Abstract
The treatment of different types of wastewater by physicochemical or biological (non-microalgal) methods could often be either inefficient or energy-intensive. Microalgae are ubiquitous microscopic organisms, which thrive in water bodies that contain the necessary nutrients. Wastewaters are typically contaminated with nitrogen, phosphorus, and other trace elements, which microalgae require for their cell growth. In addition, most of the microalgae are photosynthetic in nature, and these organisms do not require an organic source for their proliferation, although some strains could utilize organics both in the presence and absence of light. Therefore, microalgal bioremediation could be integrated with existing treatment methods or adopted as the single biological method for efficiently treating wastewater. This review paper summarized the mechanisms of pollutants removal by microalgae, microalgal bioremediation potential of different types of wastewaters, the potential application of wastewater-grown microalgal biomass, existing challenges, and the future direction of microalgal application in wastewater treatment.
1. Introduction
Global economic and societal development has led to increased water demand along with a water deficit. Reports project that by the year 2030, at current water utilization practices, globally, there could be a 40% water deficit [1]. Rampant industrialization, urbanization, and groundwater contamination are some of the major causes of water deficit. Although different technologies exist for treating wastewater, each technology has its drawbacks and advantages; therefore, it would be crucial to select one that could serve the dual purpose of effective purification and resource recovery optimization [2]. Parallel to water deficit, wastewater discharge is also rising due to increasing industrial, agricultural, and urban activities. The release of wastewater into the environment leads to the discharge of nutrients, causing eutrophication in water bodies. Additionally, the circular economy concept has now been adopted by various governmental institutions to reduce and curb the pollution caused by wastewater. However, the reuse of wastewater would require a fit-for-purpose approach that could reduce the energy and production cost associated with wastewater treatment and enhance the resource recovery from wastewater [3]. The concept of the circular economy revolves around the reclamation and recycling of resources.
Conventional wastewater treatment plants (WWTPs) are now facing challenges, as circular economy principles impose stringent pollutant limits that are necessary before discharge and reuse of wastewater into the environment. Therefore, a fit-for-purpose approach could be adopted to allow selective water reuse and water savings while reducing the cost associated with wastewater recycling [3,4]. Other drawbacks of conventional wastewater treatment processes are high energy consumption, greenhouse gas emissions, recyclable resource wastage, and excessive solid landfilling. Several technologies such as hydrothermal carbonization, anaerobic digestion, and pyrolysis are now being developed and applied to maximize energy recovery from waste biosolids to phase out from excessive solid landfilling [1,5]. These drawbacks pose a challenge to developing a sustainable waste management solution for wastewater treatment and disposal. Therefore, there is a need for alternative wastewater treatment methods that would be low carbon-emitting and high on resource recycling and consume less energy and promote biorefinery and circular economy concepts. One such alternative option could be the use of microalgae to treat industrial, agricultural, and domestic wastewaters. The potential of microalgal bioremediation of wastewater was first proposed by Oswald and Golueke as early as 1950 [6]. The microalgae could utilize the abundant sunlight that falls on the surface of wastewater as their energy requirement for growth and the simultaneous removal of pollutants. Microalgae could also grow in the arid environment and highly saline water. To produce 1 kg of microalgal biomass would require 1.83 kg of carbon dioxide—offering the potential of coupling sequestration of CO2 while treating wastewater [7]. At the end of the microalgal bioremediation of wastewater, the generated sludge would mostly comprise microalgae [8]. In the existing activated sludge process, typically the energy requirement for oxygen supply is 1 kWh/kg biochemical oxygen demand (BOD). Microalgae cultivation in wastewater does not require oxygen supply; rather, the produced microalgal biomass could be processed by anaerobic digestion to generate 1 kWh/kg BOD. From microalgal biomass, other energy recovery options could be gasification and pyrolysis for syngas and biochar production, fermentation, and hydrothermal liquefaction (HTL) of microalgal biomass to produce bioethanol, bio-butanol, and biocrude [9,10].
Microalgae are not only limited to the removal of nutrients from wastewater generated from WWTPs; there are recent studies that report the use of microalgae to remove pharmaceutical compounds and pesticides from industrial and agricultural generated wastewaters [11,12]. Therefore, microalgae’s ability to grow, assimilate, and resist toxic conditions in wastewater make it a versatile organism for treating different types of liquid effluents originating from various industrial and agricultural practices. Furthermore, with the integration of the biorefinery concept, the harvested microalgae biomass could be used to produce carbon-neutral fuels, high-value pigments, fish and animal feed, biofertilizers, bioplastics, and carbon dioxide mitigation in a cyclic manner, thereby promoting the concept of circular economy with zero waste into the environment.
Several review articles already exist on microalgal wastewater treatment; however, these review papers could be categorized based on treatment mechanisms [13,14,15,16], specific wastewater treatment [12,16,17], nutrient uptake [2,17,18,19,20], and microalgal biomass valorization [9,21,22,23]. However, commercial microalgal treatment of wastewater would require integrating multiple individual unit operations including microalgal cultivation, biomass separation, and biomass valorization. Therefore, this review paper explored all the relevant topics to cover these unit operations: (1) mechanisms involved in microalgal bioremediation of different types of wastewater, (2) techniques for cultivating microalgae in wastewater, (3) comparing different biomass dewatering techniques, and (4) various biorefinery options that are available for the valorization of microalgae biomass to different value-added products. Finally, the major challenges in microalgal bioremediation of wastewaters were pointed out, and recommendations were made to overcome these challenges.
2. Microalgal Treatment of Wastewater
2.1. Selection of Strains
Over 3000 strains of microalgae were identified [24]; the characteristics of these algae strains would vary, and so would be their wastewater treatment potential. However, the major factors of selecting a suitable strain or a mix-consortia for wastewater treatment would be (i) characteristics of wastewater, (ii) the desired level of treatment efficiency required, (iii) the cost and energy requirement of biomass harvesting, and (iv) the application of the harvested biomass.
2.2. The Necessity of Pretreatment of Wastewater
Wastewater could contain several compounds at elevated concentrations that inhibit biological (e.g., microalgal) growth [25]. Similarly, the turbidity and pH of wastewater could inhibit microalgal growth [26]. Physicochemical pretreatment of wastewater would improve the conditions for microalgal growth and reduce wastewater’s strength [27,28]. Lin et al. (2017) adopted a three-step strategy for efficient treatment of textile industry water (TWW): adsorption of toxic compounds by granular activated carbon, followed by anaerobic digestion to produce electricity and reduce the load of the TWW before cultivating microalgae in the partially treated TWW [29]. Although ammonium is the most preferred nitrogen source for microalgal growth, the ammonium tolerance limit for microalgal strains was reported as high as 1000 μmol NH4-N L−1 [20]. A pretreatment would be required to reduce the ammonium concentration in wastewater, or wastewater should be diluted to a tolerable limit where the selected strain could grow efficiently [30]. For several wastewater types (e.g., metal, mining, paper, oil, and grease), electrocoagulation was used as a pretreatment step, which could effectively remove various chemical additives, turbidity, pathogens [31,32,33]. Microalgal biomass cultivation in such pretreated wastewater could also reduce residual nutrients and turbidity from the treated wastewater [27].
2.3. Mechanism of Treating Wastewater
2.3.1. Nutrient Uptake
The treatment of wastewater having low nitrogen to organic carbon ratio (C/N) is challenging. In such wastewater, supplementation of organics is often practiced to improve bacterial nutrient removal efficiency as a source of energy. On the contrary, microalgae could utilize sunlight, the soluble inorganic carbon dioxide, nitrogen, and other nutrients to increase their cell numbers while treating wastewater. Depending on the strain type, microalgal cellular nitrogen content could range from 3–10% [34,35]. A variety of inorganic (e.g., ammonium, nitrate, nitrite, atmospheric nitrogen) and organic (e.g., urea, glycine, etc.) forms of nitrogen could be assimilated by microalgal/cyanobacterial strains, although the efficiency would again vary among strains and growth conditions. From a cost perspective, microalgal removal of phosphorus from wastewater could be a superior choice over chemical precipitation and engineered wetland based phosphorus removal [36,37]. Microalgae could also selectively consume nitro and amino groups from different aromatic compounds (e.g., aminonaphthalenes and nitrobenzonates) as nitrogen source—thereby reducing the toxicity of the original pollutants [38]. Microalgal nutrients’ removal efficiencies from different wastewaters are listed in Table 1.

Table 1.
Microalgal nutrient removal efficiencies from different wastewater.
2.3.2. Metals Adsorption/Uptake
High concentrations of heavy metals in wastewater could inhibit microalgal photosynthesis [18]. Nevertheless, microalgae efficiently concentrate the metal pollutants both internally and externally and could be adopted for metal removal from wastewaters [55]. Microalgal cells need several metals (i.e., Fe, Mn, Cu, Co, Zn, and Mo) in trace amounts as their growth requirements. However, microalgae are also capable of concentrating on various heavy metals (e.g., Cd, Hg, Ni, Zn, Fe, Cu, Pb, Cr, etc.) through different mechanisms [55]. Microalgal cell walls are comprised of carbohydrates and polysaccharides that could have multiple negatively charged (amino, hydroxyl, carboxyl, pyruvate, sulfide, phosphate, etc.) groups as active sites for adsorbing positively charged metal cations [56]. Such extracellular adsorption of heavy metals occurs very fast till a saturation level is reached [57]. Heavy metals could also get transported through the cell membrane inside the cells, thereby reducing their concentrations in wastewater [58]. Several microalgae could release various polysaccharides, also known as exopolysaccharides (EPS), into the growth media [59]. Negatively charged groups within EPS molecules are also capable of absorbing metals [60]. Both living and dead cells of microalgal are capable of metal removal, although the metal removal efficiencies for living cells are higher compared to dead cells [61]. The removal efficiencies of several metals (e.g., Cd, Cr, Cu, Pb, Hg, Ni, and Zn) by selected microalgae are listed in Table 2.

Table 2.
Microalgal removal of metals from wastewater.
2.3.3. Organic Removal
There are mainly three mechanisms (i.e., biodegradation, consumption, biosorption) that the microalgae could utilize to remove the organics from wastewater. Microalgal cell walls could have several polymers group that could offer potential sorption sites for organic pollutants; however, the removal of organics by microalgal sorption was rather low [75,76]. Although most of the microalgae are photosynthetic in nature, a group of microalgae could utilize various organics in either mixotrophic or heterotrophic mode, and this would allow mixing organic-rich stream (e.g., wastewater from food processing, glycerol from biodiesel plant, etc.) into other wastewater for combined treatment and improving the biomass and lipid yield [54,77]. Apart from bioaccumulation, microalgae, either as monoculture or consortia, could transform organic pollutants (e.g., phenolics, petroleum hydrocarbons, pesticides, polyaromatic hydrocarbons, polychlorinated bisphenyls, etc.) to other less toxic and non-toxic compounds, or even completely mineralized products (i.e., CO2) [78,79,80,81].
2.3.4. Symbiotic Relationship
Microalgae could also participate, in a symbiotic relationship with bacteria or fungi, to degrade organics [82]. As a byproduct of the photosynthesis process, microalgae produce oxygen, which could be utilized by aerobic bacteria for the mineralization of organics to carbon dioxide; microalgae would utilize this CO2, and the process would be repeated, ultimately treating wastewater [13,83]. On the other hand, bacteria produce several useful compounds such as Vitamin B12, indole-3-acetic acid, siderophores to promote microalgal growth. At times, bacteria could also regenerate or fix several inorganic nutrients (e.g., iron, nitrogen) and organics (e.g., D-glucose, Na-acetate) that are not available to microalgae for utilization [84]. Bacterial release of polysaccharides and proteins could also assist the bioflocculation of microalgae biomass [85].
2.3.5. Passive Removal of Contaminants at Elevated pH
As early as 1970, it was observed that microalgae flocculated in the waste stabilization pond, specifically on warm and sunny days when the CO2 was depleted, and pH increased [86]. Therefore, microalgae could also passively assist in removing the pollutant from wastewater. As the microalgae grow, the soluble carbonate would be consumed—giving rise to the culture pH, in case there would be no additional supply of CO2 other than atmospheric diffusion. At elevated culture pH, several pollutants could form insoluble precipitates (e.g., various metals, phosphorus)—facilitating their removal from wastewater [87], while ammonium could get converted to ammonia and escape to the atmosphere [88].
3. Microalgal Cultivation in Different Wastewaters
Typical microalgal biomass concentration in large scale open cultivation could be 0.5 g/L. Depending on the cellular composition of the microalgae, nitrogen, and phosphorus content in the biomass could be in the range of 5.4–8.7% and 0.7–1.1%, respectively [89]. Therefore, microalgae alone could potentially consume or remove 43.5 and 5.5 mg/L of nitrogen and phosphorus, respectively, from wastewater. Additionally, microalgae could also adsorb pollutants from wastewater and assist other microorganisms in removing additional contaminants. Considering wastewater would have all other necessary micro-elements. In case wastewater contains excessive TN and TP, then such wastewater needs to be diluted to an appropriate ratio before microalgal bioremediation.
3.1. Municipal Wastewater
Typical nitrogen and phosphorus concentrations in the municipal sewage wastewater (MSWW) are 21.9–28.8 and 8.2–10.4 mg/L [90]. The concentration of dissolved organics in the MSWW is usually low for the bacteria for the complete consumption of nitrogen and phosphorus [91]. Hence, after the activated sludge process (ASP), an advanced treatment process is adopted to remove excess nutrients (N, P). Microalgae could remove the residual nitrogen and phosphorus from the effluent of ASP [16,92]. However, microalgae cultivation in the MSWW could efficiently remove nitrogen, phosphorus, BOD, and heavy metals [93,94]. Microalgae could also remove pathogens from the MSWW [95].
