Procedure for Calculating the Calcium Carbonate Precipitation Potential (CCPP) in Drinking Water Supply: Importance of Temperature, Ionic Species and Open/Closed System
Abstract
1. Introduction
Aim
2. Existing Standards and Methods
3. Materials and Methods
3.1. Selection of Software and Database
3.2. Selected Water Types
3.3. Demonstration of Calculation Method
4. Results
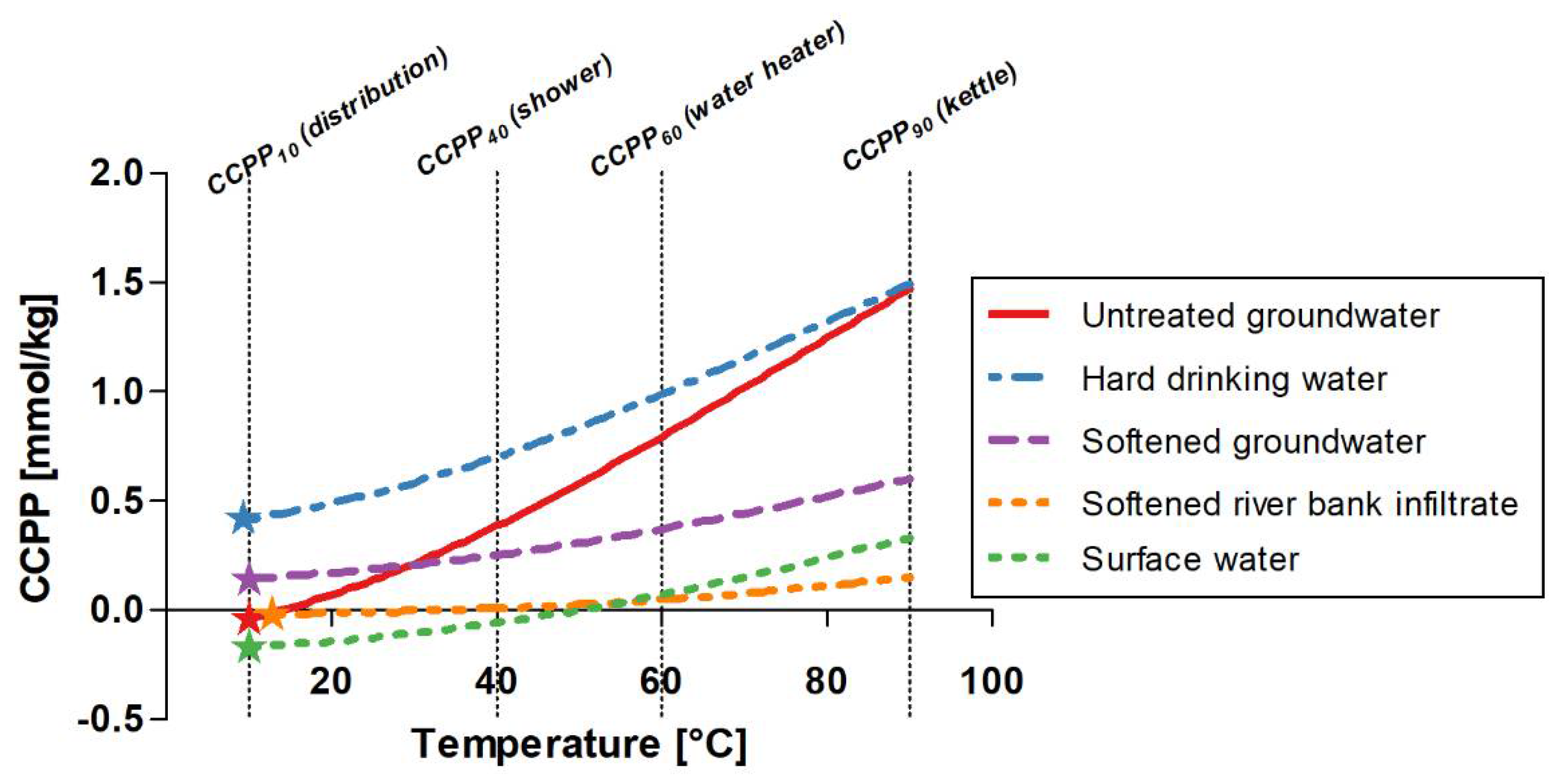
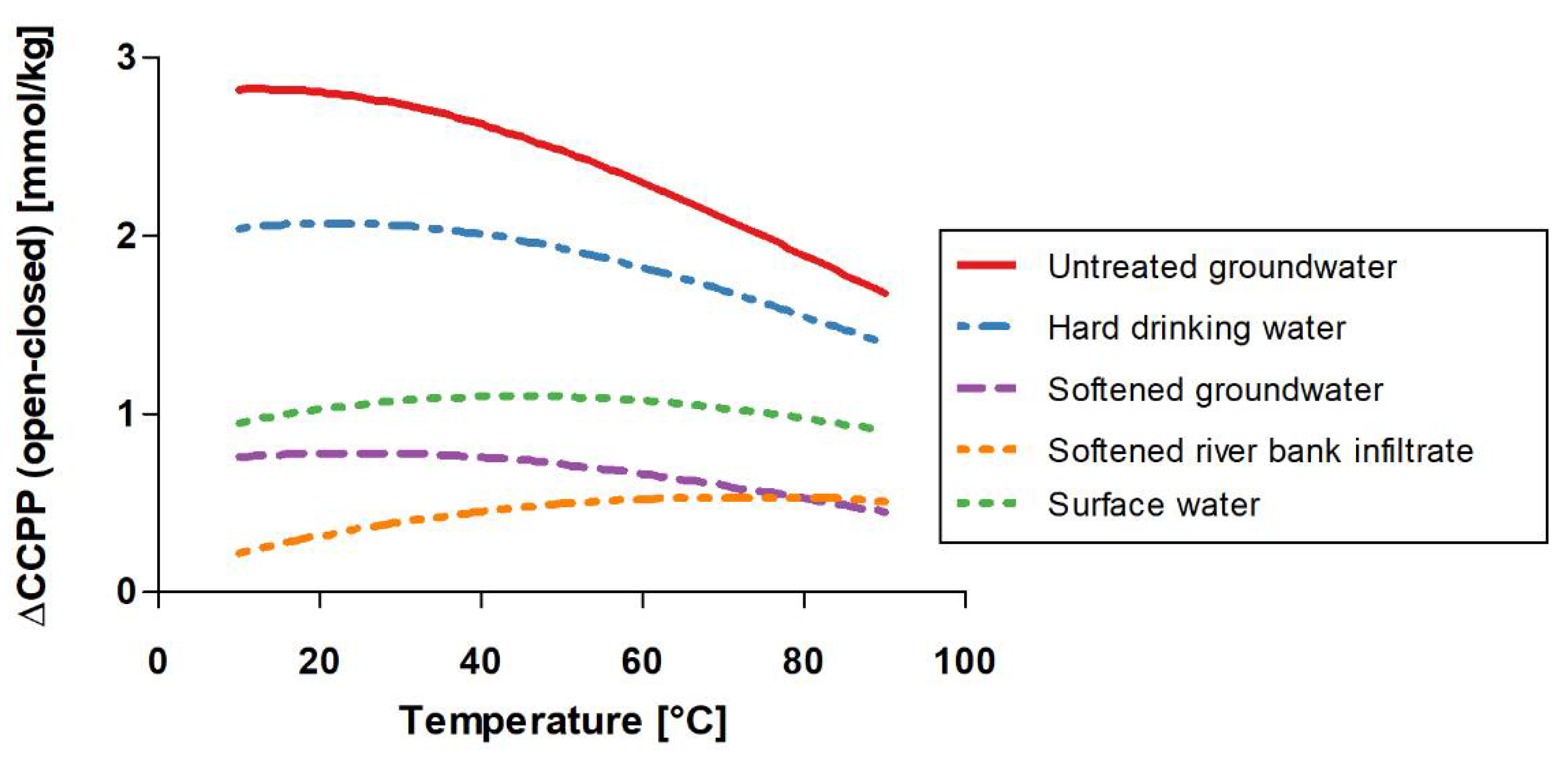
4.1. Temperature Dependency
4.2. Importance of Ionic Species
4.2.1. Compliance with DIN 38404-10
4.2.2. pH Dependency
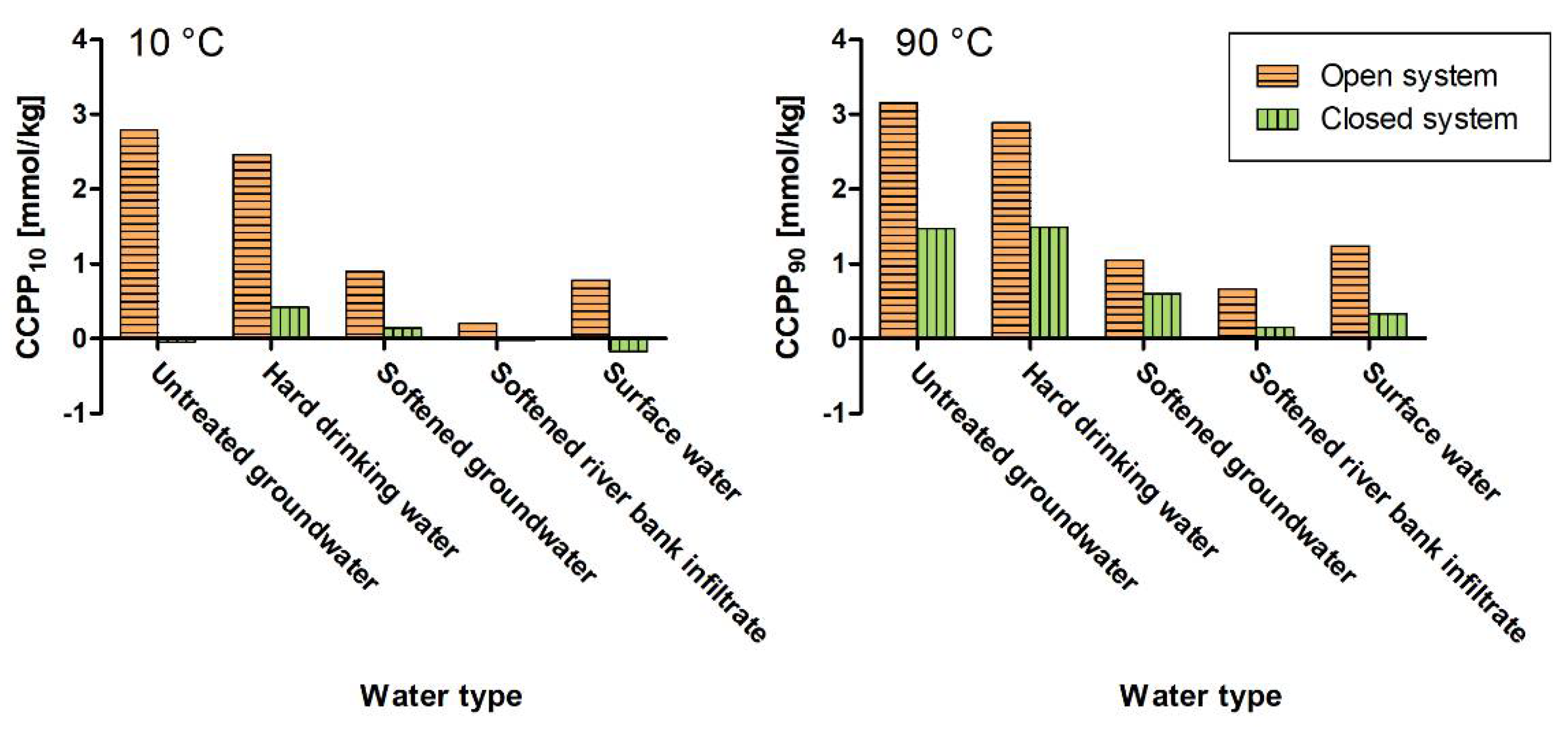
4.3. Open Versus Closed Systems
4.4. Recommended Procedure
- Use PHREEQC for calculations with the phreeqc.dat database unless compliance with DIN 38404-10 is required.
- At least include Ca, pH, temperature, alkalinity, Mg, Na, K, Cl, PO43−, NO3−, SO42− and complexes with SO42− and PO43−. Additionally, measured elements may be included if they are present in macro concentrations and their ionic species are included in the PHREEQC database file.
- Calculate CCPP at a range of temperatures to estimate the CaCO3 dissolution and precipitation potential throughout the distribution networks and at the consumer. The relevant temperatures are the sample temperature (distribution networks), 40 °C (shower), 60 °C (water heater) and 90 °C (boiling water). Using the phreeqc.dat database ensures that the solubility constants for CaCO3 are valid at temperatures 0–90 °C.
- Calculate CCPP both for open systems with atmospheric CO2 exchange to estimate the maximum CaCO3 scaling potential of drinking water and for systems without CO2 exchange to investigate the corrosion and scaling potential in closed systems.
- Report the calculation method and software along with CCPP. This should at least include the temperature, included elements and ionic species, solubility data or database, software and standard followed (if applicable).
