Aqueous Adsorption of Heavy Metals on Metal Sulfide Nanomaterials: Synthesis and Application
Abstract
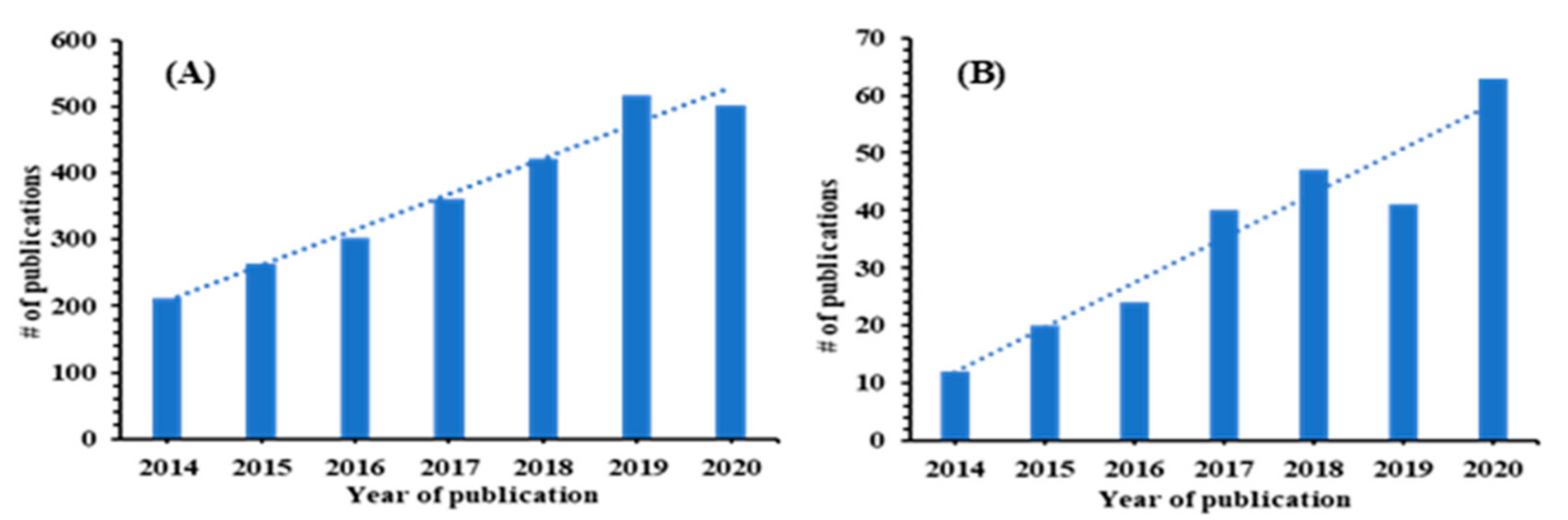
:1. Introduction
2. Structures, Preparation, and Supports of MS-NMs
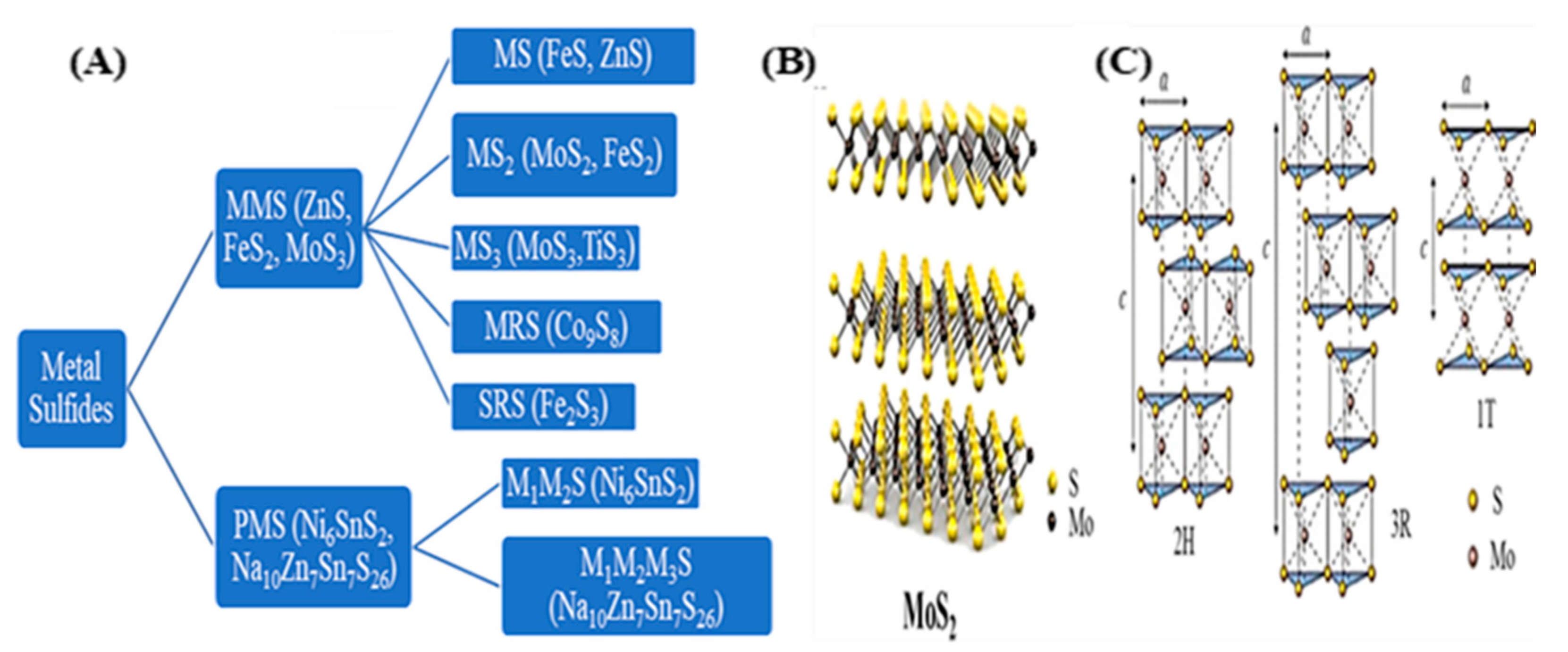
2.1. Structures
2.2. Synthesis
2.2.1. Top-Down Approach
Mechanical Exfoliation
LPE
Electrospinning
Ball Milling
2.2.2. Bottom-Up Strategy
Cation Exchange
Hydrothermal (Solvothermal) Synthesis
Chemical Vapor Deposition
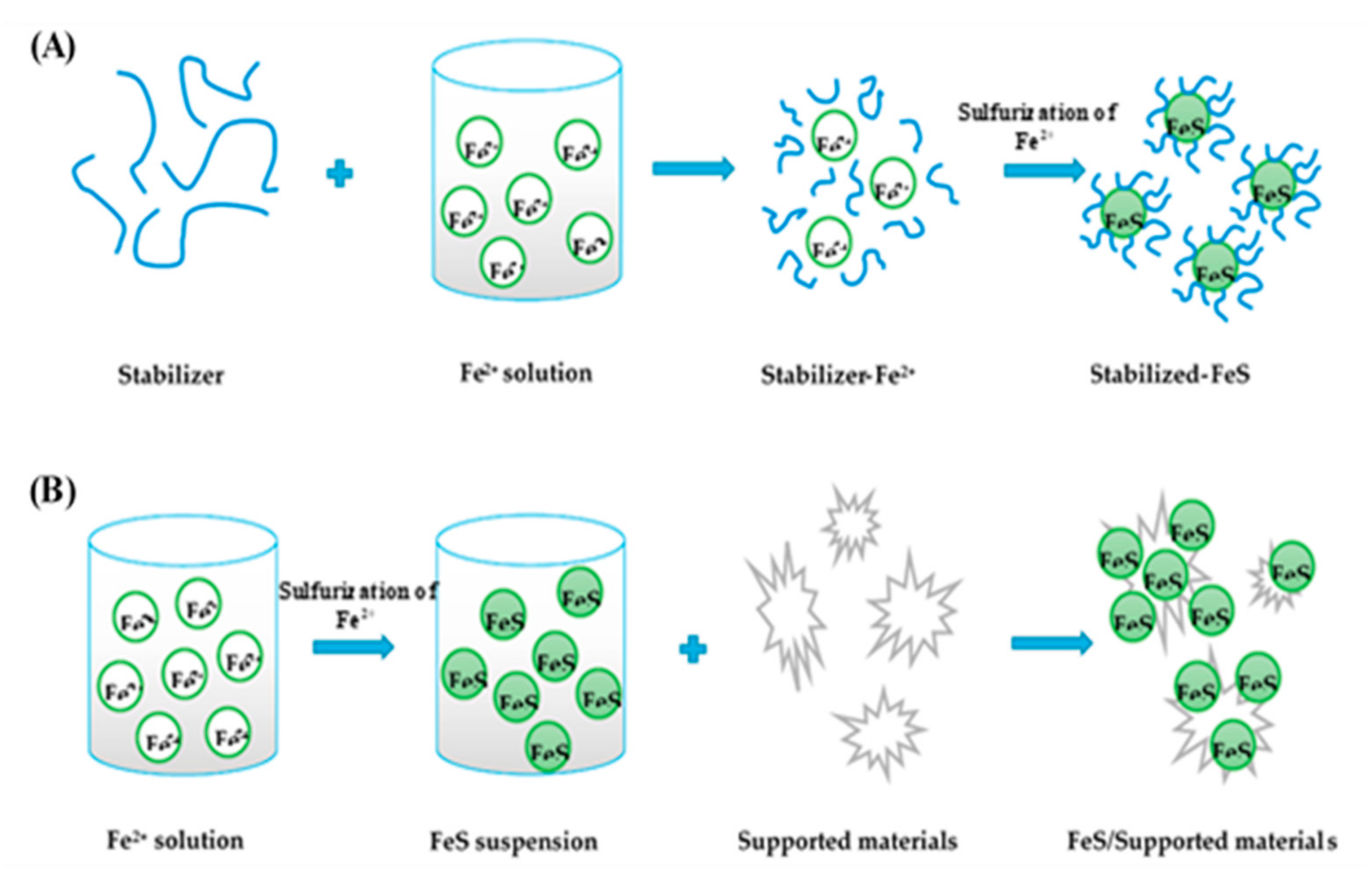
2.3. Functions and Types of Support/Stabilizing Materials for MS-NMs
2.3.1. Functions of Support/Stabilizing Materials for MSs
2.3.2. Types of Supporting Materials
Organic Support
Inorganic Support
3. Motivations for Choosing MS-NMs over Bulk Materials and Their HM Adsorption Mechanism
3.1. Motivations for Choosing MS-NMs over Bulk Materials for HM Removal from Water
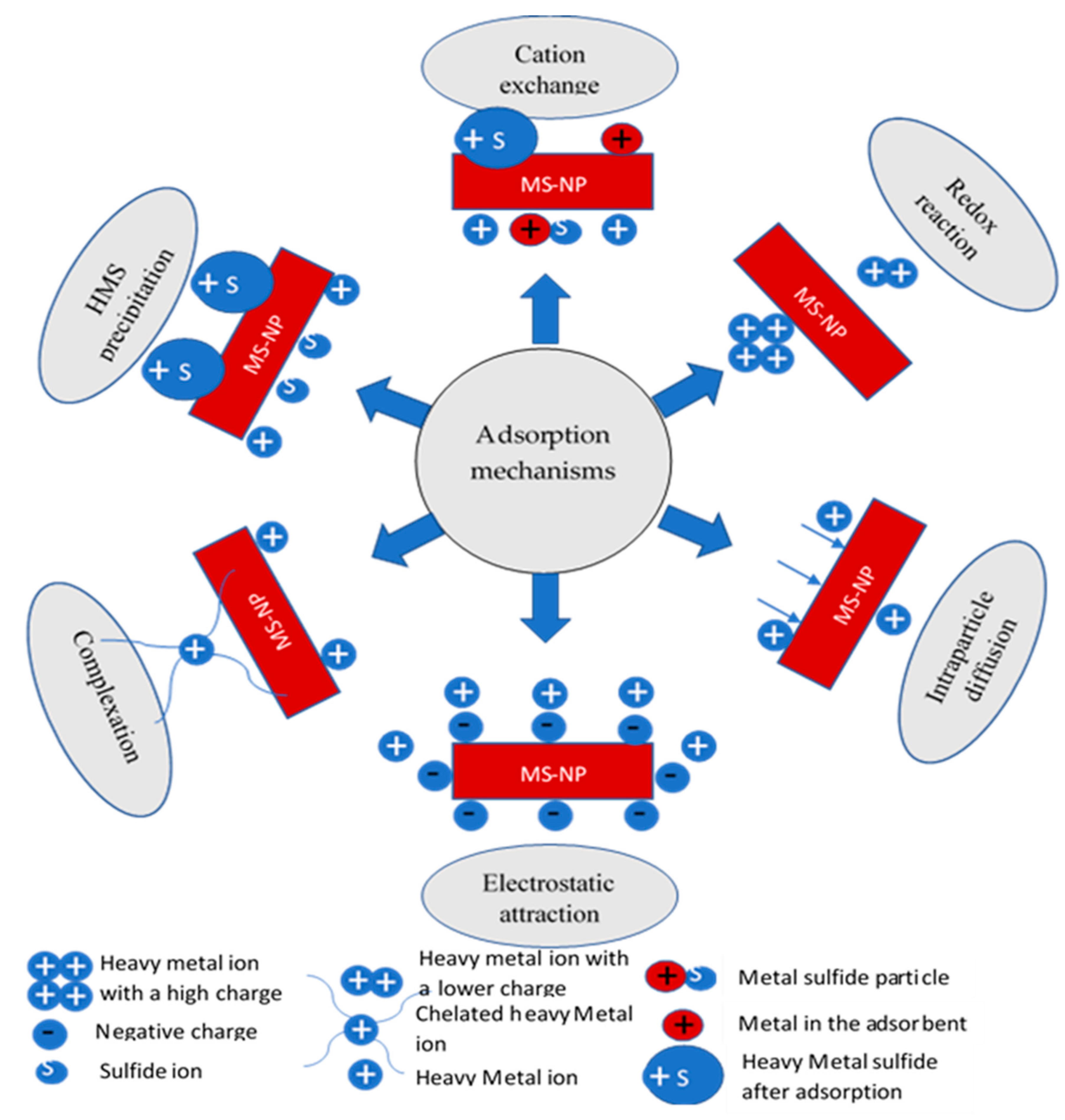
3.2. Adsorption Mechanisms of HMs on MS-NMs
4. Application of HMs Sequestration with Selected MS-NMs
4.1. Iron Sulfides
4.2. Zinc Sulfide
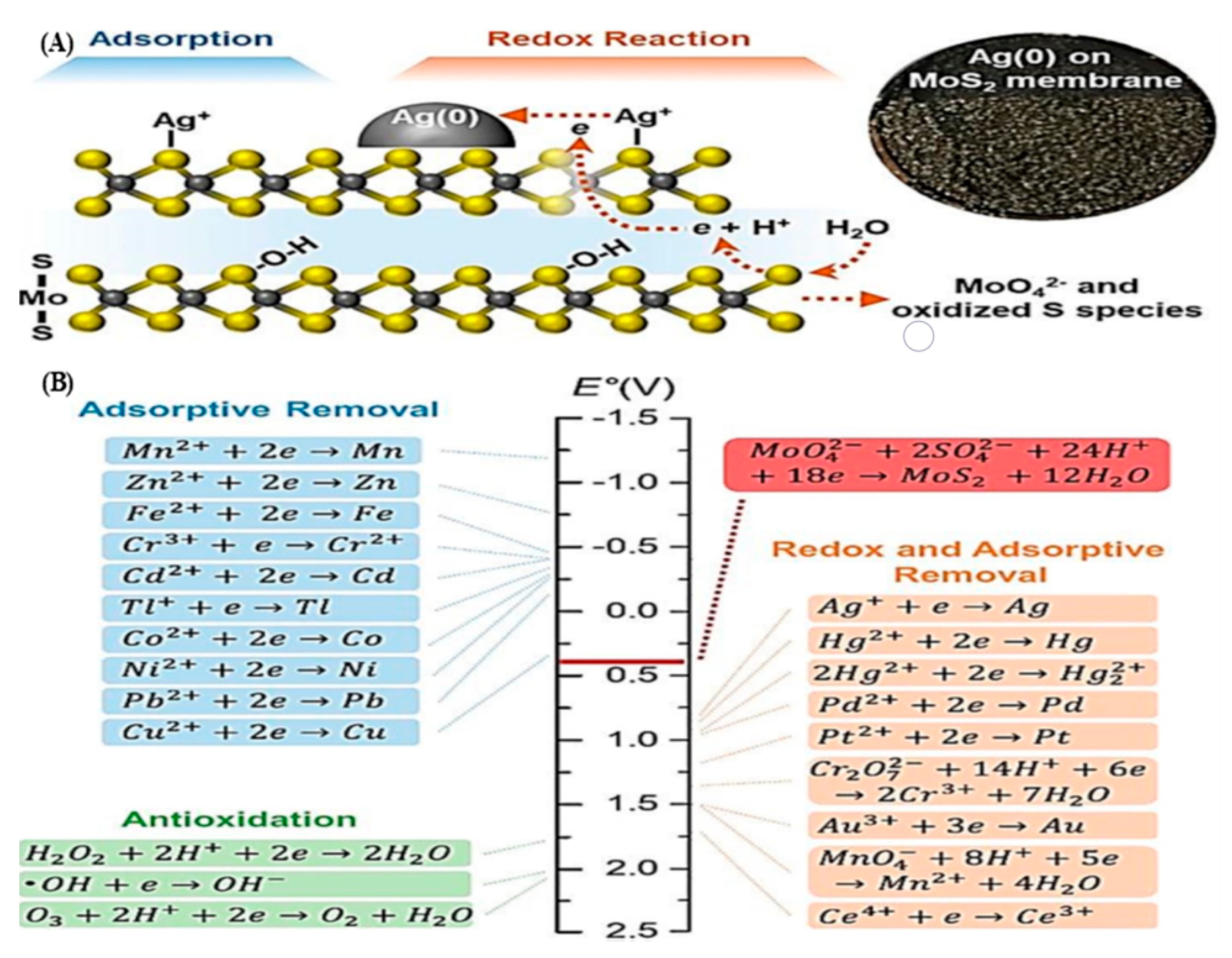
4.3. Molybdenum Sulfides
4.4. Copper Sulfides
4.5. Other MS-NMs
4.6. A Note on the Controversy of NM Usage and Long-Term Impact on the Environment
5. Conclusions and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, D.; Li, B.; Wu, J.; Liu, Y. Elemental mercury capture from industrial gas emissions using sulfides and selenides: A review. Environ. Chem. Lett. 2020, 19, 1–17. [Google Scholar] [CrossRef]
- Chen, S.; Hu, J.; Han, S.; Guo, Y.; Belzile, N.; Deng, T. A review on emerging composite materials for cesium adsorption and environmental remediation on the latest decade. Sep. Purif. Technol. 2020, 251, 117340. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Yuan, X.; Wu, Y.; Wang, H.; Tan, Y.Z.; Chew, J.W. Roles of sulfur-edge sites, metal-edge sites, terrace sites, and defects in metal sulfides for photocatalysis. Chem. Catal. 2021, 1, 44–68. [Google Scholar] [CrossRef]
- Ha, D.-H.; Caldwell, A.H.; Ward, M.J.; Honrao, S.; Mathew, K.; Hovden, R.; Koker, M.K.A.; Muller, D.A.; Hennig, R.G.; Robinson, R.D. Solid-Solid Phase Transformations Induced through Cation Exchange and Strain in 2D Heterostructured Copper Sulfide Nanocrystals. Nano Lett. 2014, 14, 7090–7099. [Google Scholar] [CrossRef]
- Fu, W.; Yang, S.; Yang, H.; Guo, B.; Huang, Z. 2D amorphous MoS 3 nanosheets with porous network structures for scavenging toxic metal ions from synthetic acid mine drainage. J. Mater. Chem. A 2019, 7, 18799–18806. [Google Scholar] [CrossRef]
- Hai, X.; Chang, K.; Pang, H.; Li, M.; Li, P.; Liu, H.; Shi, L.; Ye, J. Engineering the edges of MoS2 (WS2) crystals for direct exfoliation into monolayers in polar micromolecular solvents. J. Am. Chem. Soc. 2016, 138, 14962–14969. [Google Scholar] [CrossRef]
- Yin, L.; Hai, X.; Chang, K.; Ichihara, F.; Ye, J. Synergetic exfoliation and lateral size engineering of MoS2 for enhanced photocatalytic hydrogen generation. Small 2018, 14, 1704153. [Google Scholar] [CrossRef]
- Ma, S.; Huang, L.; Ma, L.; Shim, Y.; Islam, S.M.; Wang, P.; Zhao, L.-D.; Wang, S.; Sun, G.; Yang, X. Efficient uranium capture by polysulfide/layered double hydroxide composites. J. Am. Chem. Soc. 2015, 137, 3670–3677. [Google Scholar] [CrossRef] [PubMed]
- Ai, K.; Ruan, C.; Shen, M.; Lu, L. MoS2 nanosheets with widened interlayer spacing for high-efficiency removal of mercury in aquatic systems. Adv. Funct. Mater. 2016, 26, 5542–5549. [Google Scholar] [CrossRef]
- Wang, Z.; Mi, B. Environmental applications of 2D molybdenum disulfide (MoS2) nanosheets. Environ. Sci. Technol. 2017, 51, 8229–8244. [Google Scholar] [CrossRef]
- Sankararamakrishnan, N.; Singh, R.; Srivastava, I. Performance of novel MgS doped cellulose nanofibres for Cd (II) removal from industrial effluent–mechanism and optimization. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekaran, S.; Yao, L.; Deng, L.; Bowen, C.; Zhang, Y.; Chen, S.; Lin, Z.; Peng, F.; Zhang, P. Recent advances in metal sulfides: From controlled fabrication to electrocatalytic, photocatalytic and photoelectrochemical water splitting and beyond. Chem. Soc. Rev. 2019, 48, 4178–4280. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Wang, Y.; Li, H.; Peng, Y. The application of nanostructured transition metal sulfides as anodes for lithium ion batteries. J. Energy Chem. 2018, 27, 1536–1554. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, A.K.; Srivastava, S.K.; Raul, P.K.; Gupta, A.K.; Shrivastava, R. Graphene nanocomposites of CdS and ZnS in effective water purification. J. Nanoparticle Res. 2014, 16, 1–17. [Google Scholar] [CrossRef]
- Fang, L.; Li, L.; Qu, Z.; Xu, H.; Xu, J.; Yan, N. A novel method for the sequential removal and separation of multiple heavy metals from wastewater. J. Hazard. Mater. 2018, 342, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Chauhan, V.; Kumar, R. A comprehensive review on synthesis and applications of molybdenum disulfide (MoS2 ) material: Past and recent developments. Inorg. Chem. Commun. 2020, 121, 108200. [Google Scholar] [CrossRef]
- Lukowski, M.A.; Daniel, A.S.; Meng, F.; Forticaux, A.; Li, L.; Jin, S. Enhanced Hydrogen Evolution Catalysis from Chemically Exfoliated Metallic MoS2 Nanosheets. J. Am. Chem. Soc. 2013, 135, 10274–10277. [Google Scholar] [CrossRef]
- Li, Z.; Fan, R.; Hu, Z.; Li, W.; Zhou, H.; Kang, S.; Zhang, Y.; Zhang, H.; Wang, G. Ethanol introduced synthesis of ultrastable 1T-MoS2 for removal of Cr(VI). J. Hazard. Mater. 2020, 394, 122525. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, X.; Sun, J.; Li, D.; Yang, X. Enhanced Catalytic Activities of Surfactant-Assisted Exfoliated WS2 Nanodots for Hydrogen Evolution. Acs Nano 2016, 10, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-Y.; Liu, Q.-L. Study of the layer-dependent properties of MoS2 nanosheets with different crystal structures by DFT calculations. Catal. Sci. Technol. 2018, 8, 1867–1879. [Google Scholar] [CrossRef]