3.2. Industrial Wastewater
For several countries, the textile industry wastewater (TWW) could contribute more than 10% (as high as 30%) of the total industrial wastewater [96,97,98]. TWW often contains dyes, fats, acids, binders, salts, heavy metals (e.g., Cr, Cu, As, Zn, etc.), thickeners, and reducing agents, in addition to nitrogen and phosphorus compounds [29]. The dyes in the TWW are recalcitrant organics and pose a challenge in its remediation and simultaneously could adversely affect the quality of the receiving water bodies, even at low concentrations [15]. A number of physicochemical techniques were studied to treat TWW; however, most of these methods could be costly, energy-intensive, and inefficient [96]. Bioremediation of TWW by different microorganisms (i.e., yeast, bacteria, microalgae) was explored. Microalgae were able to utilize several dyes (e.g., Eriochrome blueSE and blackT) as a source of carbon and nitrogen [99]. While microalgae could consume the organic compounds of TWW, the organic dyes in the TWW could also be adsorbed on to the microalgal cells, thereby treating the TWW [96].
3.3. Produced Water
Produced waters from oil and gas industries contain toxic petroleum compounds, in addition to various heavy metals and added chemicals (i.e., hydrocarbons, volatile fatty acids, carbonyl group, and high molecular weight organic acids) [100]. While the produced water could be toxic to many microalgal strains, some algal strains (e.g., Chlorella sp., Dunaliella sp., Scenedesmus sp., etc.) could tolerate and grow in the produced or pretreated produced water [26,101,102]. The algae-bacterial consortium could effectively treat the produced water [26,101,103]. The feasibility of large-scale microalgal biomass production in several countries, using produced water, was recently explored [17].
3.4. The Aqueous Phase of Biomass to Energy Generation Process
The digestate of the anaerobic process often contains very high concentrations of TN, TP, and other elements; this digestate could be a source of nutrients for microalgal cultivation [104,105]. In recent times, hydrothermal liquefaction (HTL) technology is being studied as a promising technology for converting various biomass into biocrude oil. However, as a byproduct of the HTL process, nutrient-rich aqueous phase liquid (APL) is also generated. Several microalgae strains were found to effectively treat and recycle the nutrients from the APL after appropriately diluting the APL in the growth medium [51,52].
3.5. Pharmaceuticals Wastewater
Wastewater contaminated with pharmaceuticals and personal care compounds (PPCCs) poses a serious threat because of their ecotoxicity and health issues. Some of these PPCCs could not be efficiently removed by the existing activated sludge process [11]. On the contrary, microalgae have the ability to remove the PPCCs such as triclosan from wastewater, although the efficiency of removal would vary among microalgal strains and operating conditions [106,107,108]. Microalgal removal of PPCCs from wastewater could occur in three potential pathways: adsorption, accumulation, and degradation, either intracellular or extracellular [109]. Microalgae could also be used as indicators/markers to assess the toxicity of some PPCCs derivatives like triclosan in wastewater discharges and natural water bodies [110]. Microalgal adsorption efficiency for PPCCs are rather low 0–16.7% [111,112]. A few microalgae could uptake and accumulate several PPCCs, such as organic substrates and separate from the contaminated water [113]. Some of the microalgae showed a limited ability to degrade several PPCCs; however, in the presence of a suitable organic compound, the degradation rate and efficiency greatly increased [114]. Although microalgal removal of a couple of PPCCs (e.g., benzothiazole, diclofenac, OH-Benzothiazole, etc.) were affected by seasonal variation (temperature), the removal of most common PPCCs (e.g., caffeine, acetaminophen, ibuprofen, etc.) in wastewater was minimally affected by the variation in temperature [115,116]. However, as the microalgal cell gets exposure to the PPCCs, its metabolites composition needs to be characterized. Further, transformation products of these PPCCs into other products need to be identified and characterized.
3.6. Agro-Industry Wastewater
Wastewater generated from livestock (e.g., swine, piggery, cattle) and poultry industries are typically rich in nitrogen and phosphorus; microalgae were used to successfully recover nutrients and produce biomass while treating wastewaters from these industries [42,43,117,118]. Dairy industries wastewater (DWW) is generally rich in organic content with high BOD5 and COD values; therefore, mixotrophic microalgal strains (i.e., Scenedesmus sp., Tetraselmis sp., etc.) could be very efficient in treating DWW [119]. Microalgae were also used to treat wastewater from the carpet mill, brewery effluents [120,121]. Similarly, microalgae were able to treat wastewater generated from food processing industries [54,122].
3.7. Wastewater Derived from Mining Activity
Wastewaters from mines are typically acidic in nature and could contain potentially hazardous metals; because of microalgal ability to intra and/or extra cellular accumulation of various metals, these organisms could potentially be applied to treat mine wastewater [123], mainly both inside and outside the cell. Tong et al. (2015) studied the continuous removal of zinc from the leachate of a mine using microalgal biofilm [124]. Li et al. (2015) studied the combined treatment of municipal sewage water and zinc contaminated water from an abandoned mine using a microalgal biofilm of Stichococcus bacillaris [124].
3.8. Landfill Leachate
Landfill leachate often contains a high concentration of ammonia that itself could be toxic to microalgae [125]. Additionally, the leachate’s black color could interfere with the light penetration and, consequently, microalgal growth and remediation of leachate. An appropriate dilution or pretreatment would be required for the efficient treatment of the leachate. Raw leachate could be mixed with seawater or pretreated leachate for microalgal bioremediation [125,126,127]. It was demonstrated that a consortium of five microalgae could efficiently remove COD, NH3-N, and orthophosphate from aerobically treated leachate in high rate algal ponds (HRAP) [128]. At times, phosphorus concentration in the leachate could be low, and it should be supplemented as an appropriate concentration since microalgae require phosphorus for their growth [127,129].
3.9. Aquaculture Wastewater
For the intensive aquaculture system, the effluent often contains an elevated concentration of dissolved nitrogen compounds, mainly in ammonia, generated from the undigested feed and the feces [130]. While bacterial nitrification of ammonia and other nitrogenous compounds to gaseous nitrogen is a feasible option, microalgal assimilation of these nitrogenous compounds could be a sustainable alternative option, as the produced biomass could be used as superior feed ingredients [49,131,132]. In another study, microalgal-bacterial floc was applied in a sequencing batch reactor to successfully treat the aquaculture wastewater [80]; the easy separation of microalgal–bacterial floc by gravity sedimentation could reduce the overall cost of aquaculture wastewater treatment.
4. The Process of Microalgal Cultivation
4.1. Suspended Growth
The most common microalgae cultivation method in wastewater is a suspended growth system, either in open or closed systems (photobioreactor or PBR). Among various PBRs, biocoil, horizontal tubular, and vertical PBRs are commonly studied for wastewater treatment [133,134]. Microalgae cultivation in the PBR could reduce or minimize unwanted contamination, evaporation water loss, and loss of injected CO2. High volumetric biomass productivity could be achieved inside a PBR with low optical depth; as the PBR’s optical depth increases, the productivity of the biomass decreases [135,136]. In general, the cost of PBR materials could be too high for treating wastewater. Furthermore, the energy investment for mixing the culture inside a PBR could be several times higher than the calorific energy of the produced microalgal biomass [137]. On the contrary, the open cultivation of microalgae in wastewater could be very promising, despite some challenges (i.e., evaporation water loss, contamination, etc.) [138,139]. Microalgal bioremediation of wastewater was explored using earthen lagoon, concrete tanks, and raceway ponds [140,141,142]. High rate algal pond (HRAP) and corrugated raceway pond (CRP) are two of the open type cultivation systems, which were specifically designed for treating wastewater [126,143]. The depth of the open cultivation system could vary from 0.15–0.45 m [144]; since microalgae are very efficient in absorbing the light, the top layer of the culture would absorb most of the light, keeping the cells below in the dark [145]. The mixing of microalgae culture in a large-scale open system is often insufficient, and hence microalgal bioremediation in a deeper pond could be inefficient [146].
4.2. Attached Growth
To overcome the challenges of microalgal biomass harvesting from the suspended culture, microalgae cells could be immobilized or attached to a support medium where the attached cells contact wastewater, and the nutrients get absorbed and subsequently utilized by the microalgae to produce biomass [147]. The immobilized cells could be on a static surface or mounted on a rotating paddle [148]. After achieving the desired degree of bioremediation or when the biomass growth on the surface would reach a certain thickness, the support media could be removed from wastewater, and the biomass could be scraped out. Further, the remaining fraction of the cells on the biofilm could act as inoculum for the next batch [149]. A biofilm of Chlorella sp was developed in a microbial fuel cell as a biocathode to treat TWW and generate electricity [150]. Algal turf scrubber (ATS) is another configuration of attached microalgal growth system, where benthic microalgae could be grown on a solid support and wastewater would be circulated over the ATS [151]. Similarly, a floating conveyer belt of a dimpled metal sheet could be used to form an algal biofilm on its surface [152]. However, the cost involved in developing the support medium is one of the major barriers to commercially implementing this technique [95]. Furthermore, the attached biofilm could accumulate other non-algal compounds and thereby could reduce the quality of the biomass [153].
4.3. Batch vs. (Semi) Continuous
Microalgae could be cultivated, either in suspension or as attached, in either batch or (semi) continuous mode to treat wastewater. Most of the lab-scale microalgal bioremediation experiments were conducted in batch mode. Several studies demonstrated the superior performance of continuous/semi-continuous cultivation systems as compared to batch cultivation for wastewater treatment and biomass production [49,124,153]. One of the potential drawbacks of continuous cultivation is the culture crash after few cycles of microalgal growth [153]. The use of a photobioreactor for the (semi) continuous cultivation of microalgae could undermine wastewater treatment’s overall cost and energy effectiveness.
4.4. Monoculture vs. Consortia
While a vast majority of studies used monoculture of microalgal strains for the treatment of wastewater, a number of studies included a specific consortium of microalgae [154,155,156]. Since wastewaters are typically contaminated with different types of contaminants, a mixed culture of microalgal strains could be advantageous as the limitation of a strain could be overcome by others [14,157]. Indeed, many studies found that polycultures of microalgae were more efficient in treating wastewater than the treatment efficiency by monoculture [121,158]. Microalgal consortia also showed increased resistance to culture crash [159]. Microalgal consortia cultivation could also assist in biomass harvesting, especially if one or more strains produce EPS or filamentous in nature [160].
5. Harvesting of Microalgae Biomass
Removal of microalgal biomass from the treated wastewater is one of the critical processes of microalgal bioremediation of wastewater. Therefore, efficient preliminary harvesting of microalgae would also be crucial if the treated wastewater needs to be used for other purposes. Although there are multiple techniques available for the separation of biomass from the bulk of the culture, the adoption of a harvesting technique would mainly depend on the application of the produced biomass and the energy requirement per unit of biomass production. In most cases, a two-phase harvesting process is adopted to obtain a biomass paste of 20% solid content or higher. In the preliminary step, harvesting techniques like sedimentation, flocculation, filtration, etc., are used to obtain a biomass slurry (typically 1–4%), which then could be further concentrated (typically above 20%) using a centrifuge. The harvesting efficiency of microalgae grown in different wastewater is listed in Table 3. In addition, a comparison of different harvesting techniques is shown in Table 4.

Table 3.
Harvesting efficiencies of microalgae grown in different wastewaters.

Table 4.
Energy requirement in microalgae biomass harvesting.
5.1. Sedimentation
Some cells of microalgae could not stay afloat in the growth medium due to their cell size and or pH value of the medium; in either case, the cells sink to the bottom. Therefore, the sedimentation process could be applied to separate microalgae biomass as an inexpensive and variable harvesting process for large scale operations. Cells of several microalgal strains, e.g., Scenedesmus and Chlorella sp., are big enough to remain suspended in the growth medium in the absence of any mixing. Typically, the microalgal cell surface has a negative charge, which prevents cells from attaching to each other. As the microalgal culture density increase, the cells utilize the soluble carbonate, and in the process, the pH of the growth media also increases, and it could also neutralize the surface charge of the microalgae. Several inorganic compounds precipitate at elevated pH levels; this process could also initiate microalgal cells to coprecipitate. Since the gradual pH increase and natural precipitation could be time-consuming, at times, a base solution (i.e., sodium hydroxide) is added to expedite the microalgal sedimentation.
5.2. Auto-Flocculation
Several cyanobacterial strains form flocs, which are held together with the help of EPS that they produce. Several other cyanobacteria are filamentous in nature (e.g., Phormidium sp., Leptolyngbya sp., Pseudoanabaena sp.), which tangle together to form floc [173,174]. Several factors (i.e., light intensity, temperature, nutrient deprivation, etc.) could influence the EPS productivity and subsequent destabilizing microalgal cell surface leading to auto-flocculation [175].
5.3. Bio Flocculation
The co-cultivation of a non-settling microalga with a self-settling microalga could enhance the combined biomass harvesting efficiency [176]. A couple of fungus strains (e.g., Aspergillus sp.) could form gelatinous pellets, where negatively charged microalgal strain could attach and could together precipitate [177]. The available organics in wastewater could be used as a substrate by the fungus to grow and form the pellets. Similarly, bio-flocs formed by microalgae and bacteria could be removed by gravity sedimentation from the treated wastewater [178].
5.4. Coagulation–Flocculation
Multivalent metal salts (Fe, Al), and cationic polymers (both synthetic and natural) were shown to be most effective in the harvesting of microalgae from wastewater [179]. The efficacy of ferric chloride [93,133] and alum [180] was demonstrated for many microalgal strains. The addition of natural polymers (e.g., tanfloc, chitosan, tannin, starch, gamma glutamic acid, guar gum, and tamarind kernel polysaccharide), at a lower dosage, could also improve the microalgal harvesting efficiency [181]. Recent studies have demonstrated that iron and aluminum could be recovered from ferric chloride and alum harvested biomass [182,183]. In addition, the extracted metals, together with a small fraction of new coagulant, could harvest biomass from the subsequent batch of microalgal culture. As an alternative, the bacterial strain was grown separately using lignocellulosic materials to produce specific bio-flocculants (e.g., xylanase, and cellulase), which was found to be very efficient in harvesting microalgal strain (e.g., Chlorella minitussima) [184].
