Three-Dimensional Electrochemical Oxidation of Recalcitrant Dye Using Green Iron Microparticles
Abstract
:1. Introduction
2. Material and Methodology
2.1. Chemicals Used during the Process
2.2. Production of Iron Microparticles (Fe-MPs)
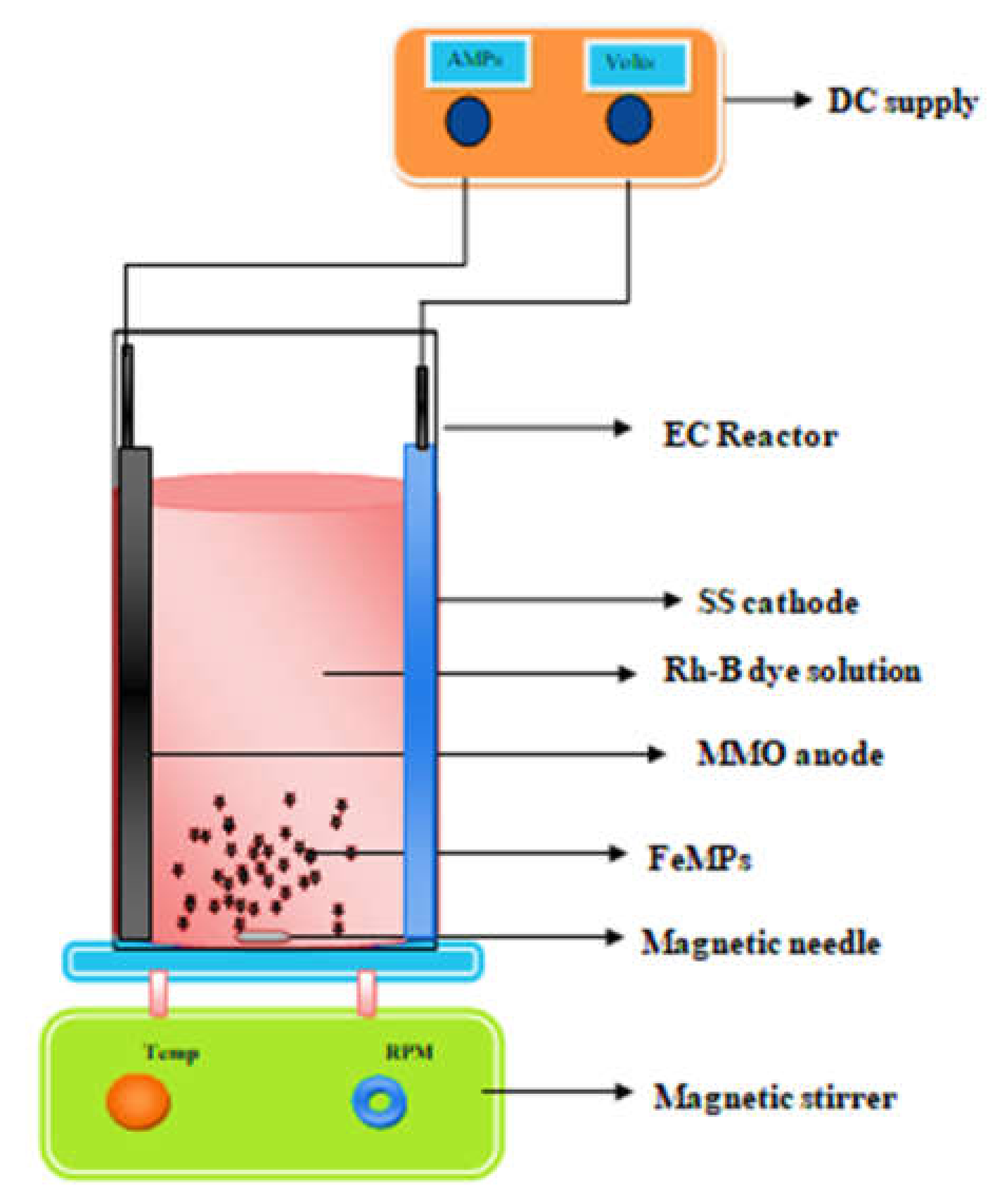
2.3. Electrochemical Experimentsin 3D Approach
3. Results and Discussion
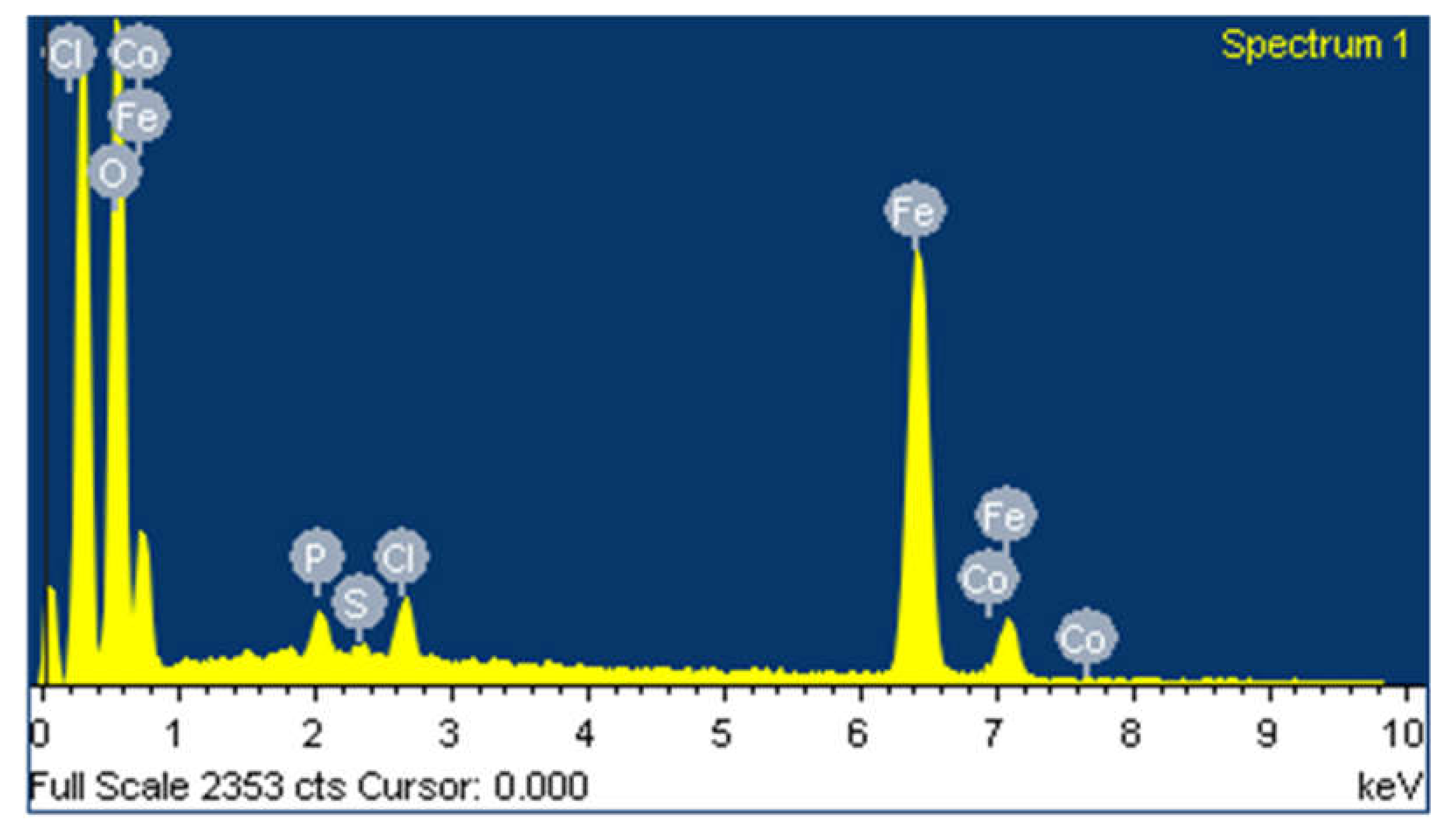
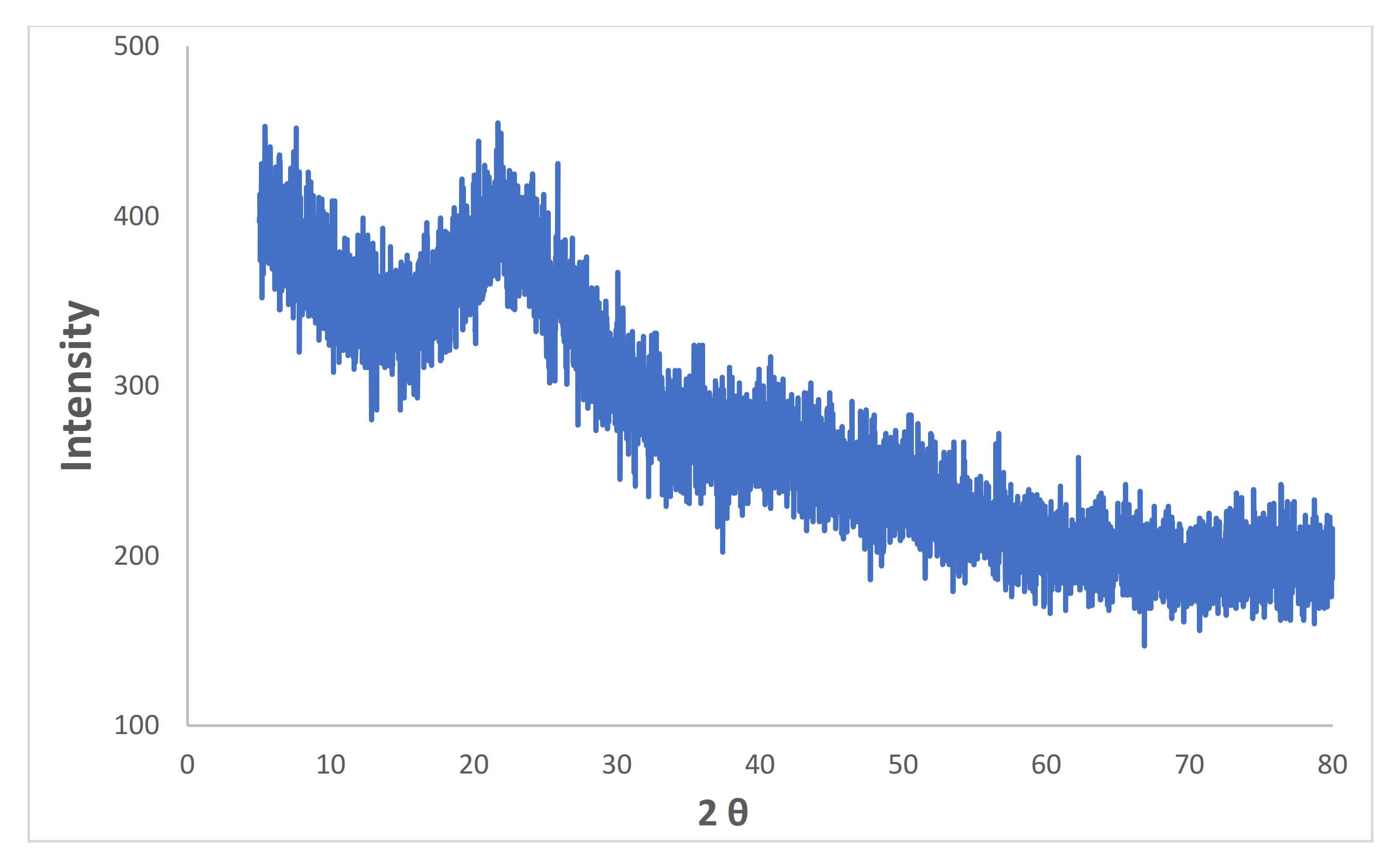
3.1. Characterization of Synthesized Iron Microparticles
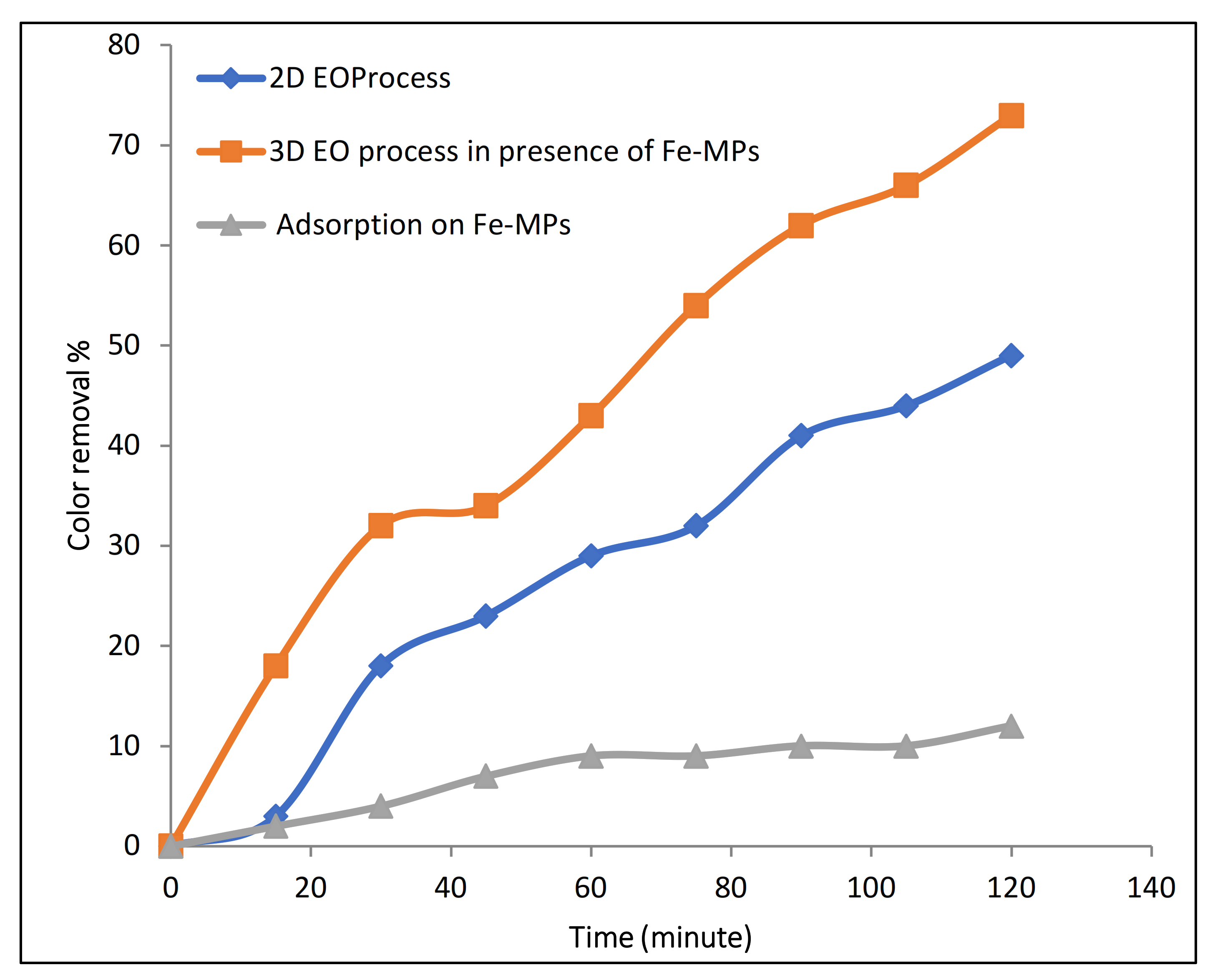
3.2. Results of 3D Electrochemical Process
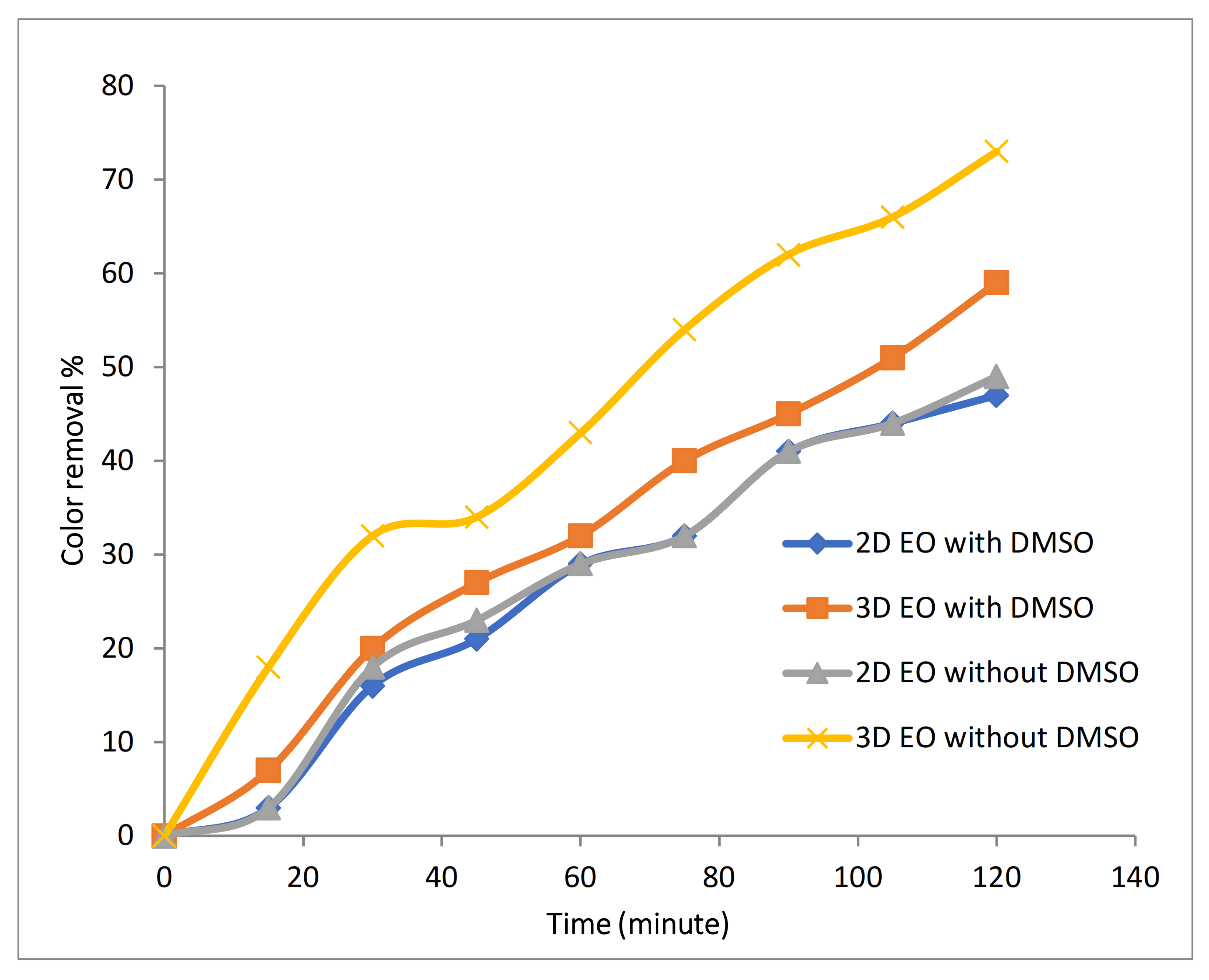
3.2.1. Evaluation of Catalytic Effect of Fe-MP as Third Electrode
3.2.2. Analysis of Free Radicals
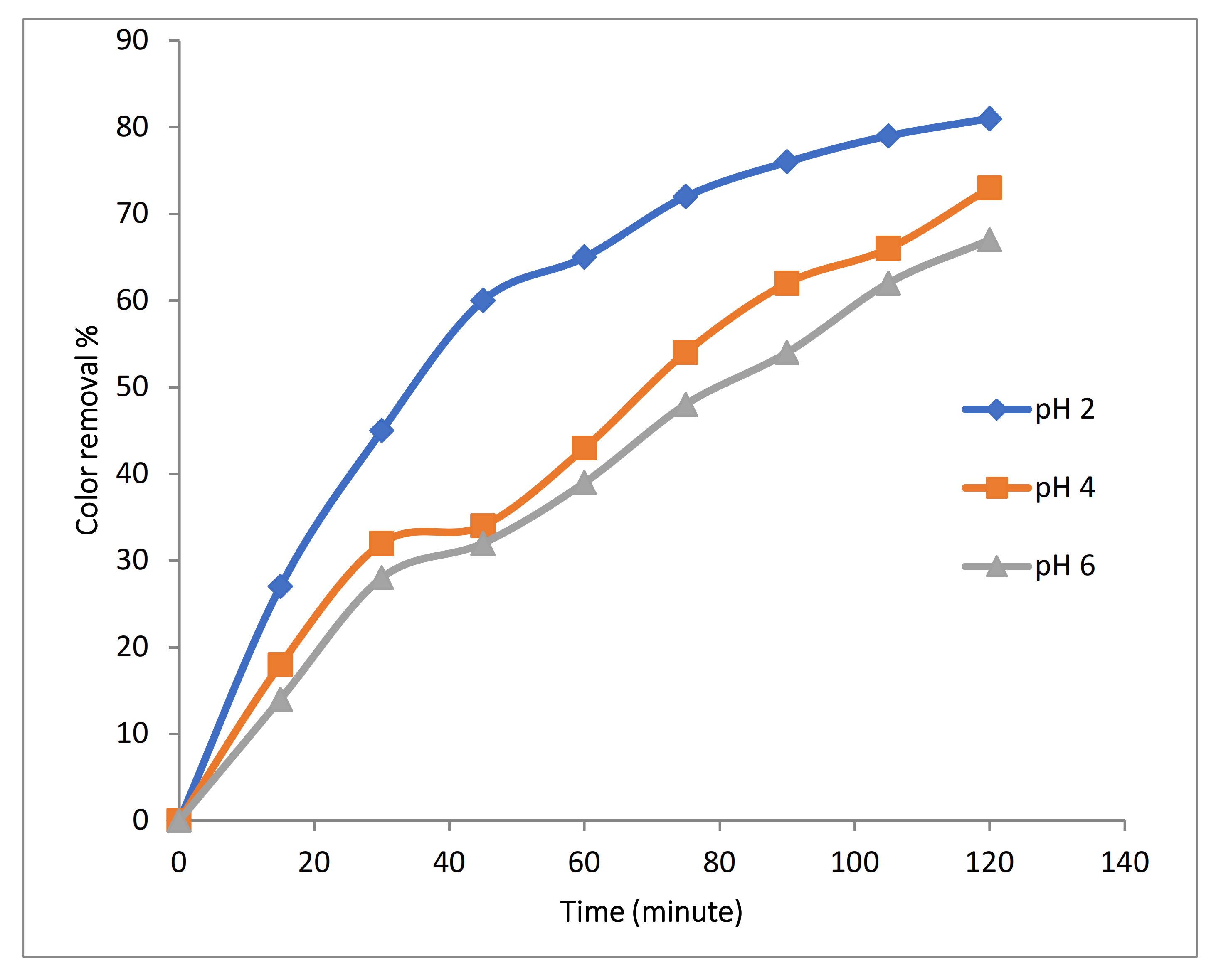
3.2.3. Effect of pH on 3D EO Process
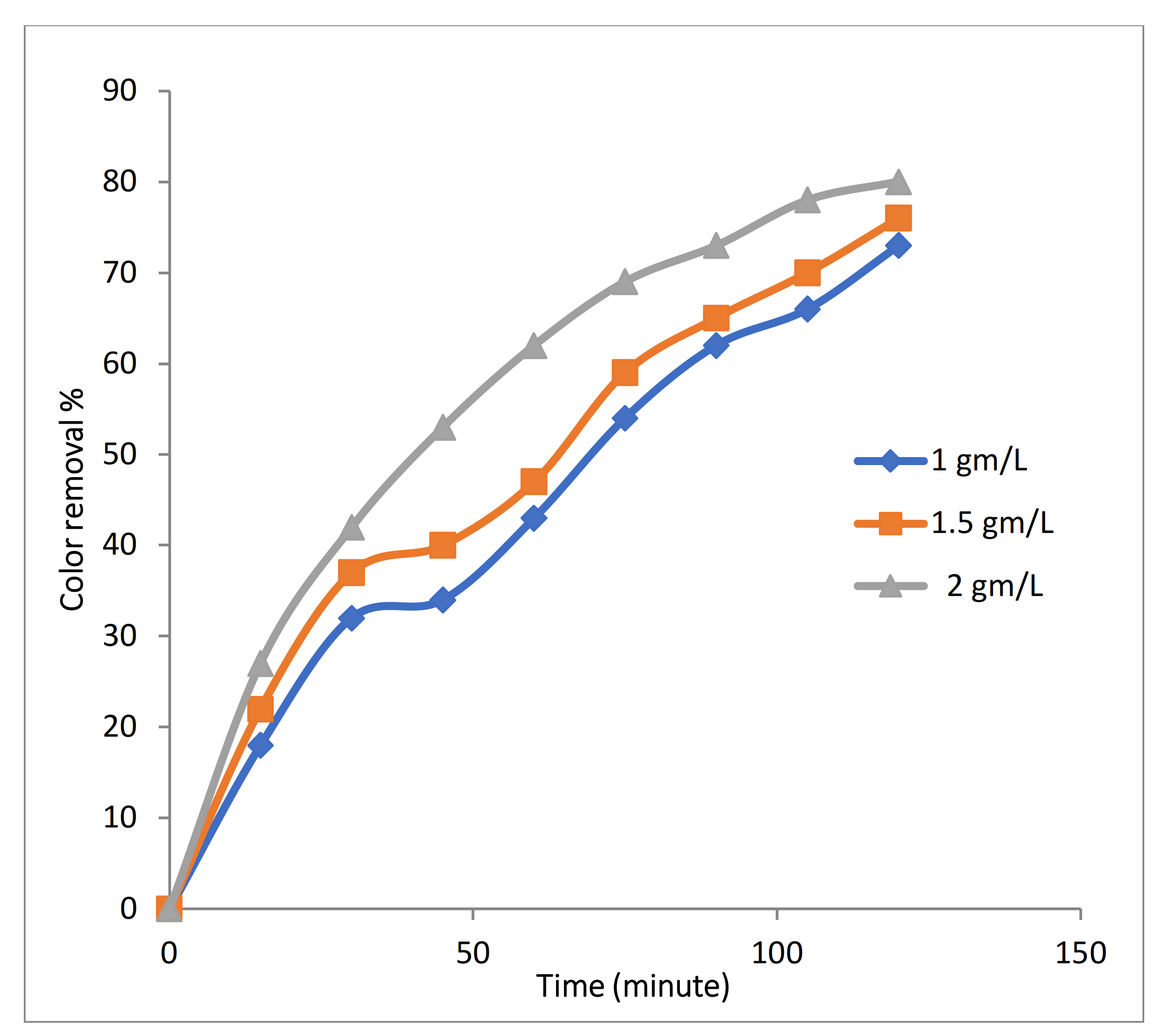
3.2.4. Effect of Fe-MP Dose on 3D EO Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Martinez-Huitle, C.A.; Brillas, E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review. Appl. Catal. B Environ. 2009, 87, 105–145. [Google Scholar] [CrossRef]
- Forgacs, E.; Cserhati, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Gutierrez, M.C.; Crespi, M. A review of electrochemical treatments for colour elimination. Color. Technol. 1999, 115, 342–345. [Google Scholar] [CrossRef]
