Controlling the Structural Robustness of Zirconium-Based Metal Organic Frameworks for Efficient Adsorption on Tetracycline Antibiotics
Abstract
:1. Introduction
2. Experimental Details
2.1. Reagents
2.2. Synthesis of Metal Organic Frameworks
2.3. Characterization of MOFs
2.4. Adsorption Experiments
2.5. Analytical Methods
2.6. Calculation Method
3. Results and Discussion
3.1. Characterizations of MOFs
3.1.1. Brunauer-Emmett-Teller Analysis
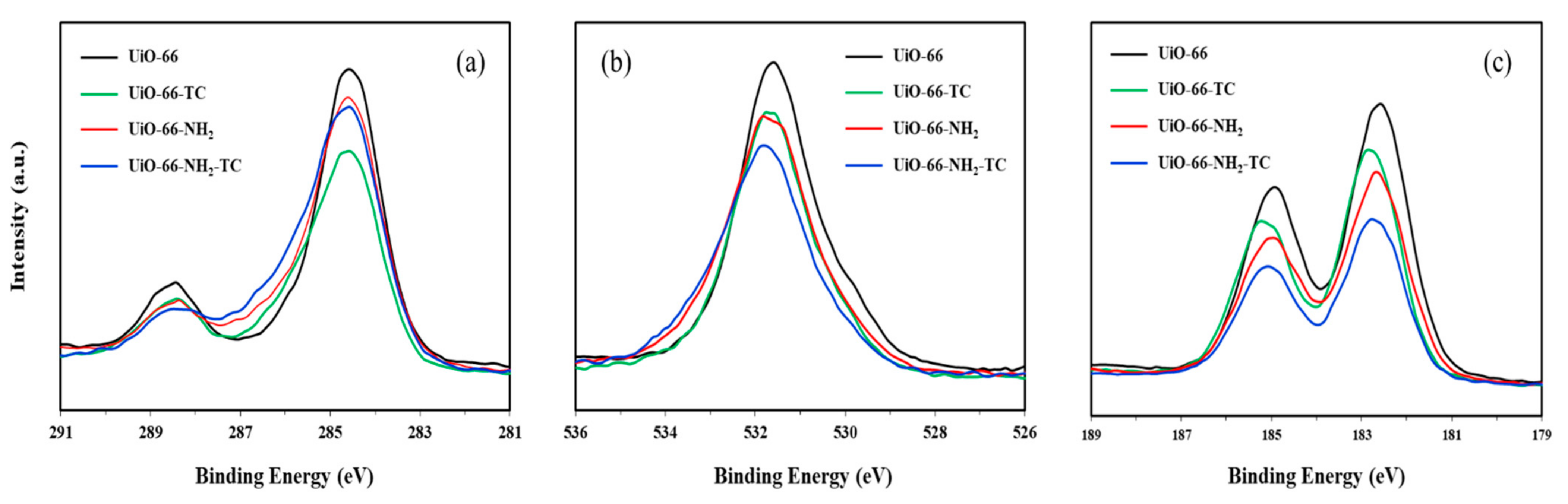
3.1.2. XPS Analysis
3.1.3. Field Emission Scanning Electron Microscopy
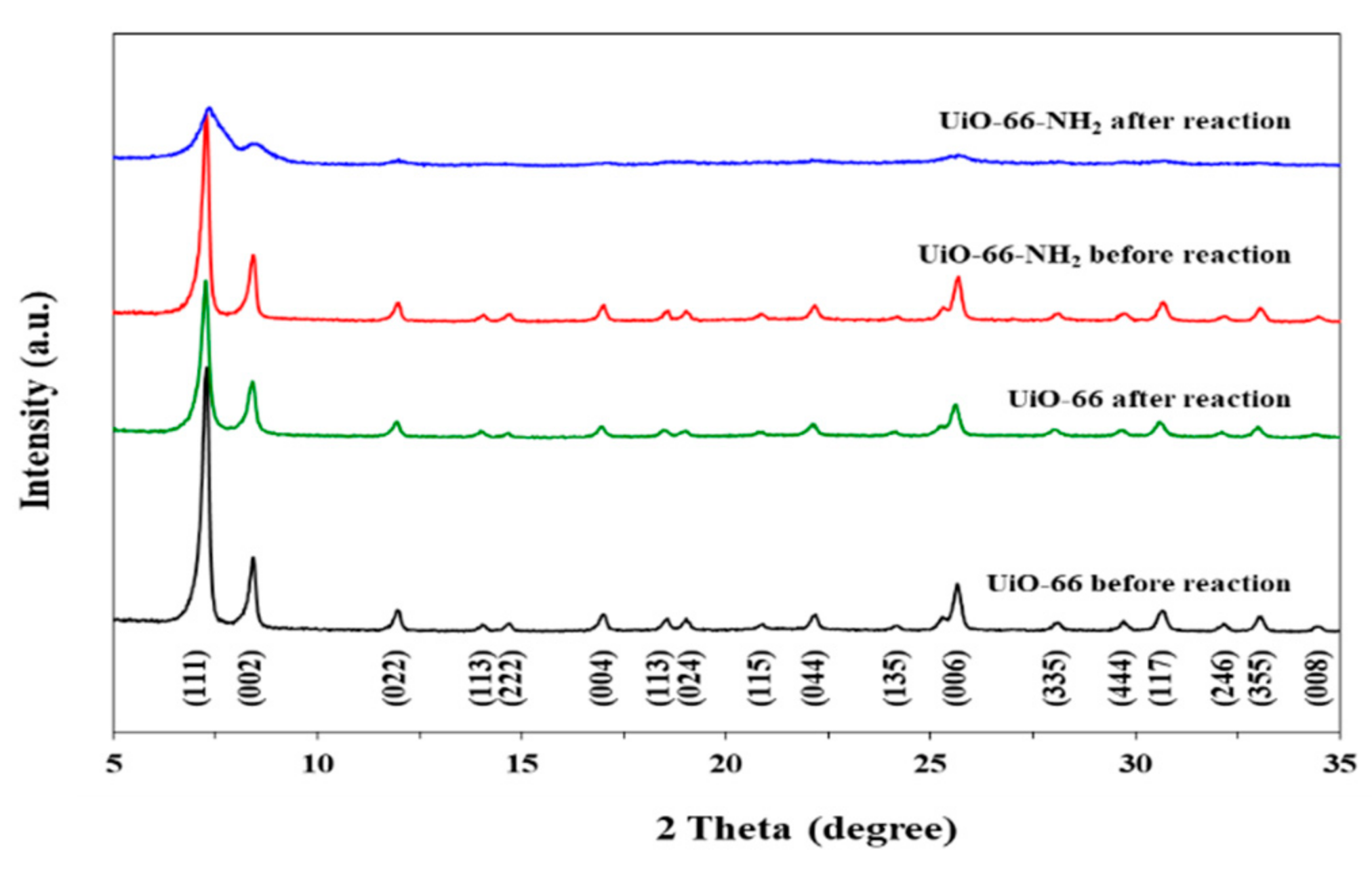
3.1.4. XRD Analysis
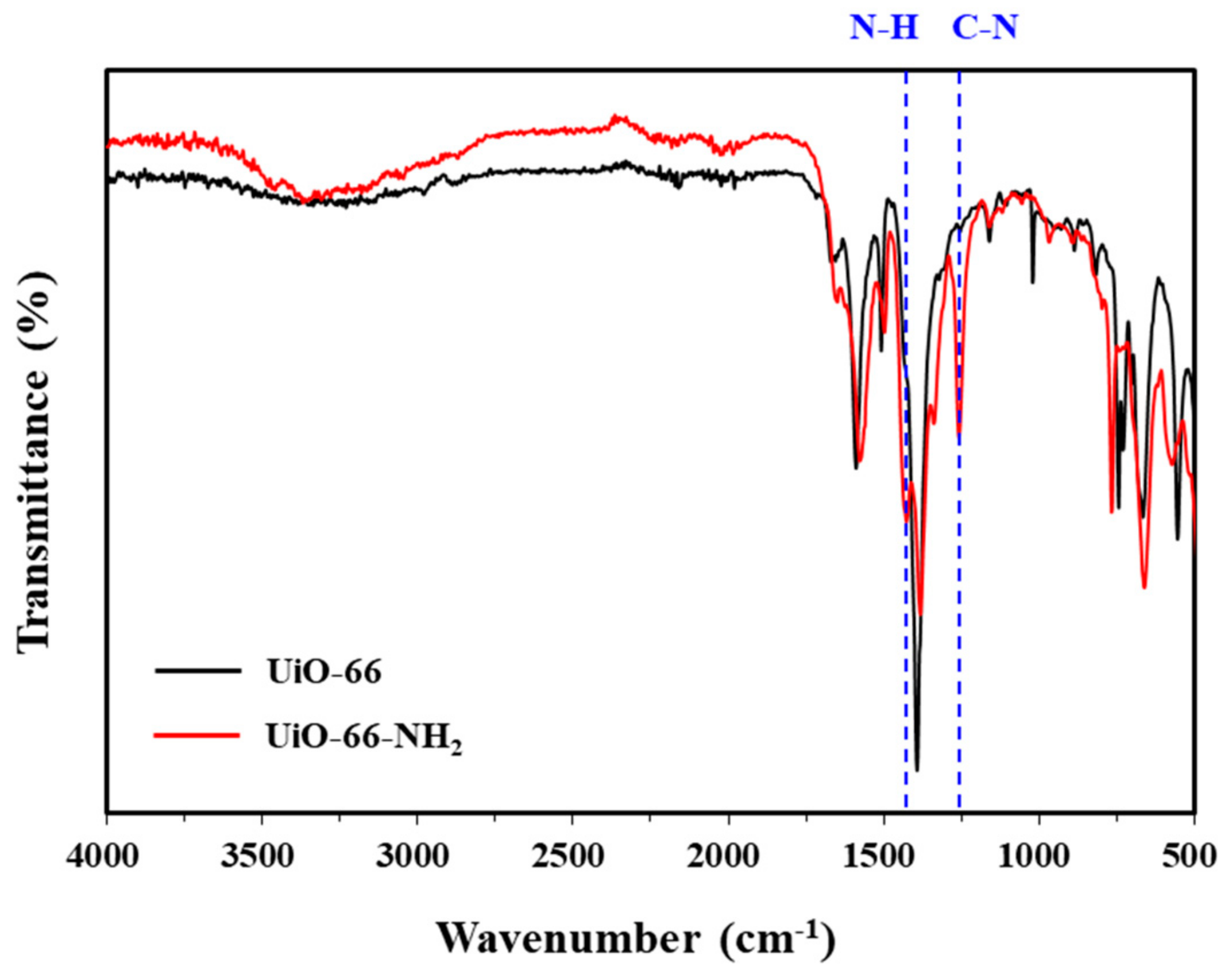
3.1.5. FT-IR Spectroscopy
3.2. Adsorption Removal of TCs
3.2.1. Batch Adsorption
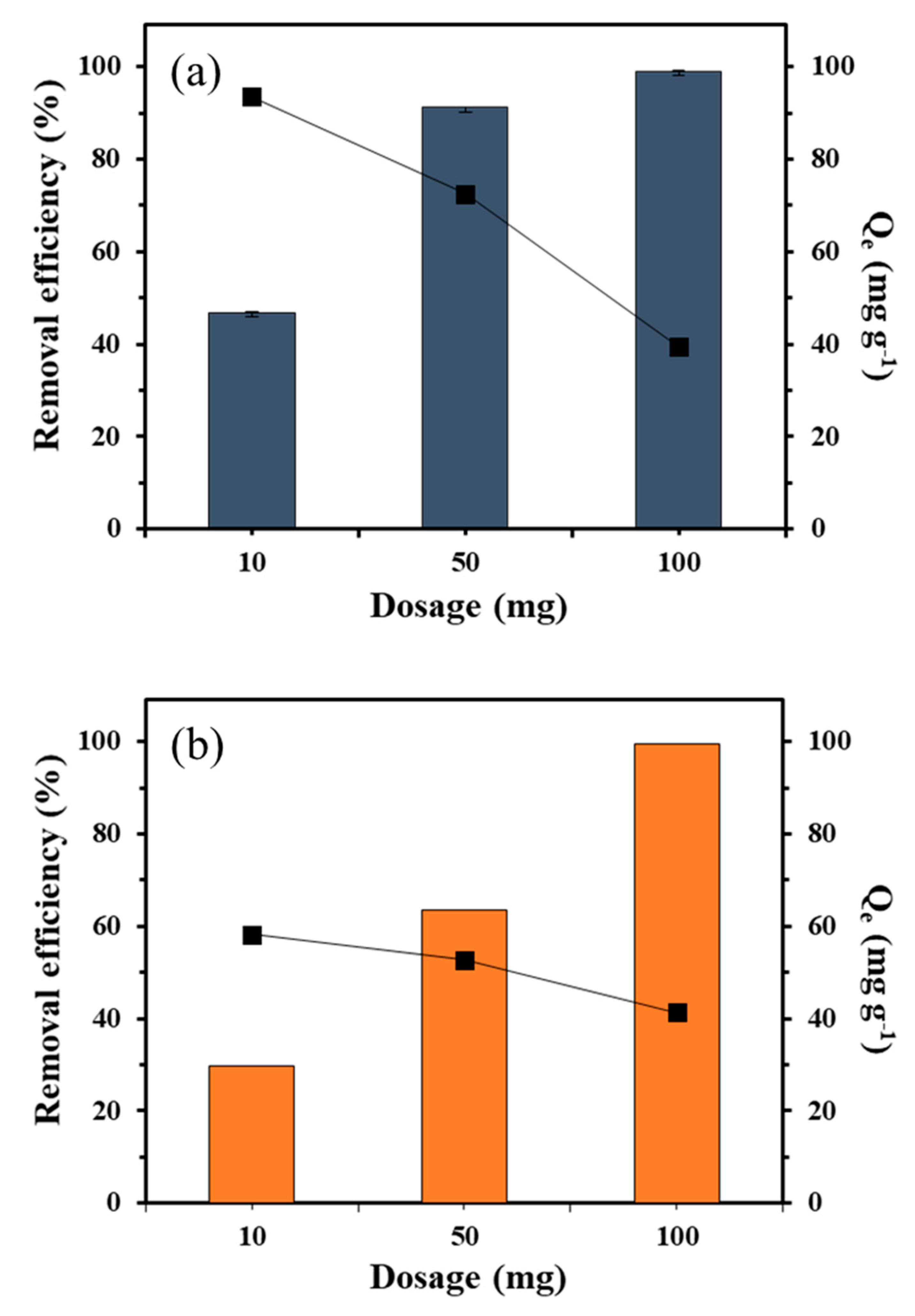
3.2.2. Effect of Adsorbent Dosage
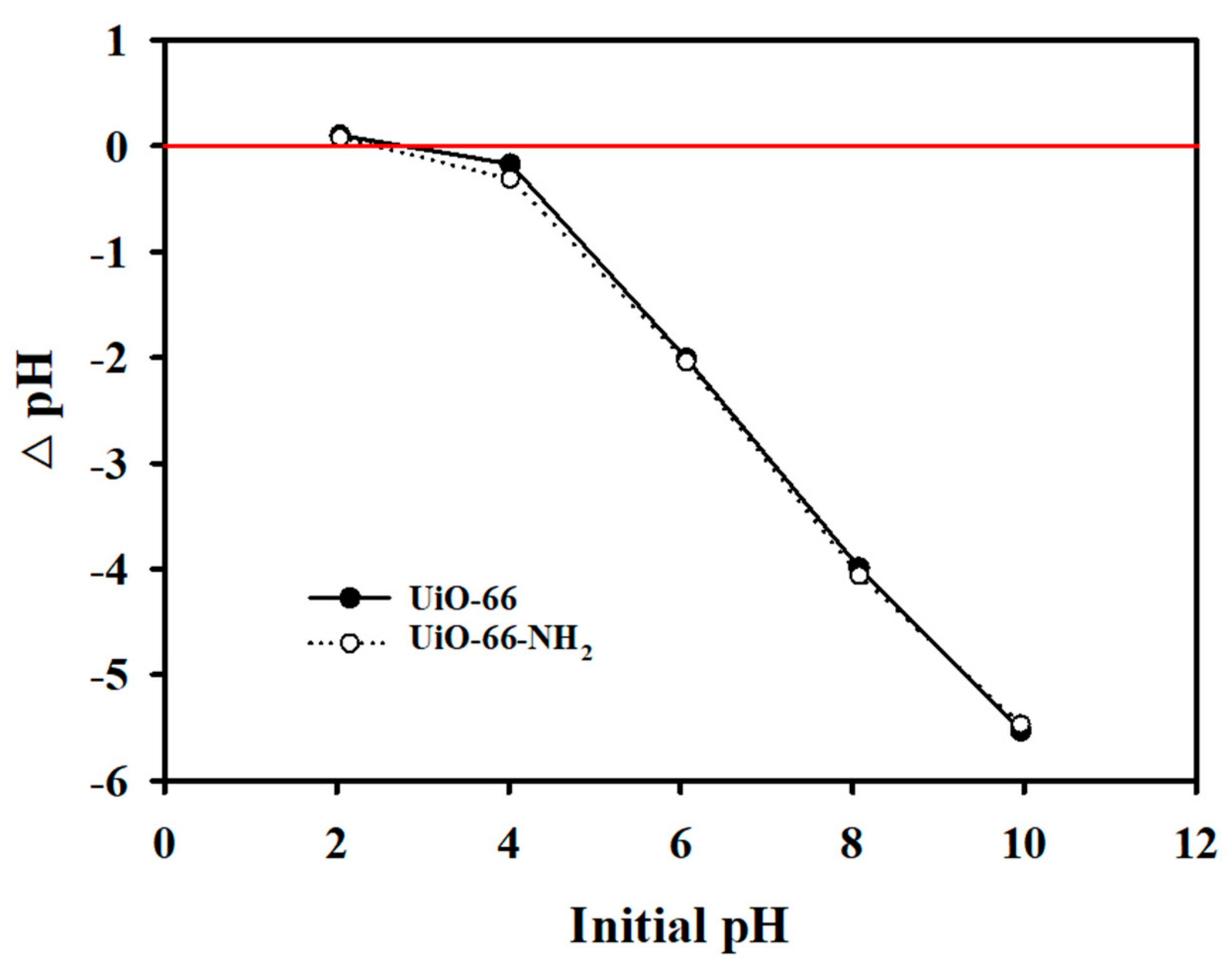
3.2.3. Effect of pH and PZC
3.2.4. Effect of Initial TC Concentration and Ionic Strength
3.2.5. Adsorption Kinetics
3.2.6. Adsorption Isotherms
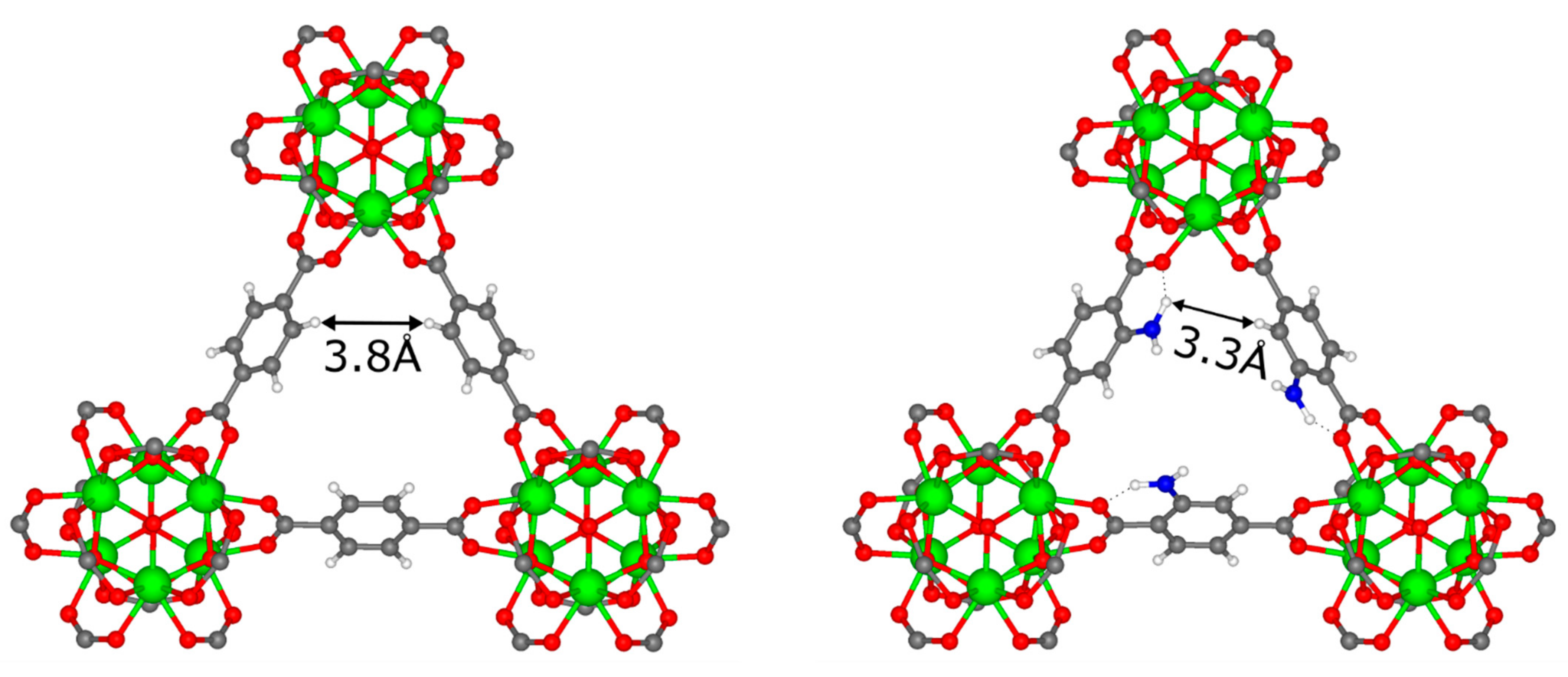
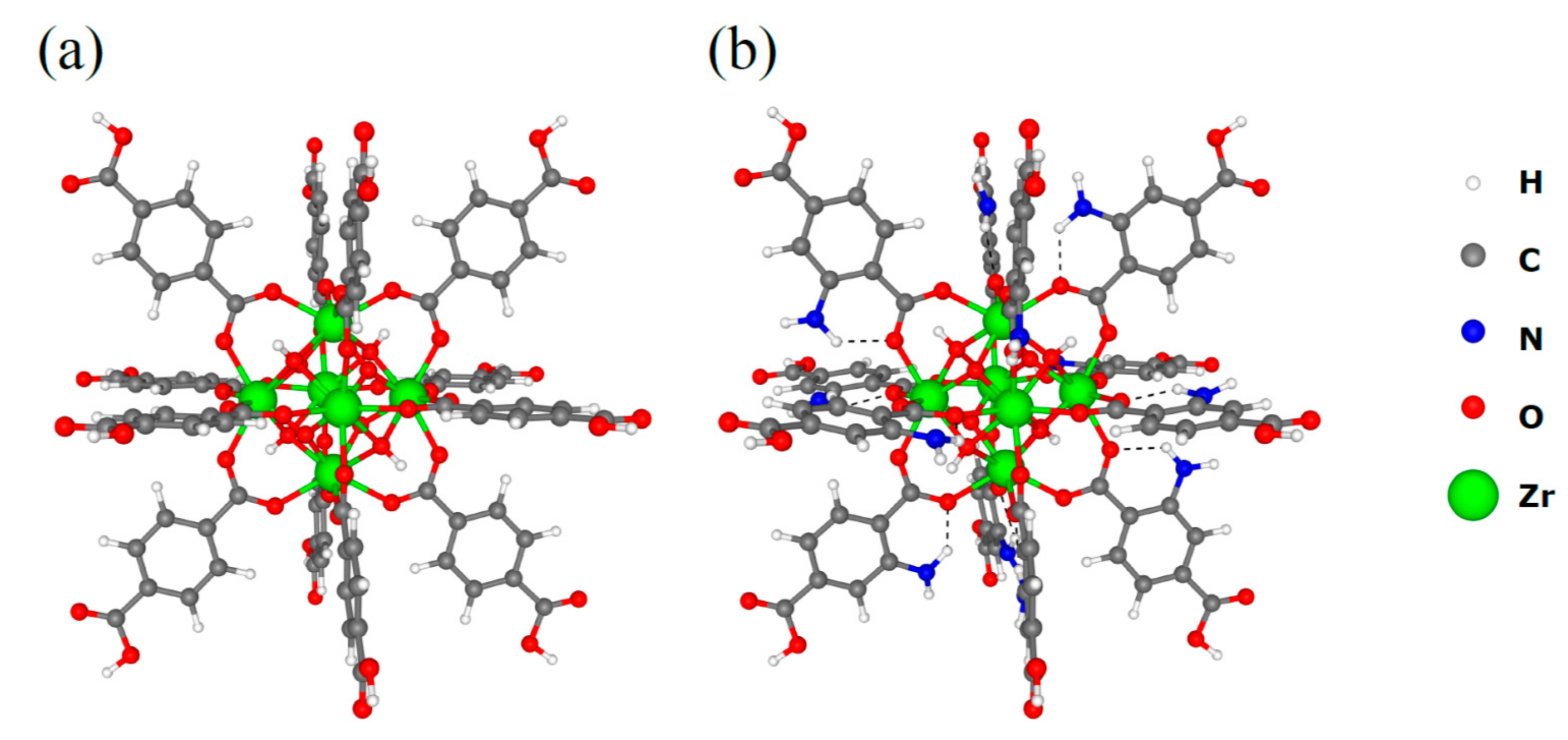
3.3. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BET | Brunauer-Emmett-Teller |
| DFT | Density functional theory |
| HPLC | High-performance liquid chromatography |
| MOF | Metal organic framework |
| OA | Oxalic acid |
| PAW | Projector-augmented wave |
| PZC | Point of zero charge |
| TGA | Temperature Gradient Analysis |
| VASP | Vienna ab initio Simulation Package |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
| BDC | Terephthalic acid (Benzene-1, 4-dicarboxylic acid, H2BDC) |
| DI | Deionized water |
| GGA | Generalized gradient approximation |
| TC | Tetracycline |
| PTFE | Polytetrafluoroethylene |
| UV | Ultraviolet |
References