5.5. Electrocoagulation
In the electrocoagulation process, the coagulants are produced in situ from the anode as the electrodes (aluminum, steel, etc.) are connected to a DC power source. In addition to low coagulants requirements, the energy requirement for the electrocoagulation process is very low (e.g., 0.3 kWh/m3) [163]. The coagulants then form large flocs of microalgal cells, which get separated either by sedimentation or floatation. During the electrocoagulation process, oxygen and hydrogen gas microbubbles are generated at the anode and cathode, respectively. These microbubbles could attach the coagulated algal cells and float to the top [185]. The efficiency of algal separation was much higher for aluminum electrodes compared to steel electrodes [186].
5.6. Flotation of Microalgae
In the floatation of microalgae harvesting, often with coagulants’ addition, microscopic bubbles are introduced to the bottom of the floatation tank, where the cells are attached to the surface of the bubbles and get destabilized; the cells rise to the top and get concentrated [187,188]. The bubble size plays an important role in the removal efficiency of algae; the smaller the bubble’s size, the higher the algae removal efficiency [189]. However, the generation of smaller-size bubbles (e.g., 10–100 μm) would be energy-intensive (e.g., 7.6 kW h/m3) [190].
5.7. Filtration
Membrane filtration of microalgae culture could potentially achieve 100% cell recovery, and unlike other modes of harvesting, membrane filtration could be applied to most of the microalgae strains—resulting in a biomass slurry where the quality of the biomass would remain intact [191]. For a filamentous strain (e.g., Arthospira sp.), a vibrating screen set up could be used to separate the biomass [192]. Tangential-flow-filtration (TFF) is another promising biomass separation technique where the culture is pumped through the TFF module, where a fraction of the clear water passes through the membrane while all the cells remain in the reduced volume of the culture, whereby the biomass density in the water increases. The concentrated culture is passed through the TFF until the desired biomass density is obtained. Biomass density, cell morphology, and salinity of the culture are some of the critical parameters that would affect the energy consumption of the membrane filtration [193]. During the TFF operation, membrane fouling would occur; the frequency and extent of membrane fouling would depend on the strain type, composition of the growth media, and mode of TFF operation. Usually, backwashing is used during the TFF operation, in small duration, for cleaning the membrane and recovering the biomass. Filtrate coming out of the TFF would have excellent quality for reuse for multiple purposes. Nevertheless, the energy demand of the filtration system could be very high, typically in the range of 0.7–2.3 kWh/m3 of culture.
6. Application of the Produced Biomass
Microalgal biomass has the potential to be a sustainable alternative feedstock compared to the feedstock typically generated from the terrestrial plant. Water and nutrients supply are two factors that would influence microalgal biomass production’s cost and energy; in this regard, the cultivation of microalgae in wastewater could offer additional benefits, although this would limit the application of produced algae biomass. Potential applications of microalgae biomass grown in different industrial wastewaters were reviewed recently [7]. A schematic of the microalgal bioremediation of wastewater and the potential applications of the produced biomass is shown in Figure 1.
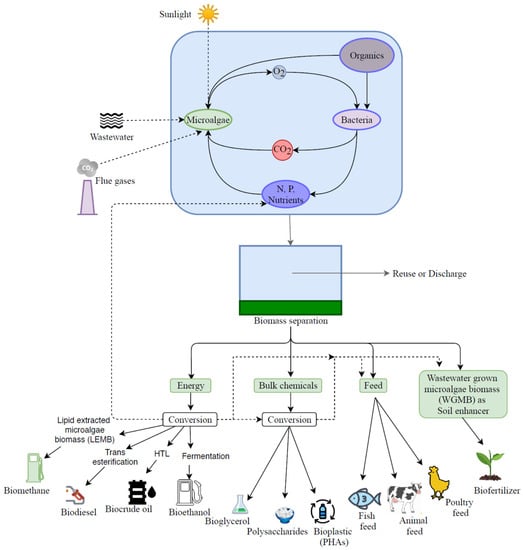
Figure 1.
Microalgal bioremediation of wastewater and the potential applications of the produced biomass.
6.1. Composition of the Microalgae Biomass
The major metabolites of microalgal biomass are protein, lipid, and carbohydrate; the relative composition of these metabolites could vary among strains and the prevailing growth conditions (i.e., nutrient composition and concentrations, duration of cultivation, light intensity, temperature, pH, salinity, etc.) [21,42]. Therefore, the metabolite production from wastewater-grown microalgae would be influenced mainly by wastewater characteristics and the strain that could efficiently treat it. The composition of metabolites of several wastewater-grown microalgae is shown in Table 5. Several microalgae could accumulate various high-value pigments (phycocyanin, phycoerythrin, β-carotene, lutein, astaxanthin, etc.) [194,195,196].

Table 5.
The metabolites of wastewater-grown microalgae.
6.2. The Necessity of Pretreatment
As the microalgae grow in wastewater, they would consume the nutrients and concentrate other contaminants in wastewater. Depending on the biomass harvesting technique, the microalgal biomass could also be contaminated with other unwanted compounds [26], which could interfere with the intended use of the produced biomass. Therefore, the removal of the coagulants would be necessary for specific applications. Although there are limited studies of metal recovery from the coagulated-flocculated biomass, there is a need to study the recovery of other organic polymers that are used to harvest the microalgae biomass.
6.3. The Potential Application of the Biomass
6.3.1. Biofertilizer
One potential application of wastewater-grown microalgae biomass (WGMB) is a soil amendment as either soil additive or biofertilizer [93]. The application of microalgal biomass in the soil could mainly enhance soil nitrogen and phosphorus content, in addition to many other plant-required minerals (Ca, K, Fe, Mn, etc.). Microalgae could also adsorb the heavy metals; however, the concentrations of heavy metals in the biomass, or anaerobic-digested biomass, were lower than the allowable concentration [205,206]. In small-scale studies, WGMB was used as a biofertilizer to grow wheat, corn, cucumber, barley, tomato, etc. [93,206,207]. Microalgal biomass is also considered as a slow-release biofertilizer [208]. Furthermore, the addition of microalgae as biofertilizer could enhance the soil organic content [209]. Microalgae biomass, depending on the species, could also introduce various plant stimulating compounds [210]. On the contrary, WGMB could accompany pathogens and other micropollutants, which could pose concerns for the application of WGMB as a soil additive [211].
6.3.2. Energy Production and Nutrients Recycling
Anaerobic digestion of WGMB was studied extensively as a means of generating biogas energy, which could offset the energy requirement at the treatment plant. The biomethane yield from WGMB would depend on the biomass’s composition; the methane conversion from carbohydrates and protein compounds was reported to be higher than the lipid molecules [212]. Hence, microalgal strain with low lipid content would be preferable for treating wastewater if biogas is the intended end product. The lipid extracted microalgae biomass (LEMB) could also be anaerobically digested to obtain biogas gas (i.e., methane); the methane yield was reported in the range of 0.3–0.504 L/g of LEMB [22,105]. The energy return on operational energy invested (EROOI) for the overall process of biogas production from wastewater grown microalgal biomass could exceed 3 [213,214]. During the AD process and the conversion of methane to electricity, CO2 would be generated, which could be integrated with the microalgal cultivation in wastewater. Several studies explored the production of biodiesel by growing lipid-rich microalgae in wastewater [96,127]. However, the microalgal biomass needs to be dried for efficient extraction of lipid from it, where drying the wet microalgae biomass could be very energy-intensive. Therefore, the anaerobic digestion of whole wet biomass could be cost and energy effective. The potential biogas yield from microalgal biomass was reported to be as high as 200–600 mL/g organic content [215]. Pyrolysis is another technique that could be applied to dried microalgae biomass to produce oil [216]. In the last decade, hydrothermal liquefaction (HTL) emerged as a potential technique to convert whole biomass into bio-oil [217]. However, unlike pyrolysis, the HTL technique doesn’t require dry biomass; instead, the water within the biomass could be used as a reaction media. Some of the biomass elements (i.e., N, P, and other trace elements) are associated with the liquid stream as a byproduct of biogas and biocrude oil; this liquid stream could contain toxic organic compounds. Microalgae could not only treat the waste stream but also recycle the nutrients of this waste stream. Table 6 summarizes the energy recovery from different wastewater-grown biomass to energy conversion techniques.

Table 6.
Energy recovered from wastewater-grown microalgal biomass using different conversion techniques.
6.3.3. Bulk Chemicals
Apart from alcohol, microalgal carbohydrates could be converted to lactic acid [227,228]. Another emerging application of microalgal biomass is the production of bioplastic [229]. A number of cyanobacterial strains could produce intracellular polyhydroxyalkanoates (PHA) with properties similar to plastic; therefore, the cultivation of specific cyanobacteria in wastewater could potentially be a potential feedstock for bioplastic [230]. As a byproduct of biodiesel production from microalgal lipid, glycerol is produced (10% of lipid weight). Microalgae and cyanobacteria produce exopolysaccharides (EPS). Microalgae EPS could be used as a gelling agent, thickener, stabilizer, and as biolubricants and anti-inflammatory agents [231].
6.3.4. Animal Feed Production
The composition of microalgae biomass would vary among strains and culture conditions. Nevertheless, the nutritional properties of several microalgae biomass could be on par or exceeding the quality of many feed ingredients. Therefore, the inclusion of microalgae biomass in feed would enhance the quality of the feed. Further, the cost of cultivating microalgae in nutrient-rich wastewater would be very low, as there would be little to no requirement for additional nutrients. For example, during sago production, organic-rich wastewater is generated; Spirulina sp. biomass produced in the anaerobically digested sago wastewater in a high rate algal pond was suitable for animal feed [198]. In the diluted swine manure wastewater, the feasibility of producing omega-3 fatty acid-rich microalgae biomass as a source of feed ingredient was explored [232]. Microalgae biomass, produced in the aquaculture effluent, could be used as an ingredient of fish feed [89,90].
6.3.5. Ingredients for the Cosmetic Industry
A number of microalgae and cyanobacteria (e.g., Chlorella sp., Dunaliella sp., Nannochloropsis sp., Spirulina sp.) produce different secondary metabolites, and the extracts of these compounds could act as a bio-based alternative feedstock for producing various cosmetics like anti-oxidants, UV-protectants, antiaging, etc. [233]. Further, the specific extracts of microalgae (e.g., Chlorella sp., Tetraselmis sp., Dunaliella sp., Emiliana sp., Noctiluca sp., etc.) are used for skin care (e.g., moisturizer, firmness, and brightness enhancer) and slimming products [234]. The extracts of several microalgal biomass (e.g., Monodus sp., Thalassiosira sp., Chaeloceros sp., Chlorococcum sp.) could help in the growth of human hair and hair follicles; hence, these were proposed as ingredients for specific formulations, which could prevent hair loss [235].
7. Challenges of Microalgal Wastewater Treatment and Future Research Direction
- In large-scale outdoor microalgal cultivation, the average biomass productivity could range from 15–30 g/m2/day [23,236,237]. Considering microalgal cellular nitrogen content around 4–6%, microalgal nitrogen uptake rate could be in the range of 0.6–1.8 g N/m2/day. Therefore, for a 20 cm deep pond, the nitrogen removal rate would be 3.0–9 mg N/L/day. On the contrary, the nitrogen removal rate in a typical activated sludge process is around 30–78 mg N/L/day, several times higher than the microalgal nitrogen removal rate [238]. Therefore, a larger footprint would be required for the microalgal bioremediation of wastewater. The other concern of microalgal bioremediation is the seasonal variability of light intensity and temperature. Unlike bacteria, microalgae would require sunlight for their cell growth; cloud covers and shorter daylight during the winter season would limit the rate of microalgal bioremediation.
- Metabolic engineering pathways for several microorganisms (e.g., yeasts and bacteria) are already well established, and these microorganisms could be tailored for specific purposes. However, more research in microalgal metabolic engineering would be needed to overcome some of the challenges of microalgal bioremediation of wastewater.
- Microalgae biomass growth rate, nutrient recycling, and wastewater treatment efficiencies would be limited in the absence of an external CO2 supply [140]. A point source of CO2 (e.g., flue gas) in the vicinity of the cultivation site would enhance the overall process.
- Microalgal biomass density for large scale open cultivation systems often does not exceed 0.5 g/L, which could be mainly attributed to the light saturation at the top layer. Keeping a deeper culture would result in reduced biomass density. Although higher flow velocity could improve the mixing and light utilization, the increment in energy requirement for increased flow velocity follows the cube law of power. Therefore, the culture depth and flow velocity must be optimized for biomass production in large-scale cultivation.
- Although a microalga could have very high efficiency in removing specific contaminant, its sudden exposure to wastewater containing a very high concentration of that contaminant (e.g., metals, toxic compounds) could be detrimental to its culture, which in turn could undermine the effectiveness of the treatment process. Therefore, an adaptation or climatization process for the strain to the target compound(s) needs to be developed on site [115].
- At times, wastewater could have a very high concentration of ammonia, which could be toxic to many microalgal strains. In some wastewaters, nitrogen could be present in aromatic compounds as nitro, amino, or other groups. If the pH value of wastewater is either very low or very high, then it should be adjusted in the appropriate range for efficient microalgal bioremediation [26]. The concentration of nitrogen and or phosphorus in wastewater could be low in wastewater, which would yield a low biomass density; the cost and energy requirement per unit of biomass separation could be very high—rendering the overall process of microalgal bioremediation non-viable.
- The ability of a single microorganism to completely degrade an aromatic or a mixture of xenobiotic pollutants is rarely observed. To achieve a better removal efficiency for such unwanted contaminants from wastewater, the combination of microalgae and other suitable microorganisms could be applied [239]. Although polycultures of microalgae could have better efficiency in treating wastewater compared to the bioremediation efficiency of monoculture, over the long term, the consistency in polyculture biomass quality could be a concern. If bio-flocculation is used to harvest any desired microalga, the bio-flocculating strain could impair the quality of the overall biomass.
