Comparison of Online Sensors for Liquid Phase Hydrogen Sulphide Monitoring in Sewer Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sensors
- Intelligent Spectral Analyzer T4 (ISA, GO Systemelektronik GmbH, Kiel, Germany). The ISA is an in-situ UV/Vis spectrometer that measures the absorbance of various substances in the ultraviolet and visible light range (200–708 nm) with a 2 nm resolution. The sensor has an adjustable optical path ranging between 1 and 20 mm [15,21]. For the wastewater application presented in this study, the optical path was set at 1 mm. Automatic cleaning of the sensor is carried out before each measurement using compressed air. As no default or global calibration is provided for the sensor, a local calibration—a site-specific calibration based on the local conditions and wastewater matrix to improve the sensor’s accuracy, was carried out beforehand using Multiple Linear Regression (MLR). Detailed information on the calibration process is presented in Pacheco Fernández et al. 2020 [15].
- OPUS (TriOS Mess- und Datentechnik GmbH, Oldenburg, Germany). The OPUS is an ultraviolet (UV) spectral sensor that measures the absorbance of various substances in the UV range. Like the ISA, both sensors are built on the principle of spectrophotometry, which uses light to measure chemical concentrations [22]. The sensor used in the study is portable, lightweight (titanium, 2 kg), and has moderate power consumption (<8 W) [7]. An optical path ranging from 0.3 to 50 mm is possible; however, given the characteristics of the wastewater used in this study, a path length of 1 mm was used. The OPUS covers a 200–360 nm spectral range with a 0.8 nm resolution utilizing a xenon flash lamp as the light source and a 256-channel high-end miniature spectrometer [7,23]. Measurements were set at 1-min intervals with the cleaning function using compressed air, activated every 10 min. To evaluate the plug-and-measure attribute of this sensor, we used the global calibration or predefined configuration provided by the manufacturer for comparison to the other online sensors.
- SulfiLoggerTM S1/X1-1020 (SulfiLogger A/S, Aarhus, Denmark). The measurement principle of the SulfiLoggerTM S1/X1-1020 (here forth referred to as SulfiLoggerTM) sensor follows the electrochemical detection of H2S. H2S is measured by a current produced when the H2S in the media (liquid or gas phase) penetrates the silicone membrane at the sensor’s tip and is subsequently electrochemically oxidized. The sensor is made of stainless steel, lightweight (0.85 kg), compact, and has a passive anti-fouling flush front [24]. The sensor’s H2S measurement range in the liquid phase is 0–5 mg L−1 with a measuring frequency of at least 1-min intervals [24]. Calibration was made by mounting the calibration cap to the sensor for a feed of calibration gas with a concentration of 1000 H2S(g) ppm.
- Reference method using ECH H2S Analyzer Cubi (ECH, Elektrochemie Halle GmbH, Halle, Germany). The reference method complies with the German standards for the determination of sulfide in wastewater (DIN 38405-27:2017-10 [6]). The measuring principle of the H2S Analyzer is based on the gas extraction of H2S from the wastewater sample. This device measures H2S gas with an amperometry sensor, where the sulfide species are first converted into H2S by acidifying the sample with phosphoric acid (4 wt.%). Before the experiments, we calibrated the device in the measurement range of 0–10 mg L−1 as total dissolved sulfide (DSt), using a standard sulfide stock solution prepared from thioacetamide [6].
