Biological Early Warning Systems: The Experience in the Gran Sasso-Sirente Aquifer
Abstract
1. Introduction
2. Materials and Methods
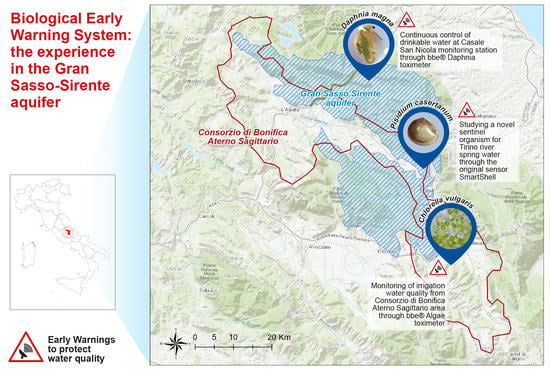
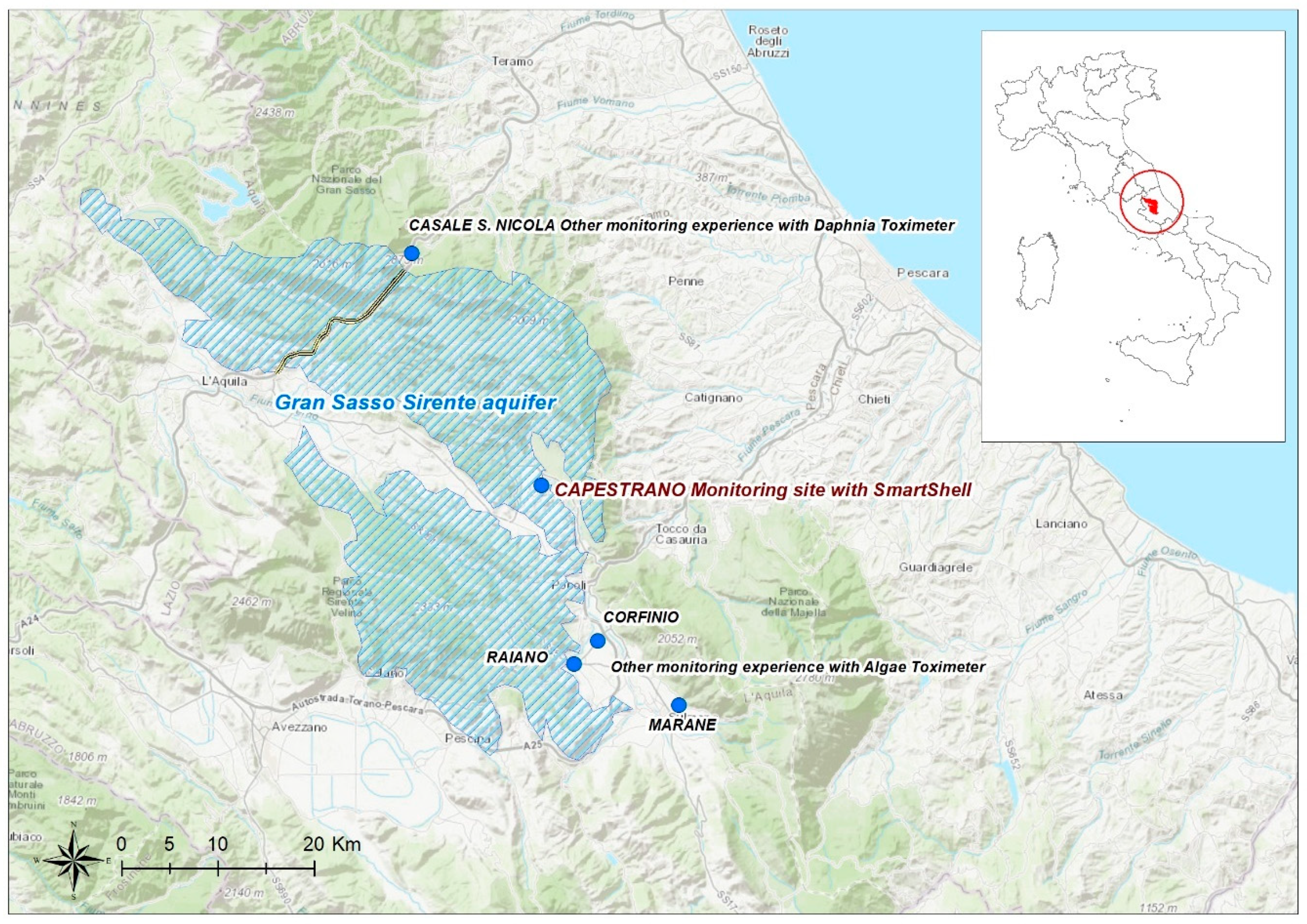
2.1. Study Area: The Gran Sasso-Sirente Aquifer (GS-S)
2.2. Monitoring Site
- Casale San Nicola monitoring station (Isola del Gran Sasso, Teramo, Italy), where groundwater collected close to highway tunnels and INFN laboratory (technically named “Ruzzo sbarramento destro + sinistro”) is continuously controlled by DTOX in the aqueduct network of the Teramo province (managed by Ruzzo Reti S.p.a.);
- Corfinio, Marane and Raiano channel and tanks (Sulmona, L’Aquila, Italy) in the irrigation network managed by Consorzio di Bonifica dell’Aterno Sagittario are monitored by ATOX.
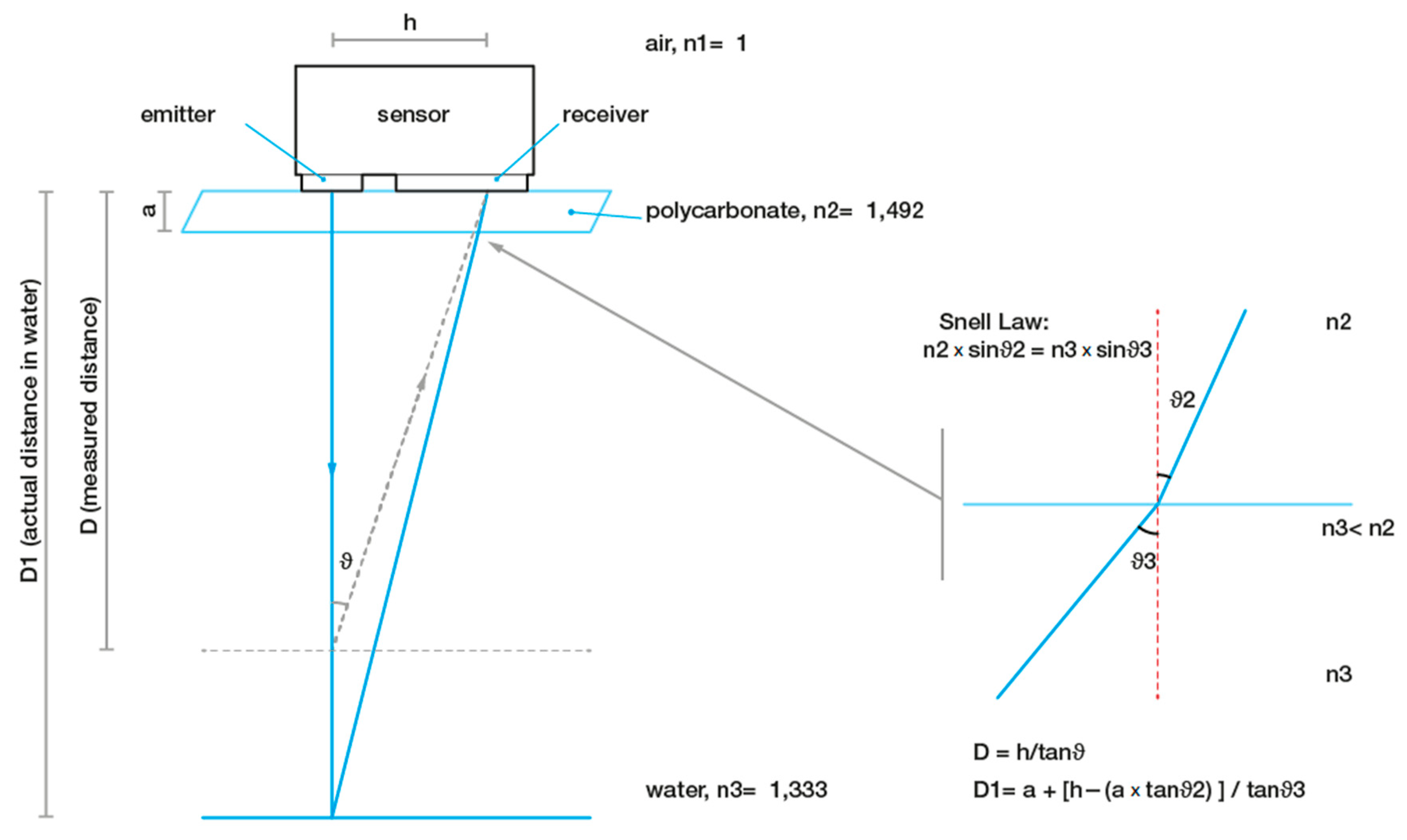
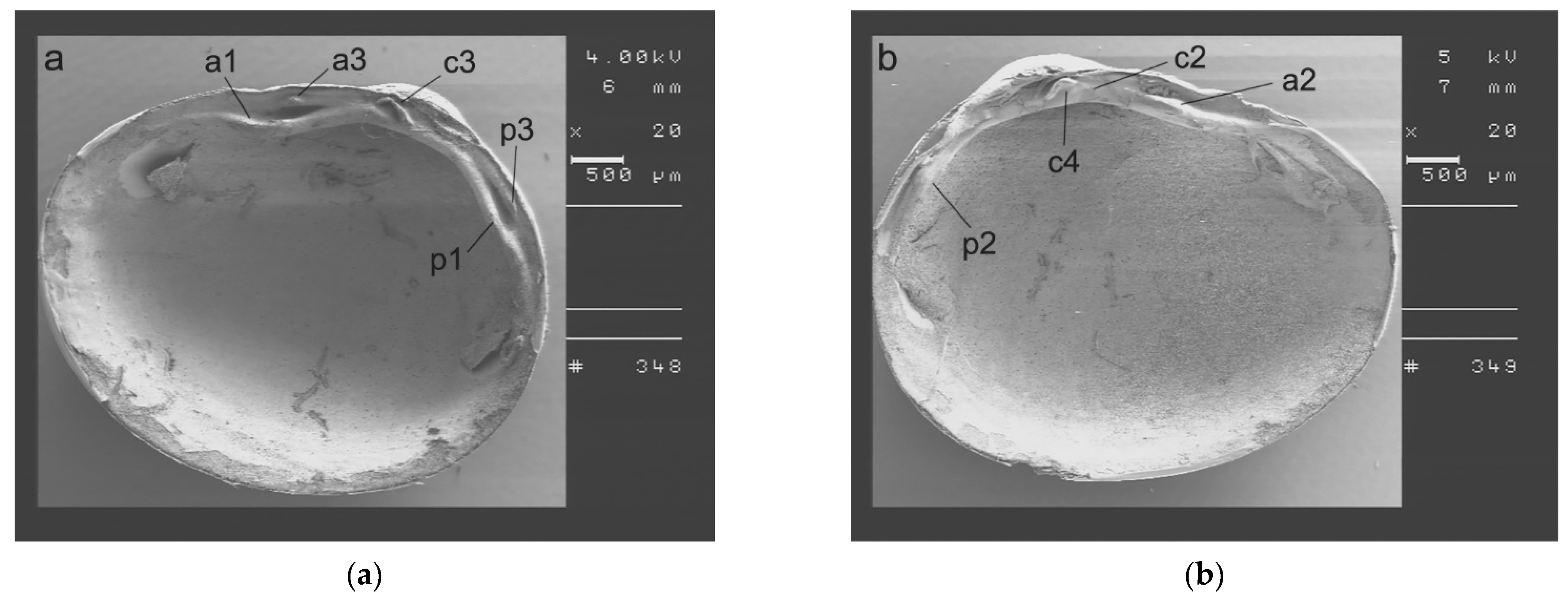
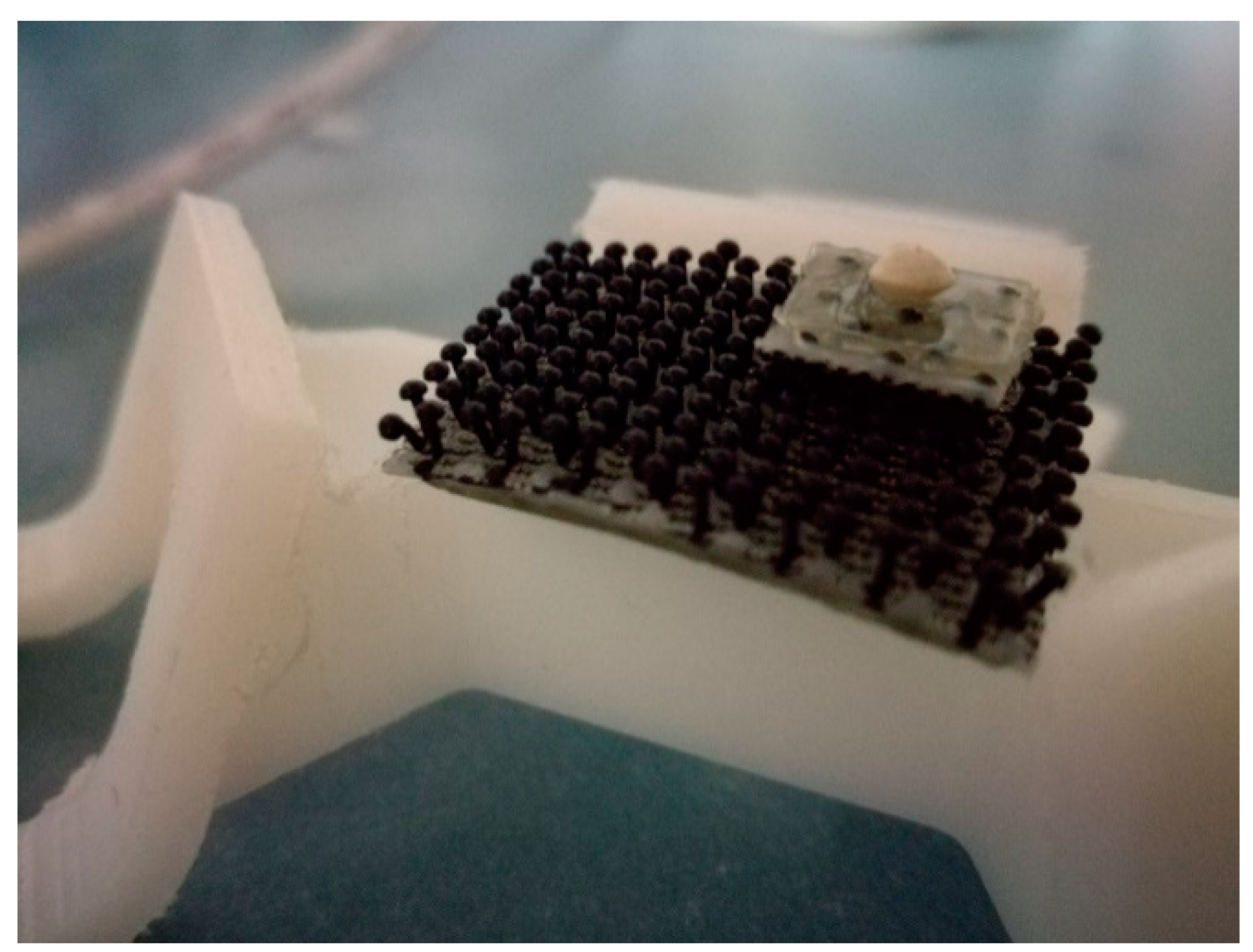
2.3. SmartShell in the Tirino River Springs
2.4. Spectral Analysis of Valve Movements
2.5. Other Biomonitoring Experiences in GS-S: DTOX in Drinkable Water of Teramo Province and ATOX for Control of Irrigation Water of L’Aquila
3. Results
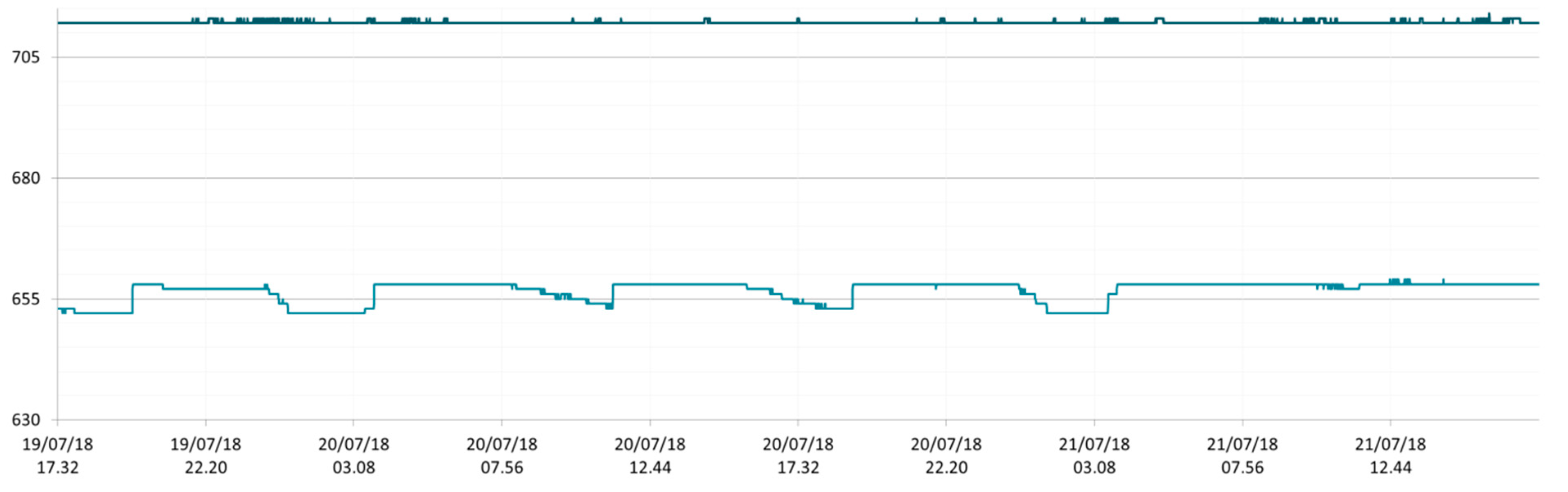
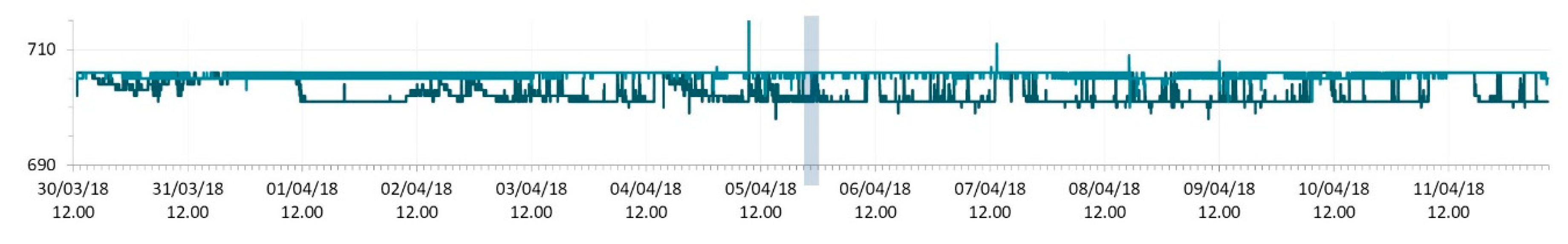
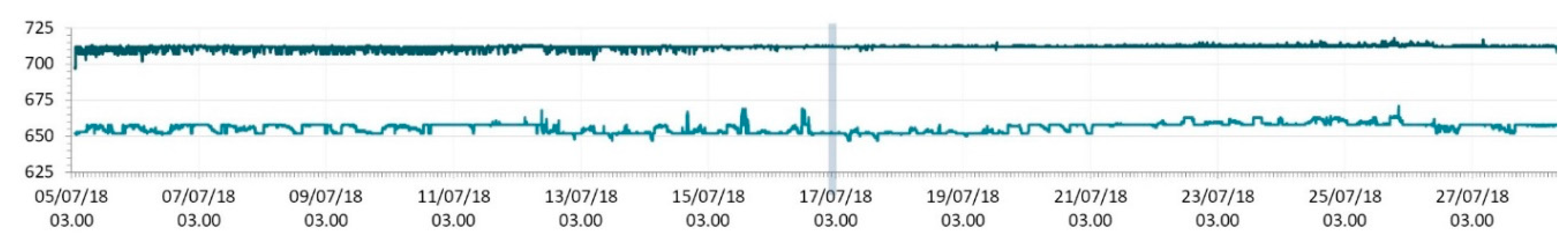
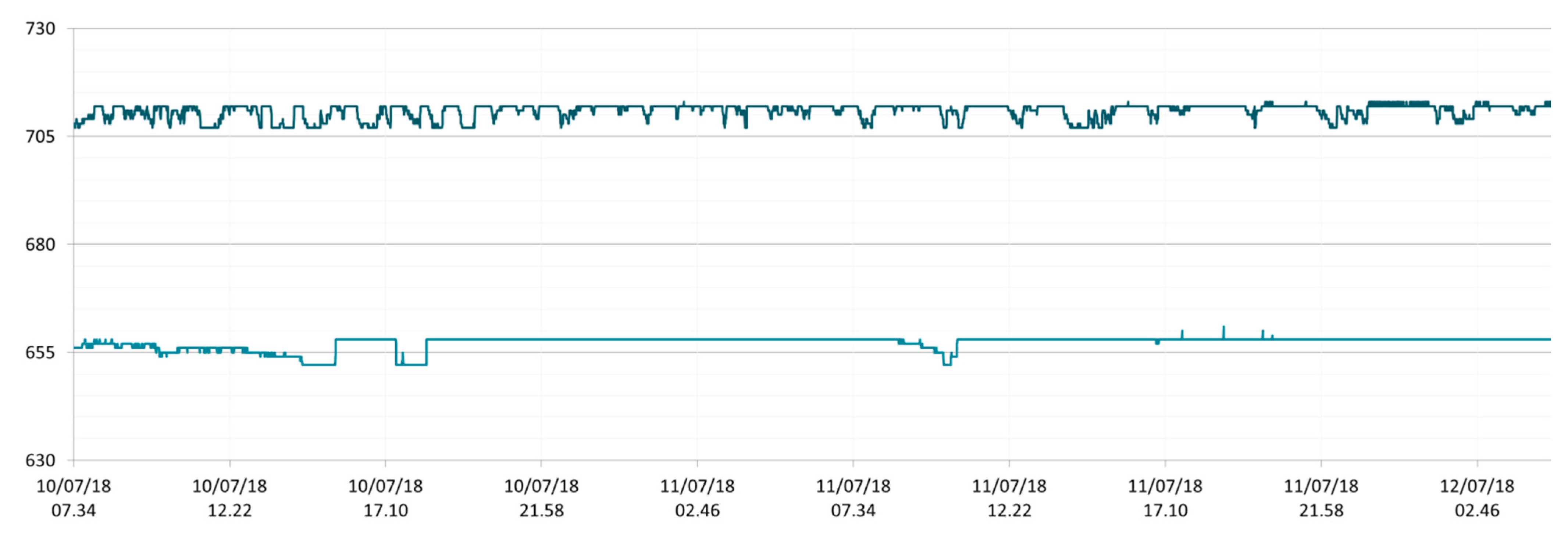
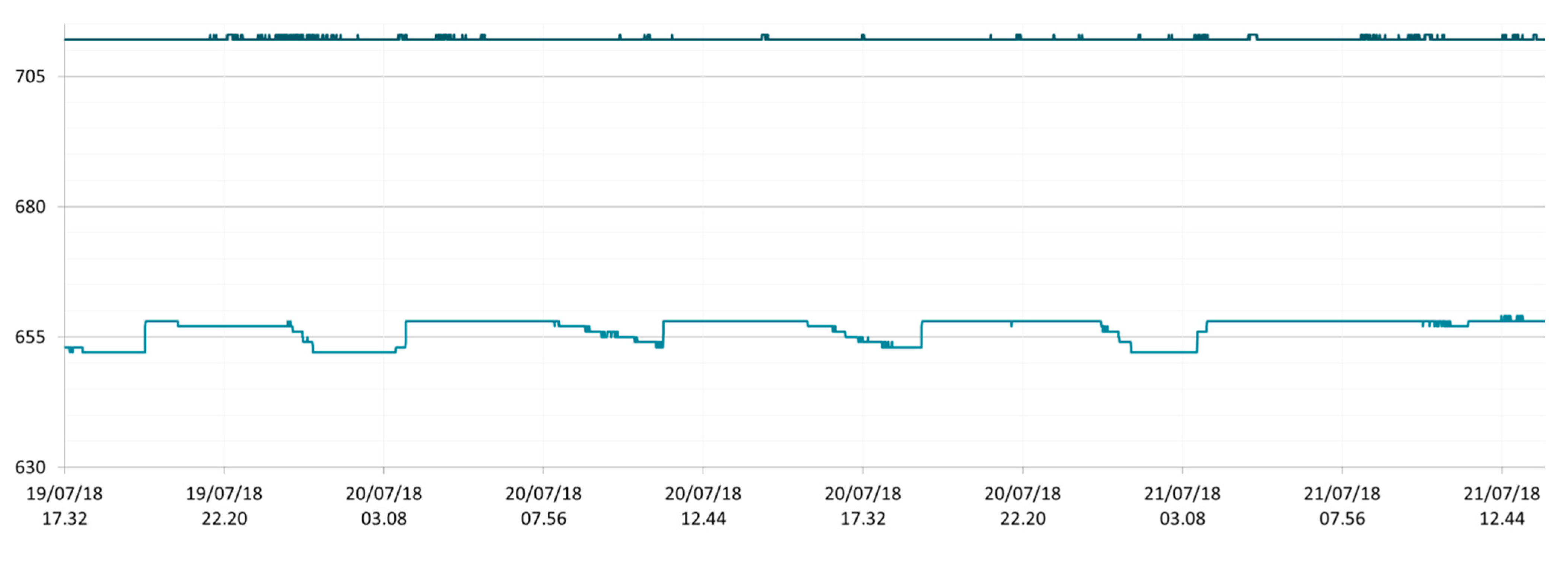
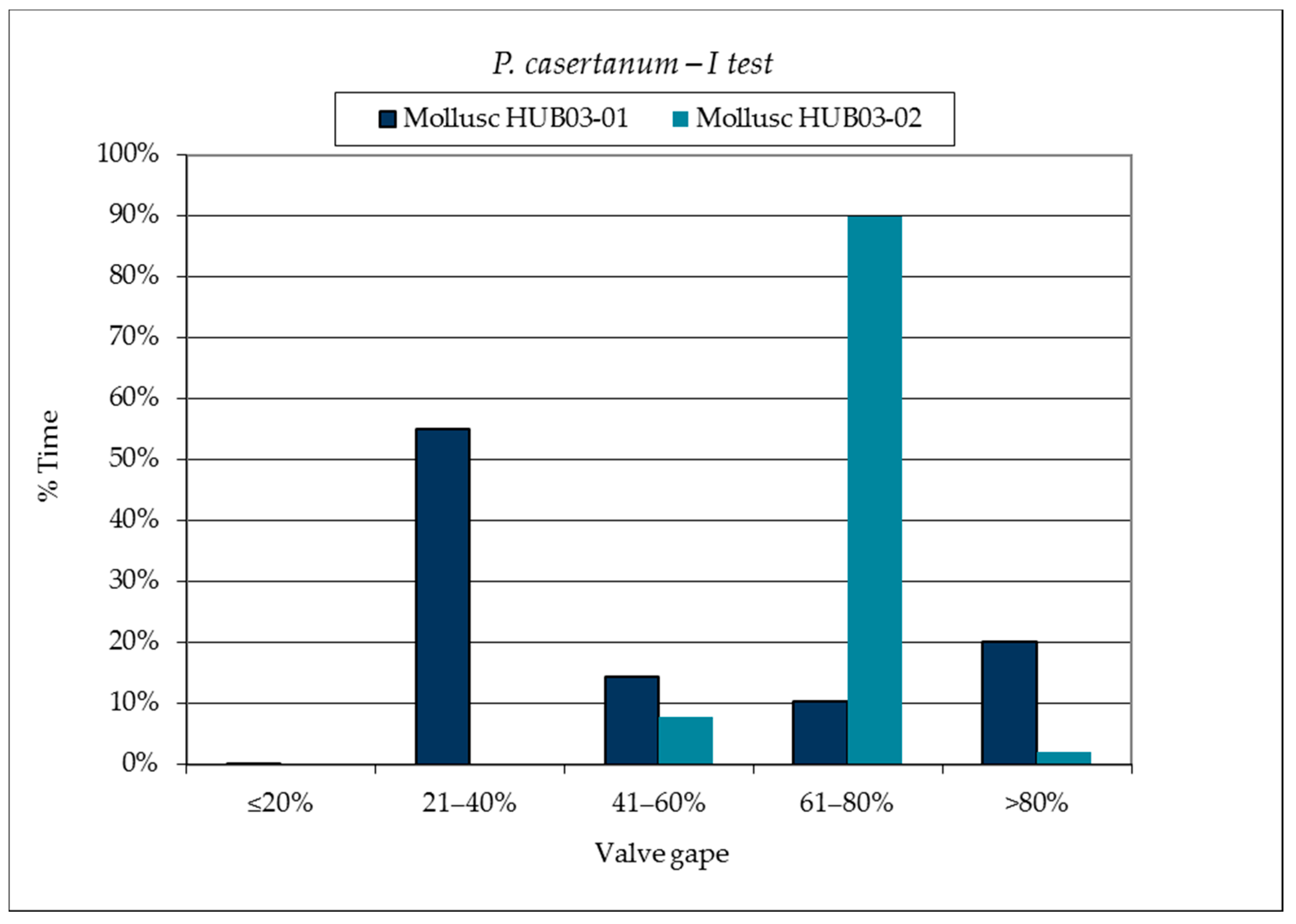
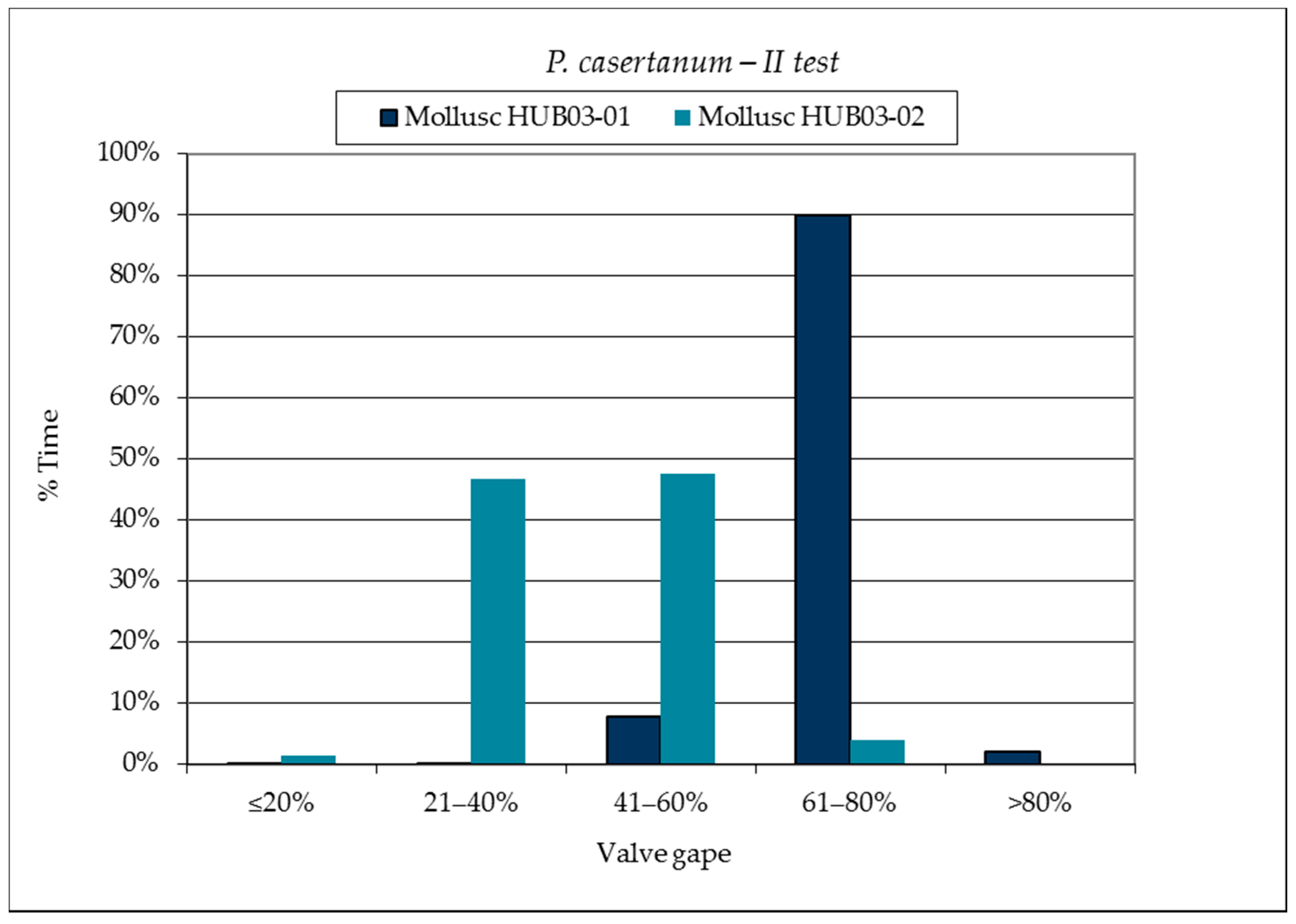
