Ion-Exchange Technology for Lactic Acid Recovery in Downstream Processing: Equilibrium and Kinetic Parameters
Abstract
:1. Introduction
- chemical synthesis from non-renewable sources, such as coal, petroleum products and natural gas. In this case, it is mainly based on the hydrolysis of lactonitrile by strong acids, but it can also include base-catalyzed degradation of sugars, oxidation of propylene glycol, hydrolysis of chloropropionic acid, oxidation of propylene by nitric acid and reaction of acetaldehyde, carbon monoxide and water at high temperatures and pressures [2,3]; and secondly by
- fermentation, from different renewable substrates, such as starchy materials (e.g., corn, maize, rice, rye, wheat, potato, barley and cassava), lignocellulosic biomass (from agricultural, agro-industrial and forestry sources), microalgae, food waste (e.g., vegetables, meat, etc.) and glycerol [4]. Low temperatures, low energy consumption, better environmental concerns and high purity are some of the advantages of the fermentative route over chemical synthesis [5]. Consequently, 90% of lactic acid production is done by fermentation, since pure lactic acid can be obtained, whereas the chemical synthesis it always gives a racemic mixture [6]. Moreover, the fermentation route is considered a “bio-refinery” alternative, which relies within the circular economy approach.
2. Materials and Methods
2.1. Reagents
2.2. Resins
2.3. Resin Selection
2.4. Sorption Process Optimization by Box–Behnken Design
2.4.1. Sorption Isotherms Models
2.4.2. Adsorption Kinetic Models
2.5. Desorption Process Optimization
2.6. Data Analysis
2.7. Analytical Methodology
2.8. Statistical Analysis
3. Results and Discussion
3.1. Ion-Exchange Resins Screening for Lactic Acid Extraction
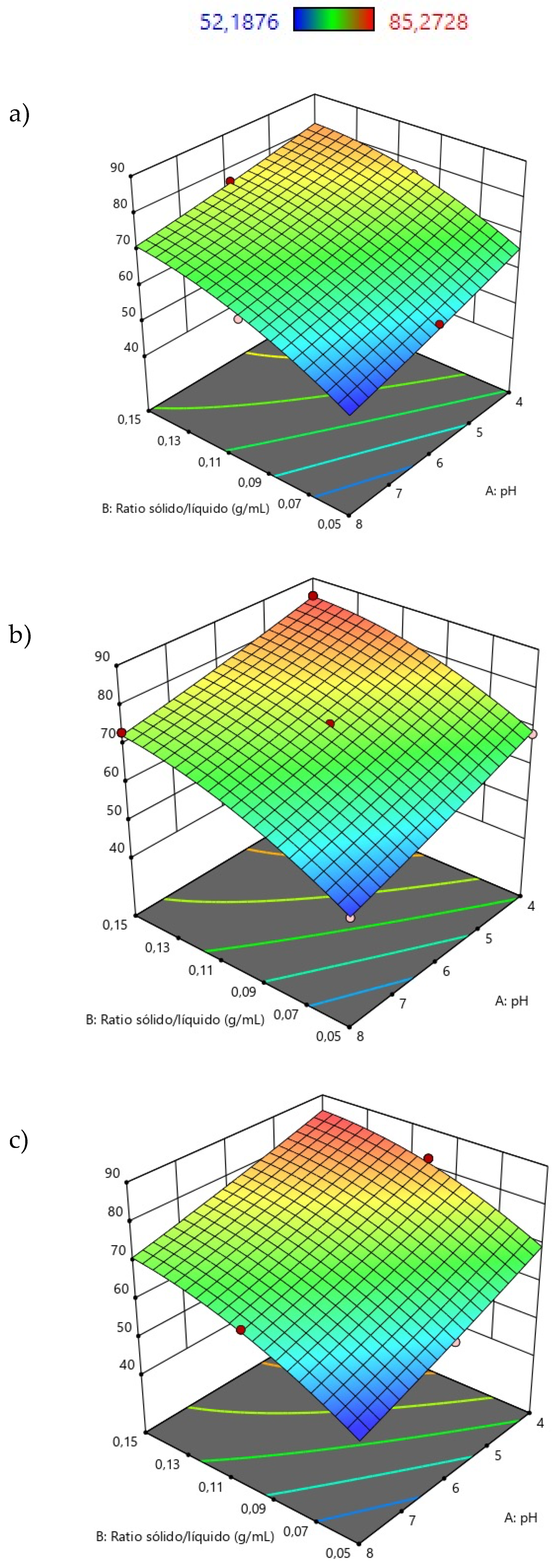
3.2. Optimization of Sorption Process for Lactic Acid Recovery Using Response Surface Methodology
- (a)
- Protonation of the tertiary amine group:P-R2Nres +H++Cl− <==> P-R2NH+ Cl− res
- (b)
- Adsorption of lactic acid (HL) and lactate anion (L−):P-R2NH+ Cl− res + L− <==> P-R2NH+ L− res + Cl− (pH > pKa(HL/L−))P-R2NH+ Cl− res + HL <==> P-R2NH+ L− res + H+Cl− (pH < pKa(HL/L−))
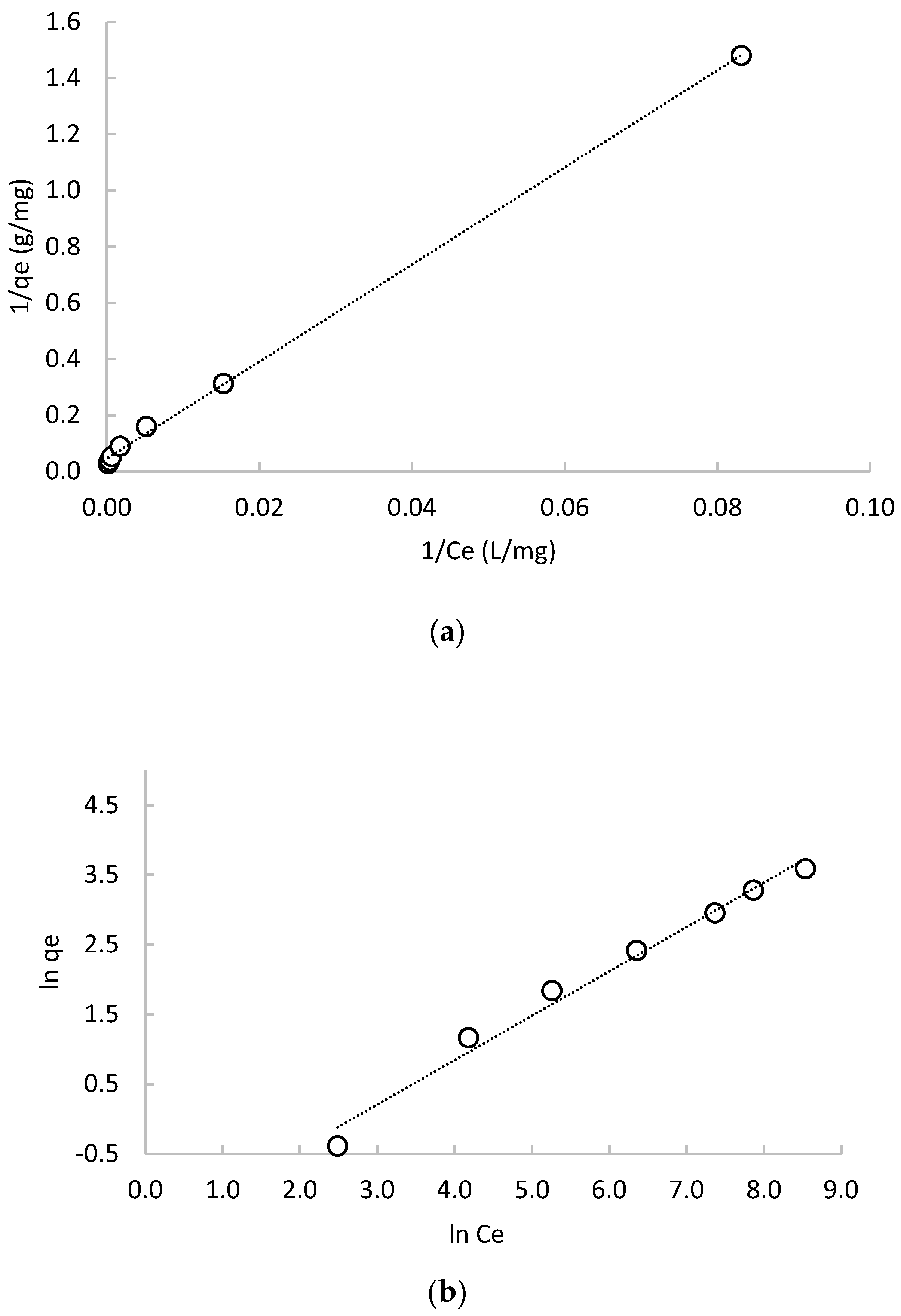
3.2.1. Lactic Acid Sorption Isotherms
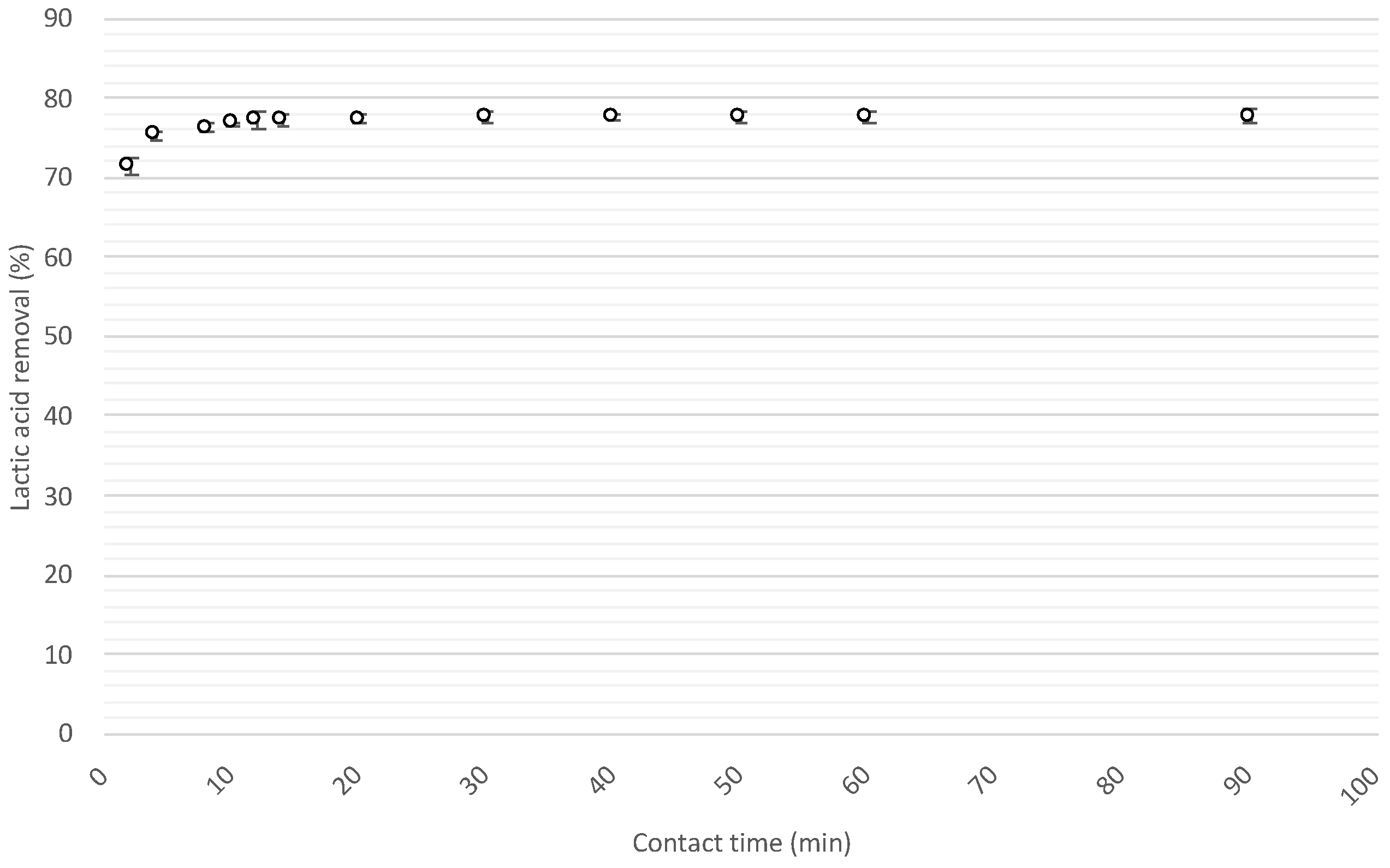
3.2.2. Lactic Acid Sorption Kinetics
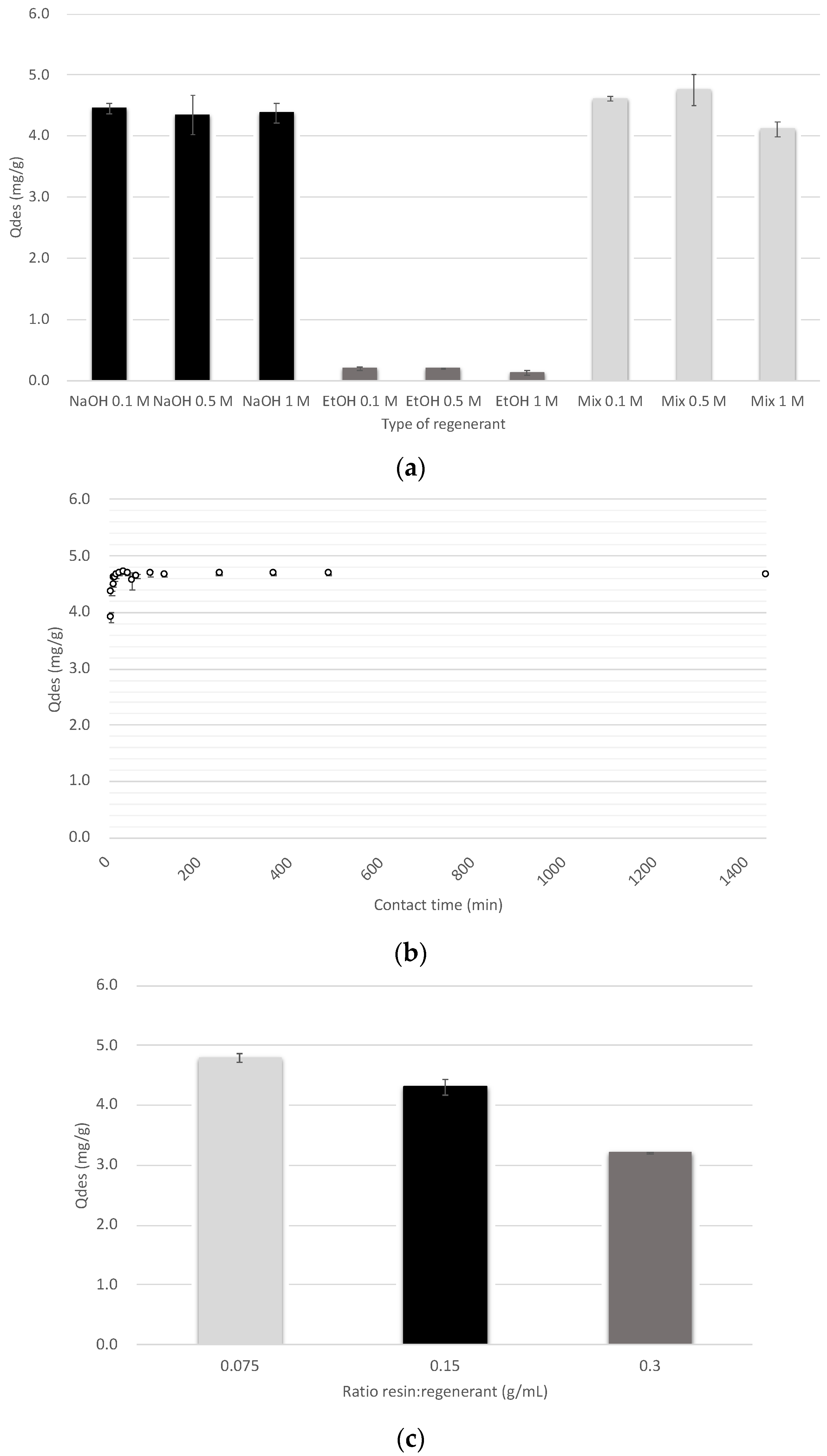