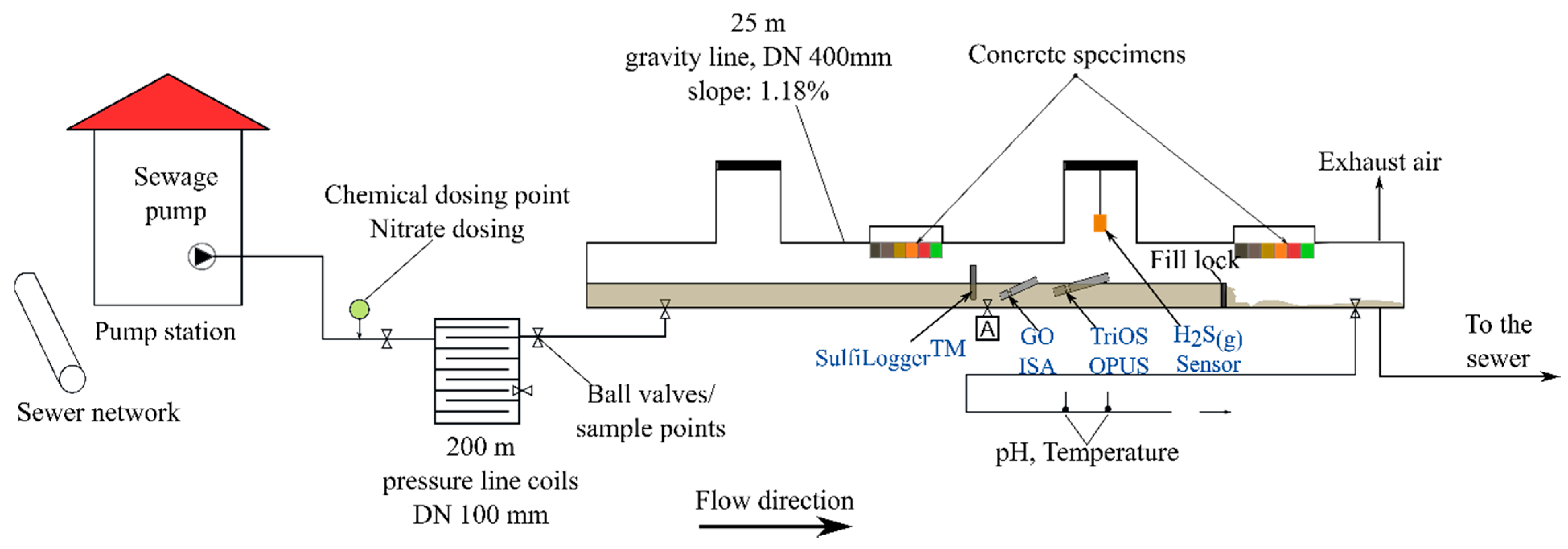
2.2. Experimental Site—Sewer Pilot Plant
2.3. Sulfide Equilibrium in Water
2.4. Experimental Conditions
2.5. Sampling, Laboratory Analysis, and Data Collection
2.6. Chemical Dosing Scheme
2.7. Assessment and Statistical Analysis for Comparison of Sensors
3. Results and Discussion
3.1. Comparison of Sensors to the Reference Method (Phase 1)
3.2. Sensors’ Performance under Different Wastewater Pump Operation (Phase 2)
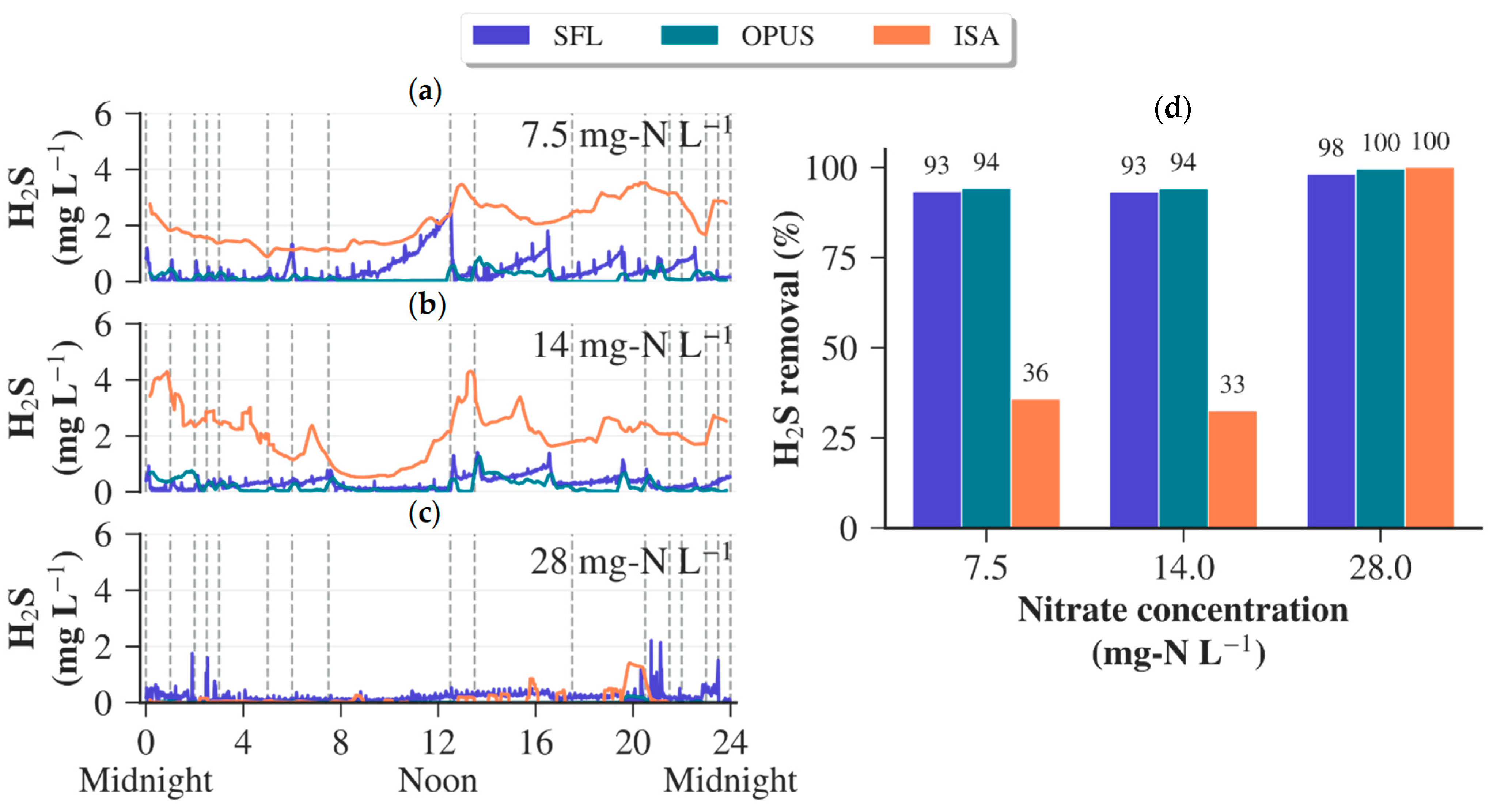
3.3. Performance of Sensors during Nitrate Dosing
3.4. Practical Applications and Challenges
3.5. Advantages and Disadvantages
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hvitved-Jacobsen, T. Sulfur Transformations in Sewer Networks: Effects, Prediction and Mitigation of Impacts. In Environmental Technologies to Treat Sulfur Pollution: Principles and Engineering, 2nd ed.; Lens, P., Ed.; IWA Publishing: London, UK, 2020; pp. 100–101. ISBN 9781789060966. [Google Scholar]
- Haaning Nielsen, A.; Vollertsen, J.; Hvitved-Jacobsen, T. Chemical Sulfide Oxidation of Wastewater—Effects of PH and Temperature. Water Sci. Technol. 2004, 50, 185–192. [Google Scholar] [CrossRef]
- Hvitved-Jacobsen, T.; Vollertsen, J.; Nielsen, A.H. Sewer Processes: Microbial and Chemical Process Engineering of Sewer Networks, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 119–120. ISBN 978-1-4398-8178-1. [Google Scholar]
- APHA American Public Health Association. Standard methods for the examination of water and wastewater. In “4500-S2− SULFIDE”, Standard Methods for the Examination of Water and Wastewater; APHA-AWWA-WEF: Washington, DC, USA, 2018. [Google Scholar] [CrossRef]