5. Discussion
6. Conclusions
- Several standards are available for CCPP calculations, but none of these consider both dissolution and precipitation of CaCO3 in drinking water systems where temperatures can reach 100 °C and the water can exchange CO2 with the atmosphere.
- CCPP is temperature-dependent and increased by 0.17–1.51 mmol/kg for five different water types when increasing the temperature from 10 °C to 90 °C, which must be considered when calculating CCPP in drinking water systems.
- The elements and ionic species included in CCPP calculations affect the results. CCPP decreased by 0.006–0.173 mmol/kg when including Mg, Na, K, SO42−, PO43− and NO3−, and associated complexes in addition to Ca, pH and alkalinity.
- CCPP10 increased by 0.22–2.82 mmol/kg when calculating CCPP for open systems in contact with atmospheric CO2. To resemble conditions in drinking water systems, CCPP should be calculated for both closed and open systems with and without CO2 exchange.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Calculating CCPP in PHREEQC

Appendix A.2. Interpreting the Output File
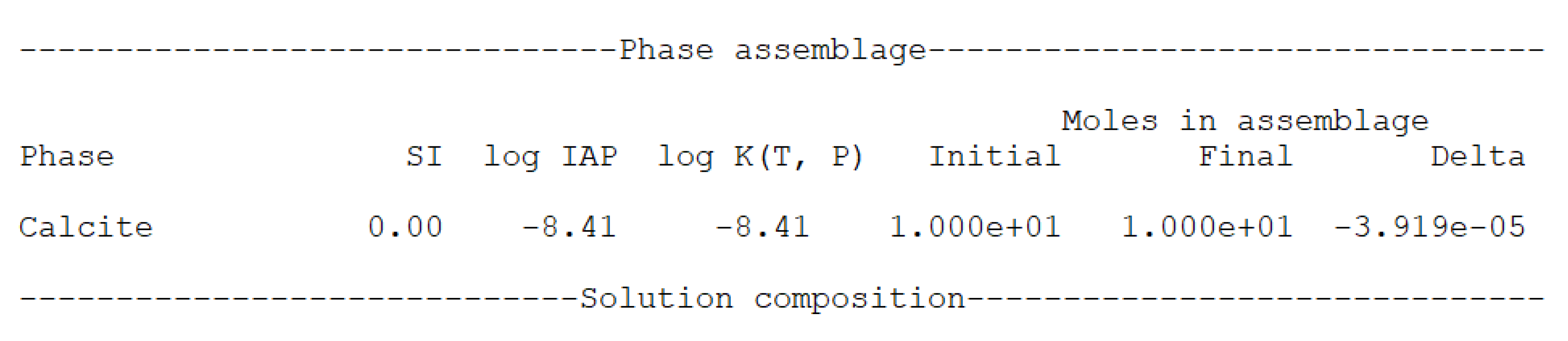
Appendix A.3. Temperature
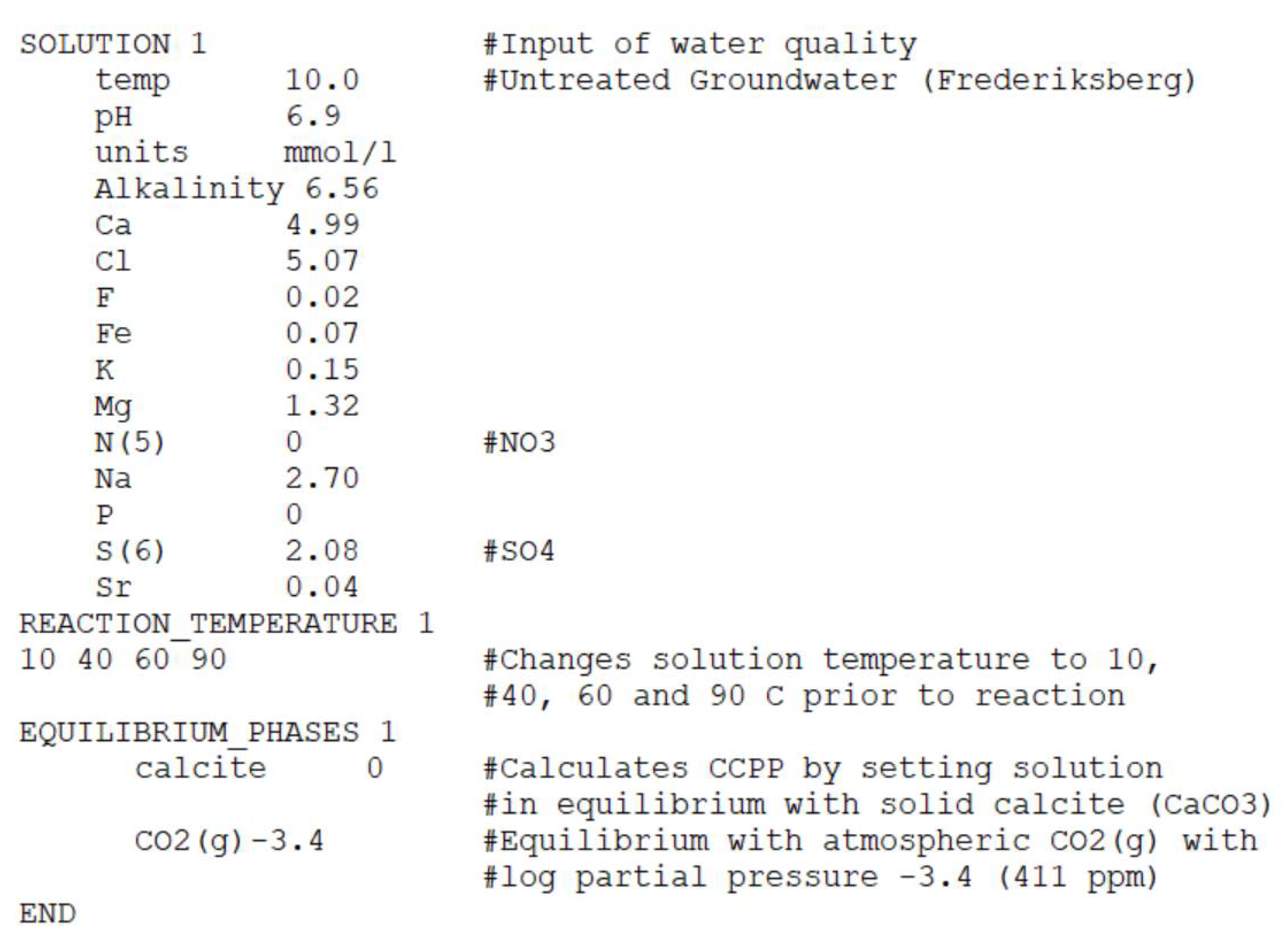
Appendix A.4. Open System
References
- Van Der Bruggen, B.; Goossens, H.; Everard, P.; Stemgée, K.; Rogge, W. Cost-benefit analysis of central softening for production of drinking water. J. Environ. Manag. 2009, 91, 541–549. [Google Scholar] [CrossRef]
