Iron Oxide (Fe3O4)-Supported SiO2 Magnetic Nanocomposites for Efficient Adsorption of Fluoride from Drinking Water: Synthesis, Characterization, and Adsorption Isotherm Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Nanoparticles and Related Nanocomposites
2.2.1. Fe3O4 Nanoparticles
2.2.2. Synthesis of Calcium Carbonate Nanoparticles (Ca-NPs)
2.2.3. Synthesis of SiO2 Nanoparticles (Si-NPs)
2.2.4. Fe-Si Nanocomposites (Fe-Si-NCs)
2.3. Screening and Optimization Studies
2.4. Adsorption Isotherms
2.5. Characterization of Fe-NPs and Fe-Si-NCs
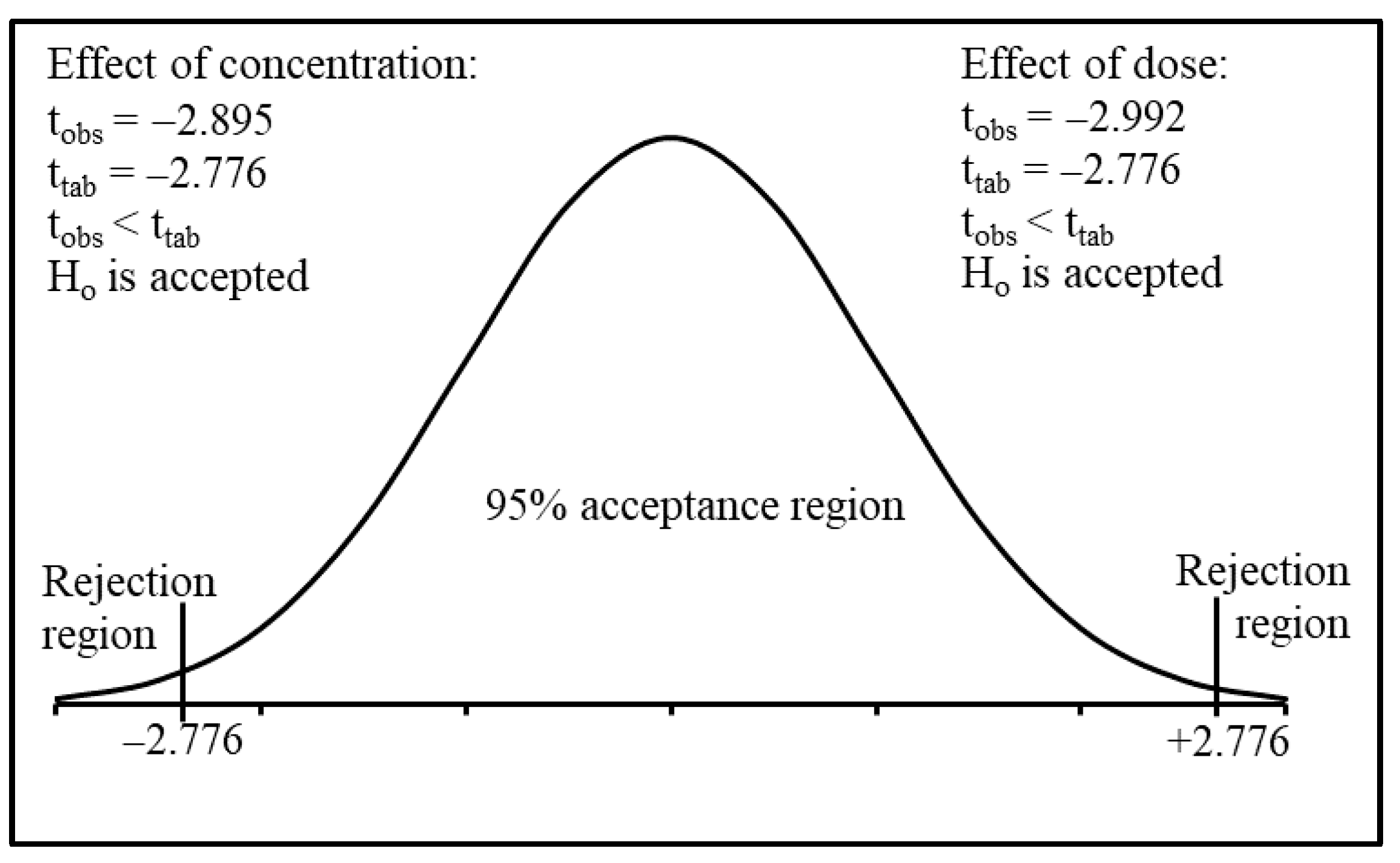
2.6. Statistical Analysis
3. Results and Discussion
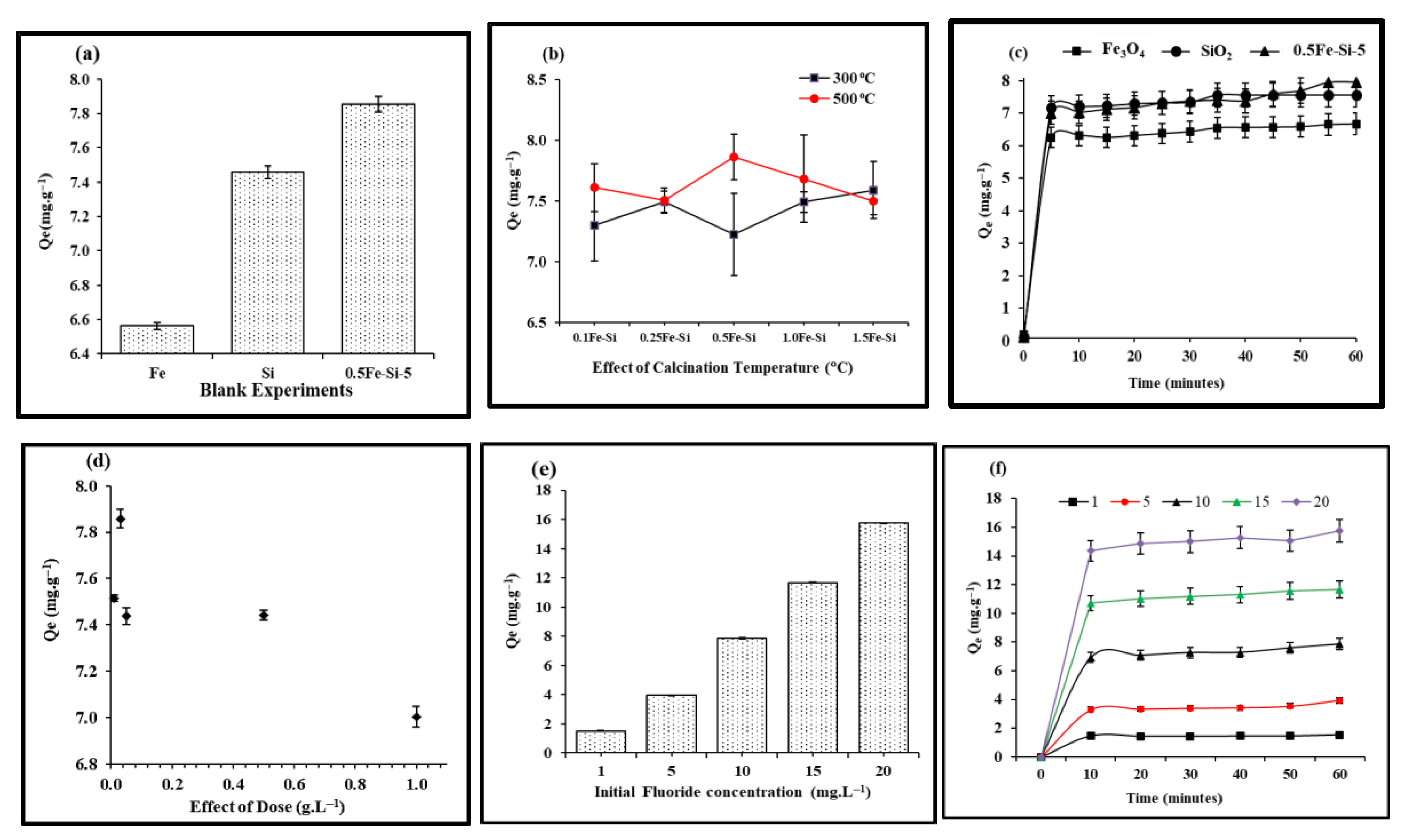
3.1. Screening and Optimization Studies
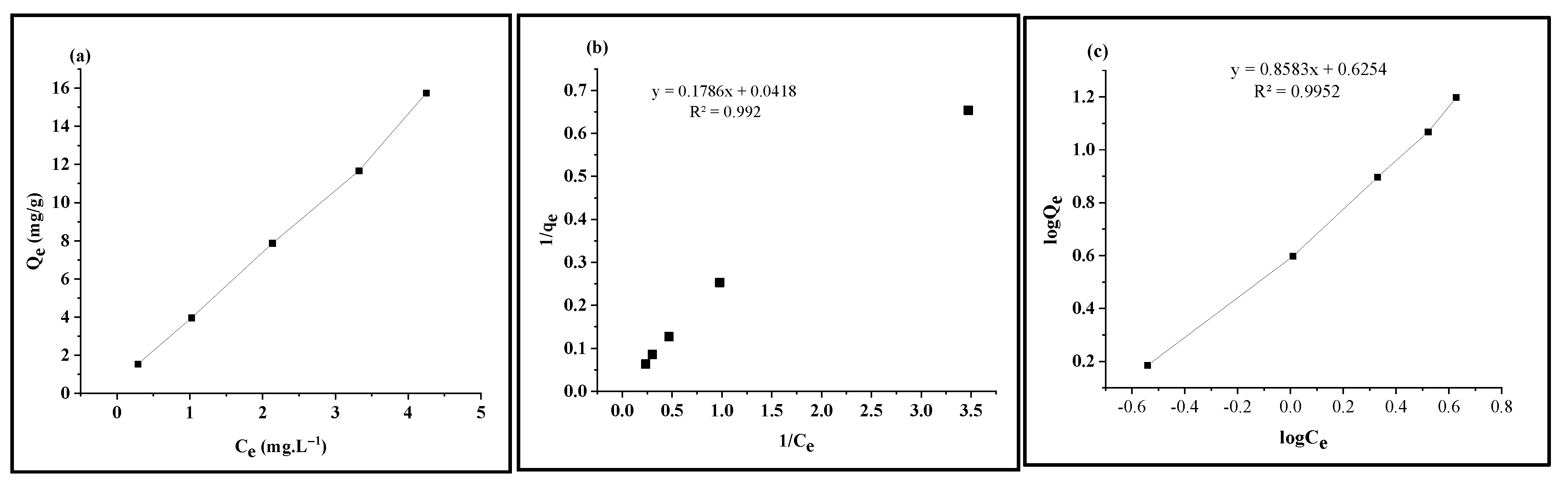
3.2. Adsorption Isotherm
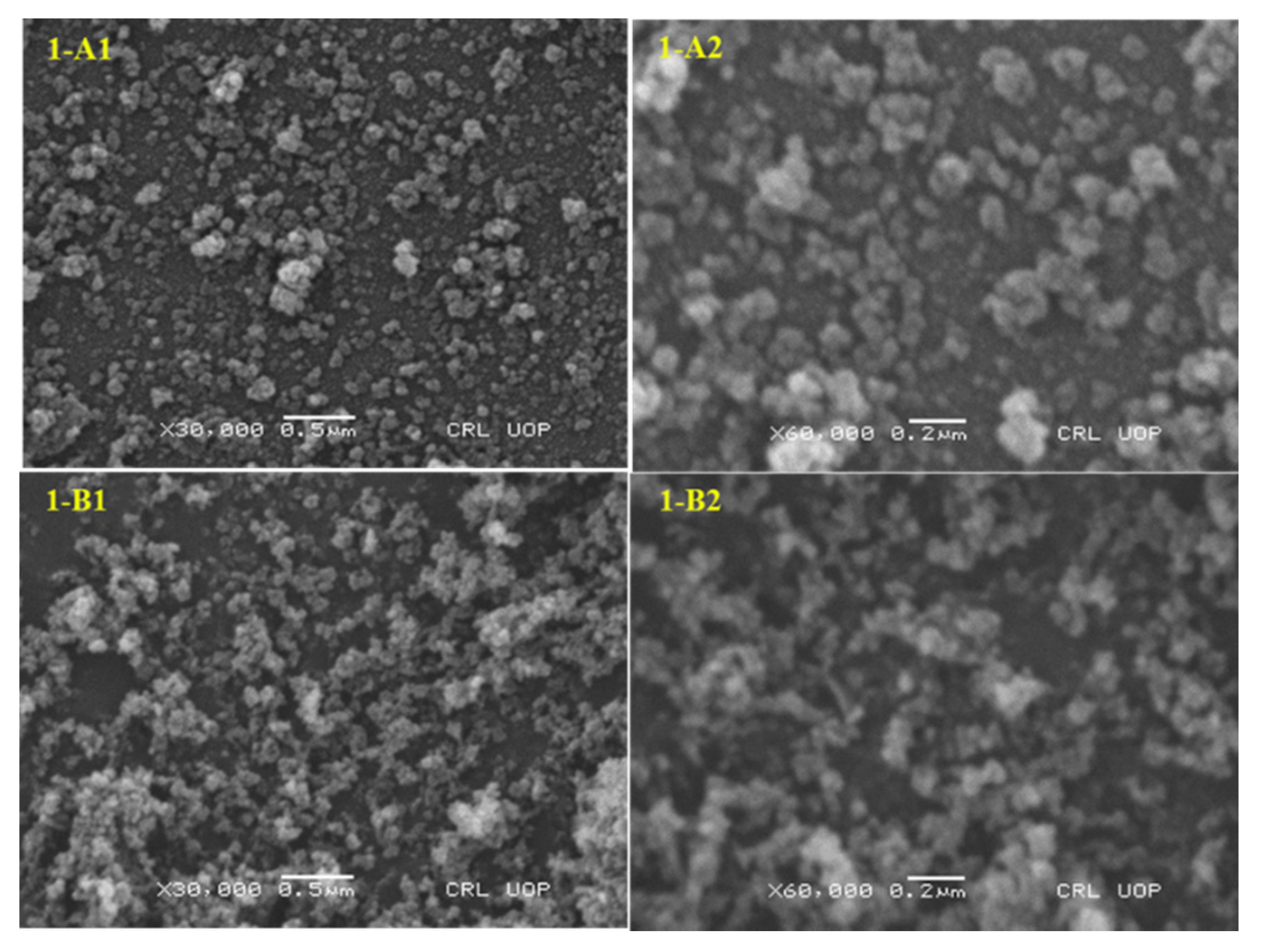
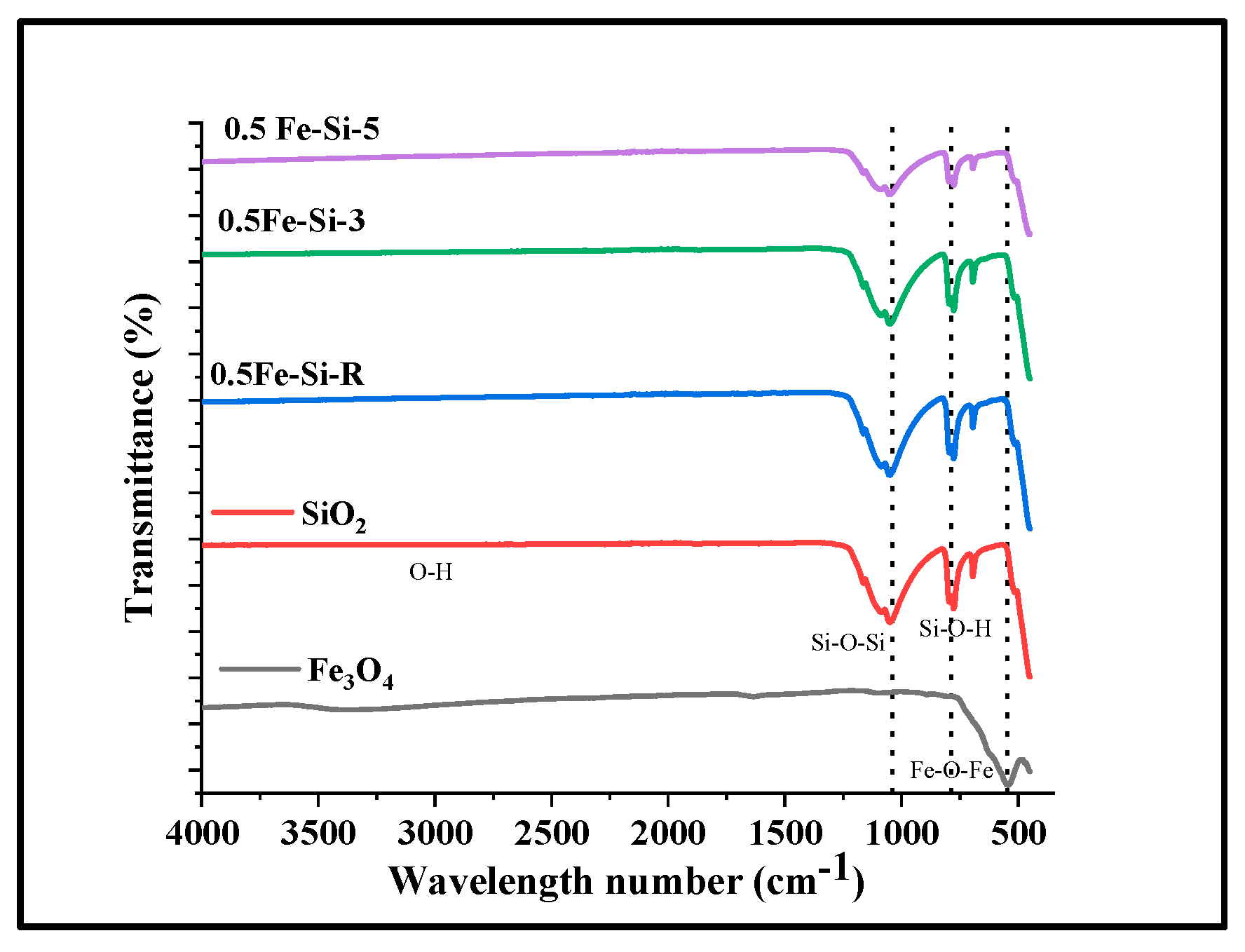
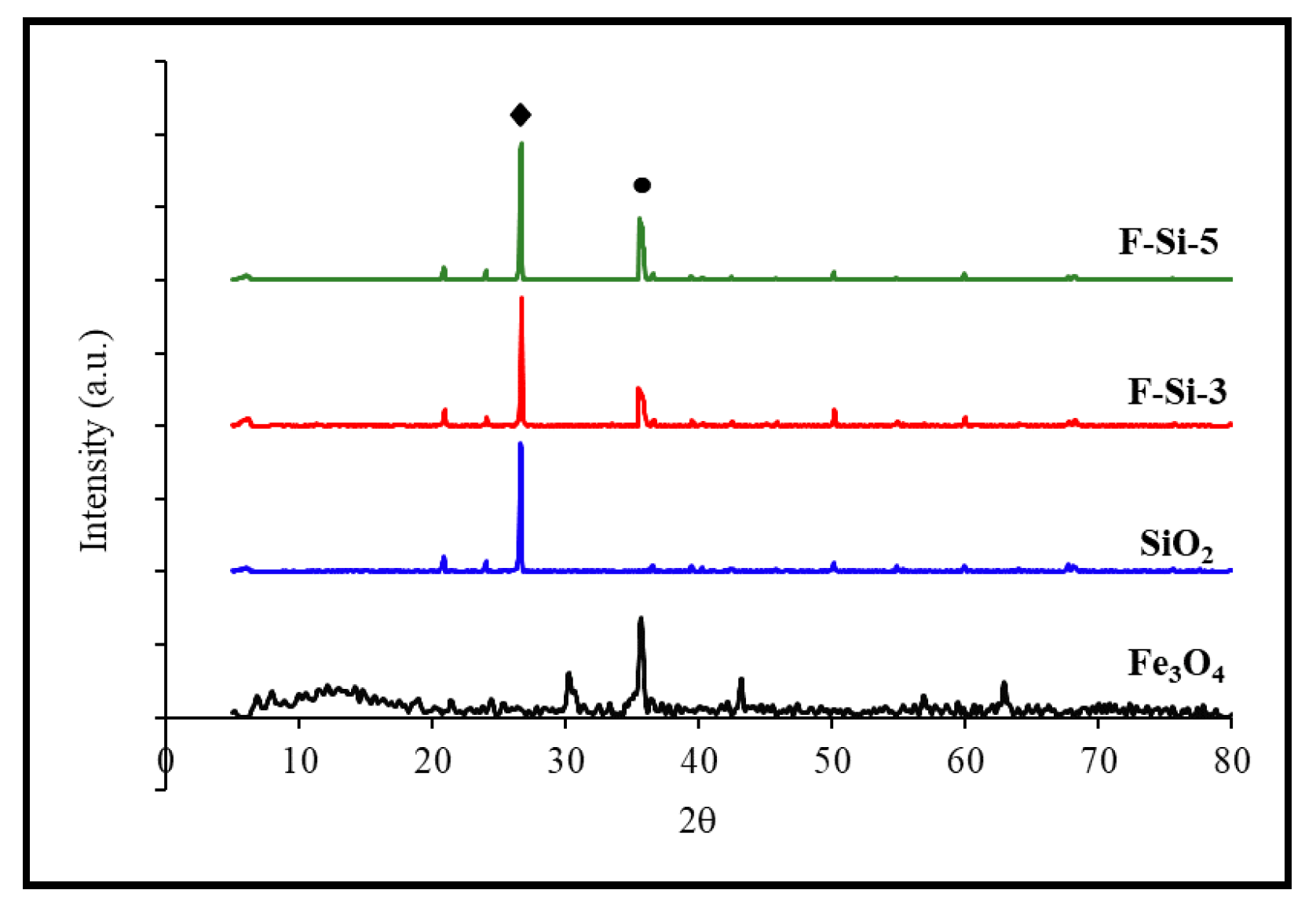
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Xu, W.; Chen, L.; Jia, Y.; Wang, L.; Huang, X.-J.; Liu, J. Excellent fluoride removal performance by CeO2–ZrO2 nanocages in water environment. Chem. Eng. J. 2013, 231, 198–205. [Google Scholar] [CrossRef]
- Wang, H.; Feng, Q.; Liu, K.; Li, Z.; Tang, X.; Li, G. Highly efficient fluoride adsorption from aqueous solution by nepheline prepared from kaolinite through alkali-hydrothermal process. J. Environ. Manag. 2017, 196, 72–79. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Carnevale Miino, M.; Torretta, V.; Rada, E.C.; Caccamo, F.M.; Sorlini, S. Adsorption of Fluorides in Drinking Water by Palm Residues. Sustainability 2020, 12, 3786. [Google Scholar] [CrossRef]
- Jiang, H.; Li, X.; Tian, L.; Wang, T.; Wang, Q.; Niu, P.; Chen, P.; Luo, X. Defluoridation investigation of Yttrium by laminated Y-Zr-Al tri-metal nanocomposite and analysis of the fluoride sorption mechanism. Sci. Total Environ. 2019, 648, 1342–1353. [Google Scholar] [CrossRef]
