Degradation of Tryptophan by UV Irradiation: Influencing Parameters and Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. UV Reactor
2.3. Experimental Procedure
2.4. Analytical Methods
3. Results
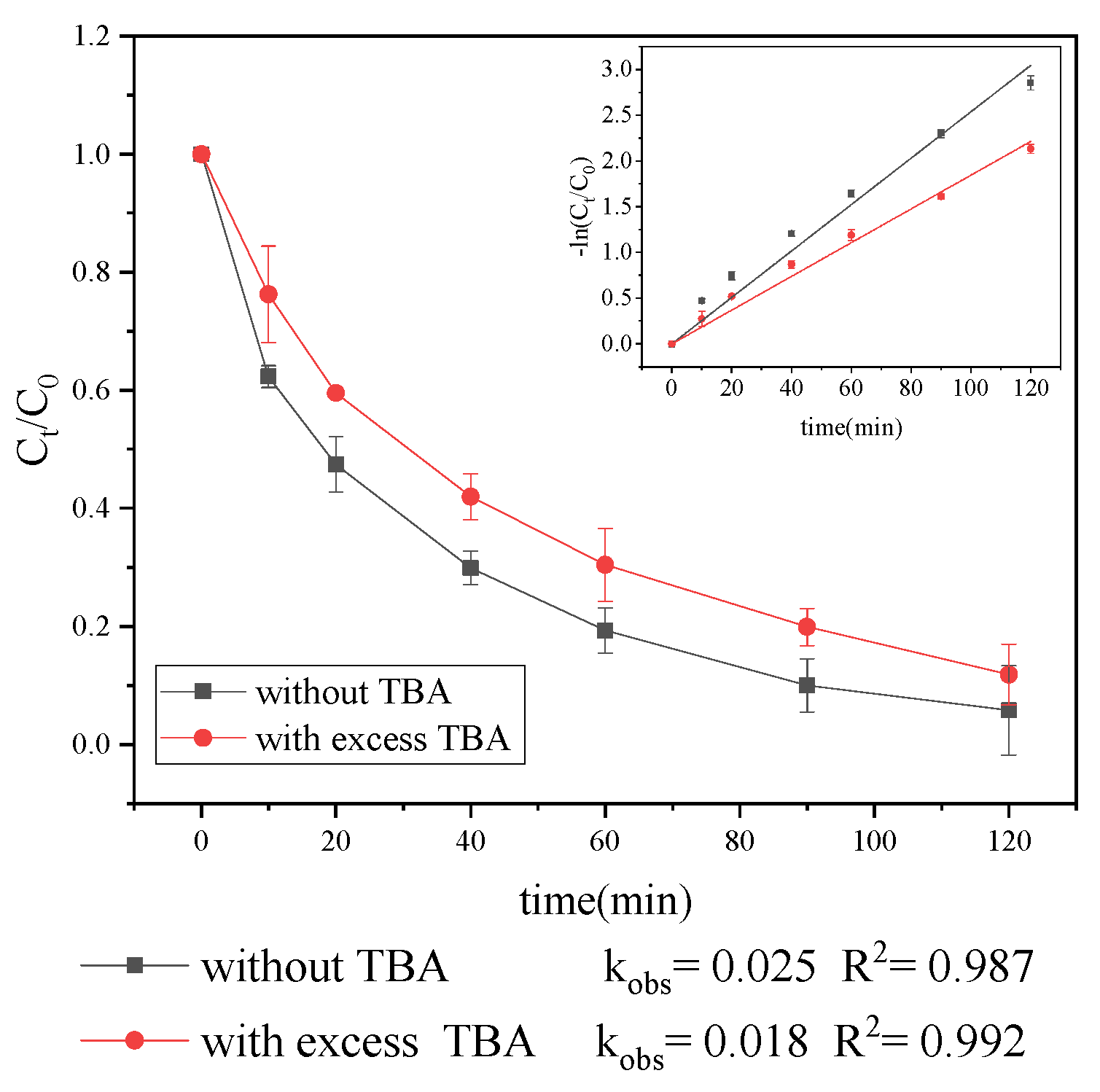
3.1. Kinetic Model of UV Degradation of Trp
e−aq + O2→·O2−
2·O2− + 2H+→H2O2 + O2
H2O2 + hv→2·OH
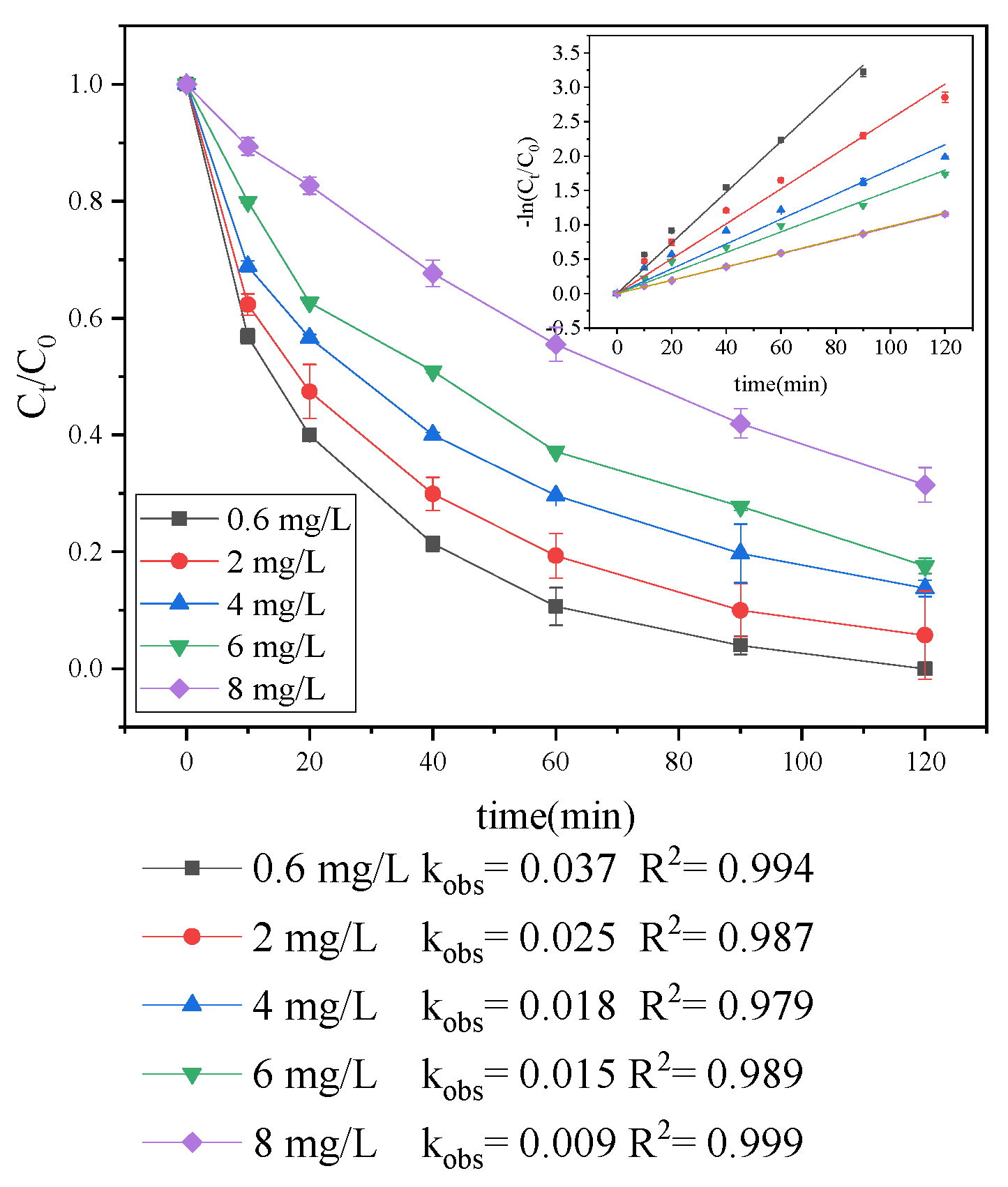
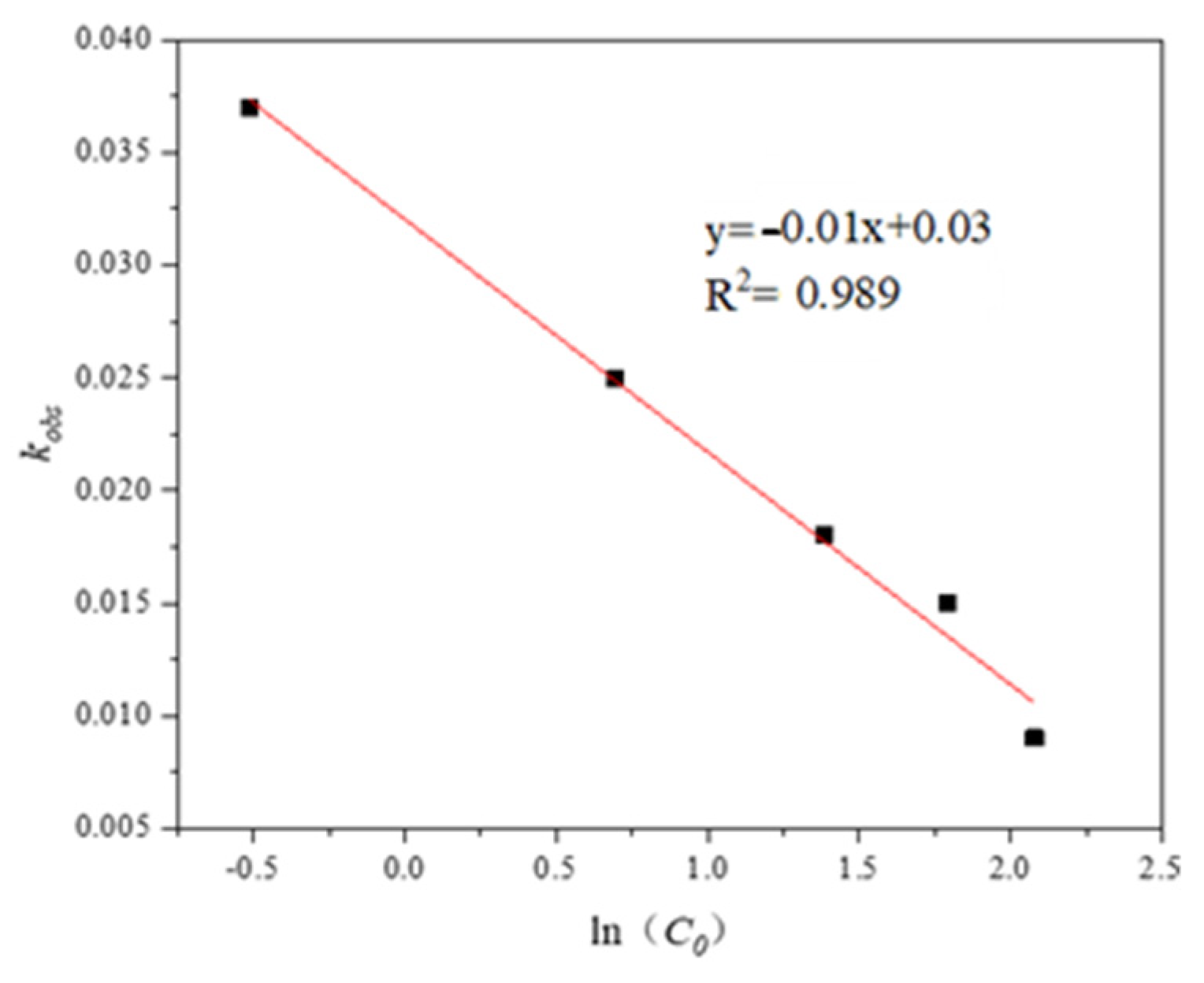
3.1.1. Effect of Initial Trp Concentration
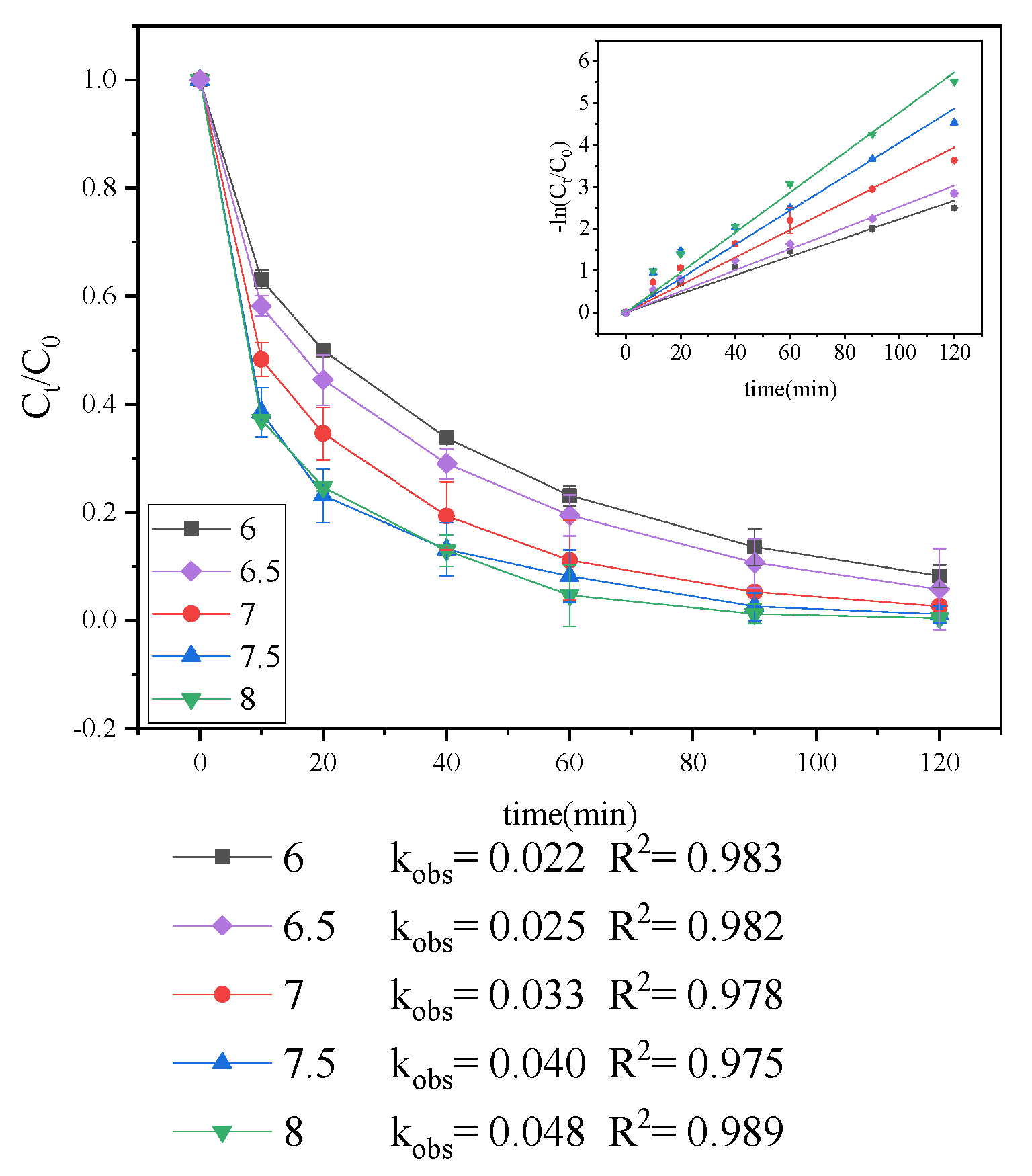
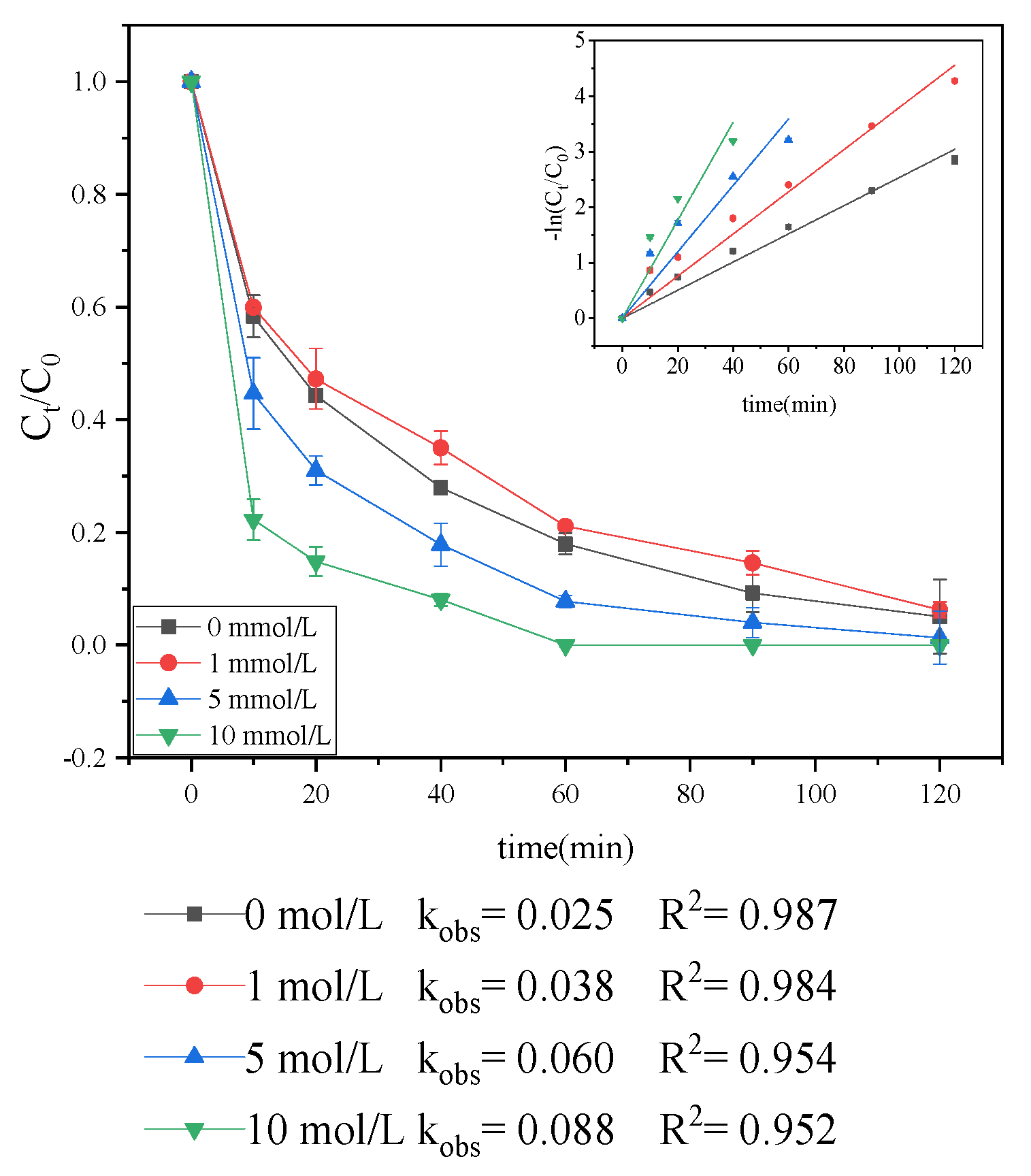
3.1.2. Effect of pH
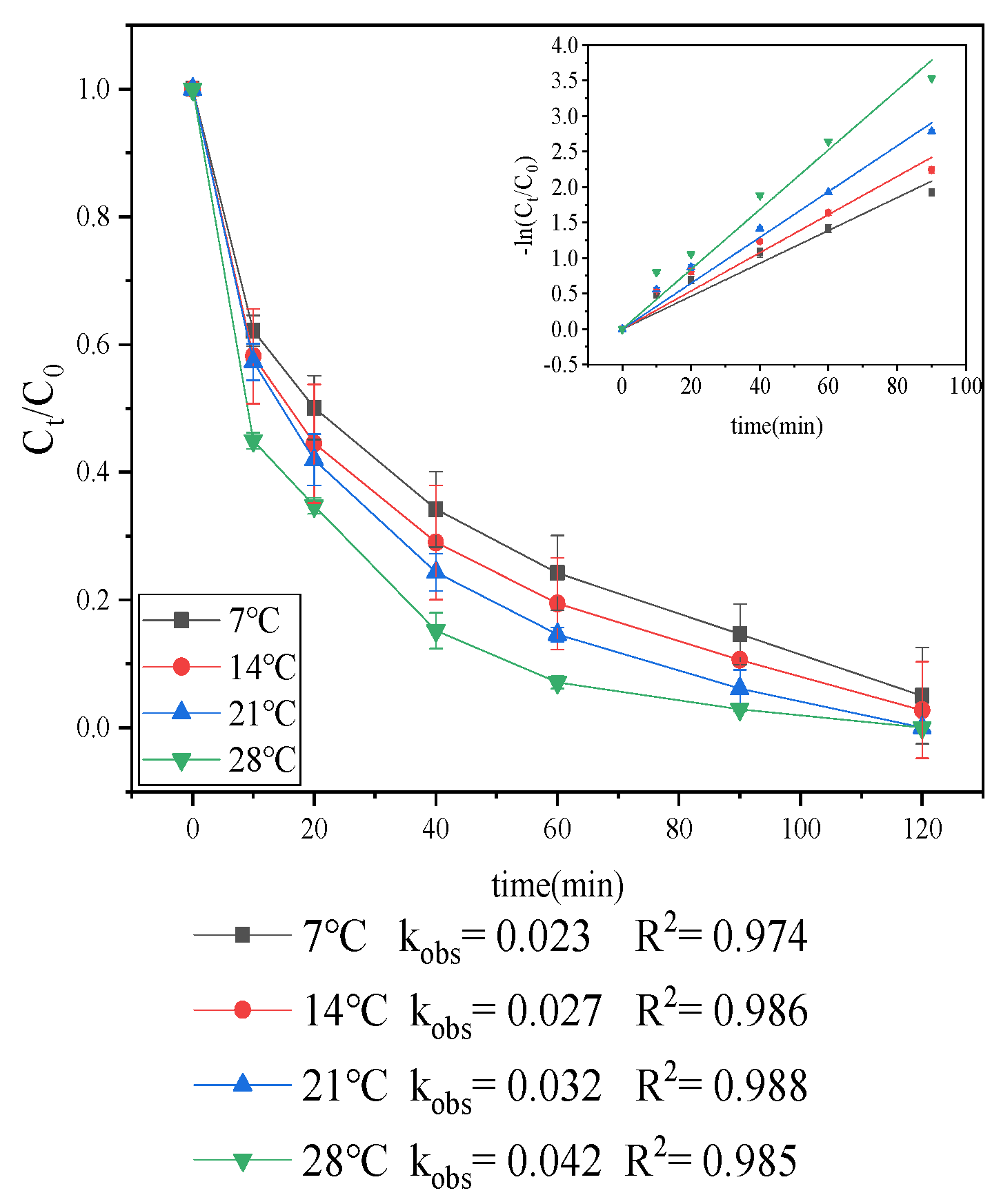
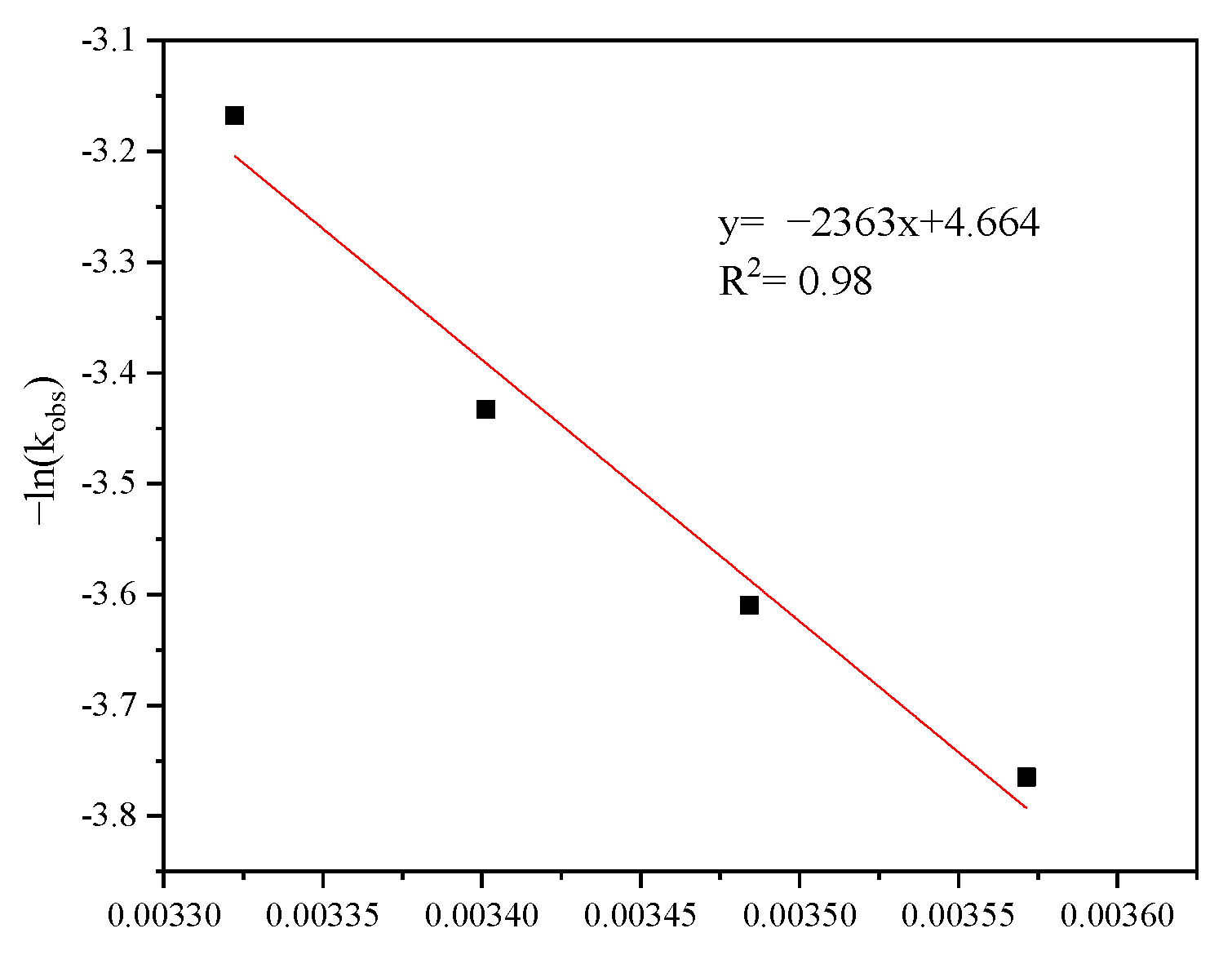
3.1.3. Effect of Temperature
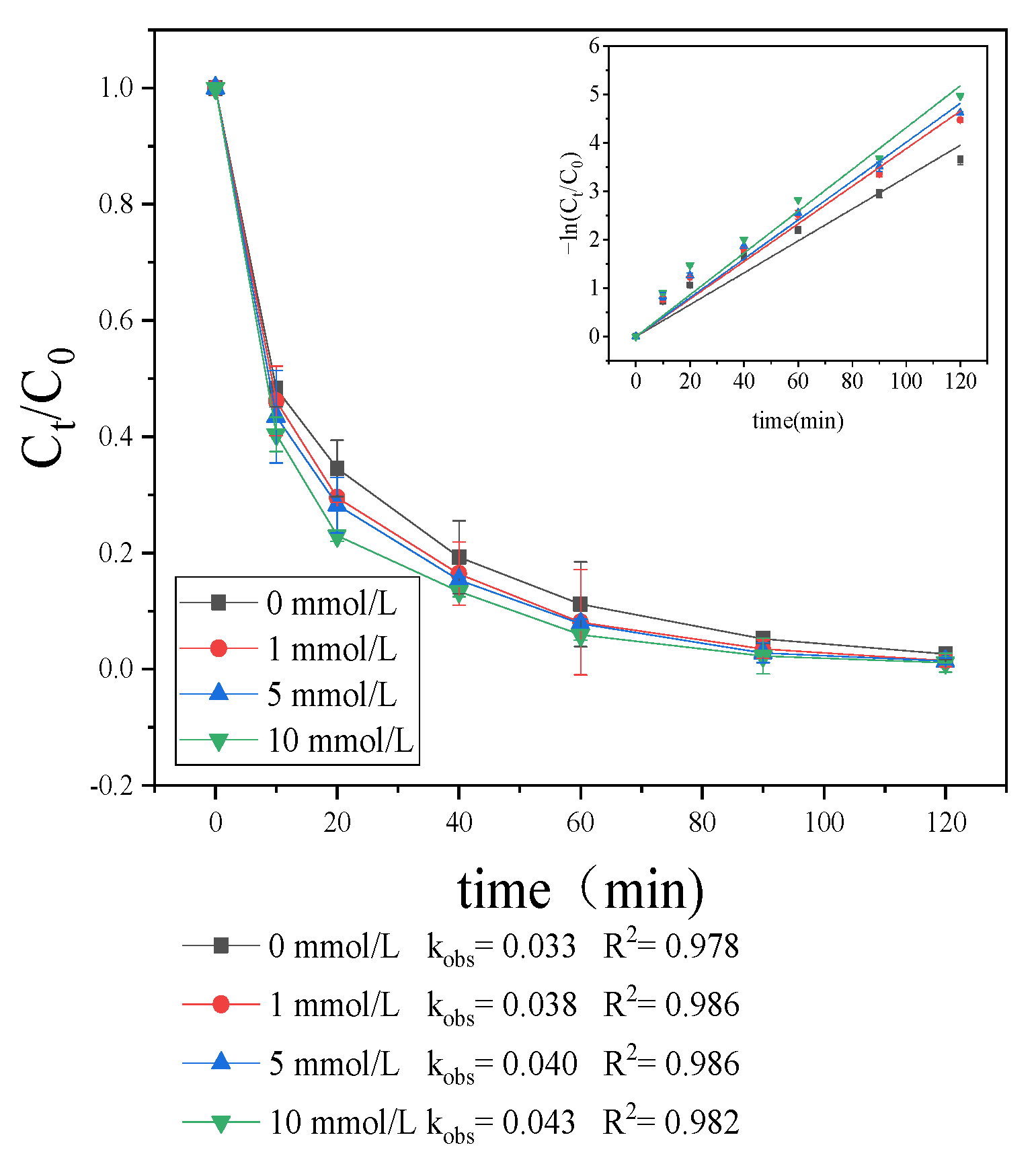
3.1.4. Effect of Water Matrix
NO3− + hv→·NO2− + 0.5O2
NO2− + hv→·ON + O−
O− + H2O→·OH + OH−
Cl− + ·OH↔HOCl−
Cl− + Cl−↔Cl2−
3.2. Mechanism of Trp Degradation
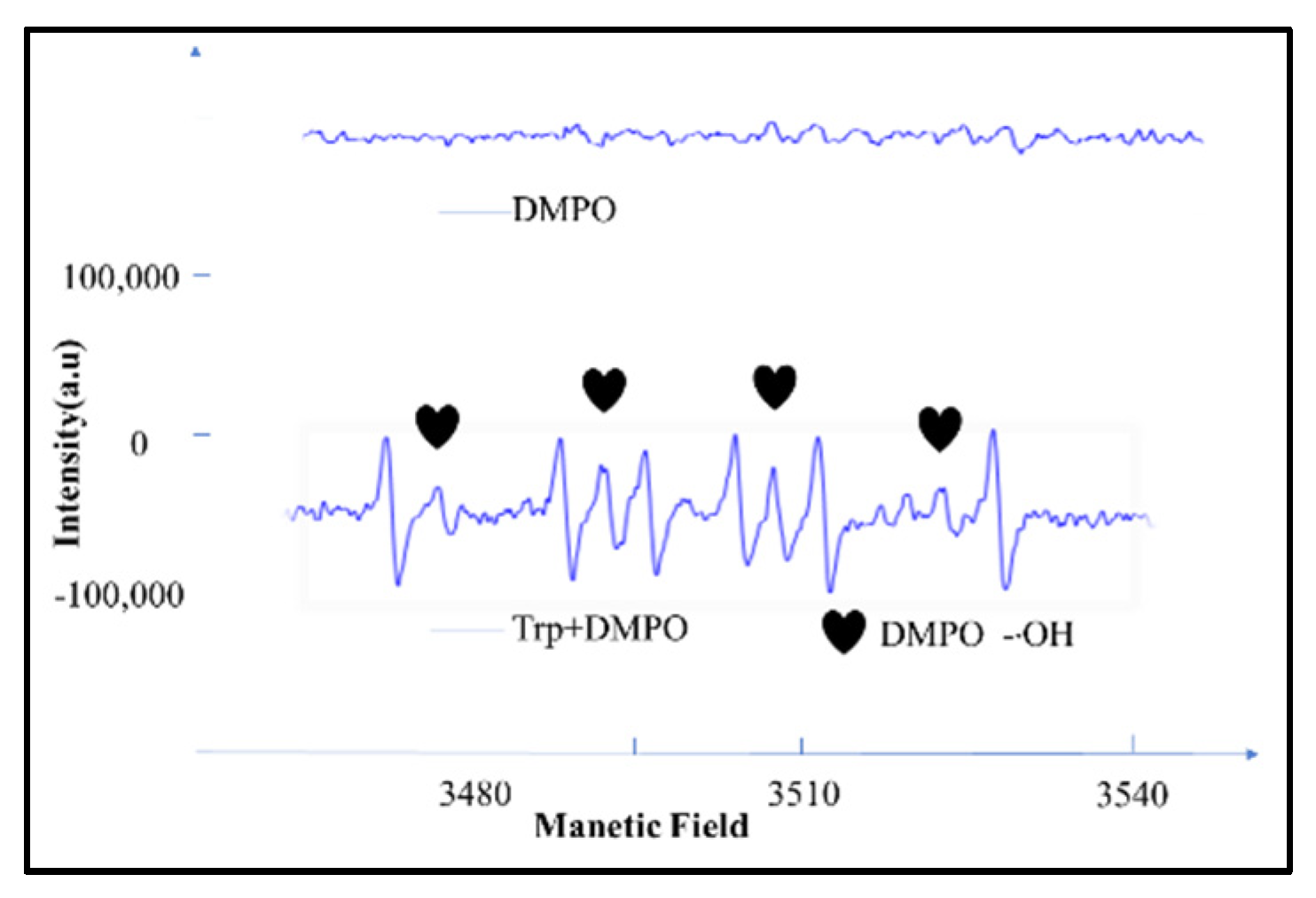
3.2.1. Generation of Radical Species
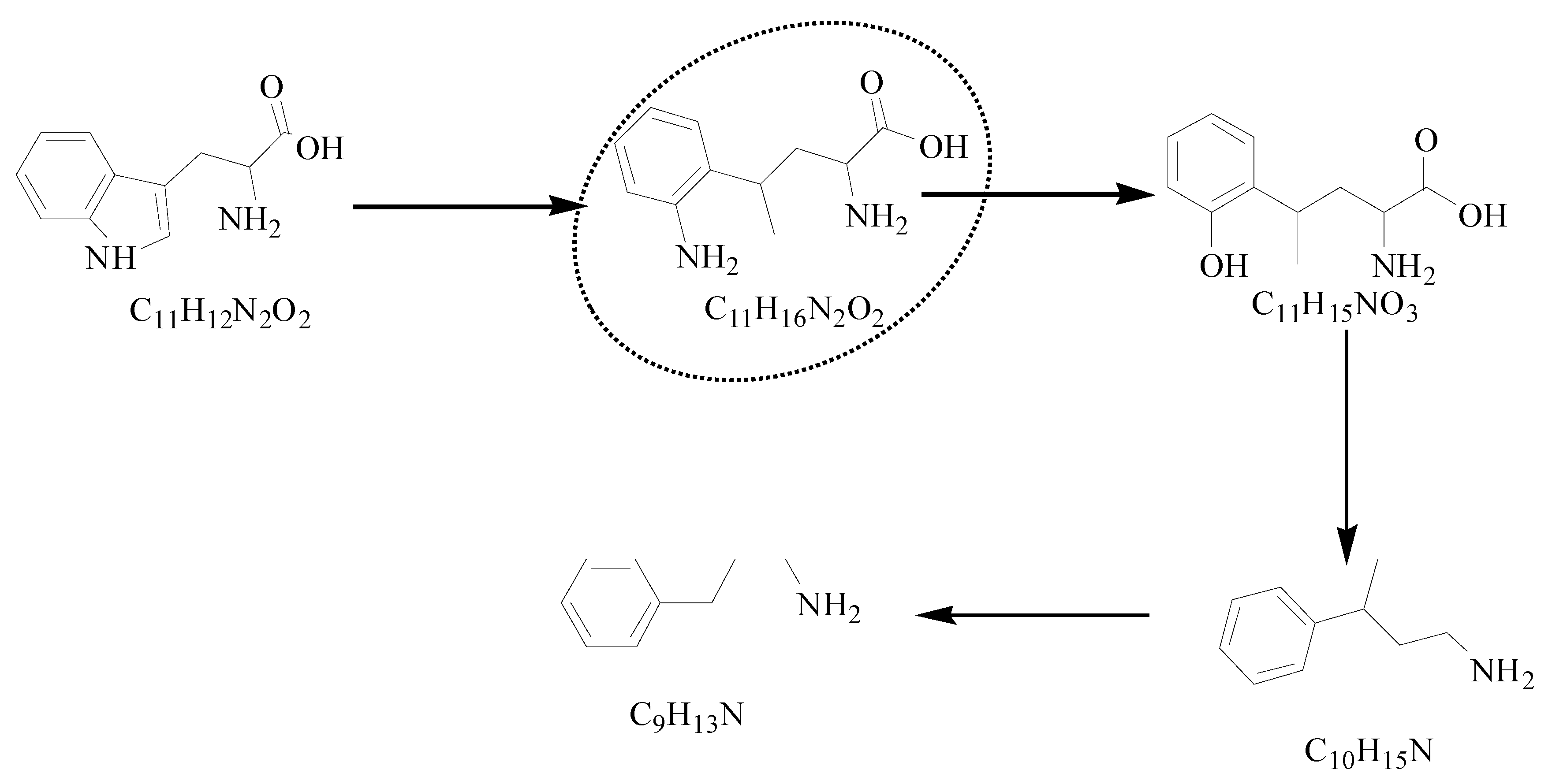
3.2.2. Degradation Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Trehy, M.L.; Yost, R.A.; Miles, C.J. Chlorination byproducts of amino acids in natural waters. Environ. Sci. Technol. 1986, 20, 1117–1122. [Google Scholar] [CrossRef]
- Cai, L.; Li, L.; Yu, S.; Guo, J.; Kuppers, S.; Dong, L. Formation of odorous by-products during chlorination of major amino acids in East Taihu Lake: Impacts of UV, UV/PS and UV/H2O2 pre-treatments. Water Res. 2019, 162, 427–436. [Google Scholar] [CrossRef]
