The Benthic Quality Index to Assess Water Quality of Lakes May Be Affected by Confounding Environmental Features
Abstract
1. Introduction
- Screening methods for Water Data Information in support of the implementation of the Water Framework Directive (SWIFT-WFD: www.swift-wfd.com) [5];
- Development and Testing of an Integrated Assessment System for the Ecological Quality of Streams and Rivers throughout Europe using Benthic Macroinvertebrates (AQUEM: www.aqem.de) [6];
- Standardisation of River Classifications: Framework method for calibrating different biological survey results against ecological quality classifications to be developed for the Water Framework Directive (STAR: www.eu-star.at/frameset.htm) [7];
- Relationships between the ecological and chemical status of surface waters (REBECCA: www.ymparisto.fi/eng/research/euproj/rebecca/homepage.html) [8];
- Water Bodies in Europe: Integrative systems to assess ecological status and recovery (WISER: www.wiser.eu) [9];
- Local hydro-morphology, habitat and RBMPs: new measures to improve ecological quality in South European rivers and lakes (INHABIT: www.life-inhabit.it);
- Managing aquatic ecosystems and water resources under multiple stresses (MARS: www.mars-project.eu) [10,11].
2. Materials and Methods
2.1. Study Area
2.2. Lake Classification
2.3. Sampling Methodology
2.4. Statistical Analyses
3. Results
3.1. Lake Classification
3.2. Single-Site Sediment and Water Descriptors
3.3. Whole-Lake Macroinvertebrates Assemblages
3.4. Environmental Effects on Macroinvertebrates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Geist, J. Integrative freshwater ecology and biodiversity conservation. Ecol. Indic. 2011, 11, 1507–1516. [Google Scholar] [CrossRef]
- Geist, J.; Hawkins, S.J. Habitat recovery and restoration in aquatic ecosystems: Current progress and future challenges. Aquat. Conserv Mar. Freshw. Ecosyst. 2016, 26, 942–962. [Google Scholar] [CrossRef]
- United Nations World Water Assessment Programme (WWAP). The United Nations World Water Development Report 2018: Nature-Based Solutions; UNESCO: Paris, France, 2018; p. 139. [Google Scholar]
- Rouillard, J.; Lago, M.; Abhold, K.; Röschel, L.; Kafyeke, T.; Mattheiß, V.; Klimmek, H. Protecting aquatic biodiversity in Europe: How much do EU environmental policies support ecosystem-based management? Ambio 2018, 47, 15–24. [Google Scholar] [CrossRef]
- Schneider, X. D66: Best Practice Guidance for Water Body Monitoring; XPRO Consulting Limited: Lefkosia, Cyprus, 2007; p. 36. Available online: https://www.xpro-consulting.com/uploads/4/9/5/5/49557869/d66-swift-best-practice.pdf (accessed on 29 June 2020).
- Hering, D.; Verdonschot, P.F.M.; Moog, O.; Sandin, L. Integrated assessment of running waters in Europe. Hydrobiologia 2004, 516, 379. [Google Scholar]
- Furse, M.; Hering, D.; Moog, O.; Verdonschot, P.; Johnson, R.K.; Brabec, K.; Gritzalis, K.; Buffagni, A.; Pinto, P.; Friberg, N.; et al. The STAR project: Context, objectives and approaches. Hydrobiologia 2006, 566, 3–29. [Google Scholar] [CrossRef]
- Friberg, N.; Skriver, J.; Larsen, S.E.; Pedersen, M.L.; Buffagni, A. Stream macroinvertebrate occurrence along gradients in organic pollution and eutrophication. Fresh. Biol. 2010, 55, 1405–1419. [Google Scholar] [CrossRef]
- Feld, C.K.; Borja, A.; Carvalho, L.; Hering, D.F. Water bodies in Europe: Integrative systems to assess ecological status and recovery—Results of the WISER project. Hydrobiologia 2013, 704, 501. [Google Scholar]
- Taipale, S.J.; Vuorio, K.; Strandberg, U.; Kahilainen, K.K.; Jarvinen, M.; Hiltunen, M.; Peltomaa, E.; Kankaala, P. Lake eutrophication and brownification downgrade availability and transfer of essential fatty acids for human consumption. Environ. Int. 2016, 96, 156–166. [Google Scholar] [CrossRef]