3.1. SmartShell in Tirino River Spring
3.2. DTOX in Drinkable Water of Teramo Province and ATOX for Control of Irrigation Water of L’Aquila
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greenwood, R.; Mills, G.A.; Roig, B. Introduction to emerging tools and their use in water monitoring. TrAC Trends Anal. Chem. 2007, 26, 263–267. [Google Scholar] [CrossRef]
- Gunatilaka, A.; Diehl, P. A Brief Review of Chemical and Biological Continuous Monitoring of Rivers in Europe and Asia. In Biomonitors and Biomarkers as Indicators of Environmental Change; Butterworth, F.M., Villalobos-Pietrini, R., Gonsebatt, M.E., Eds.; Springer: New York, NY, USA, 2001; Volume 2, pp. 9–28. [Google Scholar]
- Butterworth, F.M.; Villalobos-Pietrini, R.; Gonsebatt, M.E. Introduction. In Biomonitors and Biomarkers as Indicators of Environmental Change; Butterworth, F.M., Villalobos-Pietrini, R., Gonsebatt, M.E., Eds.; Springer: New York, NY, USA, 2001; Volume 2, pp. 1–8. [Google Scholar]
- Bae, M.; Park, Y. Biological early warning system based on the responses of aquatic organisms to disturbances: A review. Sci. Total Environ. 2014, 466–467, 635–649. [Google Scholar] [CrossRef]
- Belding, D.L. The Respiratory Movements of Fish as an Indicator of a Toxic Environment. Trans. Am. Fish. Soc. 1929, 59, 238–245. [Google Scholar] [CrossRef]
- Keys, A.B. The measurement of the respiratory exchange of aquatic animals. Biol. Bull. 1930, 59, 187–198. [Google Scholar] [CrossRef]