- Naim, M.M.; El Abd, Y.M. Removal and recovery of dyestuffs from dyeing wastewaters. Sep. Purif. Methods 2002, 31, 171–228. [Google Scholar] [CrossRef]
- Hao, O.J.; Kim, H.; Chiang, P.-C. Decolorization of wastewater. Crit. Rev. Environ. Sci. Technol. 2000, 30, 449–505. [Google Scholar] [CrossRef]
- Simond, O.; Schaller, V.; Comninellis, C. Theoretical model for the anodic oxidation of organics on metal oxide electrodes. Electrochim. Acta 1997, 42, 2009–2012. [Google Scholar] [CrossRef]
- Kotz, R.; Stucki, S.; Carcer, B. Electrochemical waste water treatment using high overvoltage anodes. Part I: Physical and electrochemical properties of SnO2 anodes. J. Appl. Electrochem. 1991, 21, 14–20. [Google Scholar] [CrossRef]
- Yu, D.; Wang, L.; Yang, T.; Yang, G.; Wang, D.; Huagang, N.; Wu, M. Tuning Lewis acidity of iron-based metal-organic frameworks for enhanced catalytic ozonation. Chem. Eng. J. 2021, 404, 127075. [Google Scholar] [CrossRef]
- Yang, T.; Yu, D.; Wang, D.; Yang, T.; Li, Z.; Wu, M.; Crittenden, J. Accelerating Fe (III) / Fe (II) cycle via Fe (II) substitution for enhancing Fenton-like performance of Fe-MOFs. Appl. Catal. B Environ. 2021, 286, 119859. [Google Scholar]