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Li, N.; Zhou, L.; Jin, X.; Owens, G.; Chen, Z. Simultaneous removal of tetracycline and oxytetracycline antibiotics from wastewater using a ZIF-8 metal organic-framework. J. Hazard. Mater. 2019, 366, 563–572. [Google Scholar] [CrossRef]
- Christian, T.; Schneider, R.J.; Färber, H.A.; Skutlarek, D.; Meyer, M.T.; Goldbach, H.E. Determination of Antibiotic Residues in Manure, Soil, and Surface Waters. Acta Hydrochim. Hydrobiol. 2003, 31, 36–44. [Google Scholar] [CrossRef]
- Göbel, A.; Thomsen, A.; McArdell, C.S.; Joss, A.; Giger, W. Occurrence and Sorption Behavior of Sulfonamides, Macrolides, and Trimethoprim in Activated Sludge Treatment. Environ. Sci. Technol. 2005, 39, 3981–3989. [Google Scholar] [CrossRef]
- Kim, S.-C.; Carlson, K. Temporal and Spatial Trends in the Occurrence of Human and Veterinary Antibiotics in Aqueous and River Sediment Matrices. Environ. Sci. Technol. 2007, 41, 50–57. [Google Scholar] [CrossRef]
- Kummerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Yu, L.-L.; Cao, W.; Wu, S.-C.; Yang, C.; Cheng, J.-H. Removal of tetracycline from aqueous solution by MOF/graphite oxide pellets: Preparation, characteristic, adsorption performance and mechanism. Ecotoxicol. Environ. Saf. 2018, 164, 289–296. [Google Scholar] [CrossRef]
- Hu, T.; Jia, Q.; He, S.; Shan, S.; Su, H.; Zhi, Y.; He, L. Novel functionalized metal-organic framework MIL-101 adsorbent for capturing oxytetracycline. J. Alloy. Compd. 2017, 727, 114–122. [Google Scholar] [CrossRef]
- Miège, C.; Choubert, J.-M.; Ribeiro, L.; Eusèbe, M.; Coquery, M. Fate of pharmaceuticals and personal care products in wastewater treatment plants—Conception of a database and first results. Environ. Pollut. 2009, 157, 1721–1726. [Google Scholar] [CrossRef] [Green Version]