- Yadav, K.K.; Gupta, N.; Kumar, V.; Khan, S.A.; Kumar, A. A review of emerging adsorbents and current demand for defluoridation of water: Bright future in water sustainability. Environ. Int. 2018, 111, 80–108. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.M.; Andrade, M.; Carreira, P.M.; Eggenkamp, H.G.M.; Graça, R.C.; Aires-Barros, L.; Antunes DA Silva, M. Chemical and isotopic signatures of Na/HCO3/CO2-rich geofluids, North Portugal. Geofluids 2006, 6, 273–287. [Google Scholar] [CrossRef]
- Sivasankar, V.; Darchen, A.; Omine, K.; Sakthivel, R. Fluoride: A world ubiquitous compound, its chemistry, and ways of contamination. In Surface Modified Carbons as Scavengers for Fluoride from Water; Springer: Berlin/Heidelberg, Germany, 2016; pp. 5–32. [Google Scholar]
- Ayoob, S.; Gupta, A.K. Performance evaluation of alumina cement granules in removing fluoride from natural and synthetic waters. Chem. Eng. J. 2009, 150, 485–491. [Google Scholar] [CrossRef]
- Rasool, A.; Farooqi, A.; Xiao, T.; Ali, W.; Noor, S.; Abiola, O.; Ali, S.; Nasim, W. A review of global outlook on fluoride contamination in groundwater with prominence on the Pakistan current situation. Environ. Geochem. Health 2018, 40, 1265–1281. [Google Scholar] [CrossRef]
- Shahid, M.K.; Kim, J.Y.; Shin, G.; Choi, Y. Effect of pyrolysis conditions on characteristics and fluoride adsorptive performance of bone char derived from bone residue. J. Water Process Eng. 2020, 37, 101499. [Google Scholar] [CrossRef]
- Huang, L.; Yang, Z.; He, Y.; Chai, L.; Yang, W.; Deng, H.; Wang, H.; Chen, Y.; Crittenden, J. Adsorption mechanism for removing different species of fluoride by designing of core-shell boehmite. J. Hazard. Mater. 2020, 394, 122555. [Google Scholar] [CrossRef]
- Meenakshi, R.C. Fluoride in drinking water and its removal. J. Hazard. Mater. 2006, 137, 456–463. [Google Scholar] [CrossRef]
- Wambu, E.W.; Ambusso, W.O.; Onindo, C.; Muthakia, G.K. Review of fluoride removal from water by adsorption using soil adsorbents—An evaluation of the status. J. Water Reuse Desalin. 2015, 6, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Chen, G.; Peng, C.; Qiao, H.; Ke, F.; Hou, R.; Li, D.; Cai, H.; Wan, X. Adsorptive removal of fluoride from drinking water using porous starch loaded with common metal ions. Carbohydr. Polym. 2017, 160, 82–89. [Google Scholar] [CrossRef]