- Xia, C.; Fu, L.; Liu, Z.; Liu, H.; Chen, L.; Liu, Y. Aquatic Toxic Analysis by Monitoring Fish Behavior Using Computer Vision: A Recent Progress. J. Toxicol. 2018, 2018. Available online: https://downloads.hindawi.com/journals/jt/2018/2591924.pdf (accessed on 29 November 2020). [CrossRef]
- Kokkali, V.; van Delft, W. Overview of commercially available bioassays for assessing chemical toxicity in aqueous samples. TrAC Trends Anal. Chem. 2014, 61, 133–155. [Google Scholar] [CrossRef]
- Kramer, K.J.M.; Jenner, H.A.; De Zwart, D. The valve movement response of mussels: A tool in biological monitoring. Hydrobiology 1989, 188, 433–434. [Google Scholar] [CrossRef]
- de Zwart, D.; Kramer, K.J.M.; Jenner, H.A. Practical experiences with the biological early warning system “mosselmonitor”. Environ. Toxicol. Water Qual. 1995, 10, 237–247. [Google Scholar] [CrossRef]
- Véró, M.; Salánki, J. Inductive attenuator for continuous registration of rhythmic and periodic activity of mussels in their natural environment. Med. Biol. Engng. 1969, 7, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Kim, J.H.; Lee, B.C.; Kim, S.D. Development of a new biomonitoring method to detect the abnormal activity of Daphnia magna using automated Grid Counter device. Sci. Total Environ. 2008, 389, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.S.; Salierno, J.D.; Gipson, G.T.; Molteno, T.C.A.; Hunter, C. A video-based movement analysis system to quantify behavioral stress responses of fish. Water Res. 2004, 38, 3993–4001. [Google Scholar] [CrossRef] [PubMed]