3.3. Optimization of Desorption Process for Lactic Acid Recovery
- (a)
- Desorption of lactic acid (HL) with NaOH:P-R2NH+ L− res + Na+OH− <==> P-R2Nres + Na+L− + H2O
- (b)
- Conversion of sodium lactate (NaL) to lactic acid (HL):Na+L− +H+Cl− <==> HL + Na+Cl−
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Komesu, A.; de Oliveira, J.A.R.; da Silva Martins, L.H.; Maciel, M.R.W.; Filho, R.M. Lactic Acid Production to Purification: A Review. BioResources 2017, 12, 4364–4383. [Google Scholar] [CrossRef]
- Datta, R.; Tsai, S.P.; Bonsignore, P.; Moon, S.H.; Frank, J.R. Technological and Economic Potential of Poly(Lactic Acid) and Lactic Acid Derivatives. FEMS Microbiol. Rev. 1995, 16, 221–231. [Google Scholar] [CrossRef]
- Gao, C.; Ma, C.; Xu, P. Biotechnological Routes Based on Lactic Acid Production from Biomass. Biotechnol. Adv. 2011, 29, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Alves de Oliveira, R.; Komesu, A.; Vaz Rossell, C.E.; Maciel Filho, R. Challenges and Opportunities in Lactic Acid Bioprocess Design—From Economic to Production Aspects. Biochem. Eng. J. 2018, 133, 219–239. [Google Scholar] [CrossRef]
- De Oliveira, R.A.; Filho, R.M.; Rossell, C.E.V. High Lactic Acid Production from Molasses and Hydrolysed Sugarcane Bagasse. Chem. Eng. Trans. 2016, 50, 307–312. [Google Scholar] [CrossRef]
- Hofvendahl, K.; Hahn-Hägerdal, B. Factors Affecting the Fermentative Lactic Acid Production from Renewable Resources. Enzyme Microb. Technol. 2000, 26, 87–107. [Google Scholar] [CrossRef]
- Reddy, G.; Altaf, M.; Naveena, B.J.; Venkateshwar, M.; Kumar, E.V. Amylolytic Bacterial Lactic Acid Fermentation—A Review. Biotechnol. Adv. 2008, 26, 22–34. [Google Scholar] [CrossRef]
- Gao, T.; Wong, Y.; Ng, C.; Ho, K. L-Lactic Acid Production by Bacillus Subtilis MUR1. Bioresour. Technol. 2012, 121, 105–110. [Google Scholar] [CrossRef]
- Castillo Martinez, F.A.; Balciunas, E.M.; Salgado, J.M.; Domínguez González, J.M.; Converti, A.; de Oliveira, R.P.S. Lactic Acid Properties, Applications and Production: A Review. Trends Food Sci. Technol. 2013, 30, 70–83. [Google Scholar] [CrossRef]
- Ouyang, J.; Ma, R.; Zheng, Z.; Cai, C.; Zhang, M.; Jiang, T. Open Fermentative Production of L-Lactic Acid by Bacillus Sp. Strain NL01 Using Lignocellulosic Hydrolyzates as Low-Cost Raw Material. Bioresour. Technol. 2013, 135, 475–480. [Google Scholar] [CrossRef]