- Keller-Lehmann, B.; Corrie, S.; Ravn, R.; Yuan, Z.; Keller, J. Preservation and Simultaneous Analysis of Relevant Soluble Sulfur Species in Sewage Samples. In Proceedings of the Proceedings of 2nd International IWA Conference on Sewer Operation and Maintenance, Vienna, Austria, 26–28 October 2006; Volume 1, pp. 339–346. [Google Scholar]
- DIN Deutsches Institut für Normung e.V. German Standard Methods for the Examination of Water, Waste Water and Sludge—Anions (Group D)—Part 27: Determination of Sulfide by Gas Extraction Method (D 27); DIN 384405-D 27:2017-10; Beuth Verlag: Berlin, Germany, 2017. [Google Scholar]
- Meyer, D.; Prien, R.D.; Rautmann, L.; Pallentin, M.; Waniek, J.J.; Schulz-Bull, D.E. In Situ Determination of Nitrate and Hydrogen Sulfide in the Baltic Sea Using an Ultraviolet Spectrophotometer. Front. Mar. Sci. 2018, 5. [Google Scholar] [CrossRef]
- Miloshova, M.; Baltes, D.; Bychkov, E. New Chalcogenide Glass Chemical Sensors for S2− and Dissolved H2S Monitoring. Water Sci. Technol. 2003, 47, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Shamsipur, M.; Tashkhourian, J.; Sharghi, H. Development of Sulfide-Selective Optode Membranes Based on Immobilization of Methylene Blue on Optically Transparent Triacetylcellulose Film. Instrum. Sci. Technol. 2005, 33, 703–714. [Google Scholar] [CrossRef]
- Sutherland-Stacey, L.; Corrie, S.; Neethling, A.; Johnson, I.; Gutierrez, O.; Dexter, R.; Yuan, Z.; Keller, J.; Hamilton, G. Continuous Measurement of Dissolved Sulfide in Sewer Systems. Water Sci. Technol. 2008, 57, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Levinn, C.M.; Pluth, M.D. Direct Comparison of Triggering Motifs on Chemiluminescent Probes for Hydrogen Sulfide Detection in Water. Sens. Actuators B Chem. 2021, 329, 129235. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Yang, S.; Tian, H.; Liang, S.; Sun, B. Dual-Function Fluorescent Probe for Detection of Hydrogen Sulfide and Water Content in Dimethyl Sulfoxide. ACS Omega 2019, 4, 10695–10701. [Google Scholar] [CrossRef]