- Tahir, M.; Rasheed, H. Fluoride in the drinking water of Pakistan and the possible risk of crippling fluorosis. Drink. Water Eng. Sci. 2013, 6, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Fakhri, A. Application of response surface methodology to optimize the process variables for fluoride ion removal using maghemite nanoparticles. J. Saudi Chem. Soc. 2014, 18, 340–347. [Google Scholar] [CrossRef]
- Pandi, K.; Periyasamy, S.; Viswanathan, N. Remediation of fluoride from drinking water using magnetic iron oxide coated hydrotalcite/chitosan composite. Int. J. Biol. Macromol. 2017, 104, 1569–1577. [Google Scholar] [CrossRef] [PubMed]
- Sivarajasekar, N.; Paramasivan, T.; Muthusaravanan, S.; Muthukumaran, P.; Sivamani, S. Defluoridation of water using adsorbents-a concise review. J. Environ. Biotechnol. Res. 2017, 6, 186–198. [Google Scholar]
- Zhang, L.e.; Huang, D.; Yang, J.; Wei, X.; Qin, J.; Ou, S.; Zhang, Z.; Zou, Y. Probabilistic risk assessment of Chinese residents’ exposure to fluoride in improved drinking water in endemic fluorosis areas. Environ. Pollut. 2017, 222, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Hussein, I.A.; Vegi, M.R. Defluoridation of drinking water using coalesced and un-coalesced mica. Appl. Water Sci. 2020, 10, 64. [Google Scholar] [CrossRef] [Green Version]
- Ndé-Tchoupé, A.I.; Crane, R.A.; Mwakabona, H.T.; Noubactep, C.; Njau, K.N. Technologies for decentralized fluoride removal: Testing metallic iron-based filters. Water 2015, 7, 6750–6774. [Google Scholar] [CrossRef] [Green Version]
- Rafique, T.; Naseem, S.; Bhanger, M.I.; Usmani, T.H. Fluoride ion contamination in the groundwater of Mithi sub-district, the Thar Desert, Pakistan. Environ. Geol. 2008, 56, 317–326. [Google Scholar] [CrossRef]
- Kumari, U.; Mishra, A.; Siddiqi, H.; Meikap, B.C. Effective defluoridation of industrial wastewater by using acid modified alumina in fixed-bed adsorption column: Experimental and breakthrough curves analysis. J. Clean. Prod. 2021, 279, 123645. [Google Scholar] [CrossRef]