- Di Giacinto, F.; Carbone, L.; Mascilongo, G.; Berti, M.; Ferri, N. A novel sensor for behavioural toxicity testing with freshwater and marine bivalves: Preliminary results. In Proceedings of the 55th Congress of the European Societies of Toxicology (EUROTOX 2019), Helsinki, Finland, 8–11 September 2019; Volume 314S1, pp. S97–S98. [Google Scholar]
- Grayman, W.M.; Males, R.M. Risk-based modeling of early warning systems for pollution accidents. Water Sci. Technol. 2002, 46, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Mascilongo, G.; Bodini, S.; Di Giacinto, F.; Berti, M.; Moscetta, E.; Zezza, D.; Moscetta, P.; Ferri, N. Comparison of the ecotoxicological effects of PPCPs on Artemia franciscana and Aliivibrio fischeri in automated and manual systems. In Proceedings of the 55th Congress of the European Societies of Toxicology (EUROTOX 2019), Helsinki, Finland, 8–11 September 2019; Volume 314S1, p. S97. [Google Scholar]
- Lopez-Roldan, R.; Kazlauskaite, L.; Ribo, J.; Riva, M.C.; González, S.; Cortina, J.L. Evaluation of an automated luminescent bacteria assay for in situ aquatic toxicity determination. Sci. Total Environ. 2012, 440, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Noack, U.; Walter, J. The algae toximeter for continuous water monitoring. Schriftenr. Ver. Wasser. Boden. Lufthyg. 1992, 89, 305–309. [Google Scholar] [PubMed]