- Dou, W.; Yang, W.; Zhao, X.; Pan, Q. Hollow cobalt sulfide for highly efficient uranium adsorption from aqueous solutions. Inorg. Chem. Front. 2019, 6, 3230–3236. [Google Scholar] [CrossRef]
- Liu, H.; You, Z.; Yang, S.; Liu, C.; Xie, X.; Xiang, K.; Wang, X.; Yan, X. High-efficient adsorption and removal of elemental mercury from smelting flue gas by cobalt sulfide. Environ. Sci. Pollut. Res. 2019, 26, 6735–6744. [Google Scholar] [CrossRef] [PubMed]
- Petrašauskienė, N.; Stokienė, R.; Žalenkienė, S.; Janickis, V. Formation of cobalt sulfide layers on polyamide 6 by sorption–diffusion method using solutions of dodecathionic acid, H2S12O6. Chemija 2015, 26, 5. [Google Scholar]
- Xia, D.; Gong, F.; Pei, X.; Wang, W.; Li, H.; Zeng, W.; Wu, M.; Papavassiliou, D.V. Molybdenum and tungsten disulfides-based nanocomposite films for energy storage and conversion: A review. Chem. Eng. J. 2018, 348, 908–928. [Google Scholar] [CrossRef]
- Vattikuti, V.P.; Shim, J.; Byon, C. 1D Bi2S3 nanorod/2D e-WS2 nanosheet heterojunction photocatalyst for enhanced photocatalytic activity. J. Solid State Chem. 2018, 258, 526–535. [Google Scholar] [CrossRef]
- Hu, L.; Song, X.-F.; Zhang, S.-L.; Zeng, H.-B.; Zhang, X.-J.; Marks, R.; Shan, D. MoS2 nanoparticles coupled to SnS2 nanosheets: The structural and electronic modulation for synergetic electrocatalytic hydrogen evolution. J. Catal. 2018, 366, 8–15. [Google Scholar] [CrossRef]
- Fu, L.; Yan, Z.; Zhao, Q.; Yang, H. Novel 2D nanosheets with potential applications in heavy metal purification: A review. Adv. Mater. Interfaces 2018, 5, 1801094. [Google Scholar] [CrossRef]
- Yu, H.; Xiao, P.; Wang, P.; Yu, J. Amorphous molybdenum sulfide as highly efficient electron-cocatalyst for enhanced photocatalytic H2 evolution. Appl. Catal. B Environ. 2016, 193, 217–225. [Google Scholar] [CrossRef]
- Shen, X.; Xia, X.; Ye, W.; Du, Y.; Wang, C. Hexagram-like CoS-MoS2 composites with enhanced activity for hydrogen evolution reaction. J. Solid State Electrochem. 2017, 21, 409–417. [Google Scholar] [CrossRef]
- Makovicky, E. Crystal Structures of Sulfides and Other Chalcogenides. Rev. Mineral. Geochem. 2006, 61, 7–125. [Google Scholar] [CrossRef]
- Xiong, Y.; Su, L.; Yang, H.; Zhang, P.; Ye, F. Fabrication of copper sulfide using a Cu-based metal organic framework for the colorimetric determination and the efficient removal of Hg 2+ in aqueous solutions. New J. Chem. 2015, 39, 9221–9227. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Z.; Wu, J.; Li, C. Copper sulfide microsphere for Hg0 capture from flue gas at low temperature. Mater. Today Commun. 2020, 25, 101188. [Google Scholar] [CrossRef]
- He, X.; Min, X.; Peng, T.; Ke, Y.; Zhao, F.; Sillanpää, M.; Wang, Y. Enhanced adsorption of antimonate by ball-milled microscale zero valent iron/pyrite composite: Adsorption properties and mechanism insight. Environ. Sci. Pollut. Res. 2020, 27, 16484–16495. [Google Scholar] [CrossRef]
- Han, Y.-S.; Lee, C.-M.; Chon, C.-M.; Kwon, J.A.; Park, J.-H.; Shin, Y.-J.; Lim, D.-H. Enhanced oxidation resistance of NaBH4-treated mackinawite (FeS): Application to Cr (VI) and As (III) removal. Chem. Eng. J. 2018, 353, 890–899. [Google Scholar] [CrossRef]
- Min, X.; Li, Y.; Ke, Y.; Shi, M.; Chai, L.; Xue, K. Fe-FeS2 adsorbent prepared with iron powder and pyrite by facile ball milling and its application for arsenic removal. Water Sci. Technol. 2017, 76, 192–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sereshti, H.; Gaikani, H.; Nodeh, H.R. The effective removal of mercury ions (Hg 2+) from water using cadmium sulfide nanoparticles doped in polycaprolactam nanofibers: Kinetic and equilibrium studies. J. Iran. Chem. Soc. 2018, 15, 743–751. [Google Scholar] [CrossRef]
- Ahmad, H.; Sharfan, I.I.B.; Khan, R.A.; Alsalme, A. Effective Enrichment and Quantitative Determination of Trace Hg2+ Ions Using CdS-Decorated Cellulose Nanofibrils. Nanomaterials 2020, 10, 2218. [Google Scholar] [CrossRef]
- Cho, G.; Park, Y.; Hong, Y.-K.; Ha, D.-H. Ion exchange: An advanced synthetic method for complex nanoparticles. Nano Converg. 2019, 6, 17. [Google Scholar] [CrossRef]
- Qiao, Z.; Shen, M.; Xiao, Y.; Zhu, M.; Mignani, S.; Majoral, J.-P.; Shi, X. Organic/inorganic nanohybrids formed using electrospun polymer nanofibers as nanoreactors. Coord. Chem. Rev. 2018, 372, 31–51. [Google Scholar] [CrossRef]
- Vaughan, D.J.; Corkhill, C.L. Mineralogy of Sulfides. Elements 2017, 13, 81–87. [Google Scholar] [CrossRef]
- Bentley, C.L.; Kang, M.; Maddar, F.M.; Li, F.; Walker, M.; Zhang, J.; Unwin, P.R. Electrochemical maps and movies of the hydrogen evolution reaction on natural crystals of molybdenite (MoS2): Basal vs. edge plane activity. Chem. Sci. 2017, 8, 6583–6593. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Kong, L.-B.; Dai, Y.-H.; Yan, K.; Shi, M.; Liu, M.-C.; Luo, Y.-C.; Kang, L. A Facile Strategy for the Preparation of MoS3 and its Application as a Negative Electrode for Supercapacitors. Chem. Asian J. 2016, 11, 2392–2398. [Google Scholar] [CrossRef]
- Ye, H.; Wang, L.; Deng, S.; Zeng, X.; Nie, K.; Duchesne, P.N.; Wang, B.; Liu, S.; Zhou, J.; Zhao, F.; et al. Amorphous MoS3 Infiltrated with Carbon Nanotubes as an Advanced Anode Material of Sodium-Ion Batteries with Large Gravimetric, Areal, and Volumetric Capacities. Adv. Energy Mater. 2017, 7, 1601602. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Q.; Jia, F.; Song, S. Adsorption of heavy metals on molybdenum disulfide in water: A critical review. J. Mol. Liq. 2019, 292, 111390. [Google Scholar] [CrossRef]
- Yadav, T.P.; Yadav, R.M.; Singh, D.P. Mechanical Milling: A Top Down Approach for the Synthesis of Nanomaterials and Nanocomposites. Nanosci. Nanotechnol. 2012, 2, 22–48. [Google Scholar] [CrossRef] [Green Version]