- Andrich, J.M.S.; Schröder, U. Sulfide Detection by Gold-Amalgam Microelectrodes in Artificial Wastewater. Chemosensors 2020, 8, 49. [Google Scholar] [CrossRef]
- Gutierrez, O.; Sutherland-Stacey, L.; Yuan, Z. Simultaneous Online Measurement of Sulfide and Nitrate in Sewers for Nitrate Dosage Optimisation. Water Sci. Technol. 2010, 61, 651–658. [Google Scholar] [CrossRef]
- Pacheco Fernández, M.; Knutz, T.; Barjenbruch, M. Multi-Parameter Calibration of a UV/Vis Spectrometer for Online Monitoring of Sewer Systems. Water Sci. Technol. 2020, 82, 927–939. [Google Scholar] [CrossRef]
- Despot, D.; Reinhold, L.; Augustyniak, A.; Barjenbruch, M. Dosing Free Nitrous Acid as an Alternative Sulphide Control Technology for Pressure Sewers in Germany. Water 2021, 13, 1015. [Google Scholar] [CrossRef]
- Madsen, P.S. Cases. SulfiLoggerTM Sensor. Available online: https://sulfilogger.com/cases (accessed on 24 May 2021).
- Jiang, G.; Sharma, K.R.; Yuan, Z. Effects of Nitrate Dosing on Methanogenic Activity in a Sulfide-Producing Sewer Biofilm Reactor. Water Res. 2013, 47, 1783–1792. [Google Scholar] [CrossRef] [PubMed]
- Firer, D.; Friedler, E.; Lahav, O. Control of Sulfide in Sewer Systems by Dosage of Iron Salts: Comparison between Theoretical and Experimental Results, and Practical Implications. Sci. Total Environ. 2008, 392, 145–156. [Google Scholar] [CrossRef]
- Stuetz, R.; Frechen, F.B. Odours in Wastewater Treatment—Measurement, Modelling and Control; IWA Publishing: Cornwall, UK, 2005; p. 123. ISBN 978-1-78040-293-2. [Google Scholar]
- Manual ISA Spectral Analyzer; GO Systemelektronik GmbH: Kiel, Germany, 2018; Available online: https://www.go-sys.de/en/downloads (accessed on 20 March 2020).