- Bownik, A. Daphnia swimming behaviour as a biomarker in toxicity assessment: A review. Sci. Total Environ. 2017, 601, 194–205. [Google Scholar] [CrossRef]
- Yuan, S.; Liang, C.; Li, W.; Letcher, R.J.; Liu, C. A comprehensive system for detection of behavioral change of D. magna exposed to various chemicals. J. Hazard. Mater. 2021, 402, 123731. [Google Scholar] [CrossRef]
- Salánki, J.; Farkas, A.; Kamardina, T.; Rózsa, K.S. Molluscs in biological monitoring of water quality. Toxicol. Lett. 2003, 140, 403–410. [Google Scholar] [CrossRef]
- Hartmann, J.T.; Beggel, S.; Auerswald, K.; Stoeckle, B.C.; Geist, J. Establishing mussel behavior as a biomarker in ecotoxicology. Aquat. Toxicol. 2016, 170, 279–288. [Google Scholar] [CrossRef]
- Hansen, P. Fish. In Bioassays: Advanced Methods and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 309–329. [Google Scholar]
- Zhang, G.; Chen, L.; Liu, Y.; Chon, T.; Ren, Z.; Wang, Z.; Zhao, J.; Zhao, Y. A new online monitoring and management system for accidental pollution events developed for the regional water basin in Ningbo, China. Water Sci. Technol. 2011, 64, 1828–1834. [Google Scholar] [CrossRef]
- Bartrand, T.; Grayman, W. Drinking Water Treatment Source Water Early Warning System State of the Science Review; EPA/600/R-17/405; U.S. Environmental Protection Agency: Washington, DC, USA, 2017; pp. 1–118.