- Okano, K.; Tanaka, T.; Ogino, C.; Fukuda, H.; Kondo, A. Biotechnological Production of Enantiomeric Pure Lactic Acid from Renewable Resources: Recent Achievements, Perspectives, and Limits. Appl. Microbiol. Biotechnol. 2010, 85, 413–423. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent Advances in Lactic Acid Production by Microbial Fermentation Processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef] [PubMed]
- López-Garzón, C.S.; Straathof, A.J.J. Recovery of Carboxylic Acids Produced by Fermentation. Biotechnol. Adv. 2014, 32, 873–904. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Ju, L.K. Adsorption Characteristics of Polyvinylpyridine and Activated Carbon for Lactic Acid Recovery from Fermentation of Lactobacillus Delbrueckii. Sep. Sci. Technol. 1998, 33, 1423–1437. [Google Scholar] [CrossRef]
- Monteagudo, J.M.; Aldavero, M. Production of L-Lactic Acid by Lactobacillus Delbrueckii in Chemostat Culture Using an Ion Exchange Resins System. J. Chem. Technol. Biotechnol. 1999, 74, 627–634. [Google Scholar] [CrossRef]
- Moldes, A.B.; Alonso, J.L.; Parajó, J.C. Resin Selection and Single-Step Production and Recovery of Lactic Acid from Pretreated Wood. Appl. Biochem. Biotechnol. Part A Enzym. Eng. Biotechnol. 2001, 95, 69–81. [Google Scholar] [CrossRef]
- Cao, X.; Yun, H.S.; Koo, Y.M. Recovery of L-(+)-Lactic Acid by Anion Exchange Resin Amberlite IRA-400. Biochem. Eng. J. 2002, 11, 189–196. [Google Scholar] [CrossRef]
- Moldes, A.B.; Alonso, J.L.; Parajó, J.C. Recovery of Lactic Acid from Simultaneous Saccharification and Fermentation Media Using Anion Exchange Resins. Bioprocess Biosyst. Eng. 2003, 25, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Gluszcz, P.; Jamroz, T.; Sencio, B.; Ledakowicz, S. Equilibrium and Dynamic Investigations of Organic Acids Adsorption onto Ion-Exchange Resins. Bioprocess Biosyst. Eng. 2004, 26, 185–190. [Google Scholar] [CrossRef]
- Tong, W.Y.; Fu, X.Y.; Lee, S.M.; Yu, J.; Liu, J.W.; Wei, D.Z.; Koo, Y.M. Purification of L(+)-Lactic Acid from Fermentation Broth with Paper Sludge as a Cellulosic Feedstock Using Weak Anion Exchanger Amberlite IRA-92. Biochem. Eng. J. 2004, 18, 89–96. [Google Scholar] [CrossRef]
- Dethe, M.J.; Marathe, K.V.; Gaikar, V.G. Adsorption of Lactic Acid on Weak Base Polymeric Resins. Sep. Sci. Technol. 2006, 41, 2947–2971. [Google Scholar] [CrossRef]