- Depending on the climate condition, the evaporation water loss could be 0.1–2 cm per day from an open cultivation system [139,240]. The evaporation water loss could increase the concentrations of pollutants in the culture, which would ultimately affect the bioremediation efficiency. The treated wastewater from the previous batch could be used, as necessary, to compensate for the evaporation water loss.
- Despite the immense potential of microalgal bioremediation of wastewater and the successful demonstration mostly at a relatively small scale (primarily indoor), long-term, large-scale demonstrations are limited. The effect of annual variations in light and temperature on the bioremediation efficiency and microalgal biomass quality needs to be studied.
- If coagulants or cross-flow filtration are used to separate microalgae biomass, it will increase the overall cost of bioremediation. Attached cell growth could eliminate the step for biomass harvesting; however, the cost of preparing the immobilization matrix could be extremely high [37]. Hence, appropriate strain or growth conditions should be used that facilitate autoflocculation or bioflocculation of the biomass.
- Unlike heterotrophic microorganisms, most of the microalgae would require carbon dioxide for their growth and biomass production. Although wastewaters typically contain nitrogen, phosphorus, and some other elements, as required by microalgae, supplying CO2 to the microalgae culture is very crucial for effective treatment of wastewater, as the diffusion of atmospheric CO2 into the microalgae culture is usually very slow and low. Coupling the flue gas, as the source of CO2, into the cultivation of microalgae in wastewater could enhance the environmental sustainability of the industry.
- Although the microalgal commercial application is mostly limited to the cultivation of microalgae in freshwater or seawater as food, feed, and nutraceuticals, there is a need to develop novel and wider applications of wastewater-grown microalgal biomass. In the context of a circular bio-economy, the bioremediation of wastewater by microalgae and the complete valorization of the produced biomass could be very promising. Earlier research efforts were mostly dedicated to microalgal bioremediation or the generation of value-added products from wastewater-grown microalgae biomass. Suitable applications should also be developed for the residual biomass after extracting or converting a fraction of the biomass.
8. Conclusions
A prolific growth rate, ability to adapt in different wastewaters and uptake nutrients or remove pollutants from wastewater, coupled with the assimilation of carbon dioxide, could make microalgal bioremediation of wastewaters very promising. However, regardless of the enormous potential of microalgal bioremediation of wastewaters, most of the experiments were limited to indoor lab-scale microalgal cultivation studies, with a limited number of strains, to determine the removal efficiencies of targeted elements or compounds. Therefore, more detailed studies would be required to select appropriate strain(s) from a broader range of algal species and understand the treatment efficiency together with long-term biomass productivity in a large-scale outdoor set up under fluctuating environments and wastewater characteristics. The role of individual microorganisms in microalgae–bacterial or microalga–microalgae consortia needs a better understanding of the long-term outdoor operation. The development of a strain and application-specific energy-saving biomass harvesting is a prerequisite for microalgal bioremediation of wastewater; in this regard, a self-settling strain or bioflocculating strain consortia should be developed. Furthermore, the harvested microalgal biomass needs to be valorized, in a multi-product biorefinery approach, to enhance the economic viability and environmental sustainability of wastewater bioremediation.
Author Contributions
Conceptualization: P.D., S.K., and H.A.-J.; investigation: S.K., M.T., and M.A.; data curation: S.K., M.T., and M.A.; writing—original draft preparation: H.A.-J., P.D., S.K., M.T., and M.A.; writing—review and editing: P.D.; visualization: S.K.; supervision: P.D.; project administration: P.D. and H.A.-J.; funding acquisition: P.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Qatar National Research Fund, grant number NPRP8-646-2-272, and the APC was funded by Qatar National Library.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Sun, Y.; Chen, Z.; Wu, G.; Wu, Q.; Zhang, F.; Niu, Z.; Hu, H.Y. Characteristics of water quality of municipal wastewater treatment plants in China: Implications for resources utilization and management. J. Clean. Prod. 2016, 131, 1–9. [Google Scholar] [CrossRef]
- Li, K.; Liu, Q.; Fang, F.; Luo, R.; Lu, Q.; Zhou, W.; Huo, S.; Cheng, P.; Liu, J.; Addy, M.; et al. Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresour. Technol. 2019, 291, 121934. [Google Scholar] [CrossRef] [PubMed]
- Capodaglio, A.G. Fit-for-purpose urban wastewater reuse: Analysis of issues and available technologies for sustainable multiple barrier approaches. Crit. Rev. Environ. Sci. Technol. 2020, 1–48. [Google Scholar] [CrossRef]
- Mathews, J.A.; Tan, H. Circular economy: Lessons from China. Nature 2016, 531, 440–442. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, G.; Tian, H. Current state of sewage treatment in China. Water Res. 2014, 66, 85–98. [Google Scholar] [CrossRef]
- Oswald, W.J.; Golueke, C.G. Biological Transformation of Solar Energy; Advances in Applied Microbiology; Umbreit, W.W., Ed.; Academic Press: Cambridge, MA, USA, 1960; Volume 2, pp. 223–262. [Google Scholar]
- Shahid, A.; Malik, S.; Zhu, H.; Xu, J.; Nawaz, M.Z.; Nawaz, S.; Asraful Alam, M.; Mehmood, M.A. Cultivating microalgae in wastewater for biomass production, pollutant removal, and atmospheric carbon mitigation: A review. Sci. Total Environ. 2020, 704, 135303. [Google Scholar] [CrossRef]
- Mohd Udaiyappan, A.F.; Abu Hasan, H.; Takriff, M.S.; Sheikh Abdullah, S.R. A review of the potentials, challenges and current status of microalgae biomass applications in industrial wastewater treatment. J. Water Process Eng. 2017, 20, 8–21. [Google Scholar] [CrossRef]
- Callegari, A.; Bolognesi, S.; Cecconet, D.; Capodaglio, A.G. Production technologies, current role, and future prospects of biofuels feedstocks: A state-of-the-art review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 384–436. [Google Scholar] [CrossRef]
- Oswald, W.J. My sixty years in applied algology. J. Appl. Phycol. 2003, 15, 99–106. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Kang, D.; Wu, C.; Wu, Y. Removal of pharmaceuticals and personal care products from wastewater using algae-based technologies: A review. Rev. Environ. Sci. Biotechnol. 2017, 16, 717–735. [Google Scholar] [CrossRef]
- Nie, J.; Sun, Y.; Zhou, Y.; Kumar, M.; Usman, M.; Li, J.; Shao, J.; Wang, L.; Tsang, D.C.W. Bioremediation of water containing pesticides by microalgae: Mechanisms, methods, and prospects for future research. Sci. Total Environ. 2020, 707, 136080. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, R.; Guieysse, B. Algal–bacterial processes for the treatment of hazardous contaminants: A review. Water Res. 2006, 40, 2799–2815. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, D.L.; Ralph, P.J. Microalgal bioremediation of emerging contaminants—Opportunities and challenges. Water Res. 2019, 164, 114921. [Google Scholar] [CrossRef] [PubMed]
- Husain, Q. Peroxidase mediated decolorization and remediation of wastewater containing industrial dyes: A review. Rev. Environ. Sci. Bio/Technol. 2010, 9, 117–140. [Google Scholar] [CrossRef]
- Wollmann, F.; Dietze, S.; Ackermann, J.-U.; Bley, T.; Walther, T.; Steingroewer, J.; Krujatz, F. Microalgae wastewater treatment: Biological and technological approaches. Eng. Life Sci. 2019, 19, 860–871. [Google Scholar] [CrossRef]
- Miazek, K.; Iwanek, W.; Remacle, C.; Richel, A.; Goffin, D. Effect of metals, metalloids and metallic nanoparticles on microalgae growth and industrial product biosynthesis: A review. Int. J. Mol. Sci. 2015, 16, 23929–23969. [Google Scholar] [CrossRef]
- Graham, E.J.S.; Dean, C.A.; Yoshida, T.M.; Twary, S.N.; Teshima, M.; Alvarez, M.A.; Zidenga, T.; Heikoop, J.M.; Perkins, G.B.; Rahn, T.A.; et al. Oil and gas produced water as a growth medium for microalgae cultivation: A review and feasibility analysis. Algal Res. 2017, 24, 492–504. [Google Scholar] [CrossRef]
- Daneshgar, S.; Callegari, A.; Capodaglio, A.G.; Vaccari, D. The potential phosphorus crisis: Resource conservation and possible escape technologies: A review. Resources 2018, 7, 37. [Google Scholar] [CrossRef]
- Cai, T.; Park, S.Y.; Li, Y. Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renew. Sustain. Energy Rev. 2013, 19, 360–369. [Google Scholar] [CrossRef]
- Markou, G.; Nerantzis, E. Microalgae for high-value compounds and biofuels production: A review with focus on cultivation under stress conditions. Biotechnol. Adv. 2013, 31, 1532–1542. [Google Scholar] [CrossRef]
- Jankowska, E.; Sahu, A.K.; Oleskowicz-Popiel, P. Biogas from microalgae: Review on microalgae’s cultivation, harvesting and pretreatment for anaerobic digestion. Renew. Sustain. Energy Rev. 2017, 75, 692–709. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Prussi, M.; Casini, D.; Tredici, M.R.; Rodolfi, L.; Bassi, N.; Zittelli, G.C.; Bondioli, P. Review of energy balance in raceway ponds for microalgae cultivation: Re-thinking a traditional system is possible. Appl. Energy 2013, 102, 101–111. [Google Scholar] [CrossRef]
- Sheehan, J. A Look Back at the U.S. Department of Energy’s Aquatic Species Program—Biodiesel from Algae. Program 1998, 328, 1–249. [Google Scholar]
- Tyagi, R.D.; Couillard, D. Toxic effects of inhibitors in biological wastewater treatment processes. Can. J. Chem. Eng. 1988, 66, 97–106. [Google Scholar] [CrossRef]
- Das, P.; AbdulQuadir, M.; Thaher, M.; Khan, S.; Chaudhary, A.K.; Alghasal, G.; Al-Jabri, H.M.S.J. Microalgal bioremediation of petroleum-derived low salinity and low pH produced water. J. Appl. Phycol. 2018, 31, 435–444. [Google Scholar] [CrossRef]
- Chen, R.; Liu, Y.; Liao, W. Using an environmentally friendly process combining electrocoagulation and algal cultivation to treat high-strength wastewater. Algal Res. 2016, 16, 330–337. [Google Scholar] [CrossRef]
- Chen, R.; Li, R.; Deitz, L.; Liu, Y.; Stevenson, R.J.; Liao, W. Freshwater algal cultivation with animal waste for nutrient removal and biomass production. Biomass Bioenergy 2012, 39, 128–138. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Nguyen, M.-L.T.; Lay, C.-H. Starch-containing textile wastewater treatment for biogas and microalgae biomass production. J. Clean. Prod. 2017, 168, 331–337. [Google Scholar] [CrossRef]
- Huang, J.; Kankanamge, N.R.; Chow, C.; Welsh, D.T.; Li, T.; Teasdale, P.R. Removing ammonium from water and wastewater using cost-effective adsorbents: A review. J. Environ. Sci. 2018, 63, 174–197. [Google Scholar] [CrossRef]
- Bellebia, S.; Kacha, S.; Bouyakoub, A.Z.; Derriche, Z. Experimental investigation of chemical oxygen demand and turbidity removal from cardboard paper mill effluents using combined electrocoagulation and adsorption processes. Environ. Prog. Sustain. Energy 2012, 31, 361–370. [Google Scholar] [CrossRef]
- Xu, X.; Zhu, X. Treatment of refectory oily wastewater by electro-coagulation process. Chemosphere 2004, 56, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Symonds, E.M.; Cook, M.M.; McQuaig, S.M.; Ulrich, R.M.; Schenck, R.O.; Lukasik, J.O.; Van Vleet, E.S.; Breitbart, M. Reduction of nutrients, microbes and personal care products in domestic wastewater by a benchtop electrocoagulation unit. Sci. Rep. 2015, 5, 9380. [Google Scholar] [CrossRef]