- Directive 2000/60/EC of the European Parliament and the Council of 23.10.2000. A Framework for Community Action in the Field of Water Policy; EU: Bruxelles, Belgium, 2000.
- Allan, I.J.; Vrana, B.; Greenwood, R.; Mills, G.A.; Roig, B.; Gonzalez, C. A “toolbox” for biological and chemical monitoring requirements for the European Union’s Water Framework Directive. Talanta 2006, 69, 302–322. [Google Scholar] [CrossRef]
- Diehl, P.; Gerke, T.; Jeuken, A.; Lowis, J.; Steen, R.; Van Steenwijk, J.; Stoks, P.; Willemsen, H. Early warning strategies and practices along the River Rhine. In The Rhine; Springer: Berlin/Heidelberg, Germany, 2006; Volume 5, pp. 99–124. [Google Scholar]
- Badino, G.; Pedon, E.; Saini, M.; Meucci, L.; Giacosa, D. Rilevazione in tempo reale della qualità dell’acqua superficiale mediante Unio elongatulus come biosensore autoctono: Ricerche e applicazione come sistema di preallarme. In Proceedings of the National Convention on Ecotoxicology, Turin, Italy, 7 July 2000. [Google Scholar]
- Giari, L.; Vincenzi, F.; Fano, E.A.; Graldi, I.; Gelli, F.; Castaldelli, G. Sensitivity to selected contaminants in a biological early warning system using Anodonta woodiana (Mollusca). Water SA 2017, 43, 200–208. [Google Scholar] [CrossRef]
- Commission Directive (EU) 2015/1787 of 6 October 2015 Amending Annexes II and III to Council Directive 98/83/EC on the Quality of Water Intended for Human Consumption; EU: Bruxelles, Belgium, 2015.
- Ministerial Decree 14 June 2017. Recepimento Della Direttiva (UE) 2015/1787 che Modifica gli Allegati II e III Della Direttiva 98/83/CE Sulla Qualita’ delle Acque Destinate al Consumo Umano. Modifica Degli Allegati II e III del Decreto Legislativo 2 Febbraio 2001, n. 31. G.U. 18-8-2017 Suppl. n. 192; Ministero della Salute: Rome, Italy, 2017.
- Regione Abruzzo. Sicurezza del bacino idrico del Gran Sasso. Available online: https://www.regione.abruzzo.it/content/sicurezza-bacino-idrico-del-gran-sasso (accessed on 29 November 2020).
- Petaccia, R.; Rusi, S. Idrogeologia delle sorgenti del Ruzzo (Gran Sasso d’Italia). Giorn. Geol. Appl. 2008, 8, 17–28. [Google Scholar]
- AUSL Teramo. Revoca del Divieto di Potabilità Dell’acqua di Rete a Teramo. Available online: https://www.abruzzo24ore.tv/documents/2014/nota_marconi_acqua_potabile.pdf. (accessed on 29 November 2020).
- Fais, A. Stato dell’irrigazione in Abruzzo. Programma Operativo Multiregionale “Ampliamento e Adeguamento Della Disponibilità e dei Sistemi di Adduzione e di Distribuzione delle Risorse Idriche Nelle Regioni dell’Obiettivo 1” Reg (CEE) n. 2081/93-QCS 1994/99 S; Istituto Nazionale di Economia Agraria (Abruzzo): Pescara, Italy, 2019; pp. 88–138. [Google Scholar]
- Di Lorenzo, T.; Cifoni, M.; Fiasca, B.; Di Cioccio, A.; Galassi, D.M.P. Ecological risk assessment of pesticide mixtures in the alluvial aquifers of central Italy: Toward more realistic scenarios for risk mitigation. Sci. Total Environ. 2018, 644, 161–172. [Google Scholar] [CrossRef]
- Testi, A.; Fanelli, G.; Crosti, R.; Castigliani, V.; D’Angeli, D. Characterizing river habitat quality using plant and animal bioindicators: A case study of Tirino River (Abruzzo Region, Central Italy). Ecol. Indic. 2012, 20, 24–33. [Google Scholar] [CrossRef]
- Boni, C.; Pianelli, A.; Pierdominici, P.; Ruisi, M. Le grandi sorgenti del fiume Tirino (Abruzzo). Boll. Soc. Geol. 2002, 121, 411–431. [Google Scholar]
- Petitta, M.; Tallini, M. Groundwater hydrodinamic of the Gran Sasso Massif (Abruzzi): New hydrological, hydrogeological and hydrochemical surveys (1994–2001). Boll. Soc. Geol. Ital. 2002, 121, 343–363. [Google Scholar]
- Di Celso, G.M.; Spizzico, M.; Tallini, M. Studio idrochimico multitemporale delle acque sorgive dell’acquifero carsico del Gran Sasso (Italia centrale) per un affinamento del modello idrochimico concettuale. Giorn. Geol. Appl. 2005, 2, 420–428. [Google Scholar]
- Celico, P.; Fabbrocino, S.; Petitta, M.; Tallini, M. Hydrogeological impact of the Gran Sasso motor-way tunnels (Central Italy). Giorn. Geol. Appl. 2005, 1, 156–157. [Google Scholar]
- Amoruso, A.; Crescentini, L.; Petitta, M.; Rusi, S.; Tallini, M. Impact of the 6 April 2009 L’Aquila earthquake on groundwater flow in the Gran Sasso carbonate aquifer, Central Italy. Hydrol. Process. 2011, 25, 1754–1764. [Google Scholar] [CrossRef]
- Boni, C.; Bono, P.; Capelli, G. Schema idrogeologico dell’Italia Centrale. Mem. Soc. Geol. Ital. 1986, 35, 991–1012. [Google Scholar]
- Scozzafava, M.; Tallini, M. Net infiltration in the Gran Sasso Massif (Central Italy): Thornthwaite water budget using the CN method (Soil Conservation Service). Hydrogeol. J. 2001, 9, 461–475. [Google Scholar] [CrossRef]
- Barbieri, M.; Boschetti, T.; Petitta, M.; Tallini, M. Stable isotopes (2H, 18O and 87Sr/86Sr) and hydrochemistry monitoring for groundwater hydrodynamics analysis in a karst aquifer (Gran Sasso, Central Italy). Appl. Geochem. 2005, 20, 2063–2081. [Google Scholar] [CrossRef]
- Celico, P. Idrogeologia dei massicci carbonatici, delle piane quaternarie e delle aree vulcaniche dell’Italia centro-meridionale: Progetti speciali per gli schemi idrici nel Mezzogiorno. Quad. Cassa Mezzog. 1983, 4, 1–225. [Google Scholar]
- Regione Abruzzo. Piano di Tutela Delle Acque. Available online: http://www.regione.abruzzo.it/pianoTutelaacque/ (accessed on 29 November 2020).