- Mons, M.; van Dijk, H.; Gatel, D.; Hesse, S.; Hofman, J.; Nguyen, M.-L.; Slaats, N. Drinking Water Hardness: Reasons and Criteria for Softening and Conditioning of Drinking Water; Global Water Research Coalition: London, UK, 2007. [Google Scholar]
- Lahav, O.; Birnhack, L. Quality criteria for desalinated water following post-treatment. Desalination 2007, 207, 286–303. [Google Scholar] [CrossRef]
- De Moel, P.J.; Van Der Helm, A.W.C.; Van Rijn, M.; Van Dijk, J.C.; Van Der Meer, W.G.J. Assessment of calculation methods for calcium carbonate saturation in drinking water for DIN 38404-10 compliance. Drink. Water Eng. Sci. 2013, 6, 115–124. [Google Scholar] [CrossRef]
- Brink, H.; Slaats, P.G.G.; Eekeren, M.W.M. Van Scaling in domestic heating equipment: Getting to know a complex phenomenon. Water Sci. Technol. 2004, 49, 129–136. [Google Scholar] [CrossRef]
- APHA/AWWA/WEF. 2330 Calcium Carbonate Saturation, Standard Methods for the Examination of Water and Wastewater. 2017, pp. 1–9. Available online: https://www.standardmethods.org/doi/10.2105/SMWW.2882.024 (accessed on 27 December 2020).
- Nederlandse Norm NEN 6533 Bepaling Van de Agressiviteit Ten Opzichte Van Calciumcarbonaat en Berekning Van de Verzadigingsindex 1990. pp. 1–6, Foreign Standard—Public. Available online: https://www.nen.nl/en/nen-6533-1990-nl-1029 (accessed on 27 December 2020).
