Preparation of Synthetic Clays to Remove Phosphates and Ibuprofen in Water
Abstract
:1. Introduction
2. Materials and Methods
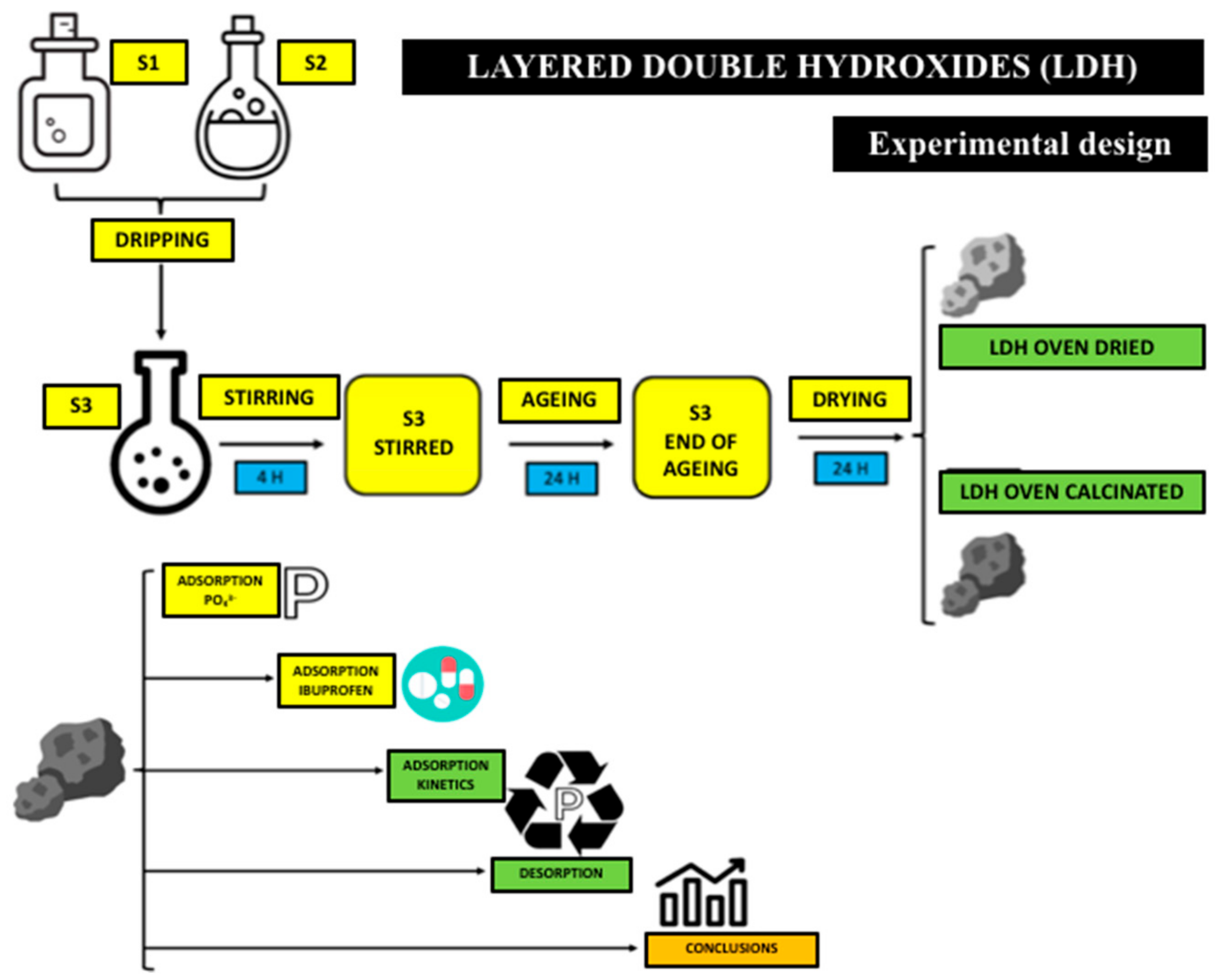
2.1. Adsorbent Production and Preparation
2.2. Materials and Solutions
2.3. Batch Adsorption Experiments
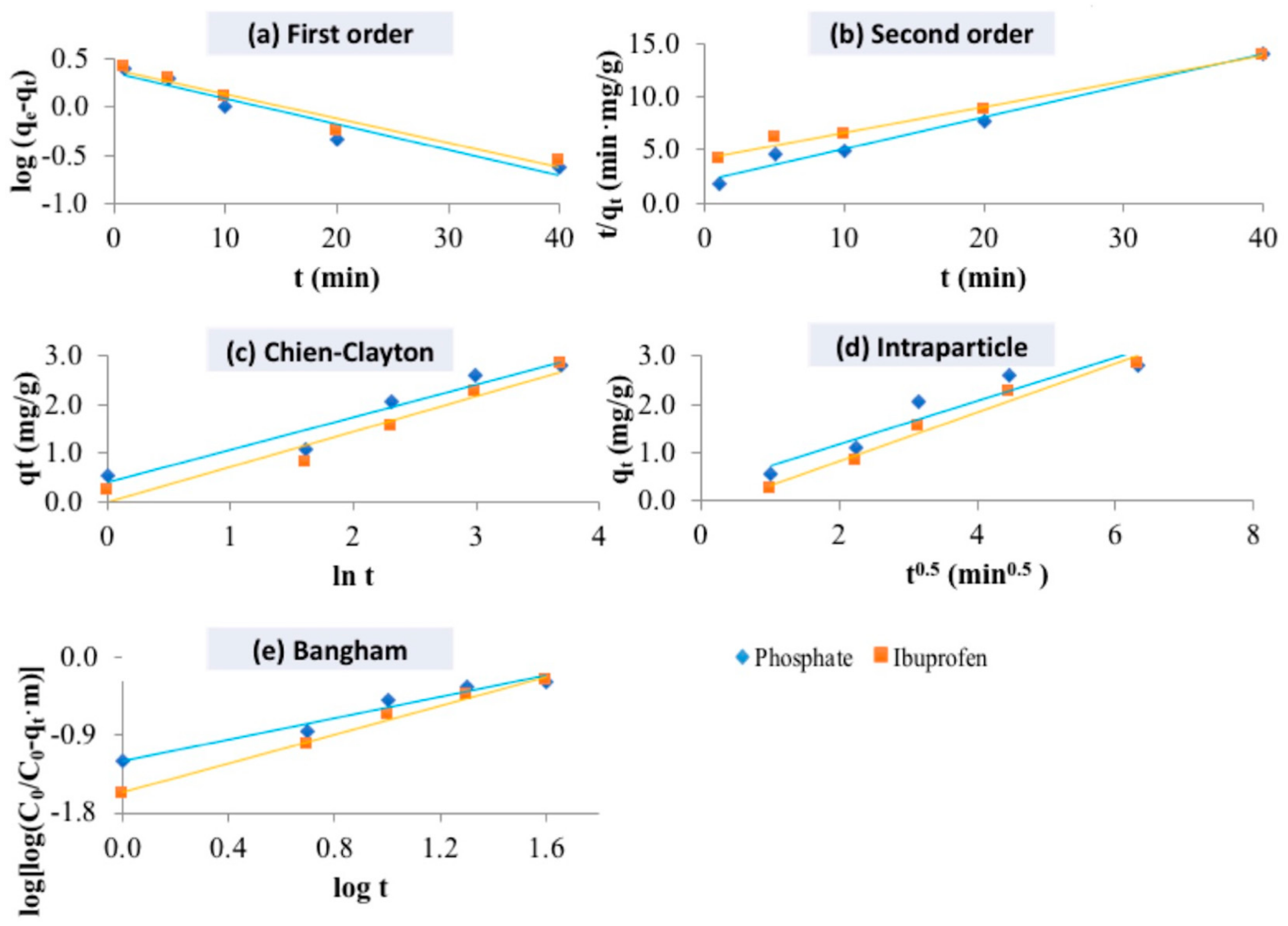
2.4. Kinetic Study of Pollutant Adsorption
2.5. Study of the Recovery of the Adsorbate
3. Results and Discussion
3.1. Formulation of an Adsorbent Composite for Removing Phosphates and Ibuprofen from Wastewater
3.2. Evaluation of the Adsorption of Phosphates and Ibuprofen in the Composite: Kinetic Study
3.3. Evaluation of the Recovery of Phosphates from the Composite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nash, D.M.; Halliwell, D.J. Tracing phosphorous transferred from grazing land to water. Water Res. 2000, 34, 1975–1985. [Google Scholar] [CrossRef]
- Devesa-Rey, R.; Iglesias, M.L.; Díaz-Fierros, F.; Barral, M.T. Total phosphorous distribution and bioavailability in the bed sediments of an Atlantic basin (Galicia, NW Spain): Spatial distribution and vertical profiles. Water Air Soil Pollut. 2009, 200, 341–352. [Google Scholar] [CrossRef]
- Li, J.; Zheng, B.; Chen, X.; Li, Z.; Xia, Q.; Wang, H.; Yang, Y.; Zhou, Y.; Yang, H. The use of constructed wetland for mitigating nitrogen and phosphorus from agricultural runoff: A review. Water 2021, 13, 476. [Google Scholar] [CrossRef]
- Álvarez-Vázquez, L.; Fernández, F.J. Optimal control of a bioreactor. Appl. Math. Comput. 2010, 216, 2559–2575. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Shukla, P.; Giri, B.S.; Chowdhary, P.; Chandra, R.; Gupta, P.; Pandey, A. Prevalence and hazardous impact of pharmaceutical and personal care products and antibiotics in environment: A review on emerging contaminants. Environ. Res. 2021, 194, 110664. [Google Scholar] [CrossRef] [PubMed]
- Pironti, C.; Ricciardi, M.; Proto, A.; Bianco, P.M.; Montano, L.; Motta, O. Endocrine-disrupting compounds: An overview on their occurrence in the aquatic environment and human exposure. Water 2021, 13, 1347. [Google Scholar] [CrossRef]
- Reyes, N.J.D.G.; Geronimo, F.K.F.; Yano, K.A.V.; Guerra, H.B.; Kim, L.-H. Pharmaceutical and personal care products in different matrices: Occurrence, pathways, and treatment processes. Water 2021, 13, 1159. [Google Scholar] [CrossRef]