- González-Ortiz, A.; Ramírez-García, J.J.; Solache-Ríos, M.J. Kinetic and Thermodynamic Behavior on the Sorption of Clindamycin from an Aqueous Medium by Modified Surface Zeolitic Tuffs. Water Air Soil Pollut. 2018, 229. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, H.; Song, F.; Huang, T.; Ji, J.; Zhong, Q.; Chu, W.; Xu, Q. UiO-66-NH2/GO Composite: Synthesis, Characterization and CO2 Adsorption Performance. Materials 2018, 11, 589. [Google Scholar] [CrossRef]
- Bautitz, I.R.; Nogueira, R.F.P. Degradation of tetracycline by photo-Fenton process—Solar irradiation and matrix effects. J. Photochem. Photobiol. A: Chem. 2007, 187, 33–39. [Google Scholar] [CrossRef]
- Kim, I.; Tanaka, H. Photodegradation characteristics of PPCPs in water with UV treatment. Environ. Int. 2009, 35, 793–802. [Google Scholar] [CrossRef]
- Peñalver, J.J.L.; Sanchez-Polo, M.; Gómez-Pacheco, C.V.; Rivera-Utrilla, J. Photodegradation of tetracyclines in aqueous solution by using UV and UV/H2O2 oxidation processes. J. Chem. Technol. Biotechnol. 2010, 85, 1325–1333. [Google Scholar] [CrossRef]
- Wang, P.; He, Y.-L.; Huang, C.-H. Reactions of tetracycline antibiotics with chlorine dioxide and free chlorine. Water Res. 2011, 45, 1838–1846. [Google Scholar] [CrossRef]
- Batt, A.L.; Kim, S.; Aga, D.S. Comparison of the occurrence of antibiotics in four full-scale wastewater treatment plants with varying designs and operations. Chemosphere 2007, 68, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Watkinson, A.; Murby, E.; Costanzo, S. Removal of antibiotics in conventional and advanced wastewater treatment: Implications for environmental discharge and wastewater recycling. Water Res. 2007, 41, 4164–4176. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Zhu, W.; Chen, F.; Wang, P.; Da, Z.; Wu, X.; Ji, H.; Yan, S.; Li, H. Commercial Diatomite for Adsorption of Tetracycline Antibiotic from Aqueous Solution. Sep. Sci. Technol. 2014, 49, 2221–2227. [Google Scholar] [CrossRef]