- Harris, D.C. Quantitative Chemical Analysis, 8th ed.; W.H. Freeman and Co.: New York, NY, USA, 2010; p. 394. ISBN 978-1-4292-1815-3. [Google Scholar]
- TriOS, G. OPUS. Available online: https://www.trios.de/en/opus.html (accessed on 2 February 2021).
- Maden, P.S. SulfiLogger H2S Sensor. SulfiLoggerTM Sensor. Available online: https://sulfilogger.com/sulfilogger-sensor (accessed on 24 May 2021).
- Yongsiri, C.; Vollertsen, J.; Rasmussen, M.; Hvitved-Jacobsen, T. Air-Water Transfer of Hydrogen Sulfide: An Approach for Application in Sewer Networks. Water Environ. Res. 2004, 76, 81–88. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Design Manual: Odor and Corrosion Control in Sanitary Sewerage Systems and Treatment Plants; U.S. Environmental Protection Agency: Washington, DC, USA, 1985.
- Sigg, L.; Stumm, W. Aquatische Chemie: Einführung in die Chemie natürlicher Gewässer; 6. Auflage; vdf Hochschulverlag: Zürich, Switzerland, 2016; p. 64. ISBN 978-3-7281-3767-8. [Google Scholar]
- Millero, F.J.; Plese, T.; Fernandez, M. The Dissociation of Hydrogen Sulfide in Seawater1. Limnol. Oceanogr. 1988, 33, 269–274. [Google Scholar] [CrossRef]
- Hershey, J.P.; Plese, T.; Millero, F.J. The PK1* for the Dissociation of H2S in Various Ionic Media. Geochim. Cosmochim. Acta 1988, 52, 2047–2051. [Google Scholar] [CrossRef]
- Brito, R.S.; Pinheiro, H.M.; Ferreira, F.; Matos, J.S.; Lourenço, N.D. In Situ UV-Vis Spectroscopy to Estimate COD and TSS in Wastewater Drainage Systems. Urban Water J. 2013, 11, 261–273. [Google Scholar] [CrossRef]
- Borrego, C.; Costa, A.M.; Ginja, J.; Amorim, M.; Coutinho, M.; Karatzas, K.; Sioumis, T.; Katsifarakis, N.; Konstantinidis, K.; De Vito, S.; et al. Assessment of Air Quality Microsensors versus Reference Methods: The EuNetAir Joint Exercise. Atmos. Environ. 2016, 147, 246–263. [Google Scholar] [CrossRef] [Green Version]
- Jolliff, J.K.; Kindle, J.C.; Shulman, I.; Penta, B.; Friedrichs, M.A.M.; Helber, R.; Arnone, R.A. Summary Diagrams for Coupled Hydrodynamic-Ecosystem Model Skill Assessment. J. Mar. Syst. 2009, 76, 64–82. [Google Scholar] [CrossRef]
- van Doorn, W.P.T.M. Methcomp: Method Comparison for Clinical Chemistry. 2020. Available online: https://github.com/wptmdoorn/methcomp (accessed on 1 March 2021).
- Rochford, P. PeterRochford/SkillMetrics. 2021. Available online: https://github.com/PeterRochford/SkillMetrics (accessed on 1 April 2021).
- McKinney, W. Data Structures for Statistical Computing in Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010; pp. 56–61. [Google Scholar]
- Reback, J.; McKinney, W.; Jbrockmendel; Van Den Bossche, J.; Augspurger, T.; Cloud, P.; Hawkins, S.; Gfyoung; Sinhrks; Roeschke, M.; et al. Pandas-Dev/Pandas: Pandas 1.2.4; Zenodo, 2021. Available online: https://zenodo.org/record/4681666#.YLTgmqgzZjU (accessed on 25 April 2021).
