131–139. [Google Scholar] [CrossRef]
- Barbot, E.; Moustier, S.; Bottero, J.Y.; Moulin, P. Coagulation and ultrafiltration: Understanding of the key parameters of the hybrid process. J. Membr. Sci. 2008, 325, 520–527. [Google Scholar] [CrossRef]
- Harif, T.; Khai, M.; Adin, A. Electrocoagulation versus chemical coagulation: Coagulation/flocculation mechanisms and resulting floc characteristics. Water Res. 2012, 46, 3177–3188. [Google Scholar] [CrossRef] [PubMed]
- Manilal, A.M.; Soloman, P.A.; Basha, C.A. Removal of Oil and Grease from Produced Water Using Electrocoagulation. J. Hazard. Toxic Radioact. Waste 2020, 24. Available online: https://ascelibrary.org/doi/abs/10.1061/%28ASCE%29HZ.2153-5515.0000463 (accessed on 3 June 2021). [CrossRef]
- Amarine, M.; Lekhlif, B.; Sinan, M.; El Rharras, A.; Echaabi, J. Treatment of nitrate-rich groundwater using electrocoagulation with aluminum anodes. Groundw. Sustain. Dev. 2020, 11, 100371. Available online: https://www.sciencedirect.com/science/article/abs/pii/S2352801X19302991 (accessed on 3 June 2021). [CrossRef]
- Tahreen, A.; Jami, M.S.; Ali, F. Role of electrocoagulation in wastewater treatment: A developmental review. J. Water Process. Eng. 2020, 37, 101440. [Google Scholar] [CrossRef]
- Vepsäläinen, M.; Sillanpää, M. Electrocoagulation in the treatment of industrial waters and wastewaters. In Advanced Water Treatment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–78. [Google Scholar] [CrossRef]
- Ghernaout, D.; Elboughdiri, N. An Insight in Electrocoagulation Process through Current Density Distribution (CDD). Open Access Libr. J. 2020, 7, 98473. [Google Scholar] [CrossRef]
- Villalobos-Lara, A.D.; Pérez, T.; Uribe, A.R.; Alfaro-Ayala, J.A.; de Jesús Ramírez-Minguela, J.; Minchaca-Mojica, J.I. CFD simulation of biphasic flow, mass transport and current distribution in a continuous rotating cylinder electrode reactor for electrocoagulation process. J. Electroanal. Chem. 2020, 858, 113807. [Google Scholar] [CrossRef]
- Hakizimana, J.N.; Gourich, B.; Chafi, M.; Stiriba, Y.; Vial, C.; Drogui, P.; Naja, J. Electrocoagulation process in water treatment: A review of electrocoagulation modeling approaches. Desalination 2017, 404, 1–21. [Google Scholar] [CrossRef]
- Safonyk, A.; Prysiazhniuk, O. Modeling and Simulation in Engineering Modeling of the Electrocoagulation Processes in Nonisothermal Conditions. Model. Simul. Eng. 2019, 2019, e9629643. [Google Scholar] [CrossRef]
- Song, P.; Song, Q.; Yang, Z.; Zeng, G.; Xu, H.; Li, X.; Xiong, W. Numerical simulation and exploration of electrocoagulation process for arsenic and antimony removal: Electric field, flow field, and mass transfer studies. J. Environ. Manag. 2018, 228, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, Z.; Ma, X.; Tang, Q.; Li, Y. Modeling of the electrocoagulation process: A study on the mass transfer of electrolysis and hydrolysis products. Chem. Eng. Sci. 2017, 165, 165–176. [Google Scholar] [CrossRef]
- Al-Barakat, H.S.; Matloub, F.K.; Ajjam, S.K.; Al-Hattab, T.A. Modeling and Simulation of Wastewater Electrocoagulation Reactor. IOP Conf. Ser.: Mater. Sci. Eng. 2020, 871, 012002. [Google Scholar] [CrossRef]
- Vázquez, A.; Nava, J.L.; Cruz, R.; Lázaro, I.; Rodríguez, I. The importance of current distribution and cell hydrodynamic analysis for the design of electrocoagulation reactors. J. Chem. Technol. Biotechnol. 2014, 89, 220–229. [Google Scholar] [CrossRef]
- Martinez-Delgadillo, S.; Mollinedo-Ponce, H.; Mendoza-Escamilla, V.; Gutiérrez-Torres, C.; Jiménez-Bernal, J.; Barrera-Diaz, C. Performance evaluation of an electrochemical reactor used to reduce Cr(VI) from aqueous media applying CFD simulations. J. Clean. Prod. 2012, 34, 120–124. [Google Scholar] [CrossRef]
- Vázquez, A.I.; Almazán, F.J.; Cruz-Diaz, M.; Delgadillo, J.A.; Lázaro, M.I.; Ojeda, C.; Rodríguez, I. Characterization of a Multiple-Channel Electrochemical Cell by Computational Fluid Dynamics (CFD) and Residence Time Distribution (RTD). ECS Trans. 2010, 29, 215. [Google Scholar] [CrossRef]
- Emamjomeh, M.M.; Sivakumar, M. Review of pollutants removed by electrocoagulation and electrocoagulation/flotation processes. J. Environ. Manag. 2009, 90, 1663–1679. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Song, J.; Kwon, A. Preliminary investigation of electrocoagulation as a substitute for chemical coagulation. Water Supply 2002, 2, 73–76. [Google Scholar] [CrossRef]
- Talaia, M.A.R. Terminal Velocity of a Bubble Rise in a Liquid Column. World Acad. Sci. Eng. Technol. 2007, 28, 264–268. [Google Scholar]
- ANSYS CFX. ANSYS CFX 19.2 Documentation. 2019. Available online: https://ansyshelp.ansys.com/ (accessed on 29 November 2019).