- Rodrigo, M.A.; Canizares, P.; Sanchez-Carretero, A.; Saez, C. Use of conductive-diamond electrochemical oxidation for wastewater treatment. Catal. Today 2010, 151, 173–177. [Google Scholar] [CrossRef]
- Canizares, P.; Saez, C.; Sanchez-Carretero, A.; Rodrigo, M.A. Influence of the characteristics of p-Si BDD anodes on the efficiency of peroxodi phosphate electrosynthesis process. Electrochem. Commun. 2008, 10, 602–606. [Google Scholar] [CrossRef]
- Brillas, E.; Sirés, I.; Arias, C.; Cabot, P.L.; Centellas, F.; Rodríguez, R.M.; Garrido, J.A. Mineralization of paracetamol in aqueous medium by anodic oxidation with a boron-doped diamond electrode. Chemosphere 2005, 58, 399–406. [Google Scholar] [CrossRef]
- Kothari, M.S.; Shah, K.A. Electrochemical oxidation for decolorization of Rhodamine-B dye using mixed metal oxide electrode: Modeling and optimization. Water Sci. Technol. 2020, 81, 720–731. [Google Scholar] [CrossRef]
- Rajeshwar, K.; Ibanez, J.G. Environmental Electrochemistry: Fundamentals and Applications in Pollution Sensors and Abatement; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Mohan, N.; Balasubramanian, N.; Subramanian, V. Electrochemical treatment of simulated textile effluent. Chem. Eng. Technol. Ind. Chem. Plant Equip. Process Eng. Biotechnol. 2001, 24, 749–753. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Removal of colour and COD from wastewater containing acid blue 22 by electrochemical oxidation. J. Hazard. Mater. 2008, 153, 83–88. [Google Scholar] [CrossRef]