- Tolonen, K.T.; Vilmi, A.; Karjalainen, S.M.; Hellsten, S.; Heino, J. Do different facets of littoral macroinvertebrate diversity show congruent patterns in a large lake system? Community Ecol. 2017, 18, 109–116. [Google Scholar] [CrossRef]
- Verdonschot, P.F. Integrated ecological assessment methods as a basis for sustainable catchment management. Hydrobiologia 2000, 422, 389–412. [Google Scholar] [CrossRef]
- Birk, S.; Bonne, W.; Borja, A.; Brucet, S.; Courrat, A.; Poikane, S.; Solimini, A.G.; Bund, W. van de Zampoukas, N.; Hering, D. Three hundred ways to assess Europe’s surface waters: An almost complete overview of biological methods to implement the water framework directive. Ecol. Indic. 2012, 18, 31–41. [Google Scholar] [CrossRef]
- Bonada, N.; Prat, N.; Resh, V.; Statzner, B. Developments in aquatic insect biomonitoring: A comparative analysis of recent approaches. Annu. Rev. Entomol. 2006, 51, 495–523. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.J.; Solan, M.; Valente, R.M. A review of approaches for classifying benthic habitats and evaluating habitat quality. J. Environ. Manag. 2004, 73, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Feio, M.J.; Poquet, J.M. Predictive models for freshwater biological assessment: Statistical approaches. Biological elements and the Iberian Peninsula experience: A review. Int. Rev. Hydrobiol. 2011, 96, 321–346. [Google Scholar] [CrossRef]
- Lindegarth, M.; Carstensen, J.; Drakare, S.; Johnson, R.K.; Sandman, A.N.; Söderpalm, A.; Wikström, S.A. Ecological assessment of swedish water bodies; development, harmonisation and integration of biological indicators. In Final Report of the Research Programme WATERS, Deliverable 1.1–4, WATERS Report No. 2016:10; Havsmiljöinstitutet: Göteborg, Sweden, 2016; Available online: https://waters.gu.se/digitalAssets/1592/1592593_waters-report-2016_10.pdf (accessed on 29 June 2020).
- McFarland, B.; Carse, F.; Sandin, L. Littoral macroinvertebrates as indicators of lake acidification within the UK. Aquatic Conserv Mar. Freshw. Ecosyst. 2010, 20, S105–S116. [Google Scholar] [CrossRef]
- Poikane, S.; Birk, S.; Böhmer, J.; Carvalho, L.; de Hoyose, C.; Gassner, H.; Hellsten, S.; Kelly, M.; Solheim, A.L.; Olin, M.; et al. A hitchhiker’s guide to European lake ecological assessment and intercalibration. Ecol. Indic. 2015, 52, 533–544. [Google Scholar] [CrossRef]
- Reyjol, Y.; Argillier, C.; Bonne, W.; Borja, A.; Buijse, A.D.; Cardoso, A.C.; Daufresne, M.; Kernan, M.; Ferreira, M.T.; Poikane, S.; et al. Assessing the ecological status in the context of the European water framework directive: Where do we go now? Sci. Total Environ. 2014, 497–498, 332–344. [Google Scholar] [CrossRef]
- Solheim, A.L.; Feld, C.K.; Birk, S.; Phillips, G.; Carvalho, L.; Morabito, G.; Mischke, U.; Willby, N.; Søndergaard, M.; Hellsten, S.; et al. Ecological status assessment of European lakes: A comparison of metrics for phytoplankton, macrophytes, benthic invertebrates and fish. Hydrobiologia 2013, 704, 57–74. [Google Scholar] [CrossRef]
- Premazzi, G.; Dalmiglio, A.; Cardoso, A.; Chiaudani, G. Lake management in Italy: The implications of the Water Framework Directive. Lakes Reserv. Res. Manag. 2003, 8, 41–59. [Google Scholar] [CrossRef]
- Rossaro, B.; Zaupa, S.; Lencioni, V.; Marziali, L.; Boggero, A. 6 Indice per la valutazione della qualità ecologica dei laghi italiani basato sulla comunità bentonica. In Indici per La Valutazione Della Qualità Ecologica dei Laghi; REPORT CNR ISE 02.13; Istituto Italiano di Idrobiologia: Verbania, Italy, 2013; pp. 93–113. Available online: http://www.ise.cnr.it/wfd (accessed on 29 June 2020).
- Boggero, A.; Zaupa, S.; Cancellario, T.; Lencioni, V.; Marziali, L.; Rossaro, B. Italian Classification Method for Macroinvertebrates in Lakes. Method Summary; Report CNR ISE, 03.16; Istituto Italiano di Idrobiologia: Verbania, Italy, 2017; p. 16. Available online: http://www.ise.cnr.it/wfd-en (accessed on 29 June 2020).