- Dotson, A.; Westerhoff, P. Occurrence and removal of amino acids during drinking water treatment. J. Am. Water Work. Assoc. 2009, 101, 101–115. [Google Scholar] [CrossRef]
- Du, P.; Zhao, H.; Cao, H.; Huang, C.H.; Liu, W.; Li, Y. Transformation of halobenzoquinones with the presence of amino acids in water: Products, pathways and toxicity. Water Res. 2017, 122, 299–307. [Google Scholar] [CrossRef] [PubMed]
- How, Z.T.; Linge, K.L.; Busetti, F.; Joll, C.A. Formation of odorous and hazardous by-products from the chlorination of amino acids. Water Res. 2018, 146, 10–18. [Google Scholar] [CrossRef]
- Bond, T.; Templeton, M.R.; Graham, N. Precursors of nitrogenous disinfection by-products in drinking water—a critical review and analysis. J. Hazard. Mater. 2012, 235, 1–16. [Google Scholar] [CrossRef]
- Shah, A.D.; Mitch, W.A. Halonitroalkanes, halonitriles, haloamides, and N-nitrosamines: A critical review of nitrogenous disinfection byproduct formation pathways. Environ. Sci. Technol. 2012, 46, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Yao, D.; Deng, Y.; Sui, M.; Gao, N. Production of trihalomethanes, haloacetaldehydes and haloacetonitriles during chlorination of microcystin-Lr and impacts of pre-oxidation on their formation. J. Hazard. Mater. 2017, 327, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Bond, T.; Henriet, O.; Goslan, E.H.; Parsons, S.A.; Jefferson, B. Disinfection byproduct formation and fractionation behavior of natural organic matter surrogates. Environ. Sci. Technol. 