- Adamoli, L. Idrogeologia del massiccio carbonatico del Gran Sasso e conseguenze idrogeologiche degli scavi autostradali. Geol. Tecn. 1990, 3, 4–15. [Google Scholar]
- Jeremy, B. Exploring Arduino: Tools and Techniques for Engineering Wizardry; Wiley Edition: Hoboken, NJ, USA, 2013. [Google Scholar]
- Redmond, K.J.; Berry, M.; Pampanin, D.M.; Andersen, O.K. Valve gape behaviour of mussels (Mytilus edulis) exposed to dispersed crude oil as an environmental monitoring endpoint. Mar. Pollut. Bull. 2017, 117, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Castagnolo, L.; Franchini, D.; Giusti, F. Bivalvi (Bivalvia). Coll. In Guide per il Riconoscimento Delle Specie Animali Delle Acque interne Italiane; Consiglio Nazionale delle Ricerca: Rome, Italy, 1980; Volume 10, pp. 1–70. [Google Scholar]
- Korniushin, A.V.; Glaubrecht, M. Anatomy and reproduction of viviparous Pisidium (Parapisidium) reticulatum Kuiper, 1966: Implications for the phylogeny of Sphaeriidae (Mollusca: Bivalvia: Heterodonta). Org. Diversity Evol. 2006, 6, 185–195. [Google Scholar] [CrossRef]
- Voode, M. Taxonomie status of Pisidium amnicum (Müller, 1774) and P. inflatum megerle von mühlfeld in porro, 1838 (Mollusea: Bivalvia: Sphaeriidae). Ruthenica 2017, 27, 39–49. [Google Scholar]
- Castagnolo, L. Gli Sphaerioidea del massiccio del Gran Sasso. In Monitoraggio Biologico del Gran Sasso; Cicolani, B., Ed.; Andromeda editrice: Colledara, Teramo, Italy, 2001; Volume 2, pp. 154–158. [Google Scholar]
- Comeau, L.A.; Babarro, J.M.F.; Longa, A.; Padin, X.A. Valve-gaping behavior of raft-cultivated mussels in the Ría de Arousa, Spain. Aquacult. Rep. 2018, 9, 68–73. [Google Scholar] [CrossRef]
- Warner, R.M. Spectral Analysis of Time-Series Data; Guilford: New York, NY, USA, 1998. [Google Scholar]
- Lechelt, M.; Blohm, W.; Kirschneit, B.; Pfeiffer, M.; Gresens, E.; Liley, J.; Holz, R.; Lüring, C.; Moldaenke, C. Monitoring of surface water by ultrasensitive Daphnia toximeter. Environ. Toxicol. 2000, 15, 390–400. [Google Scholar] [CrossRef]
- Hinkley, D.V. Inference about the change-point in a sequence of random variables. Biometrika 1970, 57, 1–17. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between quantum yield of photosynthetic electron transportand quenching of chlorophyll fluorescence. Biochim. Biophys. Act. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Englund, V.; Heino, M. Valve movement of freshwater mussel Anodonta anatina: A reciprocal transplant experiment between two lakes. Ann. Zool. Fenn. 1994, 31, 417–423. [Google Scholar] [CrossRef]
- Salanki, J.; Lukacsovics, F.; Hiripi, L. The effect of temperature variation on the rhythmic an periodic activity of the fresh water mussel (Anodonta cygnes L.). Annal. Biol. Tihany 1974, 41, 69–74. [Google Scholar]
- Morton, B.S. The rhythm of adductor activity and quiescence in Anodonta cygnea L. and Unio pictorum L. and its biological significance. For. Fun. 1970, 2, 110–120. [Google Scholar]
- Liao, C.; Jau, S.; Lin, C.; Jou, L.; Liu, C.; Liao, V.H.; Chang, F. Valve movement response of the freshwater clam Corbicula fluminea following exposure to waterborne arsenic. Ecotoxicology 2009, 18, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Morton, B. The diurnal rhythm and tidal rhythm of feeding and digestion in Ostrea edulis. Biol. J. Linn. Soc. 1971, 3, 329–342. [Google Scholar] [CrossRef]
- Ahmed, H.; Ushirobira, R.; Efimov, D.; Tran, D.; Massabuau, J. Velocity estimation of valve movement in oysters for water quality surveillance. IFAC-PapersOnLine 2015, 48, 333–338. [Google Scholar] [CrossRef]