- Fornaroli, R.; Cabrini, R.; Zaupa, S.; Bettinetti, R.; Ciampittiello, M.; Boggero, A. Quantile regression analysis as a predictive tool for lake macroinvertebrate biodiversity. Ecol. Indic. 2016, 61, 728–738. [Google Scholar] [CrossRef]
- Boggero, A.; Zaupa, S.; Rossaro, B.; Lencioni, V.; Marziali, L.; Buzzi, F.; Fiorenza, A.; Cason, M.; Giacomazzi, F.; Pozzi, S. Protocollo di Campionamento e Analisi dei Macroinvertebrati Negli Ambienti Lacustri. MATTM-APAT, Ed.; Roma, Italy, 2008. Available online: https://www.isprambiente.gov.it/files/pubblicazioni/manuali-lineeguida/metodi-biologici-acque/laghi-macroinvertebrati.pdf (accessed on 29 June 2020).
- Carpenter, S.R.; Stanley, E.H.; Vander Zanden, M.J. State of the World’s freshwater ecosystems: Physical, chemical, and biological changes. Annu. Rev. Environ. Resour. 2011, 36, 75–99. [Google Scholar] [CrossRef]
- Ward, D.; Holmes, N.; José, P. The New Rivers & Wildlife Handbook; Royal Society for the Protection of Birds: Bedfordshire, UK, 1995. [Google Scholar]
- Esteves, F.A. Fundamentos de Limnologia, 2nd ed.; Interciência/FINEP: Rio de Janeiro, Brazil, 1998. [Google Scholar]
- Galdean, N.; Callisto, M.; Barbosa, F. Lotic Ecosystems of Serra do Cipó, southeast Brazil: Water quality and a tentative classification based on the benthic macro-invertebrate community. Aquat. Ecosyst. Health 2000, 3, 545–552. [Google Scholar] [CrossRef]
- Meriläinen, J.J.; Hynynen, J.; Palomäki, A.; Mäntykoski, K.; Witick, A. Environmental history of an urban lake: A palaeolimnological study of Lake Jyväsjärvi Finland. J. Paleolimnol. 2003, 30, 387–406. [Google Scholar] [CrossRef]
- Biggs, J.; von Fumetti, S.; Kelly-Quinn, M. The importance of small waterbodies for biodiversity and ecosystem services: Implications for policy makers. Hydrobiologia 2016, 793, 3–39. [Google Scholar] [CrossRef]
- Buraschi, E.; Salerno, F.; Monguzzi, C.; Barbiero, G.; Tartari, G. Characterization of the Italian lake-types and identification of their reference sites using anthropogenic pressure factors. J. Limnol. 2005, 64, 75–84. [Google Scholar] [CrossRef]
- Vollenweider, R.; Kerekes, J. Eutrophication of Waters, Monitoring, Assessment and Control; OECD: Paris, France, 1982; p. 154. [Google Scholar]
- Tartari, G.A.; Mosello, R. Metodologie Analitiche e Controlli di Qualità nel Laboratorio Chimico Dell’istituto Italiano di Idrobiologia; Documenta; Consiglio Nazionale delle Ricerche: Roma, Italy, 1997; p. 160. [Google Scholar]
- Rowan, J.S.; Carwardine, J.; Duck, R.W.; Bragg, O.M.; Black, A.R.; Cutler, M.E.J.; Soutar, I.; Boon, P.J. Development of a technique for lake habitat survey (LHS) with applications for the European Union Water Framework Directive. Aquat. Conserv: Mar. Freshw. Ecosyst. 2006, 16, 637–657. [Google Scholar] [CrossRef]
- Dean, W.E. Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss on ignition: Comparison with other methods. J. Sediment. Petrol. 1974, 44, 242–248. [Google Scholar]
- Wentworth, C.K. A scale of grade and class terms for clastic sediments. J. Geol. 1922, 30, 377–392. [Google Scholar] [CrossRef]
- Ongley, E. Sediment measurements. In Water Quality Monitoring—A Practical Guide to the Design and Implementation of Freshwater Quality Studies and Monitoring Programmes; UNEP/WHO: London, UK, 1996; p. 15. [Google Scholar]
- Sabra, N.; Dubourguier, H.C.; Hamieh, T. Sequential extraction and particle size analysis of heavy metals in sediments dredged from the Deule Canal, France. Open Environ. Eng. J. 2011, 4, 11–17. [Google Scholar] [CrossRef]
- Wiederholm, T. Chironomidae of the Holartic region. Keys and diagnoses. Part I: Larvae. Entomol. Scand. Suppl. 1983, 19, 457. [Google Scholar]
- Timm, T. A guide to the freshwater Oligochaeta and Polychaeta of Northern and Central Europe. Lauterbornia 2009, 66, 235. [Google Scholar]
- Nocentini, A. Guide per il Riconoscimento Delle Specie Animali Delle Acque Interne Italiane; CNR: Verona, Italy, 1977–1985; Volume 29. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. Available online: https://www.R-project.org (accessed on 29 June 2020).