- Van Raalte-Drewes, M.; Brink, H.; Feij, L.; Slaats, P.; Van Soest, E.; Vaal, P.; Veenendaal, G. Scaling Propensity of Water: New Predictive Parameters. Water Intell. Online 2015, 5. [Google Scholar] [CrossRef]
- Rossum, J.R.; Merrill, D.T. An evaluation of the calcium carbonate saturation indexes. J. Am. Water Work. Assoc. 1983, 75, 95–100. [Google Scholar] [CrossRef]
- Loewenthal, R.; Morrison, I.; Wentzel, M. Control of corrosion and aggression in drinking water systems. Water Sci. Technol. 2004, 49, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Rygaard, M.; Arvin, E.; Bath, A.; Binning, P.J. Designing water supplies: Optimizing drinking water composition for maximum economic benefit. Water Res. 2011, 45, 3712–3722. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, D.-Y.; Hong, S.-H.; Kim, J.-W.; Kim, C. Development and implementation of a corrosion control algorithm based on calcium carbonate precipitation potential (CCPP) in a drinking water distribution system. J. Water Supply Res. Technol. 2008, 57, 531–539. [Google Scholar] [CrossRef][Green Version]
- Macadam, J.; Parsons, S.A. Calcium carbonate scale formation and control. Rev. Environ. Sci. Bio/Technol. 2004, 3, 159–169. [Google Scholar] [CrossRef]
- Schetters, M.J.A.; Van Der Hoek, J.P.; Kramer, O.J.I.; Kors, L.J.; Palmen, L.J.; Hofs, B.; Koppers, H. Circular economy in drinking water treatment: Reuse of ground pellets as seeding material in the pellet softening process. Water Sci. Technol. 2014, 71, 479–486. [Google Scholar] [CrossRef]
- Shi, B.; Wan, Y.; Yu, Y.; Gu, J.; Wang, G. Evaluating the chemical stability in drinking water distribution system by corrosivity and precipitation potential. Water Supply 2017, 18, 383–390. [Google Scholar] [CrossRef]
- Eggen, G.J.P.M.; Brink, H.; Nederlof, M.M. AQUACALC versie 2.0 Handleiding; 1997. Technical Manual. Available online: https://www.praktijkcodesdrinkwater.nl/opbrengst/ontharding/ (accessed on 27 December 2020).
- Mehl, V.; Johannsen, K. Calculating chemical speciation, pH, saturation index and calcium carbonate precipitation potential (CCPP) based on alkalinity and acidity using OpenModelica. J. Water Supply Res. Technol. 2017, 67, 1–11. [Google Scholar] [CrossRef]
- Höckendorf, A.; Johannsen, K.; Dorsch, T.; Reiß, M.; Flashaar, S.; Becker, O.; Bendinger, B. Method for an accelerated reproducible calcite-layering of potable water systems to validate decalcification procedures. J. Water Supply Res. Technol. 2017, 66, 75–85. [Google Scholar] [CrossRef]
- DIN DIN 38404-10. German Standard Methods for the Examination of Water, Wastewater and Sludge—Physical and Physio-Chemical Parameters (group C)—Part 10: Calculation of the Calcit Saturation of Water (C 10), Foreign Standard—Public. Available online: https://www.beuth.de/de/norm/din-38404-10/166964780 (accessed on 27 December 2020).
- Waly, T.; Kennedy, M.; Witkamp, G.-J.; Amy, G.L.; Schippers, J. The role of inorganic ions in the calcium carbonate scaling of seawater reverse osmosis systems. Desalination 2012, 284, 279–287. [Google Scholar] [CrossRef]
- Jacobson, R.L.; Langmuir, D. Dissociation constants of calcite and CaHC03+ from 0 to 50 °C. Geochim. Cosmochim. Acta 1974, 38, 301–318. [Google Scholar] [CrossRef]
- Plummer, L.; Busenberg, E. The solubilities of calcite, aragonite and vaterite in CO2-H2O solutions between 0 and 90 °C, and an evaluation of the aqueous model for the system CaCO3-CO2-H2O. Geochim. Cosmochim. Acta 1982, 46, 1011–1040. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C. Description of input and examples for PHREEQC version 3: A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. In Techniques and Methods; US Geological Survey: Menlo Park, CA, USA, 2013; p. 497. [Google Scholar]
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater and Pollution, 2nd ed.; CRC Press: Boda Raton, FL, USA, 2005. [Google Scholar]
- Vanderdeelen, J. IUPAC-NIST Solubility Data Series. 95. Alkaline Earth Carbonates in Aqueous Systems. Part 2. Ca. J. Phys. Chem. Ref. Data 2012, 41. [Google Scholar] [CrossRef]
- Tallinna Vesi Water Treatment Process. Available online: https://tallinnavesi.ee/en/ettevote/activities/water-treatment/water-treatment-process/ (accessed on 31 July 2020).