- Menter, F. Zonal Two Equation k-w Turbulence Models For Aerodynamic Flows. In Proceedings of the 23rd Fluid Dynamics, Plasmadynamics, and Lasers Conference, Orlando, FL, USA, 6–9 July 1993; Available online: https://arc.aiaa.org/doi/10.2514/6.1993-2906 (accessed on 19 May 2021).
- Sato, Y.; Sekoguchi, K. Liquid velocity distribution in two-phase bubble flow. Int. J. Multiph. Flow 1975, 2, 79–95. [Google Scholar] [CrossRef]
- de Moura, C.A.; Kubrusly, C.S. (Eds.) The Courant–Friedrichs–Lewy (CFL) Condition: 80 Years after Its Discovery; Birkhäuser: Basel, Switzerland, 2013. [Google Scholar] [CrossRef]





| Parameters | Min Intervals | Max Intervals | Typical Intervals |
|---|---|---|---|
| Current density [A/m2] | 1 | 75 | 25 |
| Inlet flow [L/h] | 1 | 1000 | 10 |
| Hydrogen gas flow [g/min] | 6 × 10−6 | 1.93 × 10−3 | 6.62 × 10−4 |
| Degassing time interval [min] | 15 | 120 | 60 |
| Degassing duration [min] | 0.1 | 2 | 1 |
| Water temperature [°C] | 15 | 25 | 20 |
| Fluid Properties | Water | Hydrogen |
|---|---|---|
| Thermodynamic State | Liquid | Gas |
| Molar Mass [kg/kmol] | 18.02 | 2.016 |
| Density [kg/m3] | 998.2 | 0.09 |
| Ref. Temperature [°C] | 20 | 20 |
| BCs | Case 1 | Case 2 | Case 3 | Case 4–6 |
|---|---|---|---|---|
| Water inlet | 0.00278 | 0.27778 | ||
| Water outlet | ||||
| Spiral Cathode | ||||
| Gas outlet | degassing |
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Retention time [s] water velocity | 486 | 67 | 8 |
| Retention time [s] superficial water velocity | 473 | 55 | 8 |
| Case 4 | Case 5 | Case 6 | |
|---|---|---|---|
| Gas holdup [%] before degassing | 0.3 | 3.76 | 0.22 |
| Gas holdup [%] after 30 s | 0.020 | 0.028 | 0.027 |
| Gas holdup [%] after 120 s | 0.019 | 0.020 | 0.020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Höhne, T.; Asl, V.F.; Ople Villacorte, L.; Herskind, M.; Momeni, M.; Al-Fayyad, D.; Taș-Köhler, S.; Lerch, A. Numerical Investigation of Degasification in an Electrocoagulation Reactor. Water 2021, 13, 2607. https://doi.org/10.3390/w13192607
Höhne T, Asl VF, Ople Villacorte L, Herskind M, Momeni M, Al-Fayyad D, Taș-Köhler S, Lerch A. Numerical Investigation of Degasification in an Electrocoagulation Reactor. Water. 2021; 13(19):2607. https://doi.org/10.3390/w13192607
Chicago/Turabian StyleHöhne, Thomas, Vahid Farhikhteh Asl, Loreen Ople Villacorte, Mark Herskind, Maryam Momeni, Douha Al-Fayyad, Sibel Taș-Köhler, and André Lerch. 2021. "Numerical Investigation of Degasification in an Electrocoagulation Reactor" Water 13, no. 19: 2607. https://doi.org/10.3390/w13192607
APA StyleHöhne, T., Asl, V. F., Ople Villacorte, L., Herskind, M., Momeni, M., Al-Fayyad, D., Taș-Köhler, S., & Lerch, A. (2021). Numerical Investigation of Degasification in an Electrocoagulation Reactor. Water, 13(19), 2607. https://doi.org/10.3390/w13192607