- Tan, T.L.; Nakajima, H.; Rashid, S.A. Adsorptive, kinetics and regeneration studies of fluoride removal from water using zirconium-based metal organic frameworks. RSC Adv. 2020, 10, 18740–18752. [Google Scholar] [CrossRef]
- Mahmood, Q.; Khan, M.S.; Riaz, N. Existing treatment—Globally in full scale plants. In Pharmaceutical Wastewater Treatment Technologies: Concepts and Implementation Strategies; Khan, N.A., Ahmed, S., Vambol, V., Vambol, S., Eds.; IWA Publishing: London, UK, 2021; p. 353. [Google Scholar] [CrossRef]
- Saikia, J.; Sikdar, Y.; Saha, B.; Das, G. Malachite nanoparticle: A potent surface for the adsorption of xanthene dyes. J. Environ. Chem. Eng. 2013, 1, 1166–1173. [Google Scholar] [CrossRef]
- Olabarrieta, J.; Monzón, O.; Belaustegui, Y.; Alvarez, J.-I.; Zorita, S. Removal of TiO2 nanoparticles from water by low pressure pilot plant filtration. Sci. Total Environ. 2018, 618, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Homaeigohar, S. Water Treatment with New Nanomaterials. Water 2020, 12, 1507. [Google Scholar] [CrossRef]
- Khan, M.S.; Shah, J.A.; Riaz, N.; Butt, T.A.; Khan, A.J.; Khalifa, W.; Gasmi, H.H.; Latifee, E.R.; Arshad, M.; Al-Naghi, A.A.A.; et al. Synthesis and Characterization of Fe-TiO2 Nanomaterial: Performance Evaluation for RB5 Decolorization and In Vitro Antibacterial Studies. Nanomaterials 2021, 11, 436. [Google Scholar] [CrossRef]
- Panneerselvam, P.; Morad, N.; Tan, K.A. Magnetic nanoparticle (Fe3O4) impregnated onto tea waste for the removal of nickel (II) from aqueous solution. J. Hazard. Mater. 2011, 186, 160–168. [Google Scholar] [CrossRef]
- Rajput, S.; Singh, L.P.; Pittman, C.U., Jr.; Mohan, D. Lead (Pb2+) and copper (Cu2+) remediation from water using superparamagnetic maghemite (γ-Fe2O3) nanoparticles synthesized by Flame Spray Pyrolysis (FSP). J. Colloid Interface Sci. 2017, 492, 176–190. [Google Scholar] [CrossRef]
- Nithya, R.; Thirunavukkarasu, A.; Sathya, A.B.; Sivashankar, R. Magnetic materials and magnetic separation of dyes from aqueous solutions: A review. Environ. Chem. Lett. 2021. [Google Scholar] [CrossRef]