- Adamakis, I.D.; Lazaridis, P.A.; Terzopoulou, E.; Torofias, S.; Valari, M.; Kalaitzi, P.; Rousonikolos, V.; Gkoutzikostas, D.; Zouboulis, A.; Zalidis, G.; et al. Cultivation, characterization, and properties of Chlorella vulgaris microalgae with different lipid contents and effect on fast pyrolysis oil composition. Environ. Sci. Pollut. Res. 2018, 25, 23018–23032. [Google Scholar] [CrossRef] [PubMed]
- Premalatha, M. Characterization of micro algal biomass through FTIR/TGA/CHN analysis: Application to Scenedesmus sp. Energy Sources Part A Recover. Util. Environ. Eff. 2015, 37, 1–8. [Google Scholar] [CrossRef]
- Pizarro, C.; Mulbry, W.; Blersch, D.; Kangas, P. An economic assessment of algal turf scrubber technology for treatment of dairy manure effluent. Ecol. Eng. 2006, 26, 321–327. [Google Scholar] [CrossRef]
- Christenson, L.; Sims, R. Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnol. Adv. 2011, 29, 686–702. [Google Scholar] [CrossRef] [PubMed]
- Luther, M. Degradation of different substituted aromatic compounds as nutrient sources by the green alga Scenedesmus obliquus. In Proceedings of the Dechema Biotechnol Conference 4, Weinheim, NY, USA, 28–30 May 1990; pp. 613–615. [Google Scholar]
- Li, Y.; Chen, Y.-F.; Chen, P.; Min, M.; Zhou, W.; Martinez, B.; Zhu, J.; Ruan, R. Characterization of a microalga Chlorella sp. well adapted to highly concentrated municipal wastewater for nutrient removal and biodiesel production. Bioresour. Technol. 2011, 102, 5138–5144. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Li, Y.; Gao, Y.; Zhao, H. Nutrients removal and recovery from saline wastewater by Spirulina platensis. Bioresour. Technol. 2017, 245, 10–17. [Google Scholar] [CrossRef]
- Wang, L.; Min, M.; Li, Y.; Chen, P.; Chen, Y.; Liu, Y.; Wang, Y.; Ruan, R. Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl. Biochem. Biotechnol. 2010, 162, 1174–1186. [Google Scholar] [CrossRef]
- Singh, M.; Reynolds, D.L.; Das, K.C. Microalgal system for treatment of effluent from poultry litter anaerobic digestion. Bioresour. Technol. 2011, 102, 10841–10848. [Google Scholar] [CrossRef]
- De Godos, I.; Vargas, V.A.; Blanco, S.; González, M.C.G.; Soto, R.; García-Encina, P.A.; Becares, E.; Muñoz, R. A comparative evaluation of microalgae for the degradation of piggery wastewater under photosynthetic oxygenation. Bioresour. Technol. 2010, 101, 5150–5158. [Google Scholar] [CrossRef] [PubMed]
- Escapa, C.; Coimbra, R.N.; Paniagua, S.; García, A.I.; Otero, M. Nutrients and pharmaceuticals removal from wastewater by culture and harvesting of Chlorella sorokiniana. Bioresour. Technol. 2015, 185, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Villar-Navarro, E.; Baena-Nogueras, R.M.; Paniw, M.; Perales, J.A.; Lara-Martín, P.A. Removal of pharmaceuticals in urban wastewater: High rate algae pond (HRAP) based technologies as an alternative to activated sludge based processes. Water Res. 2018, 139, 19–29. [Google Scholar] [CrossRef]
- Khanzada, Z.T. Phosphorus removal from landfill leachate by microalgae. Biotechnol. Rep. 2020, 25, e00419. [Google Scholar] [CrossRef] [PubMed]
- Sforza, E.; Khairallah Al Emara, M.H.; Sharif, A.; Bertucco, A. Exploitation of urban landfill leachate as nutrient source for microalgal biomass production. Chem. Eng. Trans. 2015, 43, 373–378. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, Y.; Huang, S.; Qiu, D.; Schideman, L.; Chai, X.; Zhao, Y. Characterization of microalgae-bacteria consortium cultured in landfill leachate for carbon fixation and lipid production. Bioresour. Technol. 2014, 156, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Li, C.; Yang, Z.H.; Zeng, G.M.; Feng, L.J.; Liu, J.Z.; Liu, M.; Cai, H.W. Continuous microalgae cultivation in aquaculture wastewater by a membrane photobioreactor for biomass production and nutrients removal. Ecol. Eng. 2016, 92, 55–61. [Google Scholar] [CrossRef]
- Michels, M.H.A.; Vaskoska, M.; Vermuë, M.H.; Wijffels, R.H. Growth of Tetraselmis suecica in a tubular photobioreactor on wastewater from a fish farm. Water Res. 2014, 65, 290–296. [Google Scholar] [CrossRef]
- Das, P.; AbdulQuadir, M.; Thaher, M.; Khan, S.; Chaudhary, A.K.; Al-Jabri, H. A feasibility study of utilizing hydrothermal liquefaction derived aqueous phase as nutrients for semi-continuous cultivation of Tetraselmis sp. Bioresour. Technol. 2020, 295, 122310. [Google Scholar] [CrossRef]
- Das, P.; Khan, S.; AbdulQuadir, M.; Thaher, M.; Waqas, M.; Easa, A.; Attia, E.S.M.; Al-Jabri, H. Energy recovery and nutrients recycling from municipal sewage sludge. Sci. Total Environ. 2020, 715, 136775. [Google Scholar] [CrossRef]
- Du, Z.; Hu, B.; Shi, A.; Ma, X.; Cheng, Y.; Chen, P.; Liu, Y.; Lin, X.; Ruan, R. Cultivation of a microalga Chlorella vulgaris using recycled aqueous phase nutrients from hydrothermal carbonization process. Bioresour. Technol. 2012, 126, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.-K.; Kabra, A.N.; Salama, E.-S.; Roh, H.-S.; Kim, J.R.; Lee, D.S.; Jeon, B.-H. Effect of mine wastewater on nutrient removal and lipid production by a green microalga Micratinium reisseri from concentrated municipal wastewater. Bioresour. Technol. 2014, 157, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Suresh Kumar, K.; Dahms, H.U.; Won, E.J.; Lee, J.S.; Shin, K.H. Microalgae—A promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef] [PubMed]
- Lau, P.S.; Lee, H.Y.; Tsang, C.C.K.; Tam, N.F.Y.; Wong, Y.S. Effect of Metal Interference, pH and Temperature on Cu and Ni Biosorption by Chlorella Vulgaris and Chlorella Miniata. Environ. Technol. 1999, 20, 953–961. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef]
- Blaby-Haas, C.E.; Merchant, S.S. The ins and outs of algal metal transport. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 1531–1552. [Google Scholar] [CrossRef]
- Gaignard, C.; Laroche, C.; Pierre, G.; Dubessay, P.; Delattre, C.; Gardarin, C.; Gourvil, P.; Probert, I.; Dubuffet, A.; Michaud, P. Screening of marine microalgae: Investigation of new exopolysaccharide producers. Algal Res. 2019, 44, 101711. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, H.; Cheng, S.; Zhang, W.; Zhang, X. Enhanced Microalgal Harvesting Using Microalgae-Derived Extracellular Polymeric Substance as Flocculation Aid. ACS Sustain. Chem. Eng. 2020, 8, 4069–4075. [Google Scholar] [CrossRef]
- Aksu, Z. Biosorption of Heavy Metals by Microalgae in Batch and Continuous Systems. In Wastewater Treatment with Algae; Wong, Y.-S., Tam, N.F.Y., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 37–53. [Google Scholar]
- Travieso, L.; Cañizares, R.O.; Borja, R.; Benítez, F.; Domínguez, A.R.; Dupeyrón, R.; Valiente, Y.V. Heavy metal removal by microalgae. Bull. Environ. Contam. Toxicol. 1999, 62, 144–151. [Google Scholar] [CrossRef]
- Wong, J.P.K.; Wong, Y.S.; Tam, N.F.Y. Nickel biosorption by two chlorella species, C. Vulgaris (a commercial species) and C. Miniata (a local isolate). Bioresour. Technol. 2000, 73, 133–137. [Google Scholar] [CrossRef]
- Sibi, G. Biosorption of chromium from electroplating and galvanizing industrial effluents under extreme conditions using Chlorella vulgaris. Green Energy Environ. 2016, 1, 172–177. [Google Scholar] [CrossRef]
- Pradhan, D.; Sukla, L.B.; Mishra, B.B.; Devi, N. Biosorption for removal of hexavalent chromium using microalgae Scenedesmus sp. J. Clean. Prod. 2019, 209, 617–629. [Google Scholar] [CrossRef]
- Rezaei, H. Biosorption of chromium by using Spirulina sp. Arab. J. Chem. 2016, 9, 846–853. [Google Scholar] [CrossRef]
- Chan, A.; Salsali, H.; McBean, E. Heavy Metal Removal (Copper and Zinc) in Secondary Effluent from Wastewater Treatment Plants by Microalgae. ACS Sustain. Chem. Eng. 2013, 2, 130–137. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; Geng, B. Preparation of Immobilized Sulfate-Reducing Bacteria-Microalgae Beads for Effective Bioremediation of Copper-Containing Wastewater. Water. Air. Soil Pollut. 2018, 229, 54. [Google Scholar] [CrossRef]
- Malakootian, M.; Yousefi, Z.; Limoni, Z. Removal of lead from battery industry wastewater by Chlorella vulgaris as green micro-algae (Case study: Kerman, Iran). Desalin. WATER Treat. 2019, 141, 248–255. [Google Scholar] [CrossRef]
- Kumar, R.; Goyal, D. Waste water treatment and metal (Pb2+, Zn2+) removal by microalgal based stabilization pond system. Indian J. Microbiol. 2010, 50, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Shanab, S.; Essa, A.; Shalaby, E. Bioremoval capacity of three heavy metals by some microalgae species (Egyptian isolates). Plant. Signal. Behav. 2012, 7, 392–399. [Google Scholar] [CrossRef]
- Fard, G.H.; Mehrnia, M.R. Investigation of mercury removal by Micro-Algae dynamic membrane bioreactor from simulated dental waste water. J. Environ. Chem. Eng. 2017, 5, 366–372. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, A.K.; Sikandar, M. Biosorption of Hg (II) from aqueous solution using algal biomass: Kinetics and isotherm studies. Heliyon 2020, 6, e03321. [Google Scholar] [CrossRef]
- Chong, A.M.Y.; Wong, Y.S.; Tam, N.F.Y. Performance of different microalgal species in removing nickel and zinc from industrial wastewater. Chemosphere 2000, 41, 251–257. [Google Scholar] [CrossRef]
- Torres, M.A.; Barros, M.P.; Campos, S.C.G.; Pinto, E.; Rajamani, S.; Sayre, R.T.; Colepicolo, P. Biochemical biomarkers in algae and marine pollution: A review. Ecotoxicol. Environ. Saf. 2008, 71, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiao, H.; He, N.; Sun, D.; Duan, S. Biosorption and Biodegradation of the Environmental Hormone Nonylphenol By Four Marine Microalgae. Sci. Rep. 2019, 9, 5277. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Jang, H.M.; Kan, E. Microalgal Biomass and Lipid Production on Dairy Effluent Using a Novel Microalga, Chlorella sp. Isolated from Dairy Wastewater. Biotechnol. Bioprocess. Eng. 2018, 23, 333–340. [Google Scholar] [CrossRef]
- Kobayashi, H.; Rittmann, B.E. Microbial removal of hazardous organic compounds. Environ. Sci. Technol. 1982, 16, 170A–183A. [Google Scholar] [CrossRef]
- Subashchandrabose, S.R.; Ramakrishnan, B.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Mixotrophic cyanobacteria and microalgae as distinctive biological agents for organic pollutant degradation. Environ. Int. 2013, 51, 59–72. [Google Scholar] [CrossRef]
- Godsgift Omojevwe, E.; Obasola Ezekiel, F. Microalgal-Bacterial Consortium in Polyaromatic Hydrocarbon Degradation of Petroleum Based Effluent. J. Bioremed. Biodegrad. 2016, 7, 359. [Google Scholar] [CrossRef]
- Delrue, F.; Álvarez-Díaz, P.D.; Fon-Sing, S.; Fleury, G.; Sassi, J.F. The environmental biorefinery: Using microalgae to remediate wastewater, a win-win paradigm. Energies 2016, 9, 132. [Google Scholar] [CrossRef]
- Subashchandrabose, S.R.; Ramakrishnan, B.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Consortia of cyanobacteria/microalgae and bacteria: Biotechnological potential. Biotechnol. Adv. 2011, 29, 896–907. [Google Scholar] [CrossRef]
- Ramanan, R.; Kim, B.-H.; Cho, D.-H.; Oh, H.-M.; Kim, H.-S. Algae–bacteria interactions: Evolution, ecology and emerging applications. Biotechnol. Adv. 2016, 34, 14–29. [Google Scholar] [CrossRef]
- Fuentes, J.L.; Garbayo, I.; Cuaresma, M.; Montero, Z.; González-Del-Valle, M.; Vílchez, C. Impact of microalgae-bacteria interactions on the production of algal biomass and associated compounds. Mar. Drugs 2016, 14, 100. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Laughinghouse, H.D.; Anderson, M.A.; Chen, F.; Willliams, E.; Place, A.R.; Zmora, O.; Zohar, Y.; Zheng, T.; Hill, R.T. Novel bacterial isolate from permian groundwater, capable of aggregating potential biofuel-producing microalga Nannochloropsis oceanica IMET1. Appl. Environ. Microbiol. 2012, 78, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Golueke, C.G.; Oswald, W.J. Surface Properties and Ion Exchange in Algae Removal. J. Water Pollut. Control. Fed. 1970, 42, R304–R314. [Google Scholar]