- Revelle, W. Psych: Procedures for Personality and Psychological Research; Version 1.9.12; Northwestern University: Evanston, IL, USA, 2019; Available online: https://CRAN.R-project.org/package=psych (accessed on 29 June 2020).
- Beckerman, A.P. What can modern statistical tools do for limnology. J. Limnol. 2014, 73, 161–170. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1–147. 2020. Available online: https://CRAN.R-project.org/package=nlme (accessed on 29 June 2020).
- Crawley, M.J. The R Book; John Wiley & Sons Ltd.: West Sussex, UK, 2007; p. 942. [Google Scholar]
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2018. [Google Scholar]
- Jaeger, B. r2glmm: Computes R Squared for Mixed (Multilevel) Models. R Package Version 0.1.2. 2017. Available online: https://CRAN.R-project.org/package=r2glmm (accessed on 29 June 2020).
- Mac Donald, D.D.; Ingersoll, C.G.; Berger, T.A. Development and evaluation of consensus-based sediment quality guidelines for freshwater systems. Arch. Environ. Contam. Toxicol. 2000, 39, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Bettinetti, R.; Zaupa, S.; Fontaneto, D.; Boggero, A. Biological, chemical, and ecotoxicological assessments using benthos provide different and complementary measures of lake ecological status. Water 2020, 12, 1140. [Google Scholar] [CrossRef]
- Sarukhán, J.; Whyte, A.; Hassan, R.; Scholes, R.; Ash, N.; Carpenter, S.T.; Leemans, R. Millenium ecosystem assessment: Ecosystems and human well-being. In The Millennium Ecosystem Assessment Series; Hassan, R., Scholes, R., Neville, A., Eds.; Island Press: Washington, DC, USA, 2003; p. 245. [Google Scholar]
- Free, G.; Solimini, A.; Rossaro, B.; Marziali, L.; Giacchini, R.; Paracchini, P.; Ghiani, M.; Vaccaro, S.; Gawlik, B.; Fresner, R.; et al. Modelling lake macroinvertebrate species in the shallow sublittoral, relative roles of habitat, lake morphology, aquatic chemistry and sediment composition. Hydrobiologia 2009, 133, 123–136. [Google Scholar] [CrossRef]