- John, R.P.; Nampoothiri, K.M.; Pandey, A. Acid Recovery from Cassava Bagasse Based Fermented Medium Using Anion Exchange Resins. Braz. Arch. Biol. Technol. 2008, 51, 1241–1248. [Google Scholar] [CrossRef] [Green Version]
- Quintero, J.; Acosta, A.; Mejía, C.; Ríos, R.; Torres, A.M. Purification of Lactic Acid Obtained from a Fermentative Process of Cassava Syrup Using Ion Exchange Resins. Rev. Fac. Ing. 2012, 65, 139–151. [Google Scholar]
- Bishai, M.; De, S.; Adhikari, B.; Banerjee, R. A Platform Technology of Recovery of Lactic Acid from a Fermentation Broth of Novel Substrate Zizyphus Oenophlia. 3 Biotech 2015, 5, 455–463. [Google Scholar] [CrossRef] [Green Version]
- Cui, S.; Zhao, J.; Zhang, H.; Chen, W. High-Density Culture of Lactobacillus Plantarum Coupled with a Lactic Acid Removal System with Anion-Exchange Resins. Biochem. Eng. J. 2016, 115, 80–84. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, Z.; Liu, P.; Liu, L.; Zheng, Z.; Ouyang, J. Efficient in Situ Separation and Production of L-Lactic Acid by Bacillus Coagulans Using Weak Basic Anion-Exchange Resin. Bioprocess Biosyst. Eng. 2018, 41, 205–212. [Google Scholar] [CrossRef]
- Luongo, V.; Palma, A.; Rene, E.R.; Fontana, A.; Pirozzi, F.; Esposito, G.; Lens, P.N.L. Lactic Acid Recovery from a Model of Thermotoga Neapolitana Fermentation Broth Using Ion Exchange Resins in Batch and Fixed-Bed Reactors. Sep. Sci. Technol. 2019, 54, 1008–1025. [Google Scholar] [CrossRef] [Green Version]
- Halilibrahimoğlu, N.; İnci, İ.; Baylan, N. Lactic Acid Recovery from Water by Amberlite IRA-400. Desalin. Water Treat. 2019, 172, 190–198. [Google Scholar] [CrossRef]
- Ahmad, A.; Othman, I.; Taher, H.; Banat, F. Lactic Acid Recovery from Date Pulp Waste Fermentation Broth by Ions Exchange Resins. Environ. Technol. Innov. 2021, 22, 101438. [Google Scholar] [CrossRef]
- Purolite. Purolite A100-Product Data Sheet; Purolite: Barcelona, Spain, 2007. [Google Scholar]
- Purolite. Purolite MN100-Product Data Sheet; Purolite: Barcelona, Spain, 2019. [Google Scholar]
- Purolite. Purolite A200E-Product Data Sheet; Purolite: Barcelona, Spain, 2020. [Google Scholar]
- Lewatit. Lewatit MP 64-Product Data Sheet; Lanxess: Cologne, Germany, 2011. [Google Scholar]