- Kannan, K.; Reiner, J.L.; Se, H.Y.; Perrotta, E.E.; Tao, L.; Johnson-Restrepo, B.; Rodan, B.D. Polycyclic musk compounds in higher trophic level aquatic organisms and humans from the United States. Chemosphere 2005, 61, 693–700. [Google Scholar] [CrossRef]
- Gonzalez-Gil, L.; Mauricio-Iglesias, M.; Carballa, M.; Lema, J.M. Why are organic micropollutants not fully biotransformed? A mechanistic modelling approach to anaerobic systems. Water Res. 2018, 142, 115–128. [Google Scholar] [CrossRef]
- Gonzalez-Gil, L.; Krah, D.; Ghattas, A.-K.; Carballa, M.; Wick, A.; Helmholz, L.; Lema, J.M.; Ternes, T.A. Biotransformation of organic micropollutants by anaerobic sludge enzymes. Water Res. 2019, 152, 202–214. [Google Scholar] [CrossRef]
- Jurado, A.; Vázquez-Suñé, E.; Pujades, E. Urban groundwater contamination by non-steroidal anti-inflammatory drugs. Water 2021, 13, 720. [Google Scholar] [CrossRef]
- Acosta-Herrera, A.A.; Hernández-Montoya, V.; Castillo-Borja, F.; Pérez-Cruz, M.A.; Montes-Morán, M.A.; Cervantes, F.J. Competitive adsorption of pollutants from anodizing wastewaters to promote water reuse. J. Environ. Manag. 2021, 293, 112877. [Google Scholar] [CrossRef] [PubMed]
- Das, T.K.; Scott, Q.; Bezbaruah, A.N. Montmorillonite-iron crosslinked alginate beads for aqueous phosphate removal. Chemosphere 2021, 281, 130837. [Google Scholar] [CrossRef] [PubMed]
- Vecino, X.; Devesa-Rey, R.; Moldes, A.B.; Cruz, J.M. Formulation of an alginate-vineyard pruning waste composite as a new eco-friendly adsorbent to remove micronutrients from agroindustrial effluents. Chemosphere 2014, 111, 24–31. [Google Scholar] [CrossRef]
- Vecino, X.; Devesa-Rey, R.; de Lima Stebbins, D.M.; Moldes, A.B.; Cruz, J.M.; Alcantar, N.A. Evaluation of a cactus mucilage biocomposite to remove total arsenic from water. Environ. Technol. Innov. 2016, 6, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Jawad, A.; Peng, L.; Liao, Z.; Zhou, Z.; Shahzad, A.; Ifthikar, J.; Zhao, M.; Chen, Z.; Chen, Z. Selective removal of heavy metals by hydrotalcites as adsorbents in diverse wastewater: Different intercalated anions with different mechanisms. J. Clean. Prod. 2019, 211, 1112–1126. [Google Scholar] [CrossRef]
- Zubair, M.; Daud, M.; McKay, G.; Shehzad, F.; Al-Harthi, M.A. Recent progress in layered double hydroxides (LDH)-containing hybrids as adsorbents for water remediation. Appl. Clay Sci. 2017, 143, 279–292. [Google Scholar] [CrossRef]