- Kang, J.; Liu, H.; Zheng, Y.-M.; Qu, J.; Chen, J.P. Application of nuclear magnetic resonance spectroscopy, Fourier transform infrared spectroscopy, UV–Visible spectroscopy and kinetic modeling for elucidation of adsorption chemistry in uptake of tetracycline by zeolite beta. J. Colloid Interface Sci. 2011, 354, 261–267. [Google Scholar] [CrossRef]
- Yaghi, O.; Li, G.; Li, H. Selective binding and removal of guests in a microporous metal–organic framework. Nat. Cell Biol. 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Bai, Y.; Dou, Y.; Xie, L.-H.; Rutledge, W.; Li, J.-R.; Zhou, H.-C. Zr-based metal–organic frameworks: Design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef] [PubMed]
- Carboni, M.; Abney, C.; Liu, S.; Lin, W. Highly porous and stable metal–organic frameworks for uranium extraction. Chem. Sci. 2013, 4, 2396–2402. [Google Scholar] [CrossRef]
- Chen, C.; Chen, D.; Xie, S.; Quan, H.; Luo, X.; Guo, L. Adsorption behaviors of organic micropollutants on zirconium metal−organic framework UiO-66: Analysis of surface interactions. ACS Appl. Mater. Interfaces 2017, 9, 41043–41054. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Chung, W.-C.; Wei, Z.; Gu, Z.-Y.; Jiang, H.-L.; Chen, Y.-P.; Darensbourg, D.J.; Zhou, H.-C. Construction of Ultrastable Porphyrin Zr Metal–Organic Frameworks through Linker Elimination. J. Am. Chem. Soc. 2013, 135, 17105–17110. [Google Scholar] [CrossRef]
- Gutov, O.V.; Bury, W.; Gomez-Gualdron, D.A.; Krungleviciute, V.; Fairen-Jimenez, D.; Mondloch, J.E.; Sarjeant, A.A.; Al-Juaid, S.S.; Snurr, R.Q.; Hupp, J.T.; et al. Water-stable zirconium-based metal–organic framework material with high-surface area and gas-storage capacities. Chem. Eur. J. 2014, 20, 12389–12393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondloch, J.E.; Bury, W.; Fairen-Jimenez, D.; Kwon, S.; DeMarco, E.J.; Weston, M.H.; Sarjeant, A.A.; Nguyen, S.; Stair, P.C.; Snurr, R.; et al. Vapor-Phase Metalation by Atomic Layer Deposition in a Metal–Organic Framework. J. Am. Chem. Soc. 2013, 135, 10294–10297. [Google Scholar] [CrossRef]