- Mohan, N.; Balasubramanian, N. In situ electrocatalytic oxidation of acid violet 12 dye effluent. J. Hazard. Mater. 2006, 136, 239–243. [Google Scholar] [CrossRef]
- Isarain-Chávez, E.; Baró, M.D.; Rossinyol, E.; Morales-Ortiz, U.; Sort, J.; Brillas, E.; Pellicer, E. Comparative electrochemical oxidation of methyl orange azo dye using Ti/Ir-Pb, Ti/Ir-Sn, Ti/Ru-Pb, Ti/Pt-Pd and Ti/RuO2 anodes. Electrochim. Acta 2017, 244, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Rajkumar, D.; Kim, J.G. Oxidation of various reactive dyes with in situ electro-generated active chlorine for textile dyeing industry wastewater treatment. J. Hazard. Mater. 2006, 136, 203–212. [Google Scholar] [CrossRef]
- Rajkumar, K.; Muthukumar, M. Optimization of electro-oxidation process for the treatment of Reactive Orange 107 using response surface methodology. Environ. Sci. Pollut. Res. 2012, 19, 148–160. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Gandhimathi, R.; Velmathi, S.; Sanjini, N.S. Magnetite as a heterogeneous electro Fenton catalyst for the removal of Rhodamine B from aqueous solution. RSC Adv. 2014, 4, 5698–5708. [Google Scholar] [CrossRef]