- Azimzadeh, Y.; Najafi, N.; Reyhanitabar, A.; Oustan, S.; Khataee, A.R. Modeling of phosphate removal by Mg-Al layered double hydroxide functionalized biochar and hydrochar from aqueous solutions. Iran. J. Chem. Chem. Eng. 2020, 40, 565–579. [Google Scholar] [CrossRef]
- Zhu, R.; Yan, L.; Song, W.; Ma, Z.; Jia, R.; Sun, S. Mechanochemical synthesis of Ca-Al-Fe layered double hydroxide for efficient phosphate removal from aqueous solution. Mater. Express 2021, 11, 524–532. [Google Scholar] [CrossRef]
- Kim, T.-H.; Lundehøj, L.; Nielsen, U.G. An investigation of the phosphate removal mechanism by MgFe layered double hydroxides. Appl. Clay Sci. 2020, 189, 105521. [Google Scholar] [CrossRef]
- Maia, M.A.; Dotto, G.L.; Perez-Lopez, O.W.; Gutterres, M. Phosphate removal from industrial wastewaters using layered double hydroxides. Environ. Technol. 2020, 6, 1–11. [Google Scholar] [CrossRef]
- Nava-Andrade, K.; Carbajal-Arízaga, G.G.; Obregón, S.; Rodríguez-González, V. Layered double hydroxides and related hybrid materials for removal of pharmaceutical pollutants from water. J. Environ. Manag. 2021, 288, 112399. [Google Scholar] [CrossRef]
- Santamaría, L.; Vicente, M.A.; Korili, S.A.; Gil, A. Progress in the removal of pharmaceutical compounds from aqueous solution using layered double hydroxides as adsorbents: A review. J. Environ. Chem. Eng. 2020, 8, 104577. [Google Scholar] [CrossRef]
- Rosset, M.; Sfreddo, L.W.; Perez-Lopez, O.W.; Féris, L.A. Effect of concentration in the equilibrium and kinetics of adsorption of acetylsalicylic acid on ZnAl layered double hydroxide. J. Environ. Chem. Eng. 2020, 8, 103991. [Google Scholar] [CrossRef]
- Rudzinski, W.; Plazinski, W. Theoretical description of the kinetics of solute adsorption at heterogeneous solid/solution interfaces. On the possibility of distinguishing between the diffusional and the surface reaction kinetics models. Appl. Surf. Sci. 2007, 253, 5827–5840. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl 1898, 24, 1–39. [Google Scholar]
- Plazinski, W.; Rudzinski, W.; Plazinska, A. Theoretical models of sorption kinetics including a surface reaction mechanism: A review. Adv. Colloid Interface Sci. 2009, 152, 2–13. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Azizian, S. Kinetic models of sorption: A theoretical analysis. J. Colloid Interface Sci. 2004, 276, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.H.; Clayton, W.R. Application of Elovich equation to the kinetics of phosphate release and sorption in soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetic of adsorption on carbon from solutions. J. Sanit Eng. Div. ASCE 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Islam, M.; Mishra, S.; Swain, S.K.; Patel, R.; Dey, R.K.; Naushad, M. Evaluation of Phosphate Removal Efficiency from Aqueous Solution by Polypyrrole/BOF Slag Nanocomposite. Separat Sci. Technol. 2014, 49, 2668–2680. [Google Scholar] [CrossRef]
- Acelas, N.Y.; Martin, B.D.; López, D.; Jefferson, B. Selective removal of phosphate from wastewater using hydrated metal oxides dispersed within anionic exchange media. Chemosphere 2015, 119, 1353–1360. [Google Scholar] [CrossRef]
- Aharoni, C.; Ungarish, M. Kinetics of activated chemisorption Part 2.-Theoretical models. J. Chem. Soc. Faraday Trans. 1 1977, 73, 456–464. [Google Scholar] [CrossRef]
- Sahin, O.I.; Saygi-Yalcin, B.; Saloglu, D. Adsorption of ibuprofen from wastewater using activated carbon and graphene oxide embedded chitosan–pva: Equilibrium, kinetics, and thermodynamics and optimization with central composite design. Desalin. Water Treat. 2020, 179, 396–417. [Google Scholar] [CrossRef]
- Vargues, F.; Brion, M.A.; Rosa da Costa, A.M.; Moreira, J.A.; Ribau Teixeira, M. Development of a magnetic activated carbon adsorbent for the removal of common pharmaceuticals in wastewater treatment. Int. J. Environ. Sci. Technol. 2021, 18, 2805–2818. [Google Scholar] [CrossRef]
- Wang, X.-J.; Zhu, X.-P.; Lan, L.-M.; Zuo, H.-B. Removal of chromium from laboratory wastewater using preparation-adsorption technology with a Mg/Al/Cr layered compound. RSC Adv. 2016, 6, 85595–85602. [Google Scholar] [CrossRef]
- Zhu, J.; Zeng, B.; Mo, L.; Jin, F.; Deng, M.; Zhang, Q. One-pot synthesis of Mg[sbnd]Al layered double hydroxide (LDH) using MgO and metakaolin (MK) as precursors. Appl. Clay Sci. 2021, 206, 106070. [Google Scholar] [CrossRef]
- Moustafa, D.; Mahmoud, R.; El-Salam, H.M.A.; Shehata, N. Utilization of residual zinc–iron-layered double hydroxide after methyl orange management as a new sorbent for wastewater treatment. Appl. Nanosci. 2021, 11, 709–723. [Google Scholar] [CrossRef]
- Cheng, F.; Nie, F.; Zhao, C.; Wu, X.; Lu, J.; Jiang, D.; Pan, J. Phosphorus adsorption characteristics and mechanism of biochar loaded Mg/Al-LDHs composites. Nongye Gongcheng Xuebao/Trans. Chin. Soc. Agric. Eng. 2021, 37, 226–234. [Google Scholar] [CrossRef]