- Sharma, Y.; Srivastava, V. Separation of Ni (II) ions from aqueous solutions by magnetic nanoparticles. J. Chem. Eng. Data 2010, 55, 1441–1442. [Google Scholar] [CrossRef]
- Shah, K.H.; Ali, S.; Shah, F.; Waseem, M.; Ismail, B.; Khan, R.A.; Khan, A.M.; Khan, A.R. Magnetic oxide nanoparticles (Fe3O4) impregnated bentonite clay as a potential adsorbent for Cr(III) adsorption. Mater. Res. Express 2018, 5, 096102. [Google Scholar] [CrossRef]
- Babou-Kammoe, R.; Hamoudi, S.; Larachi, F.; Belkacemi, K. Synthesis of CaCO3 nanoparticles by controlled precipitation of saturated carbonate and calcium nitrate aqueous solutions. Can. J. Chem. Eng. 2012, 90, 26–33. [Google Scholar] [CrossRef]
- Chen, J.-F.; Ding, H.-M.; Wang, J.-X.; Shao, L. Preparation and characterization of porous hollow silica nanoparticles for drug delivery application. Biomaterials 2004, 25, 723–727. [Google Scholar] [CrossRef]
- Iftikhar, A.; Khan, M.S.; Rashid, U.; Mahmood, Q.; Zafar, H.; Bilal, M.; Riaz, N. Influence of metallic species for efficient photocatalytic water disinfection: Bactericidal mechanism of in vitro results using docking simulation. Environ. Sci. Pollut. Res. 2020, 27, 39819–39831. [Google Scholar] [CrossRef]
- Riaz, N.; Hassan, M.; Siddique, M.; Mahmood, Q.; Farooq, U.; Sarwar, R.; Khan, M.S. Photocatalytic degradation and kinetic modeling of azo dye using bimetallic photocatalysts: Effect of synthesis and operational parameters. Environ. Sci. Pollut. Res. 2020, 27, 2992–3006. [Google Scholar] [CrossRef] [PubMed]
- Riaz, N.; Kait, C.F.; Man, Z.B.; Dutta, B.K.; Ramli, R.M.; Khan, M.S. Visible Light Photodegradation of Azo Dye by Cu/TiO2. In Advanced Materials Research; 2014; pp. 151–159. Available online: https://www.scientific.net/AMR.917.151 (accessed on 15 February 2021).