- Yue, L.; Wang, K.; Guo, J.; Yang, J.; Luo, X.; Lian, J.; Wang, L. Enhanced electrochemical oxidation of dye wastewater with Fe2O3 supported catalyst. J. Ind. Eng. Chem. 2014, 20, 725–731. [Google Scholar] [CrossRef]
- Rosales, E.; Iglesias, O.; Pazos, M.; Sanroman, M.A. Decolourisation of dyes under electro-Fenton process using Fe alginate gel beads. J. Hazard. Mater. 2012, 213, 369–377. [Google Scholar] [CrossRef]
- Wang, Z.; Qi, J.; Feng, Y.; Li, K.; Li, X. Fabrication and electrocatalytic performance of a novel particle electrode. Catal. Commun. 2014, 46, 165–168. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, Y.; Li, Y.; Hu, Z.; Zhou, L.; Zhou, M. Three-dimensional electrochemical process for wastewater treatment: A general review. Chem. Eng. J. 2013, 228, 455–467. [Google Scholar] [CrossRef]
- Wang, C.-T.; Hu, J.-L.; Chou, W.-L.; Kuo, Y.-M. Removal of color from real dyeing wastewater by Electro-Fenton technology using a three-dimensional graphite cathode. J. Hazard. Mater. 2008, 152, 601–606. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, L.; Yang, J.; Yu, X.; Jiang, Y.; Zhou, M. Nanoscale zero-valent iron/AC as heterogeneous Fenton catalysts in three-dimensional electrode system. Environ. Sci. Pollut. Res. 2014, 21, 8398–8405. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qi, J.; Feng, Y.; Li, K.; Li, X. Preparation of catalytic particle electrodes from steel slag and its performance in a three-dimensional electrochemical oxidation system. J. Ind. Eng. Chem. 2014, 20, 3672–3677. [Google Scholar] [CrossRef]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of nanoparticles: Technological concepts and future applications. J. Nanoparticle Res. 2008, 10, 507–517. [Google Scholar] [CrossRef]
- Shobha, G.; Moses, V.; Ananda, S. Biological synthesis of copper nanoparticles and its impact. Int. J. Pharm. Sci. Inven. 2014, 3, 6–28. [Google Scholar]
- Latha, N.; Gowri, M. Bio synthesis and characterisation of Fe3O4 nanoparticles using Caricaya Papaya leaves extract. Synthesis 2014, 3, 1551–1556. [Google Scholar]
- Pattanayak, M.; Nayak, P.L. Green synthesis and characterization of zero valent iron nanoparticles from the leaf extract of Azadirachta indica (Neem). World J. Nano Sci. Technol. 2013, 2, 6–9. [Google Scholar]
- Gottimukkala, K.S.V.; Harika, R.P.; Zamare, D. Green synthesis of iron nanoparticles using green tea leaves extract. J. Nanomed. Biother. Discov. 2017, 7, 151. [Google Scholar]