- Box, G.E.P.; Behnken, D.W. Simplex-Sum Designs: A Class of Second Order Rotatable Designs Derivable from Those of First Order. Ann. Math. Stat. 1960, 31, 838–864. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brand, G.C.; Silva, E.G.P.; Reis, P.S.; Souza, A.S.; Santos, W.N.L. Box-Behnken Design: An Alternative for the Optimization of Analytical Methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ameneiro, M.; Vecino, X.; Cruz, J.M.; Moldes, A.B. Physicochemical Study of a Bio-Based Adsorbent Made from Grape Marc. Ecol. Eng. 2015, 84, 190–193. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.M.F. Über Die Adsorption in Lösungen. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Zaverina, E.D.; Radushkevich, L.V. Sorption and Structure of Active Carbons. J. Phys. Chem. 1947, 21, 1351–1362. [Google Scholar]
- Temkin, M.I.; Pyzhev, V. Kinetics of Ammonia Synthesis on Promoted Iron Catalysts. Acta Physiochem 1940, 12, 327–356. [Google Scholar]
- Perez-Ameneiro, M.; Vecino, X.; Barbosa-Pereira, L.; Cruz, J.M.; Moldes, A.B. Removal of Pigments from Aqueous Solution by a Calcium Alginate-Grape Marc Biopolymer: A Kinetic Study. Carbohydr. Polym. 2014, 101, 954–960. [Google Scholar] [CrossRef]
- Vecino, X.; Devesa-Rey, R.; Villagrasa, S.; Cruz, J.M.; Moldes, A.B. Kinetic and Morphology Study of Alginate-Vineyard Pruning Waste Biocomposite vs. Non Modified Vineyard Pruning Waste for Dye Removal. J. Environ. Sci. 2015, 38, 158–167. [Google Scholar] [CrossRef]
- Lagergren, S. About the Theory of So-Called Adsorption of Soluble Substances. Kungliga Svenska Vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; Mckay, G. Pseudo-Second Order Model for Sorption. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Chien, S.H.; Clayton, W.R. Application of Elovich Equation to the Kinetics of Phosphate Release and Sorption in Soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Advances in Water Pollution Research. In Proceedings of the First International Conference on Water Pollution Research, Jerusalem, Israel, 18–23 June 1962; p. 231. [Google Scholar]
- Reig, M.; Vecino, X.; Hermassi, M.; Valderrama, C.; Gibert, O.; Cortina, J.L. Integration of Selectrodialysis and Solvent-Impregnated Resins for Zn(II) and Cu(II) Recovery from Hydrometallurgy Effluents Containing As(V). Sep. Purif. Technol. 2019, 229, 115818. [Google Scholar] [CrossRef]
- Cortina, J.L.; Meinhardt, E.; Roijals, O.; Martí, V. Modification and Preparation of Polymeric Adsorbents for Precious-Metal Extraction in Hydrometallurgical Processes. React. Funct. Polym. 1998, 36, 149–165. [Google Scholar] [CrossRef]