2009, 43, 5982–5989. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; He, C.S.; Qian, C.; Mu, Y. Formation of iodo-trihalomethanes (I-THMs) during disinfection with chlorine or chloramine: Impact of UV/H2O2 pre-oxidation. Sci. Total Environ. 2018, 640, 764–771. [Google Scholar] [CrossRef]
- Sarathy, S.R.; Mohseni, M. The impact of UV/H2O2 advanced oxidation on molecular size distribution of chromophoric natural organic matter. Environ. Sci. Technol. 2007, 41, 8315–8320. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Zhang, H.; Hu, C.; Liu, H.; Qu, J. Effect of ozonation on the characteristics of effluent organic matter fractions and subsequent associations with disinfection by-products formation. Sci. Total Environ. 2018, 610, 1057–1064. [Google Scholar] [CrossRef]
- Choi, Y.; Choi, Y.J. The effects of UV disinfection on drinking water quality in distribution systems. Water Res. 2010, 44, 115–122. [Google Scholar] [CrossRef]
- Wang, A.Q.; Xu, B.; Zhang, T.-Y.; Chen, Y.-Y.; Gao, N.-Y. Effect of UV irradiation and UV/chlorine processes on trichloronitromethane formation during chlorination of ronidazole. CLEAN–Soil Air Water 2017, 45, 160–163. [Google Scholar] [CrossRef]
- Szajdak, L.; Osterberg, R. Amino acids present in humic acids from soils under different cultivations. Environ. Int. 1996, 22, 331–334. [Google Scholar] [CrossRef]
- Li, C.; Lin, Q.; Dong, F.; Li, Y.; Luo, F.; Zhang, K. Formation of iodinated trihalomethanes during chlorination of amino acid in waters. Chemosphere 2019, 217, 355–363. [Google Scholar] [CrossRef]