- Vandamme, D.; Foubert, I.; Fraeye, I.; Meesschaert, B.; Muylaert, K. Flocculation of Chlorella vulgaris induced by high pH: Role of magnesium and calcium and practical implications. Bioresour. Technol. 2012, 105, 114–119. [Google Scholar] [CrossRef]
- García, J.; Mujeriego, R.; Hernández-Mariné, M. High rate algal pond operating strategies for urban wastewater nitrogen removal. J. Appl. Phycol. 2000, 12, 331–339. [Google Scholar] [CrossRef]
- Le Williams, P.J.B.; Laurens, L.M.L. Microalgae as biodiesel & biomass feedstocks: Review & analysis of the biochemistry, energetics & economics. Energy Environ. Sci. 2010, 3, 554. [Google Scholar] [CrossRef]
- Schwinn, D.E.; Dickson, B.H. Nitrogen and Phosphorus Variations in Domestic Wastewater. J. Water Pollut. Control. Fed. 1972, 44, 2059–2065. [Google Scholar]
- Yu, G.; Peng, H.; Fu, Y.; Yan, X.; Du, C.; Chen, H. Enhanced nitrogen removal of low C/N wastewater in constructed wetlands with co-immobilizing solid carbon source and denitrifying bacteria. Bioresour. Technol. 2019, 280, 337–344. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef]
- Das, P.; AbdulQuadir, M.; Thaher, M.I.; Alghasal, G.S.H.S.; Aljabri, H.M.S.J. Microalgal nutrients recycling from the primary effluent of municipal wastewater and use of the produced biomass as bio-fertilizer. Int. J. Environ. Sci. Technol. 2018, 16, 3355–3364. [Google Scholar] [CrossRef]
- Alcántara, C.; Posadas, E.; Guieysse, B.; Muñoz, R. Microalgae-based Wastewater Treatment. In Handbook of Marine Microalgae: Biotechnology Advances; Kim, S.-K., Ed.; Academic Press: Amsterdam, The Netherlands, 2015; pp. 439–455. ISBN 9780128011249. [Google Scholar]
- Hoffmann, J.P. Wastewater treatment with Suspended and Nonsuspended Algae. J. Phycol. 1998, 34, 757–763. [Google Scholar] [CrossRef]
- Fazal, T.; Mushtaq, A.; Rehman, F.; Ullah Khan, A.; Rashid, N.; Farooq, W.; Rehman, M.S.U.; Xu, J. Bioremediation of textile wastewater and successive biodiesel production using microalgae. Renew. Sustain. Energy Rev. 2018, 82, 3107–3126. [Google Scholar] [CrossRef]
- Hossain, L.; Sarker, S.K.; Khan, M.S. Evaluation of present and future wastewater impacts of textile dyeing industries in Bangladesh. Environ. Dev. 2018, 26, 23–33. [Google Scholar] [CrossRef]
- Alkaya, E.; Demirer, G.N. Sustainable textile production: A case study from a woven fabric manufacturing mill in Turkey. J. Clean. Prod. 2014, 65, 595–603. [Google Scholar] [CrossRef]
- Jinqi, L.; Houtian, L. Degradation of azo dyes by algae. Environ. Pollut. 1992, 75, 273–278. [Google Scholar] [CrossRef]
- Lu, J.; Wang, X.; Shan, B.; Li, X.; Wang, W. Analysis of chemical compositions contributable to chemical oxygen demand (COD) of oilfield produced water. Chemosphere 2006, 62, 322–331. [Google Scholar] [CrossRef]
- Mahdavi, H.; Prasad, V.; Liu, Y.; Ulrich, A.C. In situ biodegradation of naphthenic acids in oil sands tailings pond water using indigenous algae–bacteria consortium. Bioresour. Technol. 2015, 187, 97–105. [Google Scholar] [CrossRef]
- Quesnel, D.M.; Bhaskar, I.M.; Gieg, L.M.; Chua, G. Naphthenic acid biodegradation by the unicellular alga Dunaliella tertiolecta. Chemosphere 2011, 84, 504–511. [Google Scholar] [CrossRef]
- Tang, X.; He, L.Y.; Tao, X.Q.; Dang, Z.; Guo, C.L.; Lu, G.N.; Yi, X.Y. Construction of an artificial microalgal-bacterial consortium that efficiently degrades crude oil. J. Hazard. Mater. 2010, 181, 1158–1162. [Google Scholar] [CrossRef] [PubMed]
- Uggetti, E.; Sialve, B.; Latrille, E.; Steyer, J.-P. Anaerobic digestate as substrate for microalgae culture: The role of ammonium concentration on the microalgae productivity. Bioresour. Technol. 2014, 152, 437–443. [Google Scholar] [CrossRef]
- Lundquist, T.J.; Woertz, I.C.; Quinn, N.W.T.; Benemann, J.R. A Realistic Technology and Engineering Assessment of Algae Biofuel Production. Energy 2010, 1. [Google Scholar] [CrossRef]
- Xin, X.; Huang, G.; An, C.; Feng, R. Interactive Toxicity of Triclosan and Nano-TiO2 to Green Alga Eremosphaera viridis in Lake Erie: A New Perspective Based on Fourier Transform Infrared Spectromicroscopy and Synchrotron-Based X-ray Fluorescence Imaging. Environ. Sci. Technol. 2019, 53, 9884. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Huang, G.; Zhang, B. Review of aquatic toxicity of pharmaceuticals and personal care products to algae. J. Hazard. Mater. 2020, 124619. [Google Scholar] [CrossRef] [PubMed]
- Hom-Diaz, A.; Jaén-Gil, A.; Bello-Laserna, I.; Rodríguez-Mozaz, S.; Vicent, T.; Barceló, D.; Blánquez, P. Performance of a microalgal photobioreactor treating toilet wastewater: Pharmaceutically active compound removal and biomass harvesting. Sci. Total Environ. 2017, 592, 1–11. [Google Scholar] [CrossRef]
- Xiong, J.-Q.; Kurade, M.B.; Jeon, B.-H. Can Microalgae Remove Pharmaceutical Contaminants from Water? Trends Biotechnol. 2018, 36, 30–44. [Google Scholar] [CrossRef]
- Xin, X.; Huang, G.; An, C.; Raina-Fulton, R.; Weger, H. Insights into Long-Term Toxicity of Triclosan to Freshwater Green Algae in Lake Erie. Environ. Sci. Technol. 2019, 53, 2189–2198. [Google Scholar] [CrossRef]
- Peng, F.-Q.; Ying, G.-G.; Yang, B.; Liu, S.; Lai, H.-J.; Liu, Y.-S.; Chen, Z.-F.; Zhou, G.-J. Biotransformation of progesterone and norgestrel by two freshwater microalgae (Scenedesmus obliquus and Chlorella pyrenoidosa): Transformation kinetics and products identification. Chemosphere 2014, 95, 581–588. [Google Scholar] [CrossRef]
- De Wilt, A.; Butkovskyi, A.; Tuantet, K.; Leal, L.H.; Fernandes, T.V.; Langenhoff, A.; Zeeman, G. Micropollutant removal in an algal treatment system fed with source separated wastewater streams. J. Hazard. Mater. 2016, 304, 84–92. [Google Scholar] [CrossRef]
- Maes, H.M.; Maletz, S.X.; Ratte, H.T.; Hollender, J.; Schaeffer, A. Uptake, Elimination, and Biotransformation of 17α-Ethinylestradiol by the Freshwater Alga Desmodesmus subspicatus. Environ. Sci. Technol. 2014, 48, 12354–12361. [Google Scholar] [CrossRef]
- Xiong, Q.; Liu, Y.-S.; Hu, L.-X.; Shi, Z.-Q.; Cai, W.-W.; He, L.-Y.; Ying, G.-G. Co-metabolism of sulfamethoxazole by a freshwater microalga Chlorella pyrenoidosa. Water Res. 2020, 175, 115656. [Google Scholar] [CrossRef]
- Hena, S.; Gutierrez, L.; Croué, J.-P. Removal of pharmaceutical and personal care products (PPCPs) from wastewater using microalgae: A review. J. Hazard. Mater. 2021, 403, 124041. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, V.; Gutiérrez, R.; Ferrer, I.; García, J.; Bayona, J.M. Capability of microalgae-based wastewater treatment systems to remove emerging organic contaminants: A pilot-scale study. J. Hazard. Mater. 2015, 288, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Cañizares-Villanueva, R.O.; Domínguez, A.R.; Cruz, M.S.; Ríos-Leal, E. Chemical composition of cyanobacteria grown in diluted, aerated swine wastewater. Bioresour. Technol. 1995, 51, 111–116. [Google Scholar] [CrossRef]
- Park, J.; Jin, H.-F.; Lim, B.-R.; Park, K.-Y.; Lee, K. Ammonia removal from anaerobic digestion effluent of livestock waste using green alga Scenedesmus sp. Bioresour. Technol. 2010, 101, 8649–8657. [Google Scholar] [CrossRef]
- Daneshvar, E.; Zarrinmehr, M.J.; Hashtjin, A.M.; Farhadian, O.; Bhatnagar, A. Versatile applications of freshwater and marine water microalgae in dairy wastewater treatment, lipid extraction and tetracycline biosorption. Bioresour. Technol. 2018, 268, 523–530. [Google Scholar] [CrossRef]
- Subramaniyam, V.; Subashchandrabose, S.R.; Ganeshkumar, V.; Thavamani, P.; Chen, Z.; Naidu, R.; Megharaj, M. Cultivation of Chlorella on brewery wastewater and nano-particle biosynthesis by its biomass. Bioresour. Technol. 2016, 211, 698–703. [Google Scholar] [CrossRef]
- Chinnasamy, S.; Bhatnagar, A.; Hunt, R.W.; Das, K.C. Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Bioresour. Technol. 2010, 101, 3097–3105. [Google Scholar] [CrossRef]
- Nguyen, T.D.P.; Tran, T.N.T.; Le, T.V.A.; Nguyen Phan, T.X.; Show, P.-L.; Chia, S.R. Auto-flocculation through cultivation of Chlorella vulgaris in seafood wastewater discharge: Influence of culture conditions on microalgae growth and nutrient removal. J. Biosci. Bioeng. 2019, 127, 492–498. [Google Scholar] [CrossRef]
- Oyewo, O.A.; Agboola, O.; Onyango, M.S.; Popoola, P.; Bobape, M.F. Chapter 6—Current Methods for the Remediation of Acid Mine Drainage Including Continuous Removal of Metals from Wastewater and Mine Dump; Prasad, M.N.V., de Favas, P.J.C., Maiti, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 103–114. ISBN 978-0-12-812986-9. [Google Scholar]
- Li, T.; Lin, G.; Podola, B.; Melkonian, M. Continuous removal of zinc from wastewater and mine dump leachate by a microalgal biofilm PSBR. J. Hazard. Mater. 2015, 297, 112–118. [Google Scholar] [CrossRef]
- Lin, L.; Chan, G.Y.S.; Jiang, B.L.; Lan, C.Y. Use of ammoniacal nitrogen tolerant microalgae in landfill leachate treatment. Waste Manag. 2007, 27, 1376–1382. [Google Scholar] [CrossRef]
- Craggs, R.J.; McAuley, P.J.; Smith, V.J. Wastewater nutrient removal by marine microalgae grown on a corrugated raceway. Water Res. 1997, 31, 1701–1707. [Google Scholar] [CrossRef]
- Chang, H.; Fu, Q.; Zhong, N.; Yang, X.; Quan, X.; Li, S.; Fu, J.; Xiao, C. Microalgal lipids production and nutrients recovery from landfill leachate using membrane photobioreactor. Bioresour. Technol. 2019, 277, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, E.-M.; Phang, S.-M.; Chu, W.-L. Use of an algal consortium of five algae in the treatment of landfill leachate using the high-rate algal pond system. J. Appl. Phycol. 2012, 24, 953–963. [Google Scholar] [CrossRef]
- Pereira, S.F.L.; Gonçalves, A.L.; Moreira, F.C.; Silva, T.F.C.V.; Vilar, V.J.P.; Pires, J.C.M. Nitrogen removal from landfill leachate by microalgae. Int. J. Mol. Sci. 2016, 17, 1926. [Google Scholar] [CrossRef] [PubMed]
- Milhazes-Cunha, H.; Otero, A. Valorisation of aquaculture effluents with microalgae: The Integrated Multi-Trophic Aquaculture concept. Algal Res. 2017, 24, 416–424. [Google Scholar] [CrossRef]
- Tossavainen, M.; Lahti, K.; Edelmann, M.; Eskola, R.; Lampi, A.-M.; Piironen, V.; Korvonen, P.; Ojala, A.; Romantschuk, M. Integrated utilization of microalgae cultured in aquaculture wastewater: Wastewater treatment and production of valuable fatty acids and tocopherols. J. Appl. Phycol. 2019, 31, 1753–1763. [Google Scholar] [CrossRef]
- Halfhide, T.; Åkerstrøm, A.; Lekang, O.I.; Gislerød, H.R.; Ergas, S.J. Production of algal biomass, chlorophyll, starch and lipids using aquaculture wastewater under axenic and non-axenic conditions. Algal Res. 2014, 6, 152–159. [Google Scholar] [CrossRef]
- Kong, Q.; Li, L.; Martinez, B.; Chen, P.; Ruan, R. Culture of Microalgae Chlamydomonas reinhardtii in Wastewater for Biomass Feedstock Production. Appl. Biochem. Biotechnol. 2009, 160, 9. [Google Scholar] [CrossRef]
- Johnson, T.; Katuwal, S.; Anderson, G.; Gu, L.; Zhou, R.; Gibbons, W. Photobioreactor Cultivation Strategies for Microalgae and Cyanobacteria. Biotechnol. Prog. 2018, 34, 811–827. [Google Scholar] [CrossRef]
- Huang, Q.; Jiang, F.; Wang, L.; Yang, C. Design of Photobioreactors for Mass Cultivation of Photosynthetic Organisms. Engineering 2017, 3, 318–329. [Google Scholar] [CrossRef]
- Degen, J.; Uebele, A.; Retze, A.; Schmid-Staiger, U.; Trösch, W. A novel airlift photobioreactor with baffles for improved light utilization through the flashing light effect. J. Biotechnol. 2001, 92, 89–94. [Google Scholar] [CrossRef]
- Das, P.; Obbard, J.P. Incremental energy supply for microalgae culture in a photobioreactor. Bioresour. Technol. 2011, 102, 2973–2978. [Google Scholar] [CrossRef] [PubMed]
- White, R.L.; Ryan, R.A. Long-Term Cultivation of Algae in Open-Raceway Ponds: Lessons from the Field. Ind. Biotechnol. 2015, 11, 213–220. [Google Scholar] [CrossRef]
