Enhanced Transpiration by Attached Microalgae-Simulated Plants for Zero-Discharge of Reverse Osmosis Concentrated Water (WROC)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Strain
2.2. The Quality of Wastewater
2.3. Experiment Design
2.4. Analysis Method
2.4.1. Growth Index of Microalgae
2.4.2. The Water Evaporation Rate and Pollutant Concentration Monitoring
2.4.3. Statistical Analysis
3. Results and Discussion
3.1. Performance of Wastewater Treatment and Its Influence Factors
3.2. Growth of Attached Microalgae with WROC
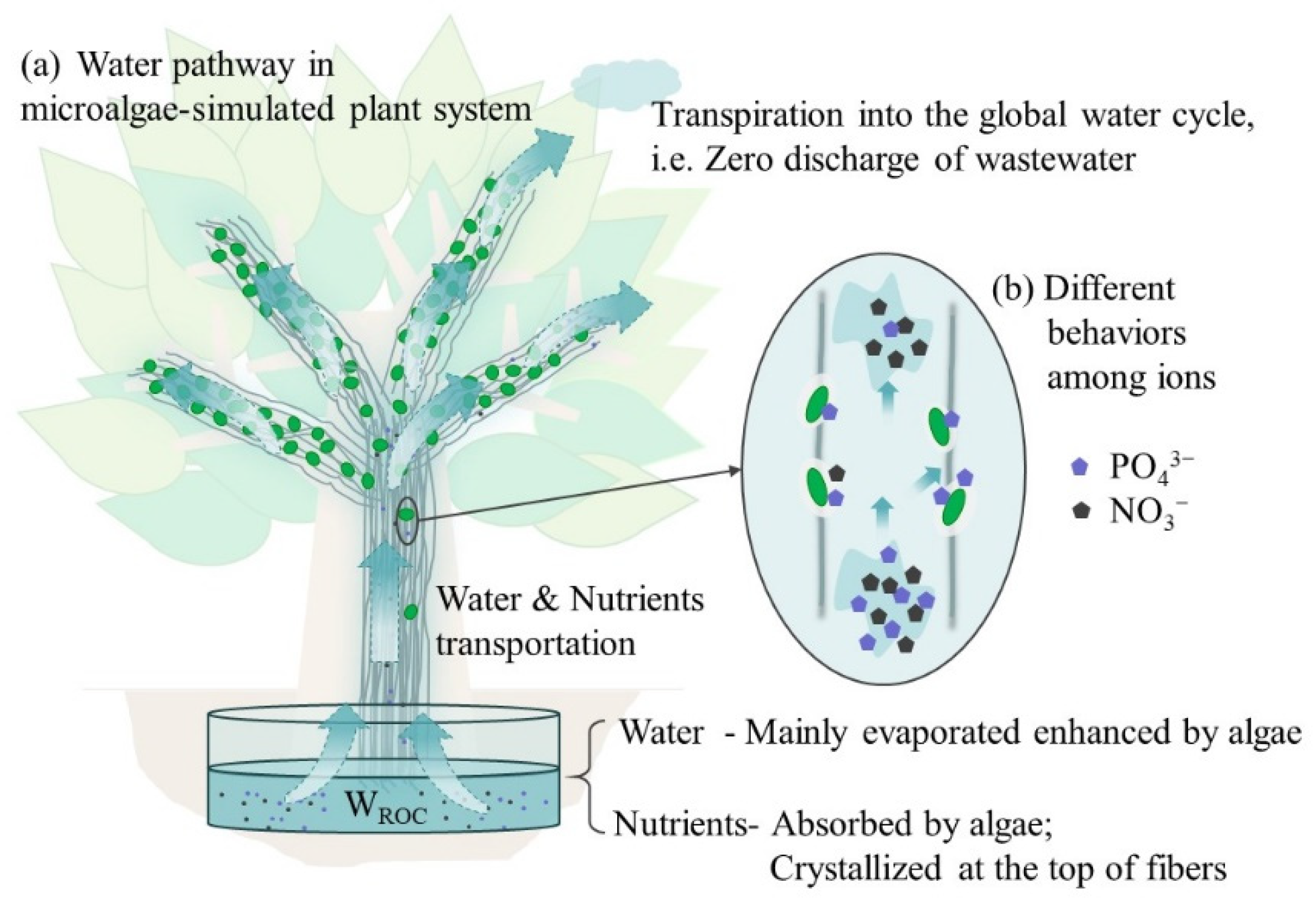
3.3. The Utilization and Distribution of Nutrients and Resource Conversion Efficiency
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hailemariam, R.H.; Woo, Y.C.; Damtie, M.M.; Kim, B.C.; Park, K.-D.; Choi, J.-S. Reverse osmosis membrane fabrication and modification technologies and future trends: A review. Adv. Colloid Interface Sci. 2020, 276, 102100. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Lienhard, J.H. How RO membrane permeability and other performance factors affect process cost and energy use: A review. Desalination 2019, 470, 114064. [Google Scholar] [CrossRef]
- Pérez-González, A.; Urtiaga, A.; Ibañez, R.; Ortiz, I. State of the art and review on the treatment technologies of water reverse osmosis concentrates. Water Res. 2012, 46, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Gu, Z.; Chen, W.; Li, Q.; Jiang, G. Removal of refractory organic pollutants in reverse-osmosis concentrated leachate by Microwave–Fenton process. Environ. Sci. Pollut. Res. 2018, 25, 28907–28916. [Google Scholar] [CrossRef]
- Deng, H. A review on the application of ozonation to NF/RO concentrate for municipal wastewater reclamation. J. Hazard. Mater. 2020, 391, 122071. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-L.; Chen, J.-Y.; Yu, J.; Liu, G.; Huang, R.-X. Analysis on emission standard-compliant technologies for treatment of reverse osmosis concentrate produced during reclamation of electroplating wastewater. Electroplat. Finish. 2014, 33, 939–942. [Google Scholar]
- Birben, N.C.; Bekbolet, M. Role of emerging contaminants on solar photocatalytic treatment of organic matter in reverse osmosis concentrate. Catal. Today 2019, 326, 101–107. [Google Scholar] [CrossRef]
- Duan, F.; Dong, W.; Tian, L.; Du, S.; Gao, M.; Wang, J. Research progress in the removing technologies of non-degradable organic substances in reverse osmosis (RO) concentrate. Ind. Water Treat. 2017, 37, 22–26. [Google Scholar] [CrossRef]
- Qiu, N.; Wang, Z.; Yang, Y.; Zhao, C.; Shi, Y. Calculation of the operating cost of sewage recycling treatment plant. China Water Wastewater 2003, 4, 97–98. [Google Scholar]