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array Programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef]
- Seabold, S.; Perktold, J. Statsmodels: Econometric and Statistical Modeling with Python. In Proceedings of the SciPy 2010 9th Python in Science Conference, Austin, TX, USA, 28–30 June 2010; pp. 92–96. [Google Scholar]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-Learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Waskom, M. Seaborn: Statistical Data Visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Giavarina, D. Understanding Bland Altman Analysis. Biochem. Medica 2015, 25, 141–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, P.F.; Petrie, A. Method Agreement Analysis: A Review of Correct Methodology. Theriogenology 2010, 73, 1167–1179. [Google Scholar] [CrossRef] [Green Version]
- McBride, G.B. A Proposal for Strength of Agreement Criteria for Lin’s Concordance Correlation Coefficient; National Institute of Water & Atmospheric Research Ltd.: Hamiltonm, New Zealand, 2005; Available online: https://www.medcalc.org/download/pdf/McBride2005.pdf (accessed on 10 April 2021).
- Langergraber, G.; Fleischmann, N.; Hofstädter, F. A Multivariate Calibration Procedure for UV/VIS Spectrometric Quantification of Organic Matter and Nitrate in Wastewater. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2003, 47, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Rieger, L.; Langergraber, G.; Thomann, M.; Fleischmann, N.; Siegrist, H. Spectral In-Situ Analysis of NO2, NO3, COD, DOC and TSS in the Effluent of a WWTP. Water Sci. Technol. 2004, 50, 143–152. [Google Scholar] [CrossRef]
- Lourenço, N.D.; Menezes, J.C.; Pinheiro, H.M.; Diniz, D. Development of PLS Calibration Models from UV-Vis Spectra for TOC Estimation at the Outlet of a Fuel Park Wastewater Treatment Plant. Environ. Technol. 2008, 29, 891–898. [Google Scholar] [CrossRef]
- Nielsen, A.H.; Vollertsen, J.; Jensen, H.S.; Madsen, H.I.; Hvitved-Jacobsen, T. Aerobic and Anaerobic Transformations of Sulfide in a Sewer System—Field Study and Model Simulations. Water Environ. Res. 2008, 80, 16–25. [Google Scholar] [CrossRef]
- Vollertsen, J.; Revilla, N.; Hvitved-Jacobsen, T.; Nielsen, A.H. Modeling Sulfides, pH and Hydrogen Sulfide Gas in the Sewers of San Francisco. Water Environ. Res. 2015, 87, 1980–1989. [Google Scholar] [CrossRef]
- Shypanski, A.H.; Yuan, Z.; Sharma, K. Influence of Pressure Main Pumping Frequency on Sulfide Formation Rates in Sanitary Sewers. Environ. Sci. Water Res. Technol. 2018, 38, 92. [Google Scholar] [CrossRef]
- Yang, W.; Vollertsen, J.; Hvitved-Jacobsen, T. Anoxic Sulfide Oxidation in Wastewater of Sewer Networks. Water Sci. Technol. 2005, 52, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Guenther, E.A.; Johnson, K.S.; Coale, K.H. Direct Ultraviolet Spectrophotometric Determination of Total Sulfide and Iodide in Natural Waters. Anal. Chem. 2001, 73, 3481–3487. [Google Scholar] [CrossRef] [PubMed]
- Pouly, F.; Touraud, E.; Buisson, J.-F.; Thomas, O. An Alternative Method for the Measurement of Mineral Sulphide in Wastewater. Talanta 1999, 50, 737–742. [Google Scholar] [CrossRef]
- Jeroschewski, P.; Steuckart, C.; Kühl, M. An Amperometric Microsensor for the Determination of H2S in Aquatic Environments. Anal. Chem. 1996, 68, 4351–4357. [Google Scholar] [CrossRef]
- Prien, R.; Meyer, D.; Sadkowiak, B. Optical Measurements of Nitrate and H2S Concentrations in Baltic Waters. In Proceedings of the Oceans 2009-Europe, Bremen, Germany, 11–14 May 2009; pp. 1–5. [Google Scholar]
- Gruber, G.; Bertrand-Krajewski, J.-L.; Beneditis, J.D.; Hochedlinger, M.; Lettl, W. Practical Aspects, Experiences and Strategies by Using UV/VIS Sensors for Long-Term Sewer Monitoring. Water Pract. Technol. 2006, 1. [Google Scholar] [CrossRef]
- Huebsch, M.; Grimmeisen, F.; Zemann, M.; Fenton, O.; Richards, K.G.; Jordan, P.; Sawarieh, A.; Blum, P.; Goldscheider, N. Technical Note: Field Experiences Using UV/VIS Sensors for High-Resolution Monitoring of Nitrate in Groundwater. Hydrol. Earth Syst. Sci. 2015, 19, 1589–1598. [Google Scholar] [CrossRef] [Green Version]
- Madsen, P.S. PowerCom Box. SulfiLoggerTM Sensor. Available online: https://sulfilogger.com/powercom-box (accessed on 24 May 2021).