- Garcia-Criado, F.; Becares, E.; Fernadez-Alaez, M. Plant-associated invertebrates and ecological quality insome Mediterranean shallow lakes: Implications for the application of the EC Water Framework Directive. Aquat. Conserv: Mar. Freshwat. Ecosyst. 2005, 15, 31–50. [Google Scholar] [CrossRef]
- Rossaro, B.; Boggero, A.; Lencioni, V.; Marziali, L.; Solimini, A. Tools for the development of a benthic quality index for Italian lakes. J. Limnol. 2006, 65, 41–51. [Google Scholar] [CrossRef][Green Version]
- Rossaro, B.; Cardoso, A.C.; Solimini, A.; Free, G.; Marziali, L.; Giacchini, R. A biotic index using benthic macroinvertebrates for Italian lakes. Ecol. Indic. 2007, 7, 412–429. [Google Scholar] [CrossRef]
- Donohue, I.; Garcia Molinos, J. Impacts of increased sediment loads on the ecology of lakes. Biol. Rev. 2009, 84, 517–531. [Google Scholar] [CrossRef]
- Dias-Silva, K.; Cabette, H.S.R.; Juen, L.; DeMarco, P. The influence of habitat integrity and physical-chemical water variables on the structure of aquatic and semi-aquatic Heteroptera. Zoologia 2010, 27, 918–930. [Google Scholar] [CrossRef]
- Jenny, H. The Soil Resource, 1st ed.; Springer: New York, NY, USA, 2008. [Google Scholar]
- Stohlgren, T.; Bachand, R.; Onami, Y.; Binkley, D. Species-environment relationships and vegetation patterns: Effects of spatial scale and tree life-stage. Plant Ecol. 1998, 135, 215–228. [Google Scholar] [CrossRef]
- Hynes, H.B.N. The stream and its valley. Verh. Internat. Verein. Theor. Angew. Limnol. 1975, 19, 1–15. [Google Scholar] [CrossRef]
- Larouche, J.R.; Bowden, W.B.; Giordano, R.; Flinn, M.B.; Crump, B.C. Microbial biogeography of Arctic streams: Exploring influences of lithology and habitat. Front. Microbiol. 2012, 3, 309. [Google Scholar] [CrossRef] [PubMed]
- McGoff, E.; Aroviita, J.; Pilotto, F.; Miler, O.; Solimini, A.G.; Porst, G.; Jurca, T.; Donohue, L.; Sandin, L. Assessing the relationship between the lake habitat survey and littoral macroinvertebrate communities in European lakes. Ecol. Ind. 2013, 25, 205–214. [Google Scholar] [CrossRef]