- Gou, J.; Zhuge, J.; Liang, F. Processing of polymer nanocomposites. In Manufacturing Techniques for Polymer Matrix Composites (PMCs); Elsevier: Amsterdam, The Netherlands, 2012; pp. 95–119. [Google Scholar]
- Shi, X.; Zhou, W.; Ma, D.; Ma, Q.; Bridges, D.; Ma, Y.; Hu, A. Electrospinning of Nanofibers and Their Applications for Energy Devices. J. Nanomater. 2015, 2015, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.A. Synthesis of Nanomaterials: Methods & Technology. In Applications of Nanomaterials in Human Health; Springer: Berlin/Heidelberg, Germany, 2020; pp. 15–21. [Google Scholar]
- Koli, S.K.; Hussain, A. Status of Electronic Waste Management in India: A Review. Adv. Treat. Tech. Ind. Wastewater 2019, 238–250. [Google Scholar] [CrossRef]
- Qiao, S.Z.; Liu, J.; Lu, G.Q.M. Synthetic chemistry of nanomaterials. In Modern Inorganic Synthetic Chemistry; Elsevier: Amsterdam, The Netherlands, 2011; pp. 479–506. [Google Scholar]
- Ottaviano, L.; Palleschi, S.; Perrozzi, F.; D’Olimpio, G.; Priante, F.; Donarelli, M.; Benassi, P.; Nardone, M.; Gonchigsuren, M.; Gombosuren, M.; et al. Mechanical exfoliation and layer number identification of MoS2 revisited. 2D Mater. 2017, 4, 045013. [Google Scholar] [CrossRef]
- Yuan, L.; Ge, J.; Peng, X.; Zhang, Q.; Wu, Z.; Jian, Y.; Xiong, X.; Yin, H.; Han, J. A reliable way of mechanical exfoliation of large scale two dimensional materials with high quality. Aip Adv. 2016, 6, 125201. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Li, X.; Guo, W.; Zhao, M.; Fan, X.; Dong, Y.; Xu, C.; Deng, J.; Fu, Y. Synthesis Methods of Two-Dimensional MoS2: A Brief Review. Crystals 2017, 7, 198. [Google Scholar] [CrossRef]
- Xie, S.; Xu, M.; Liang, T.; Huang, G.; Wang, S.; Xue, G.; Meng, N.; Xu, Y.; Chen, H.; Ma, X.; et al. A high-quality round-shaped monolayer MoS2 domain and its transformation. Nanoscale 2016, 8, 219–225. [Google Scholar] [CrossRef]
- Backes, C.; Higgins, T.M.; Kelly, A.; Boland, C.; Harvey, A.; Hanlon, D.; Coleman, J.N. Guidelines for Exfoliation, Characterization and Processing of Layered Materials Produced by Liquid Exfoliation. Chem. Mater. 2017, 29, 243–255. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, L.; Jia, F.; Li, Y.; Song, S. Removal of Cd (II) from water by using nano-scale molybdenum disulphide sheets as adsorbents. J. Mol. Liq. 2018, 263, 526–533. [Google Scholar] [CrossRef]
- Grayfer, E.D.; Kozlova, M.N.; Fedorov, V.E. Colloidal 2D nanosheets of MoS2 and other transition metal dichalcogenides through liquid-phase exfoliation. Adv. Colloid Interface Sci. 2017, 245, 40–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; von dem Bussche, A.; Qiu, Y.; Valentin, T.M.; Gion, K.; Kane, A.B.; Hurt, R.H. Chemical Dissolution Pathways of MoS2 Nanosheets in Biological and Environmental Media. Environ. Sci. Technol. 2016, 50, 7208–7217. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Zhu, B.; You, W.; Jaroniec, M.; Yu, J. A flexible bio-inspired H2-production photocatalyst. Appl. Catal. B Environ. 2018, 220, 148–160. [Google Scholar] [CrossRef]
- Mercante, L.A.; Andre, R.S.; Schneider, R.; Mattoso, L.H.C.; Correa, D.S. Free-standing SiO2/TiO2–MoS2 composite nanofibrous membranes as nanoadsorbents for efficient Pb(II) removal. New J. Chem. 2020, 44, 13030–13035. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Mezentseva, N.V.; Bobrova, L.N.; Smorygo, O.L.; Eremeev, N.F.; Fedorova, Y.E.; Bespalko, Y.N.; Skriabin, P.I.; Krasnov, A.V.; Lukashevich, A.I.; et al. Advanced Materials for Solid Oxide Fuel Cells and Membrane Catalytic Reactors. In Advanced Nanomaterials for Catalysis and Energy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 435–514. [Google Scholar] [CrossRef]
- Wang, K.; Liu, X.; Tang, J.; Wang, L.; Sun, H. Ball milled Fe0@FeS hybrids coupled with peroxydisulfate for Cr(VI) and phenol removal: Novel surface reduction and activation mechanisms. Sci. Total Environ. 2020, 739, 139748. [Google Scholar] [CrossRef]
- Iqbal, P.; Preece, J.A.; Mendes, P.M. Nanotechnology: The ‘Top-Down’ and ‘Bottom-Up’ Approaches. In Supramolecular Chemistry; Gale, P.A., Steed, J.W., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2012. [Google Scholar] [CrossRef]
- Zhang, J.; Chernomordik, B.D.; Crisp, R.W.; Kroupa, D.M.; Luther, J.M.; Miller, E.M.; Gao, J.; Beard, M.C. Preparation of Cd/Pb Chalcogenide Heterostructured Janus Particles via Controllable Cation Exchange. ACS Nano 2015, 9, 7151–7163. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Liu, J.; Wan, X.; Wang, H.; Li, Y.; Liu, J.; Rong, H.; Xu, M.; Chen, W.; Zhang, J. Phosphine ligand-mediated kinetics manipulation of aqueous cation exchange: A case study on the synthesis of Au@SnSx core–shell nanocrystals for photoelectrochemical water splitting. Chem. Commun. 2018, 54, 9993–9996. [Google Scholar] [CrossRef]
- Fenton, J.L.; Schaak, R.E. Structure-Selective Cation Exchange in the Synthesis of Zincblende MnS and CoS Nanocrystals. Angew. Chem. 2017, 129, 6564–6567. [Google Scholar] [CrossRef]
- Ma, F.-X.; Yu, L.; Xu, C.-Y.; Lou, X.W. Self-supported formation of hierarchical NiCo 2 O 4 tetragonal microtubes with enhanced electrochemical properties. Energy Environ. Sci. 2016, 9, 862–866. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Yu, L.; Lou, X.W.D. Metal Sulfide Hollow Nanostructures for Electrochemical Energy Storage. Adv. Energy Mater. 2016, 6, 1501333. [Google Scholar] [CrossRef]
- Xu, J.; Qu, Z.; Yan, N.; Zhao, Y.; Xu, X.; Li, L. Size-dependent nanocrystal sorbent for copper removal from water. Chem. Eng. J. 2016, 284, 565–570. [Google Scholar] [CrossRef]
- Powell, A.E.; Hodges, J.M.; Schaak, R.E. Preserving Both Anion and Cation Sublattice Features during a Nanocrystal Cation-Exchange Reaction: Synthesis of Metastable Wurtzite-Type CoS and MnS. J. Am. Chem. Soc. 2016, 138, 471–474. [Google Scholar] [CrossRef]