- Zazouli, M.A.; Mahvi, A.H.; Dobaradaran, S.; Barafrashtehpour, M.; Mahdavi, Y.; Balarak, D. Adsorption of fluoride from aqueous solution by modified Azolla filiculoides. Adsorption 2014, 47, 349–358. [Google Scholar]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Scherrer, P. Determination of the Internal Structure and Size of Colloidal Particles by Means of X-Rays. In Colloid Chemistry A Textbook. Chemical Technology in Single Representations; Springer: Berlin/Heidelberg, Germany, 1912; Available online: https://doi.org/10.1007/978-3-662-33915-2_7 (accessed on 21 April 2021).
- Mahramanlioglu, M.; Kizilcikli, I.; Bicer, I.O. Adsorption of fluoride from aqueous solution by acid treated spent bleaching earth. J. Fluor. Chem. 2002, 115, 41–47. [Google Scholar] [CrossRef]
- Vivek Vardhan, C.M.; Srimurali, M. Removal of fluoride from water using a novel sorbent lanthanum-impregnated bauxite. SpringerPlus 2016, 5, 1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Ahmadi, S.; Rahdar, S.; Igwegbe, C.A.; Rahdar, A.; Shafighi, N.; Sadeghfar, F. Data on the removal of fluoride from aqueous solutions using synthesized P/γ-Fe2O3 nanoparticles: A novel adsorbent. MethodsX 2019, 6, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Naghizadeh, A.; Gholami, K. Bentonite and montmorillonite nanoparticles effectiveness in removal of fluoride from water solutions. J. Water Health 2017, 15, 555–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kothari, C.R. Research Methodology: Methods and Techniques; New Age International: New Delhi, India, 2004. [Google Scholar]
- Kaushal, A.; Singh, S.K. Critical analysis of adsorption data statistically. Appl. Water Sci. 2017, 7, 3191–3196. [Google Scholar] [CrossRef] [Green Version]
- Meenakshi, S.; Viswanathan, N. Identification of selective ion-exchange resin for fluoride sorption. J. Colloid Interface Sci. 2007, 308, 438–450. [Google Scholar] [CrossRef]
- Raghav, S.; Kumar, D. Adsorption Equilibrium, Kinetics, and Thermodynamic Studies of Fluoride Adsorbed by Tetrametallic Oxide Adsorbent. J. Chem. Eng. Data 2018, 63, 1682–1697. [Google Scholar] [CrossRef]
- Shivaprasad, P.; Singh, P.K.; Saharan, V.K.; George, S. Synthesis of nano alumina for defluoridation of drinking water. Nano Struct. Nano Objects 2018, 13, 109–120. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Q.; Zhang, S.; Liu, J.; Zhou, J.; Xu, H.; Xiao, H.; Li, J. Preparation of thiol-modified Fe3O4@SiO2 nanoparticles and their application for gold recovery from dilute solution. Sep. Purif. Technol. 2013, 116, 391–397. [Google Scholar] [CrossRef]