- Garcia-March, J.R.; Jiménez, S.; Sanchis, M.A.; Monleon, S.; Lees, J.; Surge, D.; Tena-Medialdea, J. In situ biomonitoring shows seasonal patterns and environmentally mediated gaping activity in the bivalve, Pinna nobilis. Mar. Biol. 2016, 163, 1–12. [Google Scholar] [CrossRef]
- Englund, V.P.M.; Heino, M.P.; Melas, G. Field method for monitoring valve movements of bivalved molluscs. Water Res. 1994, 28, 2219–2221. [Google Scholar] [CrossRef]
- Holopainen, I.J.; Penttinen, O. Normoxic and anoxic heat output of the freshwater bivalves Pisidium and Sphaerium—I. Rhythms of spontaneous quiescence and behaviour. Oecologia 1993, 93, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora. Official Journal L 206, 22/07/1992 P. 0007-0050. 1992; EU: Bruxelles, Belgium, 2000.
- Lucentini, L.; Achene, L.; Fuscoletti, V.; Nigro Di Gregorio, F.; Pettine, P. Linee Guida per la Valutazione e Gestione del Rischio Nella Filiera Delle Acque Destinate al Consumo Umano Secondo il Modello dei Water Safety Plan; Rapporti ISTISAN 14/21; Istituto Superiore di Sanità: Rome, Italy, 2014; pp. 1–83. [Google Scholar]
- Legislative Decree of 152/2006 “Norme in Materia Ambientale” Pubblicato Nella Gazzetta Ufficiale n. 88 del 14 Aprile 2006—Supplemento Ordinario n. 96; MATTM: Rome, Italy, 2006.


| Monitoring Site | Water | BEWS Monitoring Period | Temp. (°C) | pH | Dissolved Oxygen (%) | Conductivity µS/cm |
|---|---|---|---|---|---|---|
| Capestrano | Tirino River spring | 30/03–12/04/18 – 05/07–27/07/18 | 12.30–13.50 | 6.64–7.14 | 89.7–87.4 | 668–726 |
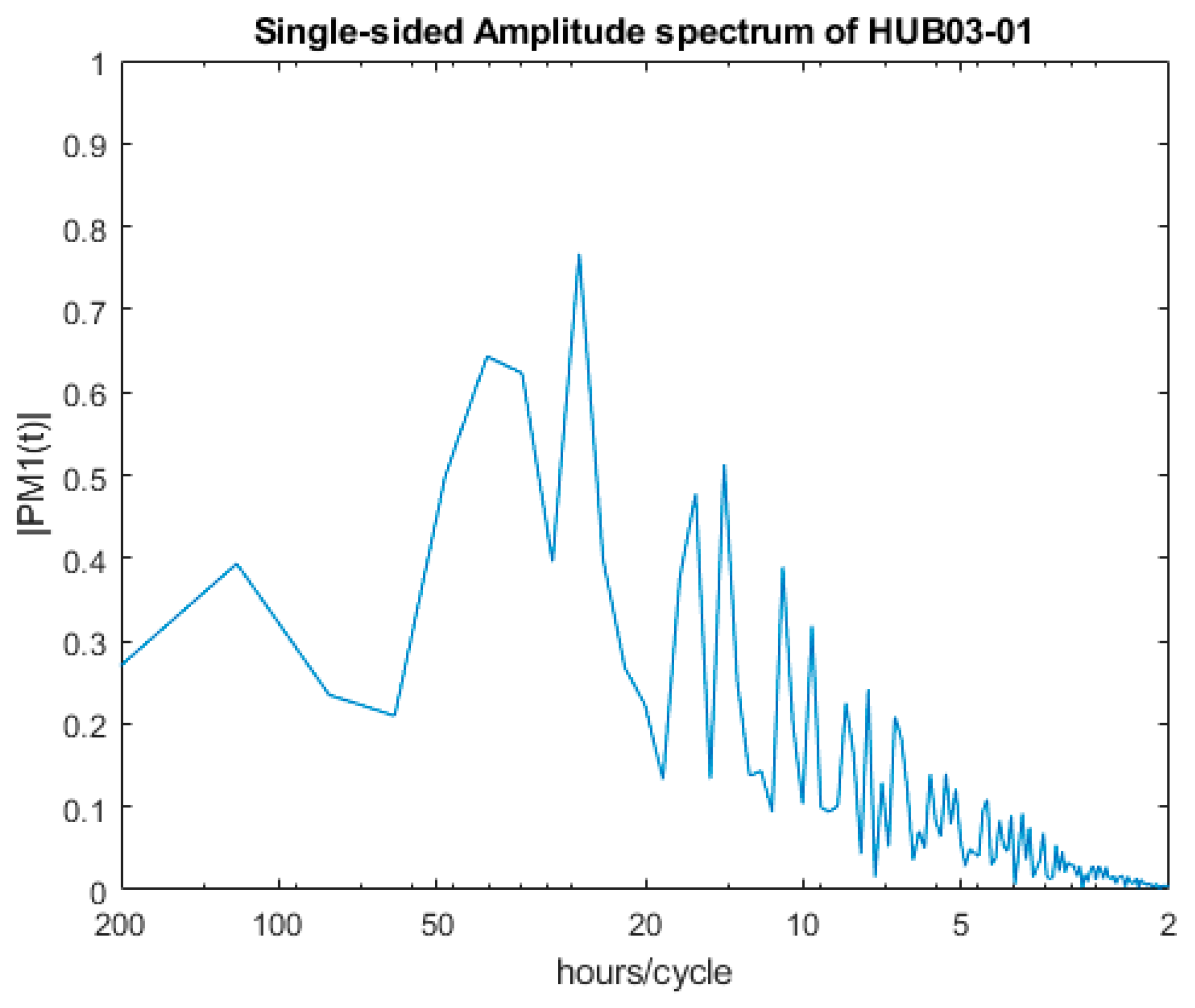
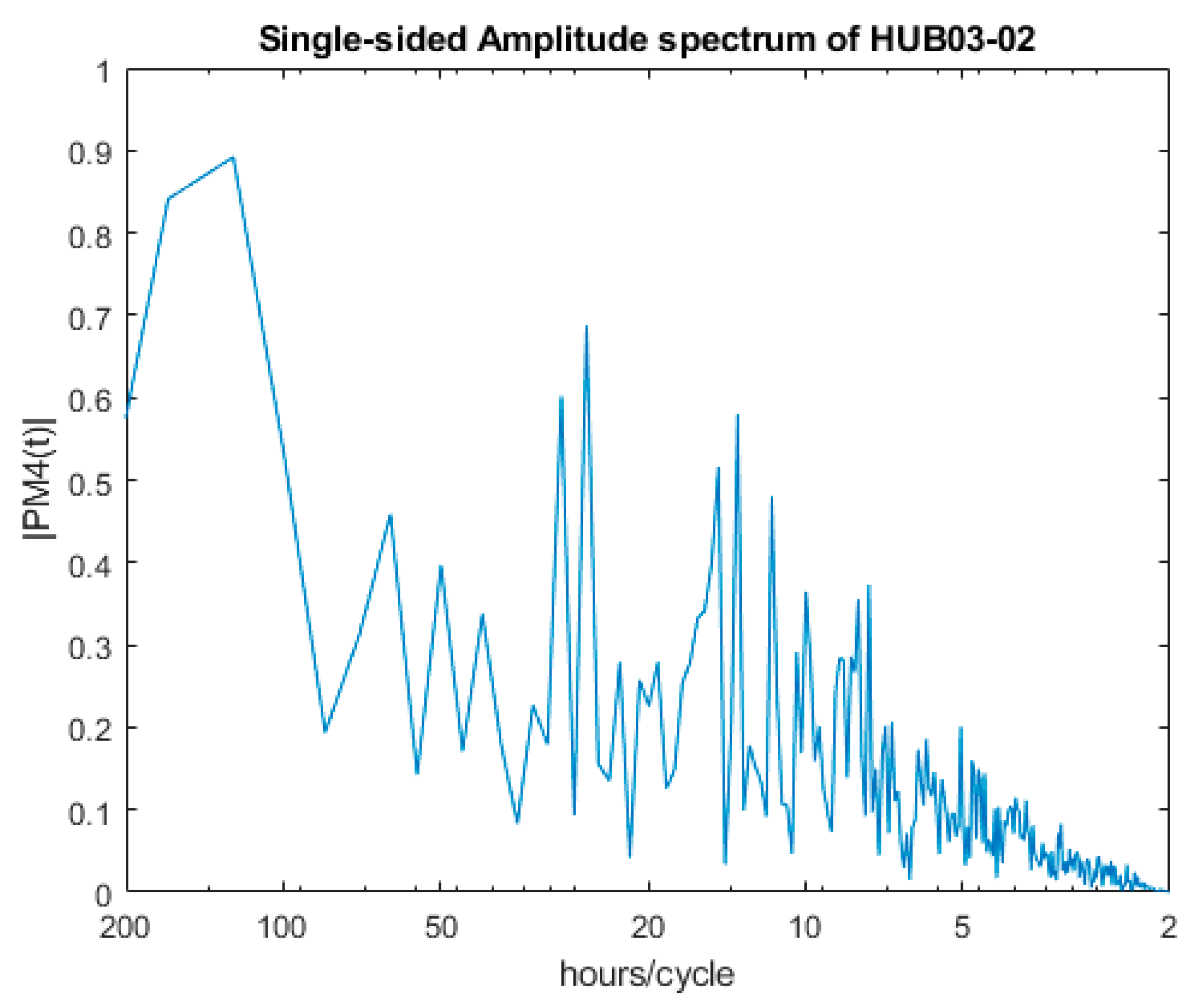
| Dominant Period | HUB03-01 | HUB03-02 |
|---|---|---|
| 1st | 25.70 | 124.00 |
| 2nd | 40.04 | 166.03 |
| 3rd | 34.33 | 26.20 |
| 4th | 14.14 | 29.29 |
| 5th | 16.02 | 13.46 |
| 6th | 10.92 | 14.65 |
| Analysing Date | Location | Maximum % Inhibition | Minimum % Inhibition |
|---|---|---|---|
| 23–26 June 20 | Marane | 0.34 | −0.94 |
| 20–27 July 20 | Corfinio | 1.40 | −0.96 |
| 27–31 July 20 | Raiano | 0.12 | −0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Giacinto, F.; Berti, M.; Carbone, L.; Caprioli, R.; Colaiuda, V.; Lombardi, A.; Tomassetti, B.; Tuccella, P.; De Iuliis, G.; Pietroleonardo, A.; et al. Biological Early Warning Systems: The Experience in the Gran Sasso-Sirente Aquifer. Water 2021, 13, 1529. https://doi.org/10.3390/w13111529
Di Giacinto F, Berti M, Carbone L, Caprioli R, Colaiuda V, Lombardi A, Tomassetti B, Tuccella P, De Iuliis G, Pietroleonardo A, et al. Biological Early Warning Systems: The Experience in the Gran Sasso-Sirente Aquifer. Water. 2021; 13(11):1529. https://doi.org/10.3390/w13111529
Chicago/Turabian StyleDi Giacinto, Federica, Miriam Berti, Luigi Carbone, Riccardo Caprioli, Valentina Colaiuda, Annalina Lombardi, Barbara Tomassetti, Paolo Tuccella, Gianpaolo De Iuliis, Adelina Pietroleonardo, and et al. 2021. "Biological Early Warning Systems: The Experience in the Gran Sasso-Sirente Aquifer" Water 13, no. 11: 1529. https://doi.org/10.3390/w13111529
APA StyleDi Giacinto, F., Berti, M., Carbone, L., Caprioli, R., Colaiuda, V., Lombardi, A., Tomassetti, B., Tuccella, P., De Iuliis, G., Pietroleonardo, A., Latini, M., Mascilongo, G., Di Renzo, L., D’Alterio, N., & Ferri, N. (2021). Biological Early Warning Systems: The Experience in the Gran Sasso-Sirente Aquifer. Water, 13(11), 1529. https://doi.org/10.3390/w13111529