- Taddei, M. When defects turn into virtues: The curious case of zirconium-based metal-organic frameworks. Coord. Chem. Rev. 2017, 343. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Yildirim, T.; Zhou, W. Exceptional Mechanical Stability of Highly Porous Zirconium Metal–Organic Framework UiO-66 and Its Important Implications. J. Phys. Chem. Lett. 2013, 4, 925–930. [Google Scholar] [CrossRef]
- Yang, Z.-H.; Cao, J.; Chen, Y.-P.; Li, X.; Xiong, W.-P.; Zhou, Y.-Y.; Zhou, C.-Y.; Xu, R.; Zhang, Y.-R. Mn-doped zirconium metal-organic framework as an effective adsorbent for removal of tetracycline and Cr(VI) from aqueous solution. Microporous Mesoporous Mater. 2019, 277, 277–285. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Huang, A.; Wan, L.; Caro, J. Microwave-assisted synthesis of well-shaped UiO-66-NH2 with high CO2 adsorption capacity. Mater. Res. Bull. 2018, 98, 308–313. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, Q.; Jiang, M.; Yao, J. Tailoring the Properties of UiO-66 through Defect Engineering: A Review. Ind. Eng. Chem. Res. 2019, 58, 17646–17659. [Google Scholar] [CrossRef]
- Cao, J.; Yang, Z.-H.; Xiong, W.-P.; Zhou, Y.-Y.; Peng, Y.-R.; Li, X.; Zhou, C.-Y.; Xu, R.; Zhang, Y.-R. One-step synthesis of Co-doped UiO-66 nanoparticle with enhanced removal efficiency of tetracycline: Simultaneous adsorption and photocatalysis. Chem. Eng. J. 2018, 353, 126–137. [Google Scholar] [CrossRef]