- Barman, D.; Ghosh, S.; Paul, S.; Dalal, B.; De, S.K. Cation Exchange-Mediated Synthesis of Library of Plasmomagnetic Nanoheterostructures: Transformation of 2-Dimensional-Shaped Fe7S8 Nanoplates to Cu–Fe–S-Based Ternary Compound. Chem. Mater. 2018, 30, 5550–5560. [Google Scholar] [CrossRef]
- Fan, Z.; Lin, L.-C.; Buijs, W.; Vlugt, T.J.H.; van Huis, M.A. Atomistic understanding of cation exchange in PbS nanocrystals using simulations with pseudoligands. Nat. Commun. 2016, 7, 11503. [Google Scholar] [CrossRef] [Green Version]
- Lei, L.; Huang, D.; Zeng, G.; Cheng, M.; Jiang, D.; Zhou, C.; Chen, S.; Wang, W. A fantastic two-dimensional MoS2 material based on the inert basal planes activation: Electronic structure, synthesis strategies, catalytic active sites, catalytic and electronics properties. Coord. Chem. Rev. 2019, 399, 213020. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, S.; Liu, Y.; Yu, Y.; Yang, W.; Xu, S.; Li, H. CoS2/GO nanocomposites for highly efficient and ppb level adsorption of Hg(II) from wastewater. J. Mol. Liq. 2021, 322, 114899. [Google Scholar] [CrossRef]
- Lim, Y.R.; Song, W.; Han, J.K.; Lee, Y.B.; Kim, S.J.; Myung, S.; Lee, S.S.; An, K.-S.; Choi, C.-J.; Lim, J. Wafer-Scale, Homogeneous MoS 2 Layers on Plastic Substrates for Flexible Visible-Light Photodetectors. Adv. Mater. 2016, 28, 5025–5030. [Google Scholar] [CrossRef]
- Fu, Y.; Li, Q.; Liu, J.; Jiao, Y.; Hu, S.; Wang, H.; Xu, S.; Jiang, B. In-situ chemical vapor deposition to fabricate Cuprous oxide/copper sulfide core-shell flowers with boosted and stable wide-spectral region photocatalytic performance. J. Colloid Interface Sci. 2020, 570, 143–152. [Google Scholar] [CrossRef]
- Yu, S.H.; Tang, Z.; Shao, Y.; Dai, H.; Wang, H.Y.; Yan, J.; Pan, H.; Chua, D.H.C. In Situ Hybridizing MoS2 Microflowers on VS2 Microflakes in a One Pot CVD Process for Electrolytic Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2019, 2, 5799–5808. [Google Scholar] [CrossRef]
- Wu, K.; Li, Z.; Tang, J.; Lv, X.; Wang, H.; Luo, R.; Liu, P.; Qian, L.; Zhang, S.; Yuan, S. Controllable defects implantation in MoS2 grown by chemical vapor deposition for photoluminescence enhancement. Nano Res. 2018, 11, 4123–4132. [Google Scholar] [CrossRef]
- Ye, G.; Gong, Y.; Lin, J.; Li, B.; He, Y.; Pantelides, S.T.; Zhou, W.; Vajtai, R.; Ajayan, P.M. Defects Engineered Monolayer MoS 2 for Improved Hydrogen Evolution Reaction. Nano Lett. 2016, 16, 1097–1103. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, T.; Sumona, M.; Gupta, B.S.; Sun, Y.; Hu, Z.; Zhan, X. Utilization of iron sulfides for wastewater treatment: A critical review. Rev. Environ. Sci. Biotechnol. 2017, 16, 289–308. [Google Scholar] [CrossRef]
- Bhattacharjee, S. Iron sulfide nanoparticles prepared using date seed extract: Green synthesis, characterization and potential application for removal of ciprofloxacin and chromium. Powder Technol. 2021, 380, 219–228. [Google Scholar] [CrossRef]
- Gong, Y.; Huang, Y.; Wang, M.; Liu, F.; Zhang, T. Application of Iron-Based Materials for Remediation of Mercury in Water and Soil. Bull. Environ. Contam. Toxicol. 2019, 102, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Calagui, M.J.C.; Senoro, D.B.; Kan, C.-C.; Salvacion, J.W.L.; Futalan, C.M.; Wan, M.-W. Adsorption of indium(III) ions from aqueous solution using chitosan-coated bentonite beads. J. Hazard. Mater. 2014, 277, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Cheng, G.; Ge, X.; Chen, M.; Wang, C.; Lou, L.; Xu, X. Aqueous Hg(II) immobilization by chitosan stabilized magnetic iron sulfide nanoparticles. Sci. Total Environ. 2018, 621, 1074–1083. [Google Scholar] [CrossRef]
- Song, X.; Ke, F.; Ge, C.; Zhang, J.; Li, S.; Li, C.; Li, J. Core–Shell Magnetic Fe3O4@ZnS Nanoparticles As Highly Efficient Adsorbents for the Removal of Pb2+ from Water. Russ. J. Phys. Chem. A 2019, 93, 522–527. [Google Scholar] [CrossRef]
- Maity, J.; Ray, S.K. Chitosan based nano composite adsorbent—Synthesis, characterization and application for adsorption of binary mixtures of Pb(II) and Cd(II) from water. Carbohydr. Polym. 2018, 182, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, J.; Liu, J.; Liu, J.; Xia, F.; Wang, C.; Dahlgren, R.A.; Liu, W. Mechanism of Cr (VI) removal by magnetic greigite/biochar composites. Sci. Total Environ. 2020, 700, 134414. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Kong, L.; Qu, Z.; Li, L.; Shen, G. Magnetic Biochar Decorated with ZnS Nanocrytals for Pb (II) Removal. ACS Sustain. Chem. Eng. 2015, 3, 125–132. [Google Scholar] [CrossRef]
- Duan, J.; Ji, H.; Liu, W.; Zhao, X.; Han, B.; Tian, S.; Zhao, D. Enhanced immobilization of U(VI) using a new type of FeS-modified Fe0 core-shell particles. Chem. Eng. J. 2019, 359, 1617–1628. [Google Scholar] [CrossRef]
- Tan, C.; Dong, Y.; Fu, D.; Gao, N.; Ma, J.; Liu, X. Chloramphenicol removal by zero valent iron activated peroxymonosulfate system: Kinetics and mechanism of radical generation. Chem. Eng. J. 2018, 334, 1006–1015. [Google Scholar] [CrossRef]
- Zhang, H.; Peng, L.; Chen, A.; Shang, C.; Lei, M.; He, K.; Luo, S.; Shao, J.; Zeng, Q. Chitosan-stabilized FeS magnetic composites for chromium removal: Characterization, performance, mechanism, and stability. Carbohydr. Polym. 2019, 214, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Gong, Y.; Ji, H.; Duan, J.; Zhao, D. Efficient removal and long-term sequestration of cadmium from aqueous solution using ferrous sulfide nanoparticles: Performance, mechanisms, and long-term stability. Sci. Total Environ. 2020, 704, 135402. [Google Scholar] [CrossRef]
- Shi, T.; Yang, D.; Yang, H.; Ye, J.; Cheng, Q. Preparation of chitosan crosslinked modified silicon material and its adsorption capability for chromium(VI). Appl. Clay Sci. 2017, 142, 100–108. [Google Scholar] [CrossRef]