- Gao, Z.; Sasaki, K.; Qiu, S. Structural Memory Effect of Mg–Al and Zn–Al layered Double Hydroxides in the Presence of Different Natural Humic Acids: Process and Mechanism. Langmuir 2018, 34, 5386–5395. [Google Scholar] [CrossRef] [PubMed]
- Zhong, P.; Ping, K.; Qiu, X.; Chen, F. Sorption of humic acid to layered double hydroxides prepared through ion thermal method. Desalin. Water Treat. 2017, 93, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Liu, S.; Zhang, H.; Qiu, Y.; Zhao, J.; Zhu, Z. Decontamination of arsenic in actual water samples by calcium containing layered double hydroxides from a convenient synthesis method. Water 2018, 10, 1150. [Google Scholar] [CrossRef] [Green Version]
- Kostić, M.; Radović, M.; Velinov, N.; Najdanović, S.; Bojić, D.; Hurt, A.; Bojić, A. Synthesis of mesoporous triple-metal nanosorbent from layered double hydroxide as an efficient new sorbent for removal of dye from water and wastewater. Ecotox. Environ. Saf. 2018, 159, 332–341. [Google Scholar] [CrossRef]
- Dasgupta, S. Controlled release of ibuprofen using Mg Al LDH nano carrier. IOP Conf. Ser. Mater. Sci. 2017, 225, 012005. [Google Scholar] [CrossRef] [Green Version]
- Kumari, P.; Pal, B.; Das, R.K. Superior adsorptive removal of eco-toxic drug diclofenac sodium by Zn–Al LDH⋅xBi2O3 layer double hydroxide composites. Appl. Clay Sci. 2021, 208, 106119. [Google Scholar] [CrossRef]
- Nuryadin, A.; Imai, T.; Kanno, A.; Yamamoto, K.; Sekine, M.; Higuchi, T. Phosphate adsorption and desorption on two-stage synthesized amorphous-ZrO2/Mg–Fe layered double hydroxide composite. Mater. Chem. Phys. 2021, 266, 124559. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, Q.; Hong, X.; Chen, J.; Liu, D. Synthesis of layered double hydroxides with nitrate and its adsorption properties of phosphate. Water Sci. Technol. 2021, 83, 100–110. [Google Scholar] [CrossRef]
- Sena, M.; Seib, M.; Noguera, D.R.; Hicks, A. Environmental impacts of phosphorus recovery through struvite precipitation in wastewater treatment. J. Clean. Prod. 2021, 280, 124222. [Google Scholar] [CrossRef]
- Onishi, B.S.D.; dos Reis Ferreira, C.S.; Urbano, A.; Santos, M.J. Modified hydrotalcite for phosphorus slow-release: Kinetic and sorption-desorption processes in clayey and sandy soils from North of Paraná state (Brazil). Appl. Clay Sci. 2020, 197, 105759. [Google Scholar] [CrossRef]
| LDHD (Oven-Dried) | LDHC (Calcined) | |||
|---|---|---|---|---|