- Rogers, J.N.; Rosenberg, J.N.; Guzman, B.J.; Oh, V.H.; Mimbela, L.E.; Ghassemi, A.; Betenbaugh, M.J.; Oyler, G.A.; Donohue, M.D. A critical analysis of paddlewheel-driven raceway ponds for algal biofuel production at commercial scales. Algal Res. 2014, 4, 76–88. [Google Scholar] [CrossRef]
- Posadas, E.; del Mar Morales, M.; Gomez, C.; Acién, F.G.; Muñoz, R. Influence of pH and CO2 source on the performance of microalgae-based secondary domestic wastewater treatment in outdoors pilot raceways. Chem. Eng. J. 2015, 265, 239–248. [Google Scholar] [CrossRef]
- Randrianarison, G.; Ashraf, M.A. Microalgae: A potential plant for energy production. Geol. Ecol. Landscapes 2017, 1, 104–120. [Google Scholar] [CrossRef]
- Park, J.B.K.; Craggs, R.J. Algal production in wastewater treatment high rate algal ponds for potential biofuel use. Water Sci. Technol. 2011, 63, 2403–2410. [Google Scholar] [CrossRef]
- Park, J.B.K.; Craggs, R.J. Wastewater treatment and algal production in high rate algal ponds with carbon dioxide addition. Water Sci. Technol. 2010, 61, 633–639. [Google Scholar] [CrossRef]
- Murthy, G.S. Chapter 18—Overview and Assessment of Algal Biofuels Production Technologies. In Biofuels; Pandey, A., Larroche, C., Ricke, S.C., Dussap, C.-G., Gnansounou, E., Eds.; Academic Press: Amsterdam, The Netherlands, 2011; pp. 415–437. ISBN 978-0-12-385099-7. [Google Scholar]
- Wang, J.; Liu, J.; Liu, T. The difference in effective light penetration may explain the superiority in photosynthetic efficiency of attached cultivation over the conventional open pond for microalgae John Sheehan. Biotechnol. Biofuels 2015, 8, 49. [Google Scholar] [CrossRef]
- Eltanahy, E.; Salim, S.; Vadiveloo, A.; Verduin, J.; Curado Andrade Pais, B.M.; Moheimani, N. Comparison between Jet and Paddlewheel Mixing for the Cultivation of Micro-algae in Anaerobic Digestate Piggery Effluent (ADPE). Algal Res. 2018, 35, 274–282. [Google Scholar] [CrossRef]
- Boelee, N.C.; Temmink, H.; Janssen, M.; Buisman, C.J.N.; Wijffels, R.H. Nitrogen and phosphorus removal from municipal wastewater effluent using microalgal biofilms. Water Res. 2011, 45, 5925–5933. [Google Scholar] [CrossRef] [PubMed]
- Roostaei, J.; Zhang, Y.; Gopalakrishnan, K.; Ochocki, A.J. Mixotrophic Microalgae Biofilm: A Novel Algae Cultivation Strategy for Improved Productivity and Cost-efficiency of Biofuel Feedstock Production. Sci. Rep. 2018, 8, 12528. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.B.; Wen, Z. Development of an attached microalgal growth system for biofuel production. Appl. Microbiol. Biotechnol. 2010, 85, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Logroño, W.; Pérez, M.; Urquizo, G.; Kadier, A.; Echeverría, M.; Recalde, C.; Rákhely, G. Single chamber microbial fuel cell (SCMFC) with a cathodic microalgal biofilm: A preliminary assessment of the generation of bioelectricity and biodegradation of real dye textile wastewater. Chemosphere 2017, 176, 378–388. [Google Scholar] [CrossRef]
- Kebede-Westhead, E.; Pizarro, C.; Mulbry, W.W. Treatment of swine manure effluent using freshwater algae: Production, nutrient recovery, and elemental composition of algal biomass at four effluent loading rates. J. Appl. Phycol. 2006, 18, 41–46. [Google Scholar] [CrossRef]
- Cao, J.; Yuan, W.; Pei, Z.J.; Davis, T.; Cui, Y.; Beltran, M. A Preliminary Study of the Effect of Surface Texture on Algae Cell Attachment for a Mechanical-Biological Energy Manufacturing System. J. Manuf. Sci. Eng. 2009, 131, 064505. [Google Scholar] [CrossRef]
- Ruiz-Marin, A.; Mendoza-Espinosa, L.G.; Stephenson, T. Growth and nutrient removal in free and immobilized green algae in batch and semi-continuous cultures treating real wastewater. Bioresour. Technol. 2010, 101, 58–64. [Google Scholar] [CrossRef]
- Renuka, N.; Sood, A.; Ratha, S.K.; Prasanna, R.; Ahluwalia, A.S. Evaluation of microalgal consortia for treatment of primary treated sewage effluent and biomass production. J. Appl. Phycol. 2013, 25, 1529–1537. [Google Scholar] [CrossRef]
- Sharma, J.; Kumar, V.; Kumar, S.S.; Malyan, S.K.; Mathimani, T.; Bishnoi, N.R.; Pugazhendhi, A. Microalgal consortia for municipal wastewater treatment—Lipid augmentation and fatty acid profiling for biodiesel production. J. Photochem. Photobiol. B Biol. 2020, 202, 111638. [Google Scholar] [CrossRef]
- Samorì, G.; Samorì, C.; Guerrini, F.; Pistocchi, R. Growth and nitrogen removal capacity of Desmodesmus communis and of a natural microalgae consortium in a batch culture system in view of urban wastewater treatment: Part I. Water Res. 2013, 47, 791–801. [Google Scholar] [CrossRef]
- Stockenreiter, M.; Haupt, F.; Seppälä, J.; Tamminen, T.; Spilling, K. Nutrient uptake and lipid yield in diverse microalgal communities grown in wastewater. Algal Res. 2016, 15, 77–82. [Google Scholar] [CrossRef]
- Qin, L.; Wang, Z.; Sun, Y.; Shu, Q.; Feng, P.; Zhu, L.; Xu, J.; Yuan, Z. Microalgae consortia cultivation in dairy wastewater to improve the potential of nutrient removal and biodiesel feedstock production. Environ. Sci. Pollut. Res. 2016, 23, 8379–8387. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Pires, J.; Simões, M. A review on the use of microalgal consortia for wastewater treatment. Algal Res. 2016, 24, 403–415. [Google Scholar] [CrossRef]
- Dong, D.; Seo, D.; Seo, S.; Lee, J.W. Flocculation of microalgae using extracellular polymeric substances (EPS) extracted from activated sludge. Membr. Water Treat. 2018, 9, 147–153. [Google Scholar] [CrossRef]
- Gutiérrez, R.; Passos, F.; Ferrer, I.; Uggetti, E.; García, J. Harvesting microalgae from wastewater treatment systems with natural flocculants: Effect on biomass settling and biogas production. Algal Res. 2015, 9, 204–211. [Google Scholar] [CrossRef]
- Madkour, A.G.M.E.; Ibrahim, H.A.H.; El-Sayed, W.M.M.; El-Moselhy, K.M. Bioflocculation technique for microalgal harvesting and wastewater nutrient recovery. Iran. J. Fish. Sci. 2020, 19, 1780–1794. [Google Scholar] [CrossRef]
- Poelman, E.; De Pauw, N.; Jeurissen, B. Potential of electrolytic flocculation for recovery of micro-algae. Resour. Conserv. Recycl. 1997, 19, 1–10. [Google Scholar] [CrossRef]
- Cheng, P.; Chen, D.; Liu, W.; Cobb, K.; Zhou, N.; Liu, Y.; Liu, H.; Wang, Q.; Chen, P.; Zhou, C.; et al. Auto-flocculation microalgae species Tribonema sp. and Synechocystis sp. with T-IPL pretreatment to improve swine wastewater nutrient removal. Sci. Total Environ. 2020, 725, 138263. [Google Scholar] [CrossRef]
- Molina Grima, E.; Belarbi, E.H.; Acién Fernández, F.G.; Robles Medina, A.; Chisti, Y. Recovery of microalgal biomass and metabolites: Process options and economics. Biotechnol. Adv. 2003, 20, 491–515. [Google Scholar] [CrossRef]
- Sim, T.-S.; Goh, A.; Becker, E.W. Comparison of centrifugation, dissolved air flotation and drum filtration techniques for harvesting sewage-grown algae. Biomass 1988, 16, 51–62. [Google Scholar] [CrossRef]
- Vandamme, D.; Pontes, S.C.V.; Goiris, K.; Foubert, I.; Pinoy, L.J.J.; Muylaert, K. Evaluation of electro-coagulation--flocculation for harvesting marine and freshwater microalgae. Biotechnol. Bioeng. 2011, 108, 2320–2329. [Google Scholar] [CrossRef] [PubMed]
- Bilad, M.R.; Vandamme, D.; Foubert, I.; Muylaert, K.; Vankelecom, I.F.J. Harvesting microalgal biomass using submerged microfiltration membranes. Bioresour. Technol. 2012, 111, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Danquah, M.K.; Ang, L.; Uduman, N.; Moheimani, N.; Forde, G.M. Dewatering of microalgal culture for biodiesel production: Exploring polymer flocculation and tangential flow filtration. J. Chem. Technol. Biotechnol. 2009, 84, 1078–1083. [Google Scholar] [CrossRef]
- Wang, S.-K.; Wang, F.; Stiles, A.R.; Guo, C.; Liu, C.-Z. Botryococcus braunii cells: Ultrasound-intensified outdoor cultivation integrated with in situ magnetic separation. Bioresour. Technol. 2014, 167, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Bosma, R.; van Spronsen, W.A.; Tramper, J.; Wijffels, R.H. Ultrasound, a new separation technique to harvest microalgae. J. Appl. Phycol. 2003, 15, 143–153. [Google Scholar] [CrossRef]
- Kim, J.; Ryu, B.-G.; Lee, Y.-J.; Han, J.-I.; Kim, W.; Yang, J.-W. Continuous harvest of marine microalgae using electrolysis: Effect of pulse waveform of polarity exchange. Bioprocess Biosyst. Eng. 2014, 37, 1249–1259. [Google Scholar] [CrossRef]
- Olguın, E.J. Phycoremediation: Key issues for cost-effective nutrient removal processes. Biotechnol. Adv. 2003, 22, 81–91. [Google Scholar] [CrossRef]
- Iasimone, F.; Seira, J.; Desmond-Le Quéméner, E.; Panico, A.; De Felice, V.; Pirozzi, F.; Steyer, J.-P. Bioflocculation and settling studies of native wastewater filamentous cyanobacteria using different cultivation systems for a low-cost and easy to control harvesting process. J. Environ. Manag. 2020, 256, 109957. [Google Scholar] [CrossRef]
- González-Fernández, C.; Ballesteros, M. Microalgae autoflocculation: An alternative to high-energy consuming harvesting methods. J. Appl. Phycol. 2012, 25, 991–999. [Google Scholar] [CrossRef]
- Alam, A.; Vandamme, D.; Chun, W.; Zhao, X.; Foubert, I.; Wang, Z.; Muylaert, K.; Yuan, Z. Bioflocculation as an innovative harvesting strategy for microalgae. Rev. Environ. Sci. Biotechnol. 2016, 15, 573–583. [Google Scholar] [CrossRef]
- Zhou, W.; Cheng, Y.; Li, Y.; Wan, Y.; Liu, Y.; Lin, X.; Ruan, R. Novel Fungal Pelletization-Assisted Technology for Algae Harvesting and Wastewater Treatment. Appl. Biochem. Biotechnol. 2012, 167, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Van Den Hende, S.; Beelen, V.; Bore, G.; Boon, N.; Vervaeren, H. Up-scaling aquaculture wastewater treatment by microalgal bacterial flocs: From lab reactors to an outdoor raceway pond. Bioresour. Technol. 2014, 159, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Udom, I.; Zaribaf, B.H.; Halfhide, T.; Gillie, B.; Dalrymple, O.; Zhang, Q.; Ergas, S.J. Harvesting microalgae grown on wastewater. Bioresour. Technol. 2013, 139, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Gani, P.; Mohamed Sunar, N.; Matias-Peralta, H.; Abdul Latiff, A.A. Effect of pH and alum dosage on the efficiency of microalgae harvesting via flocculation technique. Int. J. Green Energy 2017. [Google Scholar] [CrossRef]
- Gutiérrez, R.; Ferrer, I.; Uggetti, E.; Arnabat, C.; Salvadó, H.; García, J. Settling velocity distribution of microalgal biomass from urban wastewater treatment high rate algal ponds. Algal Res. 2016, 16, 409–417. [Google Scholar] [CrossRef]
- Das, P.; Thaher, M.I.; Abdul Hakim, M.A.Q.M.; Al-Jabri, H.M.S.J.; Alghasal, G.S.H.S. Microalgae harvesting by pH adjusted coagulation-flocculation, recycling of the coagulant and the growth media. Bioresour. Technol. 2016, 216, 824–829. [Google Scholar] [CrossRef]
- Yang, L.; Wang, L.; Zhang, H.; Li, C.; Zhang, X.; Hu, Q. A novel low cost microalgal harvesting technique with coagulant recovery and recycling. Bioresour. Technol. 2018, 266, 343–348. [Google Scholar] [CrossRef]
- Liu, C.; Hao, Y.; Jiang, J.; Liu, W. Valorization of untreated rice bran towards bioflocculant using a lignocellulose-degrading strain and its use in microalgal biomass harvest. Biotechnol. Biofuels 2017, 10, 90. [Google Scholar] [CrossRef]
- Gao, S.; Yang, J.; Tian, J.; Ma, F.; Tu, G.; Du, M. Electro-coagulation-flotation process for algae removal. J. Hazard. Mater. 2010, 177, 336–343. [Google Scholar] [CrossRef]
- Curteanu, S.; Piuleac, C.G.; Godini, K.; Azaryan, G. Modeling of electrolysis process in wastewater treatment using different types of neural networks. Chem. Eng. J. 2011, 172, 267–276. [Google Scholar] [CrossRef]
- Edzwald, J.K. Algae, Bubbles, Coagulants, and Dissolved Air Flotation. Water Sci. Technol. 1993, 27, 67–81. [Google Scholar] [CrossRef]
- Laamanen, C.A.; Ross, G.M.; Scott, J.A. Flotation harvesting of microalgae. Renew. Sustain. Energy Rev. 2016, 58, 75–86. [Google Scholar] [CrossRef]