- Roto, R.; Yusran, Y.; Kuncaka, A. Magnetic adsorbent of Fe3O4@SiO2 core-shell nanoparticles modified with thiol group for chloroauric ion adsorption. Appl. Surf. Sci. 2016, 377, 30–36. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, J.; Hu, Y.; Zhao, H.; Zhou, J.; Liu, Y.; Lou, Z.; Xu, X. Functional nanomaterials: Study on aqueous Hg(II) adsorption by magnetic Fe3O4@SiO2-SH nanoparticles. J. Taiwan Inst. Chem. Eng. 2016, 60, 394–402. [Google Scholar] [CrossRef]
- Ding, H.; Zhao, Y.; Duan, Q.; Wang, J.; Zhang, K.; Ding, G.; Xie, X.; Ding, C. Efficient removal of phosphate from aqueous solution using novel magnetic nanocomposites with Fe3O4@SiO2 core and mesoporous CeO2 shell. J. Rare Earths 2017, 35, 984–994. [Google Scholar] [CrossRef]
- Yimin, D.; Jiaqi, Z.; Danyang, L.; Lanli, N.; Liling, Z.; Yi, Z.; Xiaohong, Z. Preparation of Congo red functionalized Fe3O4@SiO2 nanoparticle and its application for the removal of methylene blue. Colloids Surf. A Physicochem. Eng. Asp. 2018, 550, 90–98. [Google Scholar] [CrossRef]
- Shamsizadeh, Z.; Ehrampoush Mohammad, H.; Dehghani Firouzabadi, Z.; Jasemi Zad, T.; Molavi, F.; Ebrahimi Ali, A.; Kamranifar, M. Fe3O4@SiO2 magnetic nanocomposites as adsorbents for removal of diazinon from aqueous solution: Isotherm and kinetic study. Pigment Resin Technol. 2020, 49, 457–464. [Google Scholar] [CrossRef]
- Asab, G.; Zereffa, E.A.; Abdo Seghne, T. Synthesis of Silica-Coated Fe3O4 Nanoparticles by Microemulsion Method: Characterization and Evaluation of Antimicrobial Activity. Int. J. Biomater. 2020, 2020, 4783612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, A.L.; Souza, D.M.; Pereira, M.C.; Fabris, J.D.; Domingues, R.Z. Synthesis and characterization of magnetic nanoparticles coated with silica through a sol-gel approach. Cerâmica 2009, 55, 420–424. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, Y.T.; Rao, K.V.; Kumari, B.S.; Kumar, V.S.S.; Pavani, T. Synthesis of Fe3O4 nanoparticles and its antibacterial application. Int. Nano Lett. 2015, 5, 85–92. [Google Scholar] [CrossRef]