- Tans, P.; Keeling, R. Trends in Atmospheric Carbon Dioxide (NOAA). Available online: https://www.esrl.noaa.gov/gmd/ccgg/trends/data.html (accessed on 11 August 2020).
- Groenendijk, M.; Van De Wetering, S.; van Nieuwenhuijze, R. Central water softening: Customer comfort is relevant in new WHO view. Water Sci. Technol. Water Supply 2008, 8, 69–74. [Google Scholar] [CrossRef]
- Godskesen, B.; Hauschild, M.; Rygaard, M.; Zambrano, K.; Albrechtsen, H.-J. Life cycle assessment of central softening of very hard drinking water. J. Environ. Manag. 2012, 105, 83–89. [Google Scholar] [CrossRef]
- Tang, C.; Merks, C.W.A.M.; Albrechtsen, H.-J. Water softeners add comfort and consume water—Comparison of selected centralised and decentralised softening technologies. Water Supply 2019, 19, 2088–2097. [Google Scholar] [CrossRef]
| NEN 6533 | DIN 38404-10 | 2330 Standard Methods | |
|---|---|---|---|
| Reference | [7] | [19] | [6] |
| Country | The Netherlands | Germany | U.S.A. |
| Applicability | Ionic strength <20 mmol/L | Drinking water | Drinking and waste water |
| Temperature | <30 °C | <60 °C 1 | 0–100 °C 2 |
| CaCO3 phases | calcite | calcite | calcite, aragonite and vaterite |
| Solubility constants | Jacobsen & Langmuir (1974) and Plummer & Busenberg (1982) [21,22] | Jacobsen & Langmuir (1974) and Plummer & Busenberg (1982) [21,22] | Plummer & Busenberg 1982 [22] |
| Ionic species | Ca2+, HCO3− and H+ | Ca2+, Mg2+, Na+, K+, Cl−, C, NO3−, SO42−, PO43− and H+ + associated complexes3 | Not specified |
| Exchange with atmospheric CO2 | No | No | No |
| Validation data | No | Yes | (Yes) 4 |
| Parameter | Unit | Untreated Groundwater 1 | Hard Drinking Water | Softened Groundwater 2 | Softened Riverbank Infiltrate 3 | Surface Water |
|---|---|---|---|---|---|---|
| DWTP | Frederiksberg | Thorsbro | Brøndbyvester | Lekkerkerk | Ülemiste | |
| Country | Denmark | Denmark | Denmark | The Netherlands | Estonia | |
| Ca | mmol/L | 4.99 | 3.74 | 1.07 | 1.07 | 1.70 |
| Mg | mmol/L | 1.32 | 0.86 | 0.91 | 0.25 | 0.33 |
| Hardness | mmol/L | 6.30 | 4.60 | 1.98 | 1.33 | 2.02 |
| Hardness | °dH | 35.3 | 25.8 | 11.1 | 7.4 | 11.3 |
| Alkalinity | mmol/L | 6.56 | 6.07 | 5.25 | 1.70 | 2.82 |
| pH | [-] | 6.90 | 7.40 | 8.00 | 7.82 | 7.25 |
| Temperature | °C | 10.0 | 10.0 | 9.3 | 12.8 | 10.0 |
| Na | mmol/L | 2.70 | 0.74 | 5.65 | 1.34 | 0.35 |
| K | mmol/L | 0.15 | 0.12 | 0.14 | 0.09 | 0.06 |
| Fe | mmol/L | 0.07 | <1.8 × 10−4 | <1.8 × 10−4 | <1.8 × 10−4 | <1.8 × 10−4 |
| Sr | mmol/L | 0.04 | 0.02 | 0.02 | - | 0.001 |
| Cl | mmol/L | 5.07 | 1.41 | 3.38 | 1.28 | 0.90 |
| F | mmol/L | 0.07 | 0.014 | 0.022 | <0.1 | 0.00 |
| PO43− | mmol/L | <3.6 × 10−5 | <3.6 × 10−5 | <3.6 × 10−5 | - | <2.1 × 10−5 |
| SO42− | mmol/L | 2.08 | 0.88 | 0.91 | 0.25 | 0.29 |
| NO3− | mmol/L | <0.002 | 0.05 | 0.03 | 0.06 | 0.04 |
| Sample date | 15.6.2018 | 3.7.2018 | 10.1.2019 | 2018 4 | 2019 4 |