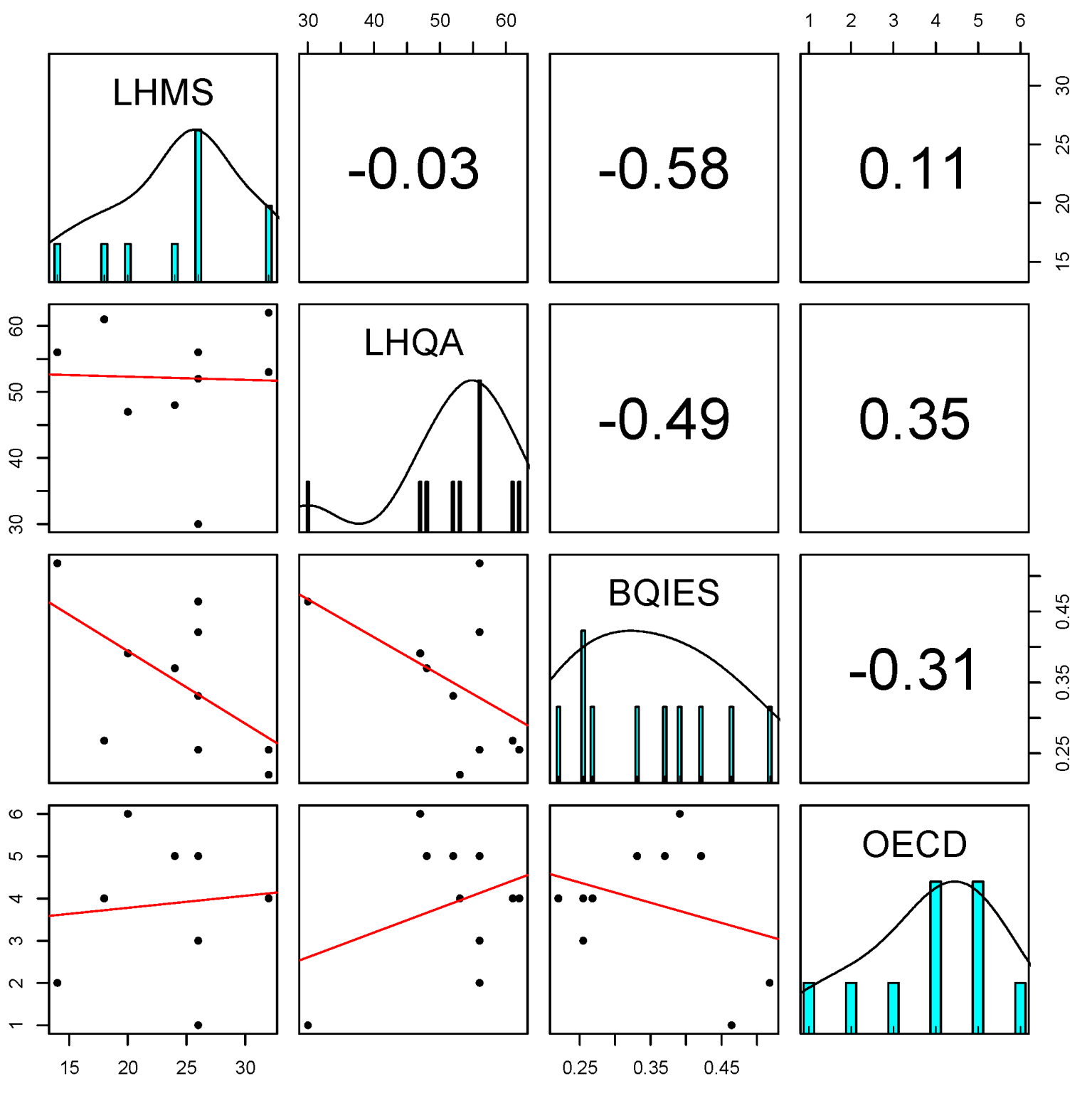
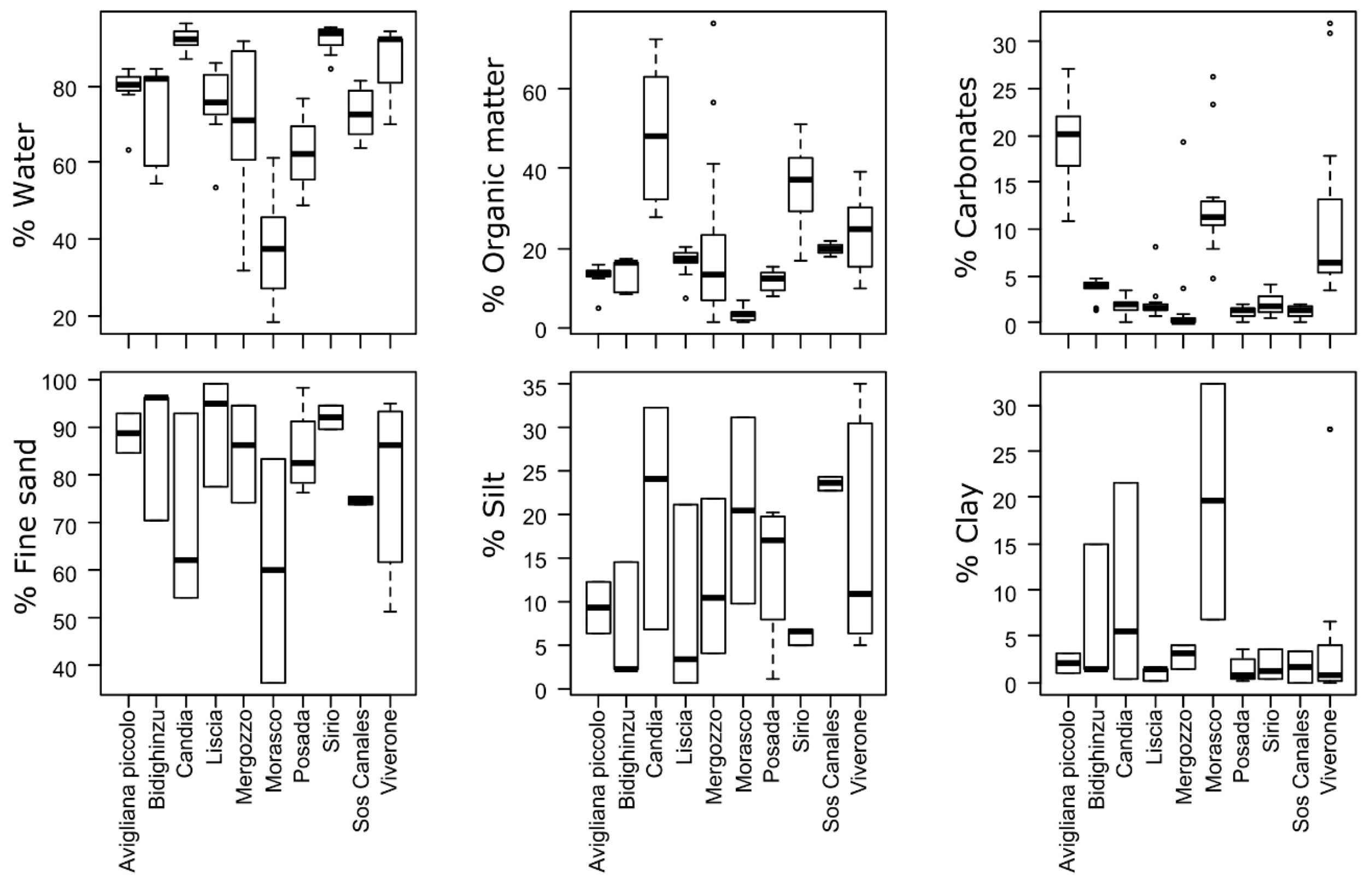

| Region | Lake Name | Lake Type | Class Type | Latitude N | Longitude E | Altitude | Lake Area | Mean Depth | Maximum Depth |
|---|---|---|---|---|---|---|---|---|---|
| Piedmont | Avigliana piccolo | Natural | AL-5 | 45°03′13″ | 07°23′30″ | 356 | 0.58 | 7.70 | 12.00 |
| Piedmont | Candia | Natural | AL-5 | 45°19′25″ | 07°54′43″ | 227 | 1.69 | 5.90 | 8.00 |
| Piedmont | Mergozzo | Natural | AL-6 | 45°57′23″ | 08°27′47″ | 194 | 1.82 | 45.60 | 73.00 |
| Piedmont | Morasco | Reservoir | AL-9 | 46°25′33″ | 08°23′48″ | 1814 | 0.57 | 31.00 | 50.00 |
| Piedmont | Sirio | Natural | AL-6 | 45°29′06″ | 07°53′05″ | 271 | 0.32 | 18.10 | 44.00 |
| Piedmont | Viverone | Natural | AL-6 | 45°24′05″ | 08°03′05″ | 230 | 5.78 | 22.50 | 50.00 |
| Sardinia | Bidighinzu | Reservoir | ME-2 | 40°33′24″ | 08°39′44″ | 330 | 1.50 | 8.40 | 30.00 |
| Sardinia | Liscia | Reservoir | ME-4 | 40°59′39″ | 09°14′37″ | 177 | 5.57 | 18.80 | 63.50 |
| Sardinia | Posada | Reservoir | ME-3 | 40°38′19″ | 09°36′28″ | 43 | 3.00 | 9.30 | 29.50 |
| Sardinia | Sos Canales | Reservoir | ME-5 | 40°33′17″ | 09°18′55″ | 709 | 0.30 | 19.70 | 47.50 |
| Lakes | O2 | TP | Chl a | Transparency | OECD | BQIESwhole-lake | LHMS | LHQA |
|---|---|---|---|---|---|---|---|---|
| Avigliana piccolo | 3 | 18.56 | 2.17 | 4.17 | oligo-mesotrophic | 0.255 | 26 | 56 |
| Bidighinzu | 3 | 259.16 | 14.67 | 0.89 | hypertrophic | 0.391 | 20 | 47 |
| Candia | 19 | 27.70 | 11.02 | 2.74 | eutrophic | 0.421 | 26 | 56 |
| Liscia | 15 | 41.97 | 6.73 | 3.04 | meso-eutrophic | 0.268 | 18 | 61 |
| Mergozzo | 75 | 4.60 | 1.98 | 7.50 | oligotrophic | 0.518 | 14 | 56 |
| Morasco | 78 | 2.20 | 0.36 | 7.33 | ultra-oligotrophic | 0.464 | 26 | 30 |
| Posada | 32 | 37.44 | 7.35 | 1.97 | eutrophic | 0.370 | 24 | 48 |
| Sirio | 3 | 41.07 | 3.70 | 5.03 | meso-eutrophic | 0.220 | 32 | 53 |