- Kong, L.; Li, Z.; Huang, X.; Huang, S.; Sun, H.; Liu, M.; Li, L. Efficient removal of Pb(II) from water using magnetic Fe3S4/reduced graphene oxide composites. J. Mater. Chem. A 2017, 5, 19333–19342. [Google Scholar] [CrossRef]
- Su, Y.; Adeleye, A.S.; Huang, Y.; Sun, X.; Dai, C.; Zhou, X.; Zhang, Y.; Keller, A.A. Simultaneous removal of cadmium and nitrate in aqueous media by nanoscale zerovalent iron (nZVI) and Au doped nZVI particles. Water Res. 2014, 63, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Bao, J.; Lu, C.; Werner, D. Reductive sequestration of chromate by hierarchical FeS@Fe-0 particles. Water Res. 2016, 102, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liang, J.; He, X.; Zhang, L.; Liu, Y. Kinetics and mechanisms of amorphous FeS2 induced Cr(VI) reduction. J. Hazard. Mater. 2016, 320, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.S.; Gusain, R.; Kumar, N. Carbon nanomaterials: Synthesis, functionalization, and properties. In Carbon Nanomaterial-Based Adsorbents for Water Purification; Elsevier: Amsterdam, The Netherlands, 2020; pp. 137–179. [Google Scholar] [CrossRef]
- Wu, Z.; Duan, Q.; Li, X.; Li, J. Mutual effects behind the simultaneous removal of toxic metals and cationic dyes by interlayer-expanded MoS 2 nanosheets. Environ. Sci. Pollut. Res. 2019, 26, 31344–31353. [Google Scholar] [CrossRef]
- Jain, K.; Patel, A.S.; Pardhi, V.P.; Flora, S.J.S. Nanotechnology in wastewater management: A new paradigm towards wastewater treatment. Molecules 2021, 26, 1797. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, S.; Gong, Y.; Zhao, D. Efficient Removal of Lead from Water Using Stabilized Iron Sulfide Nanoparticles: Effectiveness and Effects of Stabilizer. Water Air Soil Pollut. 2019, 230, 115. [Google Scholar] [CrossRef]
- Pasinszki, T.; Krebsz, M.; Chand, D.; Kótai, L.; Homonnay, Z.; Sajó, I.E.; Váczi, T. Carbon microspheres decorated with iron sulfide nanoparticles for mercury(II) removal from water. J. Mater. Sci. 2020, 55, 1425–1435. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, M.E. Nanoadsorbents for water and wastewater remediation. Sci. Total Environ. 2020, 739, 139903. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, W.; Qiu, Y.; Yi, X.; von dem Bussche, A.; Kane, A.; Gao, H.; Koski, K.; Hurt, R. Biological and environmental interactions of emerging two-dimensional nanomaterials. Chem. Soc. Rev. 2016, 45, 1750–1780. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Sim, A.; Urban, J.J.; Mi, B. Removal and recovery of heavy metal ions by two-dimensional MoS2 nanosheets: Performance and mechanisms. Environ. Sci. Technol. 2018, 52, 9741–9748. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liang, W.; Li, Y.; Wu, Y.; Chen, Y.; Xiao, W.; Zhao, L.; Zhang, J.; Li, H. Modification, application and reaction mechanisms of nano-sized iron sulfide particles for pollutant removal from soil and water: A review. Chem. Eng. J. 2019, 362, 144–159. [Google Scholar] [CrossRef]
- Zhu, K.; Chen, C.; Xu, H.; Gao, Y.; Tan, X.; Alsaedi, A.; Hayat, T. Cr(VI) Reduction and Immobilization by Core-Double-Shell Structured Magnetic Polydopamine@Zeolitic Idazolate Frameworks-8 Microspheres. ACS Sustain. Chem. Eng. 2017, 5, 6795–6802. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Manoharan, R.; Paranthaman, M. Surface protonation and electrochemical activity of oxides in aqueous solution. J. Am. Chem. Soc. 1990, 112, 2076–2082. [Google Scholar] [CrossRef]
- van Koetsem, F.; van Havere, L.; Laing, G.D. Impact of carboxymethyl cellulose coating on iron sulphide nanoparticles stability, transport, and mobilization potential of trace metals present in soils and sediment. J. Environ. Manag. 2016, 168, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Liu, Y.; Xiong, Z.; Zhao, D. Immobilization of mercury by carboxymethyl cellulose stabilized iron sulfide nanoparticles: Reaction mechanisms and effects of stabilizer and water chemistry. Environ. Sci. Technol. 2014, 48, 3986–3994. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lou, Z.; Yu, J.; Zhou, X.; Lv, D.; Zhou, J.; Baig, S.A.; Xu, X. Immobilization of mercury (II) from aqueous solution using Al2O3-supported nanoscale FeS. Chem. Eng. J. 2017, 323, 483–491. [Google Scholar] [CrossRef]
- Wang, M. Immobilization of mercury by iron sulfide nanoparticles alters mercury speciation and microbial methylation in contaminated groundwater. Chem. Eng. J. 2020, 381, 12264. [Google Scholar] [CrossRef]
- Kamba, Y.; Ueta, M.; Uddin, M.A.; Kato, Y. Enhancement of Zinc Ion Removal from Water by Physically Mixed Particles of Iron/Iron Sulfide. Water Air Soil Pollut. 2021, 232, 17. [Google Scholar] [CrossRef]
- Siyal, A.A.; Shamsuddin, M.R.; Khan, M.I.; Rabat, N.E.; Zulfiqar, M.; Man, Z.; Siame, J.; Azizli, K.A. A review on geopolymers as emerging materials for the adsorption of heavy metals and dyes. J. Environ. Manag. 2018, 224, 327–339. [Google Scholar] [CrossRef]
- Liu, L.-N.; Dai, J.-G.; Zhao, T.-J.; Guo, S.-Y.; Hou, D.-S.; Zhang, P.; Shang, J.; Wang, S.; Han, S. A novel Zn(II) dithiocarbamate/ZnS nanocomposite for highly efficient Cr6+ removal from aqueous solutions. RSC Adv. 2017, 7, 35075–35085. [Google Scholar] [CrossRef] [Green Version]
- Lee, J. Facile fabrication of Cu-exchanged ZnS nanoadsorbents for highly efficient removal of contaminants. J. Environ. Chem. Eng. 2017, 5, 4431–4440. [Google Scholar] [CrossRef]
- Li, C. Facile synthesis of nano ZnO/ZnS modified biochar by directly pyrolyzing of zinc contaminated corn stover for Pb(II), Cu(II) and Cr(VI) removals. Waste Manag. 2018, 79, 625–637. [Google Scholar] [CrossRef]