- Kaczynski, S.E.; Kieber, R.J. Hydrophobic C18 bound organic complexes of chromium and their potential impact on the geochemistry of Cr in natural-waters. Environ. Sci. Technol. 1994, 28, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, J.; Zhang, Y.; Shi, Q.; Zhang, H.; Zhang, Y.; Yang, M. Characterization of unknown iodinated disinfection byproducts during chlorination/chloramination using ultrahigh resolution mass spectrometry. Sci. Total Environ. 2016, 554, 83–88. [Google Scholar] [CrossRef]
- Beltran, F.J.; Ovejero, G.; Garcia-Araya, J.F.; Rivas, J. Oxidation of polynuclear aromatic hydrocarbons in water. 2. UV radiation and ozonation in the presence of UV radiation. Ind. Eng. Chem. Res. 1995, 34, 1607–1615. [Google Scholar] [CrossRef]
- Canonica, S.; Meunier, L.; Von Gunten, U. Phototransformation of selected pharmaceuticals during UV treatment of drinking water. Water Res. 2008, 42, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Yang, Y.; Zhou, L.; Wang, L.; Lu, J.; Ferronato, C.; Chovelon, J.M. Photodegradation of sulfasalazine and its human metabolites in water by UV and UV/peroxydisulfate processes. Water Res. 2018, 133, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Alegria, A.E.; Ferrer, A.; Sepulveda, E. Photochemistry of water-soluble quinones. production of a water-derived spin adduct. Photochem. Photobiol. 1997, 66, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Thomas-Smith, T.E.; Blough, N.V. Photoproduction of hydrated electron from constituents of natural waters. Environ. Sci. Technol. 2001, 35, 2721–2726. [Google Scholar] [CrossRef]