- Tang, F.; Sun, Y.; Shi, Y.; Li, X.; Hu, H. Cost analysis for a microfiltration-reverse osmosis (MF-RO) process applied in wastewater reclamation. Chin. J. Environ. Eng. 2013, 7, 417–421. [Google Scholar]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef]
- Liu, J.; Tian, J. Mass transfer mechanism of reverse osmosis concentrate brines with vacuum membrane distillation in arid areas. Desalin. Water Treat. 2019, 171, 18–28. [Google Scholar] [CrossRef]
- Subramani, A.; Jacangelo, J.G. Treatment technologies for reverse osmosis concentrate volume minimization: A review. Sep. Purif. Technol. 2014, 122, 472–489. [Google Scholar] [CrossRef]
- Pradhan, S.; Fan, L.; Roddick, F.A.; Shahsavari, E.; Ball, A.S.; Zhang, X. A comparative study of biological activated carbon based treatments on two different types of municipal reverse osmosis concentrates. Chemosphere 2020, 240, 124925. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Ocon, J.D.; Chong, M.N. Electrochemical oxidation remediation of real wastewater effluents—A review. Process. Saf. Environ. Prot. 2018, 113, 48–67. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, C.; Vaidyanathan, S. Microalgae: A robust “green bio-bridge” between energy and environment. Crit. Rev. Biotechnol. 2017, 38, 351–368. [Google Scholar] [CrossRef] [Green Version]
- Mantzorou, A.; Navakoudis, E.; Paschalidis, K.; Ververidis, F. Microalgae: A potential tool for remediating aquatic environments from toxic metals. Int. J. Environ. Sci. Technol. 2018, 15, 1815–1830. [Google Scholar] [CrossRef]
- Harris, J.; Viner, K.; Champagne, P.; Jessop, P.G. Advances in microalgal lipid extraction for biofuel production: A review. Biofuels Bioprod. Biorefining 2018, 12, 1118–1135. [Google Scholar] [CrossRef]
- Nagarajan, D.; Lee, D.-J.; Chen, C.-Y.; Chang, J.-S. Resource recovery from wastewaters using microalgae-based approaches: A circular bioeconomy perspective. Bioresour. Technol. 2020, 302, 122817. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wu, X.; Zou, G.; Zhou, T.; Liu, Y.; Ruan, R. Cultivation of Chlorella vulgaris in manure-free piggery wastewater with high-strength ammonium for nutrients removal and biomass production: Effect of ammonium concentration, carbon/nitrogen ratio and pH. Bioresour. Technol. 2019, 273, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Jais, N.M.B.; Mohamed, R.M.S.B.R.; Apandi, W.A.W.M.; Peralta, H.M.M. Removal of Nutrients and Selected Heavy Metals in Wet Market Wastewater by Using Microalgae Scenedesmus sp. Appl. Mech. Mater. 2015, 773–774, 1210–1214. [Google Scholar] [CrossRef] [Green Version]
- Roy, M.; Mohanty, K. A comprehensive review on microalgal harvesting strategies: Current status and future prospects. Algal Res. 2019, 44, 101683. [Google Scholar] [CrossRef]