- Rubio, J.; Souza, M.L.; Smith, R.W. Overview of flotation as a wastewater treatment technique. Miner. Eng. 2002, 15, 139–155. [Google Scholar] [CrossRef]
- Wiley, P.E.; Brenneman, K.J.; Jacobson, A.E. Improved Algal Harvesting Using Suspended Air Flotation. Water Environ. Res. 2009, 81, 702–708. [Google Scholar] [CrossRef]
- Drexler, I.L.C.; Yeh, D.H. Membrane applications for microalgae cultivation and harvesting: A review. Rev. Environ. Sci. Biotechnol. 2014, 13, 487–504. [Google Scholar] [CrossRef]
- Vonshak, A.; Richmond, A. Mass production of the blue-green alga Spirulina: An overview. Biomass 1988, 15, 233–247. [Google Scholar] [CrossRef]
- Das, P.; Thaher, M.; Khan, S.; AbdulQuadir, M.; Al-Jabri, H. The effect of culture salinity on the harvesting of microalgae biomass using pilot-scale Tangential-Flow-Filter membrane. Bioresour. Technol. 2019, 293, 122057. [Google Scholar] [CrossRef]
- Benavente-Valdés, J.R.; Aguilar, C.; Contreras-Esquivel, J.C.; Méndez-Zavala, A.; Montañez, J. Strategies to enhance the production of photosynthetic pigments and lipids in chlorophycae species. Biotechnol. Rep. 2016, 10, 117–125. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef]
- Levasseur, W.; Perré, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef]
- Ge, S.; Qiu, S.; Tremblay, D.; Viner, K.; Champagne, P.; Jessop, P.G. Centrate wastewater treatment with Chlorella vulgaris: Simultaneous enhancement of nutrient removal, biomass and lipid production. Chem. Eng. J. 2018, 342, 310–320. [Google Scholar] [CrossRef]
- Phang, S.M.; Miah, M.S.; Yeoh, B.G.; Hashim, M.A. Spirulina cultivation in digested sago starch factory wastewater. J. Appl. Phycol. 2000, 12, 395–400. [Google Scholar] [CrossRef]
- Ji, M.-K.; Yun, H.-S.; Park, S.; Lee, H.; Park, Y.-T.; Bae, S.; Ham, J.; Choi, J. Effect of food wastewater on biomass production by a green microalga Scenedesmus obliquus for bioenergy generation. Bioresour. Technol. 2015, 179, 624–628. [Google Scholar] [CrossRef]
- Behl, K.; SeshaCharan, P.; Joshi, M.; Sharma, M.; Mathur, A.; Kareya, M.S.; Jutur, P.P.; Bhatnagar, A.; Nigam, S. Multifaceted applications of isolated microalgae Chlamydomonas sp. TRC-1 in wastewater remediation, lipid production and bioelectricity generation. Bioresour. Technol. 2020, 304, 122993. [Google Scholar] [CrossRef] [PubMed]
- Do, J.M.; Jo, S.W.; Kim, I.S.; Na, H.; Lee, J.H.; Kim, H.S.; Yoon, H.S. A feasibility study of wastewater treatment using domestic microalgae and analysis of biomass for potential applications. Water 2019, 11, 2294. [Google Scholar] [CrossRef]
- Andreotti, V.; Solimeno, A.; Rossi, S.; Ficara, E.; Marazzi, F.; Mezzanotte, V.; García, J. Bioremediation of aquaculture wastewater with the microalgae Tetraselmis suecica: Semi-continuous experiments, simulation and photo-respirometric tests. Sci. Total Environ. 2020, 738, 139859. [Google Scholar] [CrossRef] [PubMed]
- Parsy, A.; Sambusiti, C.; Baldoni-Andrey, P.; Elan, T.; Périé, F. Cultivation of Nannochloropsis oculata in saline oil & gas wastewater supplemented with anaerobic digestion effluent as nutrient source. Algal Res. 2020, 50, 101966. [Google Scholar] [CrossRef]
- Cecal, A.; Humelnicu, D.; Rudic, V.; Cepoi, L.; Ganju, D.; Cojocari, A. Uptake of uranyl ions from uranium ores and sludges by means of Spirulina platensis, Porphyridium cruentum and Nostok linckia alga. Bioresour. Technol. 2012, 118, 19–23. [Google Scholar] [CrossRef]
- Doğan-Subaşı, E.; Demirer, G.N. Anaerobic digestion of microalgal (Chlorella vulgaris) biomass as a source of biogas and biofertilizer. Environ. Prog. Sustain. Energy 2016, 35, 936–941. [Google Scholar] [CrossRef]
- Mulbry, W.; Westhead, E.K.; Pizarro, C.; Sikora, L. Recycling of manure nutrients: Use of algal biomass from dairy manure treatment as a slow release fertilizer. Bioresour. Technol. 2005, 96, 451–458. [Google Scholar] [CrossRef]
- Renuka, N.; Prasanna, R.; Sood, A.; Ahluwalia, A.S.; Bansal, R.; Babu, S.; Singh, R.; Shivay, Y.S.; Nain, L. Exploring the efficacy of wastewater-grown microalgal biomass as a biofertilizer for wheat. Environ. Sci. Pollut. Res. 2016, 23, 6608–6620. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Khan, S.; Chaudhary, A.K.; AbdulQuadir, M.; Thaher, M.I.; Al-Jabri, H. Potential Applications of Algae-Based Bio-fertilizer. In Biofertilizers for Sustainable Agriculture and Environment; Giri, B., Prasad, R., Wu, Q.-S., Varma, A., Eds.; Springer International Publishing: Cham, Switherlands, 2019; pp. 41–65. ISBN 978-3-030-18933-4. [Google Scholar]
- Sampathkumar, P.; Dineshkumar, R.; Rasheeq, A.A.; Arumugam, A.; Nambi, K.S. Marine microalgal extracts on cultivable crops as a considerable bio-fertilizer: A Review. Indian J. Tradit. Knowl. 2019, 18, 849–854. [Google Scholar]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal Biostimulants and Biofertilisers in Crop Productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef]
- Koutra, E.; Economou, C.N.; Tsafrakidou, P.; Kornaros, M. Bio-Based Products from Microalgae Cultivated in Digestates. Trends Biotechnol. 2018, 36, 819–833. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Fermoso, F.G.; Rincón, B.; Bartacek, J.; Borja, R.; Jeison, D. Challenges for Cost-Effective Microalgae Anaerobic Digestion. In Biodegradation–Engineering and Technology; InTech Publisher: London, UK, 2013; pp. 139–158. [Google Scholar] [CrossRef]
- Milledge, J.; Heaven, S. Energy Balance of Biogas Production from Microalgae: Effect of Harvesting Method, Multiple Raceways, Scale of Plant and Combined Heat and Power Generation. J. Mar. Sci. Eng. 2017, 5, 9. [Google Scholar] [CrossRef]
- ter Veld, F. Beyond the Fossil Fuel Era: On the Feasibility of Sustainable Electricity Generation Using Biogas from Microalgae. Energy Fuels 2012, 26, 3882–3890. [Google Scholar] [CrossRef]
- Mudimu, O.; Rybalka, N.; Bauersachs, T.; Born, J.; Friedl, T.; Schulz, R. Biotechnological Screening of Microalgal and Cyanobacterial Strains for Biogas Production and Antibacterial and Antifungal Effects. Metabolites 2014, 4, 373–393. [Google Scholar] [CrossRef]
- Adamczyk, M.; Sajdak, M. Pyrolysis Behaviours of Microalgae Nannochloropsis gaditana. Waste Biomass Valorization 2018, 9, 2221–2235. [Google Scholar] [CrossRef]
- Vlaskin, M.S.; Chernova, N.I.; Kiseleva, S.V.; Popel’, O.S.; Zhuk, A.Z. Hydrothermal liquefaction of microalgae to produce biofuels: State of the art and future prospects. Therm. Eng. 2017, 64, 627–636. [Google Scholar] [CrossRef]
- Mishra, S.; Mohanty, K. Co-HTL of domestic sewage sludge and wastewater treatment derived microalgal biomass—An integrated biorefinery approach for sustainable biocrude production. Energy Convers. Manag. 2020, 204, 112312. [Google Scholar] [CrossRef]
- Cheng, F.; Jarvis, J.M.; Yu, J.; Jena, U.; Nirmalakhandan, N.; Schaub, T.M.; Brewer, C.E. Bio-crude oil from hydrothermal liquefaction of wastewater microalgae in a pilot-scale continuous flow reactor. Bioresour. Technol. 2019, 294, 122184. [Google Scholar] [CrossRef] [PubMed]
- Ehimen, E.A.; Connaughton, S.; Sun, Z.; Carrington, G.C. Energy recovery from lipid extracted, transesterified and glycerol codigested microalgae biomass. GCB Bioenergy 2009, 1, 371–381. [Google Scholar] [CrossRef]
- Passos, F.; Hernández-Mariné, M.; García, J.; Ferrer, I. Long-term anaerobic digestion of microalgae grown in HRAP for wastewater treatment. Effect of microwave pretreatment. Water Res. 2014, 49, 351–359. [Google Scholar] [CrossRef]
- Xu, S.; Elsayed, M.; Ismail, G.A.; Li, C.; Wang, S.; Abomohra, A.E.-F. Evaluation of bioethanol and biodiesel production from Scenedesmus obliquus grown in biodiesel waste glycerol: A sequential integrated route for enhanced energy recovery. Energy Convers. Manag. 2019, 197, 111907. [Google Scholar] [CrossRef]
- Hena, S.; Fatimah, S.; Tabassum, S. Cultivation of algae consortium in a dairy farm wastewater for biodiesel production. Water Resour. Ind. 2015, 10, 1–14. [Google Scholar] [CrossRef]
- Sotoudehniakarani, F.; Alayat, A.; McDonald, A.G. Characterization and comparison of pyrolysis products from fast pyrolysis of commercial Chlorella vulgaris and cultivated microalgae. J. Anal. Appl. Pyrolysis 2019, 139, 258–273. [Google Scholar] [CrossRef]
- Sarkar, O.; Agarwal, M.; Naresh Kumar, A.; Venkata Mohan, S. Retrofitting hetrotrophically cultivated algae biomass as pyrolytic feedstock for biogas, bio-char and bio-oil production encompassing biorefinery. Bioresour. Technol. 2015, 178, 132–138. [Google Scholar] [CrossRef]
- Ungureanu, N.; Vladut, V.; Biris, S.-S. Capitalization of Wastewater-Grown Algae in Bioethanol Production; Engineering for Rural Development: Jelgava, Latvia, 2020; pp. 1859–1864. [Google Scholar] [CrossRef]
- Talukder, M.M.R.; Das, P.; Wu, J.C. Microalgae (Nannochloropsis salina) biomass to lactic acid and lipid. Biochem. Eng. J. 2012, 68, 109–113. [Google Scholar] [CrossRef]
- Nguyen, C.M.; Kim, J.-S.; Hwang, H.J.; Park, M.S.; Choi, G.J.; Choi, Y.H.; Jang, K.S.; Kim, J.-C. Production of l-lactic acid from a green microalga, Hydrodictyon reticulum, by Lactobacillus paracasei LA104 isolated from the traditional Korean food, makgeolli. Bioresour. Technol. 2012, 110, 552–559. [Google Scholar] [CrossRef]
- López Rocha, C.J.; Álvarez-Castillo, E.; Estrada Yáñez, M.R.; Bengoechea, C.; Guerrero, A.; Orta Ledesma, M.T. Development of bioplastics from a microalgae consortium from wastewater. J. Environ. Manag. 2020, 263, 110353. [Google Scholar] [CrossRef]
- Arias, D.M.; García, J.; Uggetti, E. Production of polymers by cyanobacteria grown in wastewater: Current status, challenges and future perspectives. New Biotechnol. 2020, 55, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Zheng, Y. Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol. Adv. 2016, 34, 1225–1244. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Hu, B.; Li, Y.; Min, M.; Mohr, M.; Du, Z.; Chen, P.; Ruan, R. Mass Cultivation of Microalgae on Animal Wastewater: A Sequential Two-Stage Cultivation Process for Energy Crop and Omega-3-Rich Animal Feed Production. Appl. Biochem. Biotechnol. 2012, 168, 348–363. [Google Scholar] [CrossRef] [PubMed]
- Shahid, A.; Khan, F.; Ahmad, N.; Farooq, M.; Mehmood, M.A. Microalgal carbohydrates and proteins: Synthesis, extraction, applications, and challenges. In Microalgae Biotechnology for Food, Health and High Value Products; Alam, M.A., Xu, J.-L., Wang, Z., Eds.; Springer: Singapore, 2020; pp. 433–468. ISBN 9789811501692. [Google Scholar]
- Couteau, C.; Coiffard, L. Chapter 15—Microalgal Application in Cosmetics. In Microalgae in Health and Disease Prevention; Levine, I.A., Fleurence, J., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 317–323. ISBN 978-0-12-811405-6. [Google Scholar]
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; de Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae—A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Campbell, P.K.; Beer, T.; Batten, D. Life cycle assessment of biodiesel production from microalgae in ponds. Bioresour. Technol. 2011, 102, 50–56. [Google Scholar] [CrossRef]
- Sturm, B.S.M.; Lamer, S.L. An energy evaluation of coupling nutrient removal from wastewater with algal biomass production. Appl. Energy 2011, 88, 3499–3506. [Google Scholar] [CrossRef]
- Nourmohammadi, D.; Esmaeeli, M.B.; Akbarian, H.; Ghasemian, M. Nitrogen removal in a full-scale domestic wastewater treatment plant with activated sludge and trickling filter. J. Environ. Public Health 2013, 2013, 504705. [Google Scholar] [CrossRef]
- Semple, K.T.; Cain, R.B.; Schmidt, S. Biodegradation of aromatic compounds by microalgae. FEMS Microbiol. Lett. 1999, 170, 291–300. [Google Scholar] [CrossRef]
- Yang, H.; He, Q.; Hu, C. Feasibility of biodiesel production and CO2 emission reduction by Monoraphidium dybowskii LB50 under semi-continuous culture with open raceway ponds in the desert area. Biotechnol. Biofuels 2018, 11, 82. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).