- Katz, M.J.; Brown, Z.J.; Colón, Y.J.; Siu, P.W.; Scheidt, K.; Snurr, R.; Hupp, J.T.; Farha, O.K. A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 2013, 49, 9449–9451. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter 1996, 54. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B Condens. Matter 1994, 50. [Google Scholar] [CrossRef] [Green Version]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA: A three-dimensional visualization system for electronic and structural analysis. J. Appl. Crystallogr. 2008, 41, 653–658. [Google Scholar] [CrossRef]
- Zhang, Y.; Ruan, Q.; Peng, Y.; Han, G.; Huang, H.; Zhong, C. Synthesis of hierarchical-pore metal-organic framework on liter scale for large organic pollutants capture in wastewater. J. Colloid Interface Sci. 2018, 525, 39–47. [Google Scholar] [CrossRef]
- Tong, X.; Yang, Z.; Feng, J.; Li, Y.; Zhang, H. BiOCl/UiO-66 composite with enhanced performance for photo-assisted degradation of dye from water. Appl. Organomet. Chem. 2018, 32. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Hu, X.; Yu, H. Highly stable and ultrasensitive chlorogenic acid sensor based on metal–organic frameworks/titanium dioxide nanocomposites. Analyst 2016, 141, 4647–4653. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.Y.; Seo, J.Y.; Kim, H.-J.; Pai, S.J.; Do, X.H.; Yoon, H.G.; Hwang, S.S.; Han, S.S.; Baek, K.-Y. Facile control of defect site density and particle size of UiO-66 for enhanced hydrolysis rates: Insights into feasibility of Zr(IV)-based metal-organic framework (MOF) catalysts. Appl. Catal. B Environ. 2019, 245, 635–647. [Google Scholar] [CrossRef]
- Duan, L.; Li, L.; Xu, Z.; Chen, W. Adsorption of tetracycline to nano-NiO: The effect of coexisting Cu(ii) ions and environmental implications. Environ. Sci. Process Impacts 2014, 16, 1462–1468. [Google Scholar] [CrossRef]