- Mckay, G.; Rosario-Ortiz, F.L. Temperature dependence of the photochemical formation of hydroxyl radical from dissolved organic matter. Environ. Sci. Technol. 2015, 49, 4147–4154. [Google Scholar] [CrossRef]
- Freuze, I.; Brosillon, S.; Arlot, J.; Laplanche, A.; Tozza, D.; Cavard, J. Impact of UV-irradiation on the formation of odorous chloroaldimines in drinking water. Chemosphere 2006, 63, 1660–1666. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.X.; Xu, B.; Lin, Y.L.; Hu, C.Y.; Zhang, T.Y.; Gao, N.Y. Photodegradation kinetics of iopamidol by UV irradiation and enhanced formation of iodinated disinfection by-products in sequential oxidation processes. Water Res. 2014, 58, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, W.; Bolton, J.R.; Blatchley, E.R., III; Qang, Z. Organic pollutant degradation in water by the vacuum-ultraviolet/ultraviolet/H2O2 process: Inhibition and enhancement roles of H2O2. Environ. Sci. Technol. 2019, 53, 912–918. [Google Scholar] [CrossRef]
- Xie, P.; Yue, S.; Ding, J.; Wan, Y.; Li, X.; Ma, J.; Wang, Z. Degradation of organic pollutants by Vacuum-Ultraviolet (VUV): Kinetic model and efficiency. Water Res. 2018, 133, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Guo, K.; Fang, J.; Yang, X.; Xiao, H.; Hou, S.; Kong, X.; Shang, C.; Yang, X.; Meng, F.; et al. Factors affecting the roles of reactive species in the degradation of micropollutants by the UV/chlorine process. Water Res. 2017, 126, 351–360. [Google Scholar] [CrossRef]