| Sos Canales | 6 | 31.67 | 6.66 | 2.80 | eutrophic | 0.331 | 26 | 52 |
| Viverone | 59 | 80.13 | 3.42 | 5.62 | meso-eutrophic | 0.255 | 32 | 62 |
| Environmental metrics | Parameters | Predictor | t-Value | p-Value |
|---|---|---|---|---|
| sediment chemistry | water content | (intercept) | 12.6 | <0.001 |
| depth | 2.9 | 0.007 | ||
| season | 0.6 | 0.547 | ||
| organic | (intercept) | 5.2 | <0.001 | |
| depth | −1.2 | 0.238 | ||
| season | −0.1 | 0.965 | ||
| inorganic | (intercept) | 17.0 | <0.001 | |
| depth | 1.6 | 0.114 | ||
| season | 1.1 | 0.299 | ||
| carbonates | (intercept) | 3.5 | 0.002 | |
| depth | −1.0 | 0.329 | ||
| season | −2.3 | 0.031 | ||
| sediment texture | fine sand | (intercept) | 22.0 | <0.001 |
| depth | 3.1 | 0.005 | ||
| season | −6.6 | <0.001 | ||
| silt | (intercept) | 12.3 | <0.001 | |
| depth | −2.7 | 0.011 | ||
| season | −5.5 | 0.001 | ||
| clay | (intercept) | 3.1 | <0.001 | |
| depth | −1.6 | 0.019 | ||
| season | −0.1 | 0.598 | ||
| water features | temperature | (intercept) | 12.5 | <0.001 |
| depth | −5.3 | <0.001 | ||
| season | 3.4 | 0.002 | ||
| oxygen | (intercept) | 9.5 | <0.001 | |
| depth | −3.6 | 0.002 | ||
| season | −1.5 | 0.140 | ||
| pH | (intercept) | 49.7 | <0.001 | |
| depth | −3.1 | 0.005 | ||
| season | −0.1 | 0.940 | ||
| conductivity | (intercept) | 5.2 | <0.001 | |
| depth | 1.5 | 0.142 | ||
| season | 1.5 | 0.146 | ||
| alkalinity | (intercept) | 3.3 | 0.002 | |
| depth | 0.9 | 0.358 | ||
| season | 0.3 | 0.767 | ||
| TP | (intercept) | 0.9 | 0.334 | |
| depth | 2.2 | 0.035 | ||
| season | 0.5 | 0.614 | ||
| TN | (intercept) | 4.5 | <0.001 | |
| depth | 2.4 | 0.023 | ||
| season | −0.9 | 0.361 |
| Response | Predictor | χ2 | p-Value | R2 |
|---|---|---|---|---|
| richness (R2 = 0.81) | PC axis sediment chemistry | 0.0 | 0.9587 | 0.00 |
| PC axis sediment texture | 0.7 | 0.3903 | 0.02 | |
| PC axis water features | 0.4 | 0.5486 | 0.01 | |
| depth | 27.5 | <0.0001 | 0.60 | |
| OECD | 0.1 | 0.7851 | 0.00 | |
| LHMS | 9.5 | 0.0021 | 0.28 | |