- Wang, C.; Yin, H.; Bi, L.; Su, J.; Zhang, M.; Lyu, T.; Cooper, M.; Pan, G. Highly efficient and irreversible removal of cadmium through the formation of a solid solution. J. Hazard. Mater. 2020, 384, 121461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alamolhodaei, N.; Eshghi, H.; Massoudi, H. Nano-sized ZnS functionalized with dioxa-dithio ligands for removal of Pb(II) from aqueous solution. Inorg. Nano Met. Chem. 2019, 49, 100–106. [Google Scholar] [CrossRef]
- Malakar, A.; Das, B.; Sengupta, S.; Acharya, S.; Ray, S. ZnS nanorod as an efficient heavy metal ion extractor from water. J. Water Process Eng. 2014, 3, 74–81. [Google Scholar] [CrossRef]
- Jia, F.; Zhang, X.; Song, S. AFM study on the adsorption of Hg 2+ on natural molybdenum disulfide in aqueous solutions. Phys. Chem. Chem. Phys. 2017, 19, 3837–3844. [Google Scholar] [CrossRef]
- Zhi, L.; Zuo, W.; Chen, F.; Wang, B. 3D MoS2 Composition Aerogels as Chemosensors and Adsorbents for Colorimetric Detection and High-Capacity Adsorption of Hg2+. ACS Sustain. Chem. Eng. 2016, 4, 3398–3408. [Google Scholar] [CrossRef]
- Aghagoli, M.J.; Beyki, M.H.; Shemirani, F. Application of dahlia-like molybdenum disulfide nanosheets for solid phase extraction of Co(II) in vegetable and water samples. Food Chem. 2017, 223, 8–15. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Wen, T.; Liu, X.; Wang, Y.; Yang, H.; Sun, J.; Feng, J.; Dong, S.; Sun, J. Highly effective remediation of Pb(II) and Hg(II) contaminated wastewater and soil by flower-like magnetic MoS2 nanohybrid. Sci. Total Environ. 2020, 699, 134341. [Google Scholar] [CrossRef]
- Tan, L.; Liu, Y.; Meng, F.; Wu, P.; Xia, Y.; Tang, Y. 3D hierarchical defect-rich C@MoS 2 nanosheet arrays developed on montmorillonite with enhanced performance in Pb(II) removal. Environ. Sci. Nano 2020, 7, 3088–3099. [Google Scholar] [CrossRef]
- Cai, W.; Dionysiou, D.D.; Fu, F.; Tang, B. CTAB–intercalated molybdenum disulfide nanosheets for enhanced simultaneous removal of Cr(VI) and Ni(II) from aqueous solutions. J. Hazard. Mater. 2020, 396, 122728. [Google Scholar] [CrossRef]
- Zhao, X.; Li, J.; Mu, S.; He, W.; Zhang, D.; Wu, X.; Wang, C.; Zeng, H. Efficient removal of mercury ions with MoS2-nanosheet-decorated PVDF composite adsorption membrane. Environ. Pollut. 2021, 268, 115705. [Google Scholar] [CrossRef]
- Huang, S.; You, Z.; Jiang, Y.; Zhang, F.; Liu, K.; Liu, Y.; Chen, X.; Lv, Y. Fabrication of Ultrathin MoS2 Nanosheets and Application on Adsorption of Organic Pollutants and Heavy Metals. Processes 2020, 8, 504. [Google Scholar] [CrossRef]
- Fu, W.; Ji, G.; Chen, H.; Yang, S.; Yang, H.; Guo, B.; Huang, Z. Engineering Anion Resin based Amorphous Molybdenum Sulphide Composite for Treatment of Authentic Acid Mine Drainage. J. Environ. Chem. Eng. 2020, 8, 104072. [Google Scholar] [CrossRef]
- Li, X.; Li, Q.; Linghu, W.; Shen, R.; Zhao, B.; Dong, L.; Alsaedi, A.; Hayat, T.; Wang, J.; Liu, J. Sorption properties of U(VI) and Th(IV) on two-dimensional Molybdenum Disulfide (MoS2) nanosheets: Effects of pH, ionic strength, contact time, humic acids and temperature. Environ. Technol. Innov. 2018, 11, 328–338. [Google Scholar] [CrossRef]
- Sun, H.; Wu, T.; Zhang, Y.; Ng, D.H.; Wang, G. Structure-enhanced removal of Cr (vi) in aqueous solutions using MoS 2 ultrathin nanosheets. New J. Chem. 2018, 42, 9006–9015. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Z.; Zhao, G.; Zhang, Z.; Xu, C.; Liu, Y.; Chen, J.; Zhuang, L.; Haya, T.; Wang, X. Enhanced adsorption of U(VI) and 241 Am(III) from wastewater using Ca/Al layered double hydroxide@carbon nanotube composites. J. Hazard. Mater. 2018, 347, 67–77. [Google Scholar] [CrossRef]
- Hu, B.; Hu, Q.; Li, X.; Pan, H.; Tang, X.; Chen, C.; Huang, C. Rapid and highly efficient removal of Eu(III) from aqueous solutions using graphene oxide. J. Mol. Liq. 2017, 229, 6–14. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, R.; Huo, Y.; Ai, Y.; Gu, P.; Wang, X.; Li, Q.; Yu, S.; Chen, Y.; Yu, Z. Efficient elimination of Cr (VI) from aqueous solutions using sodium dodecyl sulfate intercalated molybdenum disulfide. Ecotoxicol. Environ. Saf. 2019, 175, 251–262. [Google Scholar] [CrossRef]
- Liu, C.; Jia, F.; Wang, Q.; Yang, B.; Song, S. Two-dimensional molybdenum disulfide as adsorbent for high-efficient Pb(II) removal from water. Appl. Mater. Today 2017, 9, 220–228. [Google Scholar] [CrossRef]
- Jia, F.; Liu, C.; Yang, B.; Zhang, X.; Yi, H.; Ni, J.; Song, S. Thermal Modification of the Molybdenum Disulfide Surface for Tremendous Improvement of Hg 2+ Adsorption from Aqueous Solution. ACS Sustain. Chem. Eng. 2018, 6, 9065–9073. [Google Scholar] [CrossRef]
- Feng, B.; Yao, C.; Chen, S.; Luo, R.; Liu, S.; Tong, S. Highly efficient and selective recovery of Au(III) from a complex system by molybdenum disulfide nanoflakes. Chem. Eng. J. 2018, 350, 692–702. [Google Scholar] [CrossRef]
- Chen, B.; Bi, H.; Ma, Q.; Tan, C.; Cheng, H.; Chen, Y.; He, X.; Sun, L.; Lim, T.-T.; Huang, L.; et al. Preparation of graphene-MoS2 hybrid aerogels as multifunctional sorbents for water remediation. Sci. China Mater. 2017, 60, 1102–1108. [Google Scholar] [CrossRef] [Green Version]
- Pandey, S.; Fosso-Kankeu, E.; Spiro, M.J.; Waanders, F.; Kumar, N.; Ray, S.S.; Kim, J.; Kang, M. Equilibrium, kinetic, and thermodynamic studies of lead ion adsorption from mine wastewater onto MoS2-clinoptilolite composite. Mater. Today Chem. 2020, 18, 100376. [Google Scholar] [CrossRef]
- Dong, L.; Yang, J.; Mou, Y.; Sheng, G.; Wang, L.; Linghu, W.; Asiri, A.M.; Alamry, K.A. Effect of various environmental factors on the adsorption of U(VI) onto biochar derived from rice straw. J. Radioanal. Nucl. Chem. 2017, 314, 377–386. [Google Scholar] [CrossRef]
- Wang, P.; Yin, L.; Wang, J.; Xu, C.; Liang, Y.; Yao, W.; Wang, X.; Yu, S.; Chen, J.; Sun, Y.; et al. Superior immobilization of U(VI) and 243Am(III) on polyethyleneimine modified lamellar carbon nitride composite from water environment. Chem. Eng. J. 2017, 326, 863–874. [Google Scholar] [CrossRef]