| Ionic Species Included in CCPP Calculations | Untreated Groundwater | Hard Drinking Water | Softened Groundwater | Softened Riverbank Infiltrate | Surface Water | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10 °C | 90 °C | 10 °C | 90 °C | 10 °C | 90 °C | 10 °C | 90 °C | 10 °C | 90 °C | |
| Ca, pH, alkalinity | 0.138 | 1.667 | 0.486 | 1.587 | 0.198 | 0.690 | −0.015 | 0.171 | −0.158 | 0.364 |
| DIN standard (1) | −0.035 | 1.479 | 0.416 | 1.495 | 0.145 | 0.602 | −0.020 | 0.151 | −0.170 | 0.334 |
| DIN + F, Fe, Sr | −0.039 | 1.472 | 0.416 | 1.493 | 0.145 | 0.602 | −0.020 | 0.151 | −0.170 | 0.334 |
| mmol/L | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Validation data | CCPP | −0.145 | −0.032 | −0.163 | −1.111 | −0.140 | −0.030 | −0.055 | −0.115 | 0.071 | −0.003 |
| DIN database | CCPP | −0.145 | −0.032 | −0.162 | −1.110 | −0.140 | −0.030 | −0.055 | −0.115 | 0.071 | −0.003 |
| ΔCCPP | 0.000 | 0.000 | −0.001 | −0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| phreeqc.dat database | CCPP | −0.142 | −0.032 | −0.142 | −1.109 | −0.136 | −0.027 | −0.055 | −0.112 | 0.078 | 0.004 |
| ΔCCPP | −0.003 | 0.000 | −0.021 | −0.003 | −0.004 | −0.003 | 0.000 | −0.003 | −0.007 | −0.007 |
| Untreated Groundwater | Hard Drinking Water | Softened Groundwater | Softened Riverbank Infiltrate | Surface Water | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 °C | 90 °C | 10 °C | 90 °C | 10 °C | 90 °C | 10 °C | 90 °C | 10 °C | 90 °C | ||
| CCPP at observed pH | −0.04 | 1.47 | 0.42 | 1.49 | 0.14 | 0.60 | −0.02 | 0.15 | −0.17 | 0.33 | |
| pH ± 0.01 | ΔCCPP | 0.02 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 |
| pH ± 0.05 | ΔCCPP | 0.10 | 0.04 | 0.04 | 0.02 | 0.01 | 0.01 | 0.01 | 0.00 | 0.04 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.; Godskesen, B.; Aktor, H.; Rijn, M.v.; Kristensen, J.B.; Rosshaug, P.S.; Albrechtsen, H.-J.; Rygaard, M. Procedure for Calculating the Calcium Carbonate Precipitation Potential (CCPP) in Drinking Water Supply: Importance of Temperature, Ionic Species and Open/Closed System. Water 2021, 13, 42. https://doi.org/10.3390/w13010042
Tang C, Godskesen B, Aktor H, Rijn Mv, Kristensen JB, Rosshaug PS, Albrechtsen H-J, Rygaard M. Procedure for Calculating the Calcium Carbonate Precipitation Potential (CCPP) in Drinking Water Supply: Importance of Temperature, Ionic Species and Open/Closed System. Water. 2021; 13(1):42. https://doi.org/10.3390/w13010042
Chicago/Turabian StyleTang, Camilla, Berit Godskesen, Henrik Aktor, Marlies van Rijn, John B. Kristensen, Per S. Rosshaug, Hans-Jørgen Albrechtsen, and Martin Rygaard. 2021. "Procedure for Calculating the Calcium Carbonate Precipitation Potential (CCPP) in Drinking Water Supply: Importance of Temperature, Ionic Species and Open/Closed System" Water 13, no. 1: 42. https://doi.org/10.3390/w13010042
APA StyleTang, C., Godskesen, B., Aktor, H., Rijn, M. v., Kristensen, J. B., Rosshaug, P. S., Albrechtsen, H.-J., & Rygaard, M. (2021). Procedure for Calculating the Calcium Carbonate Precipitation Potential (CCPP) in Drinking Water Supply: Importance of Temperature, Ionic Species and Open/Closed System. Water, 13(1), 42. https://doi.org/10.3390/w13010042