- Smith Guide to Measuring pH in Challenging Processes. Available online: https://www.watertechonline.com/home/article/14172589/guide-to-measuring-ph-in-challenging-processes (accessed on 25 May 2021).


 ) and off (
) and off (  ) periods of the intermittent scenario (a). Barplots comparing the median H2S measurements of the sensors during constant flow scenario (b). The dashed lines (---) mark the mean H2S recorded by the sensors for the different wastewater operation.
) periods of the intermittent scenario (a). Barplots comparing the median H2S measurements of the sensors during constant flow scenario (b). The dashed lines (---) mark the mean H2S recorded by the sensors for the different wastewater operation.
 ) and off (
) and off (  ) periods of the intermittent scenario (a). Barplots comparing the median H2S measurements of the sensors during constant flow scenario (b). The dashed lines (---) mark the mean H2S recorded by the sensors for the different wastewater operation.
) periods of the intermittent scenario (a). Barplots comparing the median H2S measurements of the sensors during constant flow scenario (b). The dashed lines (---) mark the mean H2S recorded by the sensors for the different wastewater operation.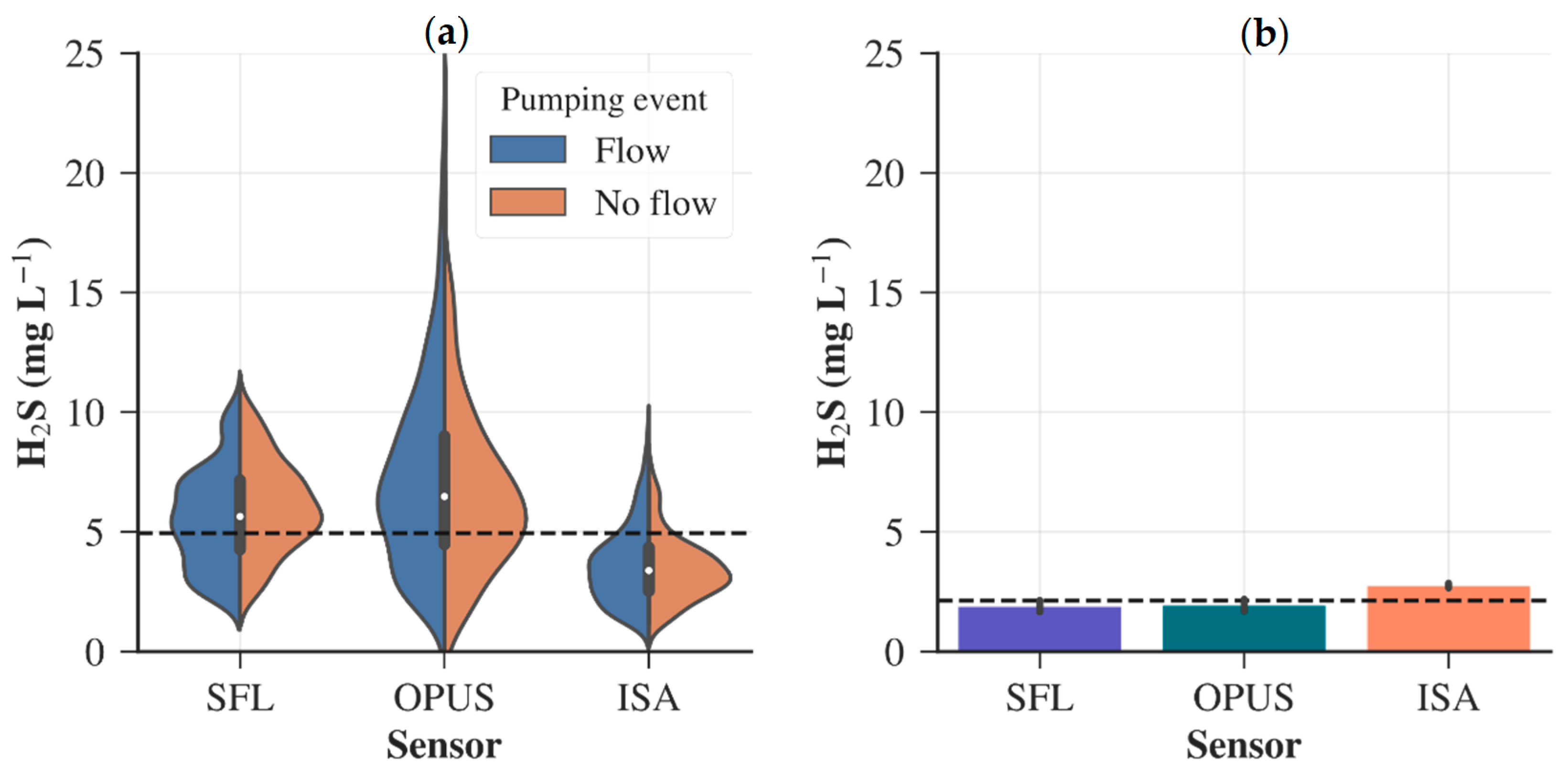
| Sensor | Sensor Type | Sulfide Species Measured (Main, (Converted)) | Measurement Range (mg L−1) |
|---|---|---|---|
| ISA | UV/Vis spectrometer | HS−, (H2S, DSt) Dependent on the calibration | Dependent on the reference method used for calibration 0–10 DSt mg L−1 (Same as the reference method used in this study) |
| OPUS | UV spectrometer | HS−, (H2S, DSt) | Dependent on the reference method used for calibration Calibration made by the manufacturer |
| SulfiLoggerTM | Micro-electrochemical (Clark-type) | H2S, (DSt) | 0–5 H2S mg L−1 |
| Test Application | Aim | Key Interest |
|---|---|---|