- Jadbabaei, N.; Ye, T.; Shuai, D.; Zhang, H. Development of Palladium-Resin Composites for Catalytic Hydrodechlorination of 4-Chlorophenol. Appl. Catal. B Environ. 2017, 205, 576–586. [Google Scholar] [CrossRef]
- Cortina, J.L.; Kautzmann, R.M.; Gliese, R.; Sampaio, C.H. Extraction Studies of Aurocyanide Using Macronet Adsorbents: Physico-Chemical Characterization. React. Funct. Polym. 2004, 60, 97–107. [Google Scholar] [CrossRef]
- Pürschel, M.; Worch, E.; Ender, V. Uptake of NOM Fractions by Anion-Exchange Resins in Demineralization Plants. Desalin. Water Treat. 2014, 52, 2987–2995. [Google Scholar] [CrossRef]
- Urbanowska, A.; Kabsch-Korbutowicz, M. The Efficiency of Macroporous Polystyrene Ion-Exchange Resins in Natural Organic Matter Removal from Surface Water. E3S Web Conf. 2017, 22. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Li, A.; Wang, J.; Lu, Y.; Zhou, Y. A Novel Aminated Polymeric Adsorbent for Removing Refractory Dissolved Organic Matter from Landfill Leachate Treatment Plant. J. Environ. Sci. 2009, 21, 1089–1095. [Google Scholar] [CrossRef]
- Urbanowska, A.; Kabsch-Korbutowicz, M. Isolation and Fractionation of Humic Substances Present in Water with the Use of Anion-Exchange Resins and Ultrafiltration. Brazilian J. Chem. Eng. 2018, 35, 1211–1217. [Google Scholar] [CrossRef]
- McKay, G.; Blair, H.S.; Gardner, J.R. Adsorption of Dyes on Chitin. I. Equilibrium Studies. J. Appl. Polym. Sci. 1982, 27, 3043–3057. [Google Scholar] [CrossRef]
- Kadirvelu, K.; Namasivayam, C. Agricultural By-Product as Metal Adsorbent: Sorption of Lead(II) from Aqueous Solution onto Coirpith Carbon. Environ. Technol. 2000, 21, 1091–1097. [Google Scholar] [CrossRef]
- Sivakumar, P.; Palanisamy, P.N. Adsorption Studies of Basic Red 29 by a Non-Conventional Activated Carbon Prepared from Euphorbia Antiquorum L. Int. J. ChemTech Res. 2009, 1, 502–510. [Google Scholar]
- Ndiaye, B.; Bustos, G.; Calvar, S.; Vecino, X.; Cruz, J.M.; Moldes, A.B.; Pérez-Cid, B. Selective Adsorption Capacity of Grape Marc Hydrogel for Adsorption of Binary Mixtures of Dyes. Water. Air. Soil Pollut. 2020, 231. [Google Scholar] [CrossRef]
- Evangelista, R.L.; Nikolov, Z.L. Recovery and Purification of Lactic Acid from Fermentation Broth by Adsorption. Appl. Biochem. Biotechnol. Part A Enzym. Eng. Biotechnol. 1996, 57–58, 471–480. [Google Scholar] [CrossRef] [Green Version]
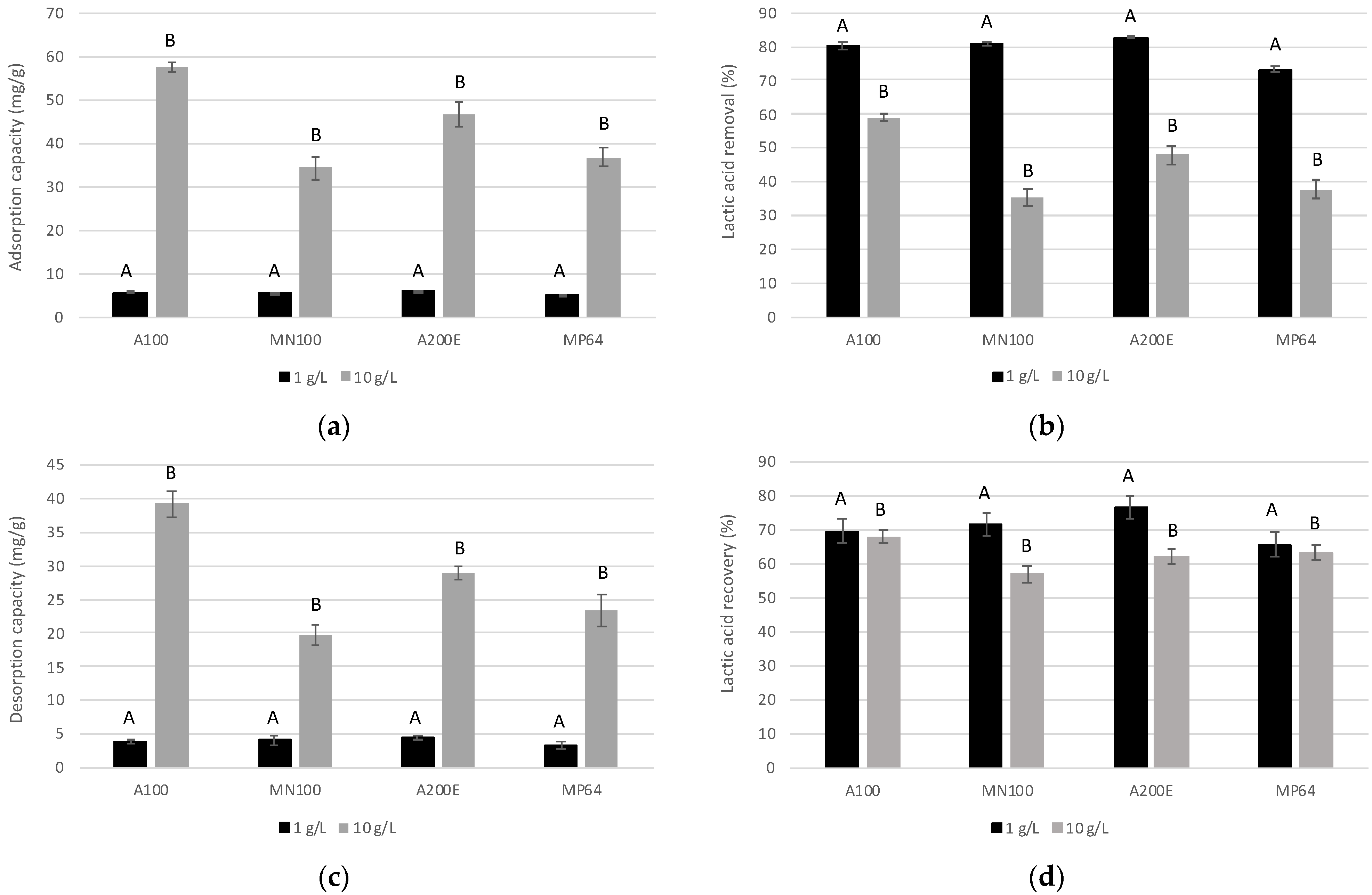