- Shagholani, H.; Ghoreishi, S.M.; Mousazadeh, M. Improvement of interaction between PVA and chitosan via magnetite nanoparticles for drug delivery application. Int. J. Biol. Macromol. 2015, 78, 130–136. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction; Addison-Wesley Publishing: Boston, MA, USA, 1956. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarwar, A.; Wang, J.; Khan, M.S.; Farooq, U.; Riaz, N.; Nazir, A.; Mahmood, Q.; Hashem, A.; Al-Arjani, A.-B.F.; Alqarawi, A.A.; et al. Iron Oxide (Fe3O4)-Supported SiO2 Magnetic Nanocomposites for Efficient Adsorption of Fluoride from Drinking Water: Synthesis, Characterization, and Adsorption Isotherm Analysis. Water 2021, 13, 1514. https://doi.org/10.3390/w13111514
Sarwar A, Wang J, Khan MS, Farooq U, Riaz N, Nazir A, Mahmood Q, Hashem A, Al-Arjani A-BF, Alqarawi AA, et al. Iron Oxide (Fe3O4)-Supported SiO2 Magnetic Nanocomposites for Efficient Adsorption of Fluoride from Drinking Water: Synthesis, Characterization, and Adsorption Isotherm Analysis. Water. 2021; 13(11):1514. https://doi.org/10.3390/w13111514
Chicago/Turabian StyleSarwar, Amna, Jin Wang, Muhammad Saqib Khan, Umar Farooq, Nadia Riaz, Abdul Nazir, Qaisar Mahmood, Abeer Hashem, Al-Bandari Fahad Al-Arjani, Abdulaziz A. Alqarawi, and et al. 2021. "Iron Oxide (Fe3O4)-Supported SiO2 Magnetic Nanocomposites for Efficient Adsorption of Fluoride from Drinking Water: Synthesis, Characterization, and Adsorption Isotherm Analysis" Water 13, no. 11: 1514. https://doi.org/10.3390/w13111514
APA StyleSarwar, A., Wang, J., Khan, M. S., Farooq, U., Riaz, N., Nazir, A., Mahmood, Q., Hashem, A., Al-Arjani, A.-B. F., Alqarawi, A. A., & Abd_Allah, E. F. (2021). Iron Oxide (Fe3O4)-Supported SiO2 Magnetic Nanocomposites for Efficient Adsorption of Fluoride from Drinking Water: Synthesis, Characterization, and Adsorption Isotherm Analysis. Water, 13(11), 1514. https://doi.org/10.3390/w13111514