- Hoffmann, J.P. Wastewater Treatment with Suspended and Nonsuspended Algae. J. Phycol. 1998, 34, 757–763. [Google Scholar] [CrossRef]
- Xu, X.-Q.; Wang, J.-H.; Zhang, T.-Y.; Dao, G.-H.; Wu, G.; Hu, H.-Y. Attached microalgae cultivation and nutrients removal in a novel capillary-driven photo-biofilm reactor. Algal Res. 2017, 27, 198–205. [Google Scholar] [CrossRef]
- Zhuang, L.-L.; Azimi, Y.; Yu, D.; Wu, Y.-H.; Hu, H.-Y. Effects of nitrogen and phosphorus concentrations on the growth of microalgae Scenedesmus. LX1 in suspended-solid phase photobioreactors (ssPBR). Biomass Bioenergy 2018, 109, 47–53. [Google Scholar] [CrossRef]
- Maeng, S.K.; Khan, W.; Park, J.W.; Han, I.; Yang, H.S.; Song, K.G.; Choi, W.J.; Kim, S.; Woo, H.; Kim, H.-C. Treatment of highly saline RO concentrate using Scenedesmus quadricauda for enhanced removal of refractory organic matter. Desalination 2018, 430, 128–135. [Google Scholar] [CrossRef]
- Maeng, S.K.; You, S.H.; Nam, J.-Y.; Ryu, H.; Timmes, T.C.; Kim, H.-C. The growth of Scenedesmus quadricauda in RO concentrate and the impacts on refractory organic matter, Escherichia coli, and trace organic compounds. Water Res. 2018, 134, 292–300. [Google Scholar] [CrossRef]
- Laguerre, O.; Lecoq, L.; Zoz, F.; Guyot, S.; Beney, L.; Flick, D. Influence of the air humidity on the drying of a liquid droplet on a solid plate and on bacterial destruction. J. Food Eng. 2017, 212, 76–86. [Google Scholar] [CrossRef]
- Shokri-Kuehni, S.M.S.; Rad, M.N.; Webb, C.; Shokri, N. Impact of type of salt and ambient conditions on saline water evaporation from porous media. Adv. Water Resour. 2017, 105, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Goncalves, E.C.; Koh, J.; Zhu, N.; Yoo, M.-J.; Chen, S.; Matsuo, T.; Johnson, J.V.; Rathinasabapathi, B. Nitrogen starvation-induced accumulation of triacylglycerol in the green algae: Evidence for a role for ROC40, a transcription factor involved in circadian rhythm. Plant J. 2016, 85, 743–757. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.K.; Mühlroth, A.; Jouhet, J.; Maréchal, E.; Alipanah, L.; Kissen, R.; Brembu, T.; Bones, A.M.; Winge, P. The Myb-like transcription factor phosphorus starvation response (PtPSR) controls conditional P acquisition and remodelling in marine microalgae. New Phytol. 2019, 225, 2380–2395. [Google Scholar] [CrossRef] [Green Version]
- Miras, A.M.; López-Rosales, L.; Cerón-García, M.C.; Sánchez-Mirón, A.; Olivera-Gálvez, A.; Garcia-Camacho, F.; Molina-Grima, E. Acclimation of the microalga Amphidinium carterae to different nitrogen sources: Potential application in the treatment of marine aquaculture effluents. Environ. Boil. Fishes 2020, 32, 1075–1094. [Google Scholar] [CrossRef]