| Resin | Type | Functional Group (Capacity) | Ionic Form | Matrix | Reference |
|---|---|---|---|---|---|
| A100 | Weak base macroporous | Tertiary amine (1.3 eq./L) | Free base | Polystyrene crosslinked with divinylbenzene | [30] |
| MN100 | Free base macroporous | Tertiary amine (0.1 eq./L) | Free base | Hyper-crosslinked polystyrene–divinylbenzene | [31] |
| A200E | Strong base gel | Quaternary ammonium (1.3 eq./L) | Cl− | Polystyrene crosslinked with divinylbenzene | [32] |
| MP64 | Weak base macroporous | Tertiary/quaternary amine (1.3 eq./L) | Free base/Cl− | Polystyrene | [33] |
| Independent Variables | |||
|---|---|---|---|
| Units | Range of Variation | ||
| pH | - | 4–8 | |
| Resin/solution ratio | g/mL | 0.05–0.15 | |
| Contact time | h | 1–9 | |
| Dimensionless, coded independent variables | |||
| Nomenclature | Definition | Range of variation | |
| pH | x1 | (x1−6)/2 | (−1,1) |
| Resin/solution ratio | x2 | (x2−0.10)/0.05 | (−1,1) |
| Contact time | x3 | (x3−5)/4 | (−1,1) |
| Dependent Variables | |||
| Nomenclature | Units | ||
| Adsorption capacity | y1 | mg/g | |
| Lactic acid removal | y2 | % | |
| Desorption capacity | y3 | mg/g | |
| Lactic acid recovery | y4 | % | |
| Sorption Process | Desorption Process | ||||
|---|---|---|---|---|---|
| Initial Lactic Acid (g/L) | Solid/Liquid Ratio (g/mL) | Contact Time | Ratio Solid/Liquid (g/mL) | Contact Time | Parameter Tested |
| 1 | 0.15 | 30 min | 0.15 | 30 min | Type and concentration of the regenerant |
| 0.15 | 2 min–24 h | Contact time | |||
| 0.075–0.3 | 30 min | Solid/liquid ratio in desorption | |||
| Experiment | Independent Variables (Coded) | Dependent Variables | |||||
|---|---|---|---|---|---|---|---|
| x1 | x2 | x3 | y1 | y2 | y3 | y4 | |
| 1 | −1 | −1 | 0 | 27.89 | 68.50 | 20.22 | 72.52 |
| 2 | 0 | 0 | 0 | 13.90 | 73.39 | 11.47 | 82.52 |
| 3 | −1 | 0 | 1 | 16.62 | 82.30 | 12.33 | 74.22 |
| 4 | 0 | 0 | 0 | 13.80 | 72.75 | 11.23 | 81.39 |
| 5 | 1 | 0 | −1 | 13.26 | 63.67 | 11.57 | 87.26 |
| 6 | 0 | −1 | 1 | 22.49 | 59.24 | 18.79 | 83.55 |
| 7 | 1 | 0 | 1 | 13.45 | 64.97 | 11.83 | 88.01 |
| 8 | −1 | 1 | 0 | 11.54 | 85.27 | 8.79 | 76.18 |
| 9 | 0 | 0 | 0 | 13.72 | 72.35 | 11.93 | 86.97 |
| 10 | 0 | −1 | −1 | 22.72 | 59.74 | 19.08 | 83.97 |
| 11 | 0 | 1 | 1 | 9.71 | 76.77 | 8.04 | 82.85 |
| 12 | −1 | 0 | −1 | 15.60 | 76.62 | 12.78 | 81.94 |
| 13 | 0 | 1 | −1 | 9.66 | 76.52 | 8.15 | 84.35 |