- Sharma, V.; Sharma, J. Electron microscopy study of green synthesized zero valent Iron nanoparticle. Int. J. Eng. Technol. Sci. Res. 2017, 4, 654–658. [Google Scholar]
- Kouhbanani, M.A.J.; Beheshtkhoo, N.; Taghizadeh, S.; Amani, A.M.; Alimardani, V. One-step green synthesis and characterization of iron oxide nanoparticles using aqueous leaf extract of Teucrium polium and their catalytic application in dye degradation. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 015007. [Google Scholar] [CrossRef]
- Xiao, Z.; Yuan, M.; Yang, B.; Liu, Z.; Huang, J.; Sun, D. Plant-mediated synthesis of highly active iron nanoparticles for Cr (VI) removal: Investigation of the leading biomolecules. Chemosphere 2016, 150, 357–364. [Google Scholar] [CrossRef]
- Ban, A.; Schafer, A.; Wendt, H. Fundamentals of electrosorption on activated carbon for wastewater treatment of industrial effluents. J. Appl. Electrochem. 1998, 28, 227–236. [Google Scholar] [CrossRef]
- Wu, Z.; Cong, Y.; Zhou, M.; Tan, T. p-Nitrophenol abatement by the combination of electrocatalysis and activated carbon. Chem. Eng. J. 2005, 106, 83–90. [Google Scholar] [CrossRef]
- Navalon, S.; Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Heterogeneous Fenton catalysts based on activated carbon and related materials. ChemSusChem 2011, 4, 1712–1730. [Google Scholar] [CrossRef]