- Kube, M.; Spedding, B.; Gao, L.; Fan, L.; Roddick, F. Nutrient removal by alginate-immobilized Chlorella vulgaris: Response to different wastewater matrices. J. Chem. Technol. Biotechnol. 2020, 95, 1790–1799. [Google Scholar] [CrossRef]
- Fang, F.; Lu, W.-T.; Shan, Q.; Cao, J.-S. Characteristics of extracellular polymeric substances of phototrophic biofilms at different aquatic habitats. Carbohydr. Polym. 2014, 106, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Salama, Y.; Chennaoui, M.; Sylla, A.; Mountadar, M.; Rihani, M.; Assobhei, O. Characterization, structure, and function of extracellular polymeric substances (EPS) of microbial biofilm in biological wastewater treatment systems: A review. Desalin. Water Treat. 2016, 57, 16220–16237. [Google Scholar] [CrossRef]
- Goldman, J.C.; McCarthy, J.J.; Peavey, D.G. Growth rate influence on the chemical composition of phytoplankton in oceanic waters. Nat. Cell Biol. 1979, 279, 210–215. [Google Scholar] [CrossRef]





| Fraction | Concentration (mg·L−1) | Fraction | Concentration (mg·L−1) |
|---|---|---|---|
| C6H12O6 | 93.7 | A5 solution | |
| NaNO3 | 182.0 | H3BO3 | 2.9 |
| KH2PO4·3H2O | 77.5 | MnCl2·4H2O | 1.8 |
| MgCl2·6H2O | 253.7 | ZnSO4·7H2O | 0.22 |
| KCl | 133.7 | Na2MoO4·2H2O | 0.39 |
| NaHCO3 | 263.0 | CuSO4·5H2O | 0.08 |
| NaSO4 | 443.8 | Co(NO3)2·6H2O | 0.05 |
| NaCl | 243.0 |
| Dilution Concentration of WROC | “I” Type without Microalgae (L·m−2·Day−1) | “Y” Type with Microalgae (L·m−2·Day−1) | “Y” Type without Microalgae (L·m−2·Day−1) | “I” Type with Microalgae (L·m−2·Day−1) |
|---|---|---|---|---|
| 20% | N.D | 3.05 ± 0.14 | N.D | 3.93 ± 0.08 |
| 40% | N.D | 3.05 ± 0.09 | N.D | 4.29 ± 0.07 |
| 60% | N.D | 5.07 ± 0.12 | N.D | 3.22 ± 0.11 |
| 80% | N.D | 3.22 ± 0.08 | N.D | 2.15 ± 0.06 |
| 100% | N.D | 3.05 ± 0.10 | N.D | 2.15 ± 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Yang, Y.; Yang, T.; Shi, Q.; Zhuang, L.-L. Enhanced Transpiration by Attached Microalgae-Simulated Plants for Zero-Discharge of Reverse Osmosis Concentrated Water (WROC). Water 2021, 13, 2058. https://doi.org/10.3390/w13152058
Yu H, Yang Y, Yang T, Shi Q, Zhuang L-L. Enhanced Transpiration by Attached Microalgae-Simulated Plants for Zero-Discharge of Reverse Osmosis Concentrated Water (WROC). Water. 2021; 13(15):2058. https://doi.org/10.3390/w13152058
Chicago/Turabian StyleYu, Huifang, Yanan Yang, Ting Yang, Qi Shi, and Lin-Lan Zhuang. 2021. "Enhanced Transpiration by Attached Microalgae-Simulated Plants for Zero-Discharge of Reverse Osmosis Concentrated Water (WROC)" Water 13, no. 15: 2058. https://doi.org/10.3390/w13152058
APA StyleYu, H., Yang, Y., Yang, T., Shi, Q., & Zhuang, L.-L. (2021). Enhanced Transpiration by Attached Microalgae-Simulated Plants for Zero-Discharge of Reverse Osmosis Concentrated Water (WROC). Water, 13(15), 2058. https://doi.org/10.3390/w13152058