| 14 | 1 | 1 | 0 | 10.19 | 73.32 | 8.13 | 79.78 |
| 15 | 1 | −1 | 0 | 21.73 | 52.19 | 17.76 | 81.72 |
| Langmuir Isotherm | Freundlich Isotherm | |||||
| r2 | qm | kL | RL | r2 | n | kF |
| (mg/g) | (mg/L) | (g/L) | (L/g) | |||
| 0.999 | 22.05 | 0.003 | 0.035–0.770 | 0.984 | 1.572 | 0.182 |
| Dubinin–Radushkevich Isotherm | Temkin Isotherm | |||||
| r2 | qm | E | r2 | B1 | kT | |
| (mg/g) | (mol2/J2) | (kJ/mol) | (J/mol) | (L/mg) | ||
| 0.662 | 13.02 | 76.771 | 0.081 | 0.858 | 5.599 | 0.034 |
| Pseudo-1st-Order Kinetic Model | |||
| K1 (L/g·min) | qe exp (mg/g) | qe calc (mg/g) | r2 |
| 0.063 | 5.45 | 0.13 | 0.774 |
| Pseudo-2nd-Order Kinetic Model | |||
| K2 (L/g·min) | qe exp (mg/g) | qe calc (mg/g) | r2 |
| 1.470 | 5.45 | 5.46 | 1.000 |
| Chien–Clayton Kinetic Model | |||
| α (mg/g·min) | β (g/mg) | r2 | |
| 6 × 1020 | 9.81 | 0.705 | |
| Intraparticle Diffusion Model | |||
| KP (mg/g·min0.5) | C | r2 | |
| 0.160 | 4.87 | 0.844 | |
| Type of Regenerant | Lactic Acid Recovery (%) | Regenerant Solution Cost * (€/L) |
|---|---|---|
| NaOH 0.1 M | 84.0 ± 1.9 | 0.16 |
| NaOH 0.5 M | 85.6 ± 0.1 | 0.81 |
| NaOH 1 M | 82.3± 3.2 | 1.62 |
| Ethanol 0.1 M | 3.6 ± 0.5 | 0.24 |
| Ethanol 0.5 M | 3.8 ± 0.1 | 1.21 |
| Ethanol 1 M | 2.3 ± 0.7 | 2.42 |
| Mixture NaOH/Ethanol 0.1 M | 86.7 ± 0.7 | 0.31 |
| Mixture NaOH/Ethanol 0.5 M | 89.3 ± 2.5 | 1.57 |
| Mixture NaOH/Ethanol 1 M | 77.5 ± 2.3 | 3.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vecino, X.; Reig, M.; Valderrama, C.; Cortina, J.L. Ion-Exchange Technology for Lactic Acid Recovery in Downstream Processing: Equilibrium and Kinetic Parameters. Water 2021, 13, 1572. https://doi.org/10.3390/w13111572
Vecino X, Reig M, Valderrama C, Cortina JL. Ion-Exchange Technology for Lactic Acid Recovery in Downstream Processing: Equilibrium and Kinetic Parameters. Water. 2021; 13(11):1572. https://doi.org/10.3390/w13111572
Chicago/Turabian StyleVecino, X., M. Reig, C. Valderrama, and J. L. Cortina. 2021. "Ion-Exchange Technology for Lactic Acid Recovery in Downstream Processing: Equilibrium and Kinetic Parameters" Water 13, no. 11: 1572. https://doi.org/10.3390/w13111572
APA StyleVecino, X., Reig, M., Valderrama, C., & Cortina, J. L. (2021). Ion-Exchange Technology for Lactic Acid Recovery in Downstream Processing: Equilibrium and Kinetic Parameters. Water, 13(11), 1572. https://doi.org/10.3390/w13111572