- Bergman, J.; Venemalm, L. Efficient synthesis of 2-chloro-, 2-bromo-, and 2-iodoindole. J. Org. Chem. 1992, 57, 2495–2497. [Google Scholar] [CrossRef]
- Xie, P.; Zou, Y.; Jiang, S.; Wang, J.; Zhang, L.; Wang, Z.; Yue, S.; Feng, X. Degradation of imipramine by vacuum ultraviolet (VUV) System: Influencing parameters, mechanisms, and variation of acute toxicity. Chemosphere 2019, 233, 282–291. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·Oh/·O−) in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Graham, N.J.D. Degradation of atrazine by manganese-catalysed ozonation—influence of radical scavengers. Water Res. 2000, 34, 3822–3828. [Google Scholar] [CrossRef]
- Tan, C.; Gao, N.; Zhou, S.; Xiao, Y.; Zhuang, Z. Kinetic study of acetaminophen degradation by UV-based advanced oxidation processes. Chem. Eng. J. 2014, 253, 229–236. [Google Scholar] [CrossRef]
- Lai, W.W.P.; Hsu, M.H.; Lin, A.Y.C. The role of bicarbonate anions in methotrexate degradation via UV/TiO2: Mechanisms, reactivity and increased toxicity. Water Res. 2017, 112, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.E.; Aldrich, J.E.; Bisby, R.H.; Cundall, R.B.; Redpath, J.L.; Willson, R.L. Selective free radical reactions with proteins and enzymes: Reactions of inorganic radical anions with amino acids. Radiat. Res. 1972, 49, 278–289. [Google Scholar] [CrossRef]