- Qin, Q.; Wu, X.; Chen, L.; Jiang, Z.; Xu, Y. Simultaneous removal of tetracycline and Cu(ii) by adsorption and coadsorption using oxidized activated carbon. RSC Adv. 2018, 8, 1744–1752. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Geng, J.; Wang, X.; Gu, X.; Gao, S. Adsorption of tetracycline onto goethite in the presence of metal cations and humic substances. J. Colloid Interface Sci. 2011, 361, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xin, Y.; Yan, Q.; Pan, X.; Xin, S.; Huang, X.; Chen, Q.; Liu, G. Adsorption Characteristics of Tetracycline onto Biochars as Affected by Solution Chemistry Conditions and Ball Milling Treatment. Water Air Soil Pollut. 2020, 231. [Google Scholar] [CrossRef]
- Fang, Y.; Wen, J.; Zeng, G.; Jia, F.; Zhang, S.; Peng, Z.; Zhang, H. Effect of mineralizing agents on the adsorption performance of metal–organic framework MIL-100(Fe) towards chromium(VI). Chem. Eng. J. 2018, 337, 532–540. [Google Scholar] [CrossRef]
- Wang, D.; Jia, F.; Wang, H.; Chen, F.; Fang, Y.; Dong, W.; Zeng, G.; Li, X.; Yang, Q.; Yuan, X. Simultaneously efficient adsorption and photocatalytic degradation of tetracycline by Fe-based MOFs. J. Colloid Interface Sci. 2018, 519, 273–284. [Google Scholar] [CrossRef]
- Luu, C.L.; Van Nguyen, T.T.; Nguyen, T.; Hoang, T.C. Synthesis, characterization and adsorption ability of UiO-66-NH2. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Ge, Y.; Wang, Y.; Chen, X.; Li, F.; Xuan, H.; Li, X. Adsorption of Cr(VI) on nano Uio-66-NH2 MOFs in water. Environ. Technol. 2018, 39, 1937–1948. [Google Scholar] [CrossRef] [PubMed]

| Sample | UiO-66 | UiO-66-NH2 |
|---|---|---|
| BET | 1581 m2 g−1 | 1415 m2 g−1 |
| Total pore volume (p/p0 = 0.990) | 0.66 cm3 g−1 | 0.59 cm3 g−1 |
| Mean pore diameter | 1.7931 nm | 1.8759 nm |
| Adsorption Kinetic Equations | pH | Constants | UiO-66 | UiO-66-NH2 |
|---|---|---|---|---|
| Pseudo-first-order rate equation | 3 | Qe (mg g−1) | 7.48 × 101 | 6.55 × 101 |
| K1 (min−1) | 2.60 × 10−2 | 1.04 × 10−2 | ||
| R2 | 0.88 | 0.86 | ||