- Wei, L.; Guo, S.; Yan, G.; Chen, C.; Jiang, X. Electrochemical pretreatment of heavy oil refinery wastewater using a three-dimensional electrode reactor. Electrochim. Acta 2010, 55, 8615–8620. [Google Scholar] [CrossRef]
- Zhou, M.; Lei, L. The role of activated carbon on the removal of p-nitrophenol in an integrated three-phase electrochemical reactor. Chemosphere 2006, 65, 1197–1203. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kothari, M.S.; Aly Hassan, A.; Shah, K.A. Three-Dimensional Electrochemical Oxidation of Recalcitrant Dye Using Green Iron Microparticles. Water 2021, 13, 1925. https://doi.org/10.3390/w13141925
Kothari MS, Aly Hassan A, Shah KA. Three-Dimensional Electrochemical Oxidation of Recalcitrant Dye Using Green Iron Microparticles. Water. 2021; 13(14):1925. https://doi.org/10.3390/w13141925
Chicago/Turabian StyleKothari, Manisha S., Ashraf Aly Hassan, and Kosha A. Shah. 2021. "Three-Dimensional Electrochemical Oxidation of Recalcitrant Dye Using Green Iron Microparticles" Water 13, no. 14: 1925. https://doi.org/10.3390/w13141925
APA StyleKothari, M. S., Aly Hassan, A., & Shah, K. A. (2021). Three-Dimensional Electrochemical Oxidation of Recalcitrant Dye Using Green Iron Microparticles. Water, 13(14), 1925. https://doi.org/10.3390/w13141925