- Guo, Z.-B.; Lin, Y.-L.; Xu, B.; Hu, C.Y.; Huang, H.; Zhang, T.-Y.; Chu, W.-H.; Gao, N.-Y. Factors affecting THM, HAN and HNM formation during UV-chlor(am)ination of drinking water. Chem. Eng. J. 2016, 306, 1180–1188. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Zhang, J.; Li, C.; Tian, F.X.; Gao, N.Y. Comparative evaluation of metoprolol degradation by UV/chlorine and UV/H2O2 processes. Chemosphere 2020, 243, 125325. [Google Scholar] [CrossRef]
- Armstrong, D.A.; Huie, R.E.; Koppenol, W.H.; Lymar, S.V.; Merenyi, G.; Neta, P.; Ruscic, B.; Stanbury, D.M.; Steenken, S.; Wardman, P. Standard electrode potentials involving radicals in aqueous solution: Inorganic radicals (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1139–1150. [Google Scholar] [CrossRef]
- Guan, Y.H.; Ma, J.; Li, X.C.; Fang, J.Y.; Chen, L.W. Influence of pH on the formation of sulfate and hydroxyl radicals in the UV/peroxymonosulfate system. Environ. Sci. Technol. 2011, 45, 9308–9314. [Google Scholar] [CrossRef]
- Guo, K.; Wu, Z.; Yan, S.; Yao, B.; Song, W.; Hua, Z.; Zhang, X.; Kong, X.; Li, X.; Fang, J. Comparison of the UV/CHLORINE and UV/H2O2 processes in the degradation of PPCPs in simulated drinking water and wastewater: Kinetics, radical mechanism and energy requirements. Water Res. 2018, 147, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.B.; Lin, Y.L.; Xu, B.; Huang, H.; Zhang, T.Y.; Tian, F.X.; Gao, N.Y. Degradation of chlortoluron during UV irradiation and UV/chlorine processes and formation of disinfection by-products in sequential chlorination. Chem. Eng. J. 2016, 283, 412–419. [Google Scholar] [CrossRef]