| 6 | Qe (mg g−1) | 6.24 × 101 | 3.47 × 101 | |
| K1 (min−1) | 3.25 × 10−2 | 1.57 × 10−2 | ||
| R2 | 0.72 | 0.77 | ||
| Pseudo-second-order rate equation | 3 | Qe (mg g−1) | 7.91 × 101 | 6.24 × 101 |
| K2 (q mg−1 min−1) | 5.09 × 10−4 | 3.87 × 10−4 | ||
| R2 | 0.95 | 0.92 | ||
| 6 | Qe (mg g−1) | 6.73 × 101 | 3.63 × 101 | |
| K2 (q mg−1 min−1) | 6.80 × 10−4 | 7.45 × 10−4 | ||
| R2 | 0.84 | 0.83 |
| Adsorption Isotherm Model | Constants | UiO-66 | UiO-66-NH2 |
|---|---|---|---|
| Langmuir | Qm (mg g−1) | 9.36 × 101 | 7.65 × 101 |
| KL (L mg−1) | 3.84 | 1.57 | |
| R2 | 0.98 | 0.97 | |
| Freundlich | KF (mg g−1) | 4.92 × 101 | 4.78 × 101 |
| 1/n | 1.61 × 10−1 | 1.17 × 10−1 | |
| R2 | 0.89 | 0.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-G.; Choi, K.; Lee, K.; Lee, S.; Jung, K.-W.; Choi, J.-W. Controlling the Structural Robustness of Zirconium-Based Metal Organic Frameworks for Efficient Adsorption on Tetracycline Antibiotics. Water 2021, 13, 1869. https://doi.org/10.3390/w13131869
Kim H-G, Choi K, Lee K, Lee S, Jung K-W, Choi J-W. Controlling the Structural Robustness of Zirconium-Based Metal Organic Frameworks for Efficient Adsorption on Tetracycline Antibiotics. Water. 2021; 13(13):1869. https://doi.org/10.3390/w13131869
Chicago/Turabian StyleKim, Hee-Gon, Keunsu Choi, Kibong Lee, Soonjae Lee, Kyung-Won Jung, and Jae-Woo Choi. 2021. "Controlling the Structural Robustness of Zirconium-Based Metal Organic Frameworks for Efficient Adsorption on Tetracycline Antibiotics" Water 13, no. 13: 1869. https://doi.org/10.3390/w13131869
APA StyleKim, H.-G., Choi, K., Lee, K., Lee, S., Jung, K.-W., & Choi, J.-W. (2021). Controlling the Structural Robustness of Zirconium-Based Metal Organic Frameworks for Efficient Adsorption on Tetracycline Antibiotics. Water, 13(13), 1869. https://doi.org/10.3390/w13131869