| C0 (mg/L) | Ct (mg ) | Adsorption (%) | Ct (mg ) | Adsorption (%) |
| 2.5 | 1.1 | 55.4 | 1.4 | 57.8 |
| 5 | 2.6 | 47.8 | 3.6 | 71.2 |
| 10 | 5.0 | 49.8 | 7.3 | 73.5 |
| 20 | 8.6 | 56.8 | 15.5 | 77.3 |
| LDHD (Oven-Dried) | LDHC (Calcined) | |||
|---|---|---|---|---|
| C0 (mg/L) | Ct (mg ibuprofen/L) | Adsorption (%) | Ct (mg ibuprofen/L) | Adsorption (%) |
| 2.5 | 0.8 | 32.0 | 1.3 | 52.0 |
| 5 | 2.2 | 44.0 | 2.2 | 44.0 |
| 10 | 2.7 | 27.0 | 6.9 | 69.0 |
| 20 | 7.5 | 37.5 | 13.5 | 67.5 |
| LDHD (Oven-Dried) | LDHC (Calcined) | |||||||
|---|---|---|---|---|---|---|---|---|
| Phosphate | Ibuprofen | Phosphate | Ibuprofen | |||||
| C0 (mg/L) | Ct (mg/L) | Adsorption (%) | Ct (mg/L) | Adsorption (%) | Ct (mg/L) | Adsorption (%) | Ct (mg/L) | Adsorption (%) |
| 2.5 | 1.0 | 38.0 | 1.5 | 58.0 | 1.2 | 48.0 | 1.8 | 72.0 |
| 5 | 2.3 | 46.8 | 3.2 | 64.0 | 3.4 | 68.0 | 2.4 | 48.0 |
| 10 | 4.9 | 48.9 | 4.5 | 45.0 | 6.9 | 69.0 | 6.7 | 67.0 |
| 20 | 7.0 | 35.0 | 10.1 | 50.5 | 14.3 | 71.5 | 14.1 | 70.5 |
| Kinetic Model | Kinetic Parameters | LHDC | |
|---|---|---|---|
| Phosphate | Ibuprofen | ||
| Pseudo-first-order | qe exp (mg/g) | 2.822 | 2.860 |
| qe calc (mg/g) | 2.238 | 2.882 | |
| k1 (1/min) | 0.061 | 0.082 | |
| r2 | 0.928 | 0.998 | |
| Pseudo-second-order | qe exp (mg/g) | 2.822 | 2.860 |
| qe calc (mg/g) | 3.347 | 4.137 | |
| k2 (1/min) | 0.043 | 0.014 | |
| r2 | 0.982 | 0.989 | |
| Chien–Clayton model | α | 1.219 | 0.734 |
| β | 1.489 | 1.375 | |
| r2 | 0.940 | 0.948 | |
| Intraparticle diffusion model | kp | 0.453 | 0.509 |
| C | 0.268 | -0.202 | |
| r2 | 0.898 | 0.978 | |
| Bangham model | α | 0.620 | 0.838 |
| k0 | 0.003 | 0.002 | |
| r2 | 0.963 | 0.994 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devesa-Rey, R.; del Val, J.; Feijoo, J.; González-Coma, J.P.; Castiñeira, G.; González-Gil, L. Preparation of Synthetic Clays to Remove Phosphates and Ibuprofen in Water. Water 2021, 13, 2394. https://doi.org/10.3390/w13172394
Devesa-Rey R, del Val J, Feijoo J, González-Coma JP, Castiñeira G, González-Gil L. Preparation of Synthetic Clays to Remove Phosphates and Ibuprofen in Water. Water. 2021; 13(17):2394. https://doi.org/10.3390/w13172394
Chicago/Turabian StyleDevesa-Rey, Rosa, Jesús del Val, Jorge Feijoo, José P. González-Coma, Gonzalo Castiñeira, and Lorena González-Gil. 2021. "Preparation of Synthetic Clays to Remove Phosphates and Ibuprofen in Water" Water 13, no. 17: 2394. https://doi.org/10.3390/w13172394