- Watts, R.J.; Teel, A.L. Hydroxyl radical and non-hydroxyl radical pathways for trichloroethylene and perchloroethylene degradation in catalyzed H2O2 propagation systems. Water Res. 2019, 159, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Fadda, A.; Barberis, A.; Sanna, D. Influence of pH, buffers and role of quinolinic acid, a novel iron chelating agent, in the determination of hydroxyl radical scavenging activity of plant extracts by Electron Paramagnetic Resonance (EPR). Food Chem. 2018, 240, 174–182. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Fei, W.; Ji, J.; Yang, Y. Degradation of Tryptophan by UV Irradiation: Influencing Parameters and Mechanisms. Water 2021, 13, 2368. https://doi.org/10.3390/w13172368
Zhang K, Fei W, Ji J, Yang Y. Degradation of Tryptophan by UV Irradiation: Influencing Parameters and Mechanisms. Water. 2021; 13(17):2368. https://doi.org/10.3390/w13172368
Chicago/Turabian StyleZhang, Kejia, Weicheng Fei, Jingxuan Ji, and Yulong Yang. 2021. "Degradation of Tryptophan by UV Irradiation: Influencing Parameters and Mechanisms" Water 13, no. 17: 2368. https://doi.org/10.3390/w13172368
APA StyleZhang, K., Fei, W., Ji, J., & Yang, Y. (2021). Degradation of Tryptophan by UV Irradiation: Influencing Parameters and Mechanisms. Water, 13(17), 2368. https://doi.org/10.3390/w13172368