| LHQA | 3.8 | 0.0512 | 0.13 | |
| BQIESwhole-lake | 0.1 | 0.7200 | 0.01 | |
| season | 5.1 | 0.0768 | 0.04 | |
| SDI (R2 = 0.70) | PC axis sediment chemistry | 2.0 | 0.1592 | 0.09 |
| PC axis sediment texture | 0.3 | 0.6050 | 0.01 | |
| PC axis water features | 0.2 | 0.6789 | 0.01 | |
| depth | 24.6 | <0.0001 | 0.55 | |
| OECD | 0.6 | 0.4214 | 0.04 | |
| LHMS | 5.3 | 0.0209 | 0.21 | |
| LHQA | 0.0 | 0.8764 | 0.00 | |
| BQIESwhole-lake | 0.1 | 0.8197 | 0.00 | |
| season | 3.3 | 0.1964 | 0.04 | |
| BQIESsingle-site (R2 = 0.78) | PC axis sediment chemistry | 5.5 | 0.0191 | 0.26 |
| PC axis sediment texture | 0.0 | 0.8685 | 0.00 | |
| PC axis water features | 0.0 | 0.9900 | 0.00 | |
| depth | 20.7 | <0.0001 | 0.57 | |
| OECD | 0.5 | 0.4872 | 0.03 | |
| LHMS | 6.2 | 0.0131 | 0.22 | |
| LHQA | 0.1 | 0.7734 | 0.00 | |
| BQIESwhole-lake | 0.0 | 0.8681 | 0.00 | |
| season | 8.3 | 0.0159 | 0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boggero, A.; Zaupa, S.; Bettinetti, R.; Ciampittiello, M.; Fontaneto, D. The Benthic Quality Index to Assess Water Quality of Lakes May Be Affected by Confounding Environmental Features. Water 2020, 12, 2519. https://doi.org/10.3390/w12092519
Boggero A, Zaupa S, Bettinetti R, Ciampittiello M, Fontaneto D. The Benthic Quality Index to Assess Water Quality of Lakes May Be Affected by Confounding Environmental Features. Water. 2020; 12(9):2519. https://doi.org/10.3390/w12092519
Chicago/Turabian StyleBoggero, Angela, Silvia Zaupa, Roberta Bettinetti, Marzia Ciampittiello, and Diego Fontaneto. 2020. "The Benthic Quality Index to Assess Water Quality of Lakes May Be Affected by Confounding Environmental Features" Water 12, no. 9: 2519. https://doi.org/10.3390/w12092519
APA StyleBoggero, A., Zaupa, S., Bettinetti, R., Ciampittiello, M., & Fontaneto, D. (2020). The Benthic Quality Index to Assess Water Quality of Lakes May Be Affected by Confounding Environmental Features. Water, 12(9), 2519. https://doi.org/10.3390/w12092519