- Peng, W.; Li, H.; Liu, Y.; Song, S. A review on heavy metal ions adsorption from water by graphene oxide and its composites. J. Mol. Liq. 2017, 230, 496–504. [Google Scholar] [CrossRef]
- Zhuang, Y.-T.; Zhang, X.; Wang, D.-H.; Yu, Y.-L.; Wang, J.-H. Three-dimensional molybdenum disulfide/graphene hydrogel with tunable heterointerfaces for high selective Hg(II) scavenging. J. Colloid Interface Sci. 2018, 514, 715–722. [Google Scholar] [CrossRef]
- Guo, J.; Tian, H.; He, J. Integration of CuS nanoparticles and cellulose fibers towards fast, selective and efficient capture and separation of mercury ions. Chem. Eng. J. 2021, 408, 127336. [Google Scholar] [CrossRef]
- Van der Stam, W.; Gudjonsdottir, S.; Evers, W.H.; Houtepen, A.J. Switching between Plasmonic and Fluorescent Copper Sulfide Nanocrystals. J. Am. Chem. Soc. 2017, 139, 13208–13217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, C.; Chen, S.; Wang, L.; Deng, H.; Tong, S. Low cost and rapid fabrication of copper sulfides nanoparticles for selective and efficient capture of noble metal ions. Chem. Eng. J. 2019, 373, 1168–1178. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, H.; Xu, L.; Wang, X.; Chen, M. Laser-induced photochemical synthesis of fibrous-shaped CuO@CuS nanoporous structures for enhanced electrostatic adsorption of negatively charged contaminants from wastewater. Opt. Mater. Express 2017, 7, 3863. [Google Scholar] [CrossRef]
- Ray, C. Evolution of tubular copper sulfide nanostructures from copper(i)–metal organic precursor: A superior platform for the removal of Hg(ii) and Pb(ii) ions. RSC Adv. 2015, 5, 12446–12453. [Google Scholar] [CrossRef]
- Sharifpour, E.; Khafri, H.Z.; Ghaedi, M.; Asfaram, A.; Jannesar, R. Isotherms and kinetic study of ultrasound-assisted adsorption of malachite green and Pb2+ ions from aqueous samples by copper sulfide nanorods loaded on activated carbon: Experimental design optimization. Ultrason. Sonochem. 2018, 40, 373–382. [Google Scholar] [CrossRef]
- Hu, M.; Tian, H.; He, J. Unprecedented Selectivity and Rapid Uptake of CuS Nanostructures toward Hg(II) Ions. ACS Appl. Mater. Interfaces 2019, 11, 19200–19206. [Google Scholar] [CrossRef] [PubMed]
- Cantu, J.; Valle, J.; Flores, K.; Gonzalez, D.; Valdes, C.; Lopez, J.; Padilla, V.; Alcoutlabi, M.; Parsons, J. Investigation into the thermodynamics and kinetics of the binding of Cu2+ and Pb2+ to TiS2 nanoparticles synthesized using a solvothermal process. J. Environ. Chem. Eng. 2019, 7, 103463. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Yu, L.; Lou, X.W.D. Embedding CoS2 nanoparticles in N-doped carbon nanotube hollow frameworks for enhanced lithium storage properties. Nano Res. 2017, 10, 4298–4304. [Google Scholar] [CrossRef]
- Andrea, J.; Santana, W.; Santos, N.L.; Silva, L.O.B.; Virgens, C.F. Removal of mercury(II) ions in aqueous solution using the peel biomass of Pachira aquatica Aubl: Kinetics and adsorption equilibrium studies. Environ. Monit. Assess. 2016, 188, 293. [Google Scholar] [CrossRef]
- Raj, R.; Dalei, K.; Chakraborty, J.; Das, S. Extracellular polymeric substances of a marine bacterium mediated synthesis of CdS nanoparticles for removal of cadmium from aqueous solution. J. Colloid Interface Sci. 2016, 462, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Liu, W.; Mao, Y.; Zhang, L.; Cheng, J.; Gong, M.; Zhao, H.; Dai, L.; Zhang, S.; Zhao, Q. Adsorption behavior of copper ions from aqueous solution onto graphene oxide–CdS composite. Chem. Eng. J. 2015, 259, 603–610. [Google Scholar] [CrossRef]
- Golkhah, S.; Mousavi, H.Z.; Shirkhanloo, H.; Khaligh, A. Removal of Pb(II) and Cu(II) Ions from Aqueous Solutions by Cadmium Sulfide Nanoparticles. Int. J. Nanosci. Nanotechnol. 2017, 13, 105–117. [Google Scholar]
- Ghani, N.R.N.A.; Jami, M.S.; Alam, M.Z. The role of nanoadsorbents and nanocomposite adsorbents in the removal of heavy metals from wastewater: A review and prospect. Pollution 2021, 7, 153–179. [Google Scholar]
- Kurniawan, T.A.; Sillanpää, M.E.; Sillanpää, M. Nanoadsorbents for remediation of aquatic environment: Local and practical solutions for global water pollution problems. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1233–1295. [Google Scholar] [CrossRef]
| Synthetic Techniques | Merits | Demerits | Product Quality | Reference |
|---|---|---|---|---|
| Top to bottom approaches | ||||
| Mechanical exfoliation | Facile synthesis | Low productivity | Perfect surface; high crystallinity | [12,44] |
| L.P./chemical exfoliation | Facile synthesis with high-yield; generally single-layer products | Strict operating environment; long reaction times | Small size and Few-layers thickness | [3,12,44] |
| Ball milling | Low cost, scalable, facile synthesis, relatively low cost, high productivity | Process-induced damages such as irregular shape product | More crystal defects | [45,46] |
| Electrospinning | Controlled formation of complex structures; a variety of fiber size (from submicron to nanometer diameters); flexible materials with a variety of functionalities | High temperatures; expensive to produce large diameter and challenging to generate <10 nm diameter fibers | Highly rough surfaces | [12,47] |
| Bottom-up techniques | ||||
| Chemical vapor deposition | Adjustable thickness; considerable area growth; rapid reaction rate; flexible design and easy incorporation of new technology to existing producing unit | High-temperature requirement; complex process; usually toxic, explosive, corrosive, and costly precursors | High crystal quality | [3,12,44] |
| Hydrothermal (solvothermal) synthesis | Easy to operate; ability to synthesize unstable substances near the melting point; large crystals of high quality. | High energy consumption and cost of equipment; relatively long production cycle | Numerous defects | [3,12,44] |