| (1) Intermittent flow | To determine sensors’ ability to detect H2S in an intermittently operated pressure sewer. To establish baseline conditions, i.e., when no H2S control measures are implemented. | Diurnal sulfide profiles—daily variation and influence of other key parameters, e.g., residence time, soluble COD. |
| (2) Continuous flow | To determine the sensors’ response during the continuous operation of the wastewater pump under normal wastewater conditions. | Sulfide levels for a case where longer pumping periods are used instead of shorter intermittent intervals—influence of pumping interval on sulfide production. |
| (3) Calcium nitrate dosing | To determine the sensors’ response under anoxic conditions. | Anoxic sulfide oxidation, the impact of varying nitrate concentration, effect of pH increase due to denitrification on sulfide detection. |
| Sensor | Advantages | Disadvantages |
|---|---|---|
| ISA |
|
|
| OPUS |
|
|
| SulfiLoggerTM |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Despot, D.; Pacheco Fernández, M.; Barjenbruch, M. Comparison of Online Sensors for Liquid Phase Hydrogen Sulphide Monitoring in Sewer Systems. Water 2021, 13, 1876. https://doi.org/10.3390/w13131876
Despot D, Pacheco Fernández M, Barjenbruch M. Comparison of Online Sensors for Liquid Phase Hydrogen Sulphide Monitoring in Sewer Systems. Water. 2021; 13(13):1876. https://doi.org/10.3390/w13131876
Chicago/Turabian StyleDespot, Daneish, Micaela Pacheco Fernández, and Matthias Barjenbruch. 2021. "Comparison of Online Sensors for Liquid Phase Hydrogen Sulphide Monitoring in Sewer Systems" Water 13, no. 13: 1876. https://doi.org/10.3390/w13131876
APA StyleDespot, D., Pacheco Fernández, M., & Barjenbruch, M. (2021). Comparison of Online Sensors for Liquid Phase Hydrogen Sulphide Monitoring in Sewer Systems. Water, 13(13), 1876. https://doi.org/10.3390/w13131876