| Cation exchange | Easily, efficiently, and morphologically tuned products; precise control of the NP phase; rapid reaction rates; high yield | Relative high cost of some chemicals | Numerous defects | [12,38] |
| Support | Effect of Support | HM [I0 (mg/L)] | Ads.Dose (g/L) | % Rem. | Max. Ads. Cap. (mg/g) | SSA (m2/g) | Adsorption Mechanism | pH | Reference |
|---|---|---|---|---|---|---|---|---|---|
| ZVI and PS | Increase recovery rate | Cr6+ (35) | 0.50 | 97.0 | 67.9 | 19.5 | Electrostatic attraction and redox reaction | 3–9 | [62] |
| CM | Prevents iron leaching | Hg2+ (40) | 0.33 | 85.8 | 104 a | 72–278 | Chemical and physical adsorption | 6.5 | [102] |
| CMC | Stabilizes FeS | Cd2+ (1) | 0.10 | 93.0 | 497.5 mg/L b | 44.5 | Precipitation and surface complexation | 7.0 | [92] |
| Date seed extract | Reduces iron sulfate and stabilize FeS | Cr6+ (4.5) | 0.5 | 97.0 | 8.50 | 51.0 | Redox reaction and precipitation | 7.0 | [80] |
| Biochar | Provides functional groups | Cr6+ (20) | 0.80 | 93.0 | 23.25 | 17.6 | Electrostatic attraction and redox reaction | 5.2 | [87] |
| CMC | Stabilizes FeS | Pb2+ (5.0) | 0.05 | 98.0 | 77.0 | - | Surface complexation and precipitation | 7.0 | [101] |
| ZVI | Activates FeS | Sb5+ (100) | 0.5 | ≥99.18 | 214.0 | 1.65 | Chemisorption | 2.6–10.6 | [33] |
| ZVI | Activates FeS | As3+ As5+ (100) | 2.0 | 94.5 (89.3) | 101 c 58.3 L/mg d | - | Surface complexation | 3–10 | [35] |
| Chitosan | Stabilizes FeS and provides functional groups | Cr6+/Cr3+ (50) | 2.0 | 90 (total Cr) | 119 | 14.1 | Redox reaction, surface complexation and precipitation | 3.0 | [91] |
| NaBH4 | Limits FeS oxidation in air | Cr6+ (50) As5+ (100) | 0.1 1.0 | 70 56 | 350 28 | - | Redox reaction and precipitation Surface complexation | 7.0 | [34] |
| rGO | Stabilizes Fe3S4 | Pb2+ | 0.75 | 99.5 | 285.7 | 80.96 | Precipitation and surface adsorption | 3–6 | [94] |
| Adsorbent | Size (nm) | HM | Max. Ads. Cap. and/or Removal Efficiency | Main Ads. Mechanism | Reference |
|---|---|---|---|---|---|
| ZnS/alpha-Al2O3 | 20–25 | Hg2+, Cu2+ Pb2+ Cd2+ | 99.9% 99.9% 90.8% 66.3% | Cation exchange | [15] |
| ZnDTC/ZnS | 1–2 | Cr6+ | 73.2 mg/g; 99.9% | Cation exchange | [115] |
| Fe3O4@ZnS | 750 | Pb2+ | 272 mg/g; 91% | Chemisorption | [85] |
| Cu/ZnS | 9.0 | Co2+ Ni2+ | 57.0 mg/g 53.1 mg/g | Electrostatic attraction | [116] |
| ZnO/ZnS/biochar | 51.6–69.0 (ZnO + ZnS) | Pb2+ Cu2+ Cr6+ | 135.8 mg/g 91.2 mg/g 24.5 mg/g | Surface complexation | [117] |
| ZnS NPs | - | Cd2+ | 401 mg/g; >99% | Precipitation | [118] |
| Biochar/ZnS | 7–8 | Pb2+ | 368 mg/g | - | [88] |
| Dioxa-dithio /ZnS | 33 | Pb2+ | 99.6% | Surface complexation Electrostatic attraction | [119] |
| ZnS/alpha-Al2O3 | 20–30 30–40 50–65 | Cu2+ | 650 mg/g; 98.7% 97.0% 38.5% | Cation exchange | [69] |
| ZnS/ODA | Length: 5 Diameter: 1.2 | Fe2+ Fe3+ Pb2+ | - | Cation exchange Surface complexation Precipitation | [120] |
| Adsorbent | HM | pH | Co-Existing Ions/Effect | % Rem. | Specific Ads. Mechanism | Kinetic Model/Isotherm | Ref |
|---|---|---|---|---|---|---|---|
| 2D amorphous MoS3 | Cu2+ Cd2+ Hg2+ | 6.0 | Al3+, Fe2+ Mg2+, Ca2+ (% rem. of HM still >90 after 4 cycles) | >99.5 | Single-layer adsorption | PSO/Langmuir | [25] |
| C@MoS2/MMT | Pb2+ | 6.0 | Cu2+, Cd2+, Zn2+, Cr6+ (CI have little effect) | >95.0 | Electrostatic interaction, surface diffusion and formation of PbMoO4. | PSO/Langmuir | [125] |
| MoS2/CTAB | Cr6+ Ni2+ | 7.0 | SiO32−, SO42−, CO32−, Mg2+, Ca2+, Na2+ (CI have no effects on Cr6+ adsorption but affected the Ni2+ removal significantly) | >99.9 | Redox reaction; Electrostatic attraction and outer surface complexation | PSO/Langmuir | [126] |
| P-PVDF/MoS2 | Hg2+ | 4.5–6.0 | Pb2, Cu2+, Cd2+, Hg2+, SO42− CH3COO−, H2PO4−, Cl−, NO3− (little effect: <3% fall in rem. rate) | >95.0 | Chelation and precipitation | PSO/Langmuir | [127] |
| 1T-MoS2 | Cr6+ | 6.0 | Na+, K+, Cu2+, Mg2+, Ca2+, SO42− CO32−, Cl−, NO3−, PO43−, AsO3− (no effect) | >99.9 | Redox rxn | PSO/Langmuir | [18] |
| Ultrathin MoS2 | Cd2+ Cu2+ Ag+ | 6.0 | - | 185.2 a 169.5 70.4 | Physical hole-filling effects and electrostatic interactions | PSO/Langmuir | [128] |
| MoS2/Fe3O4 | Hg2+ | 5.0 | Cu2+, Cd2+, Zn2+, Mg2+ (little effect only on Pb2+) | >99.9 | Soft–soft interaction cation exchange and electrostatic interaction | PSO/Langmuir | [124] |
| A500-MoS4 | Pb2+ Hg2+ | 5.0 | Na+, Cu2+, Mg2+, Ca2+ (CI have no effect at pH < 1) | >99.5 | Soft-soft interaction | PSO/Langmuir | [129] |
| MoS2 NSs | U6+ Th4+ | >7.5 6.0 | Na+, K+, Mg2+, ClO4−, Cl−, NO3− (Effects of CI are significantly higher on U6+ than on Th4+) | 492.72 b 454.72 | Electrostatic interaction/inner-sphere surface complexation | PSO/Freundlich | [130] |
| D-MoS2 | Cr6+ | 6.0 | SO42−, Cl−, NO3−, H2PO4−, Na+, Cu2+, Mg2+, Ca2+, Ni2+ (No effects, except for highly concentrated Cu2+ which lowers Cr6+ rem.) | >99.9 | Electrostatic interaction and redox rxn | PSO/Langmuir | [131] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kromah, V.; Zhang, G. Aqueous Adsorption of Heavy Metals on Metal Sulfide Nanomaterials: Synthesis and Application. Water 2021, 13, 1843. https://doi.org/10.3390/w13131843
Kromah V, Zhang G. Aqueous Adsorption of Heavy Metals on Metal Sulfide Nanomaterials: Synthesis and Application. Water. 2021; 13(13):1843. https://doi.org/10.3390/w13131843
Chicago/Turabian StyleKromah, Varney, and Guanghui Zhang. 2021. "Aqueous Adsorption of Heavy Metals on Metal Sulfide Nanomaterials: Synthesis and Application" Water 13, no. 13: 1843. https://doi.org/10.3390/w13131843
APA StyleKromah, V., & Zhang, G. (2021). Aqueous Adsorption of Heavy Metals on Metal Sulfide Nanomaterials: Synthesis and Application. Water, 13(13), 1843. https://doi.org/10.3390/w13131843






