Reactive Silica Traces Manure Spreading in Alluvial Aquifers Affected by Nitrate Contamination: A Case Study in a High Plain of Northern Italy
Abstract
1. Introduction
2. Materials and Methods
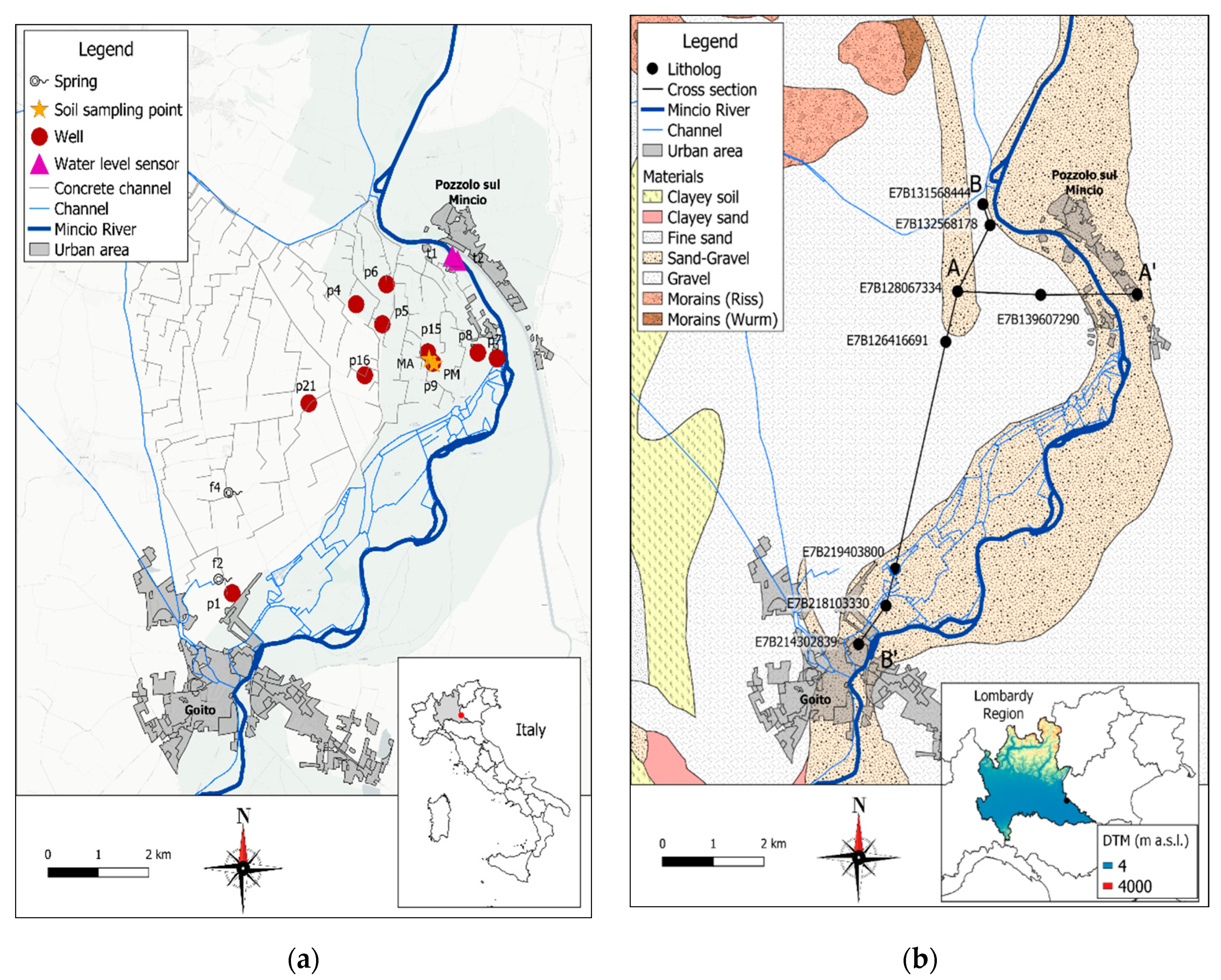
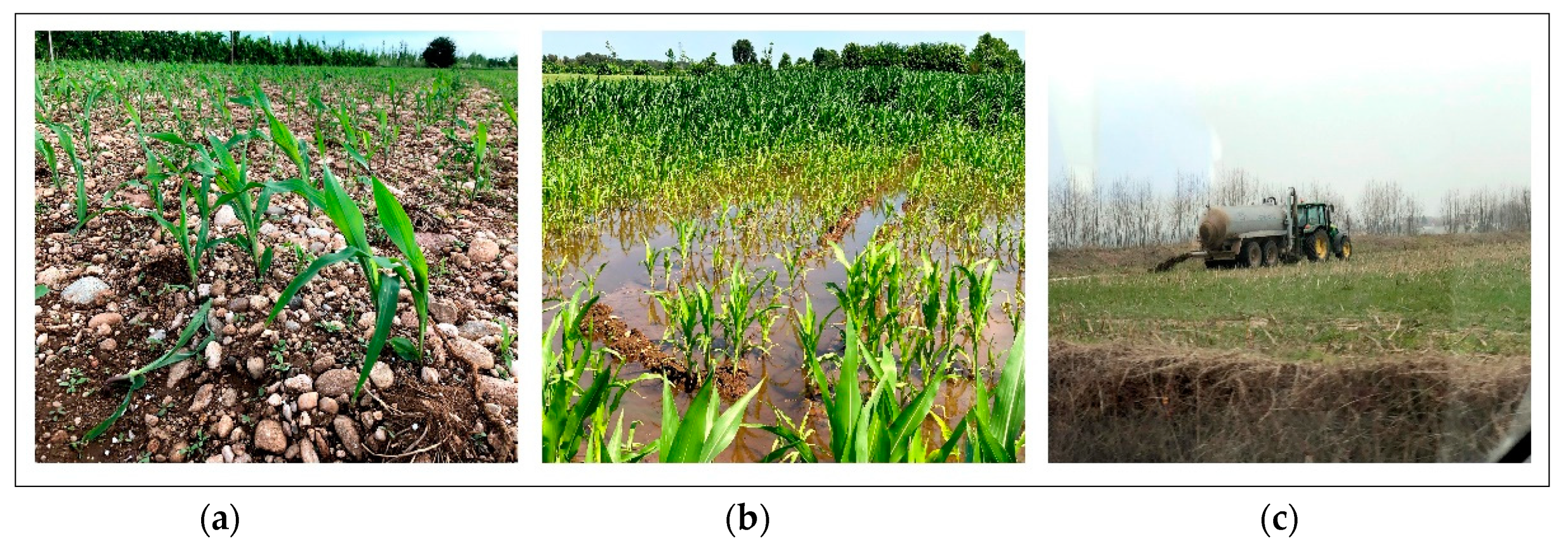
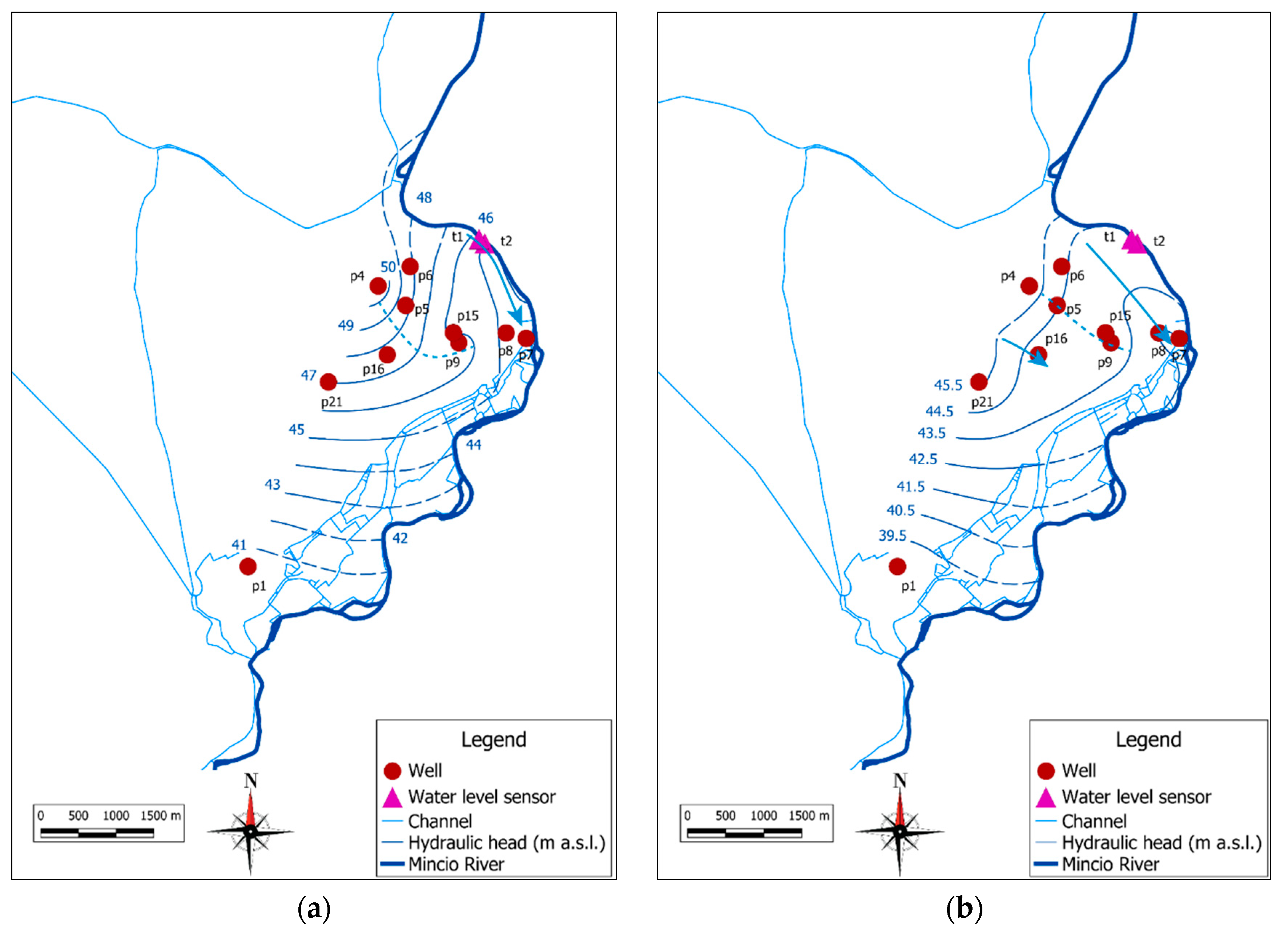
2.1. Study Area
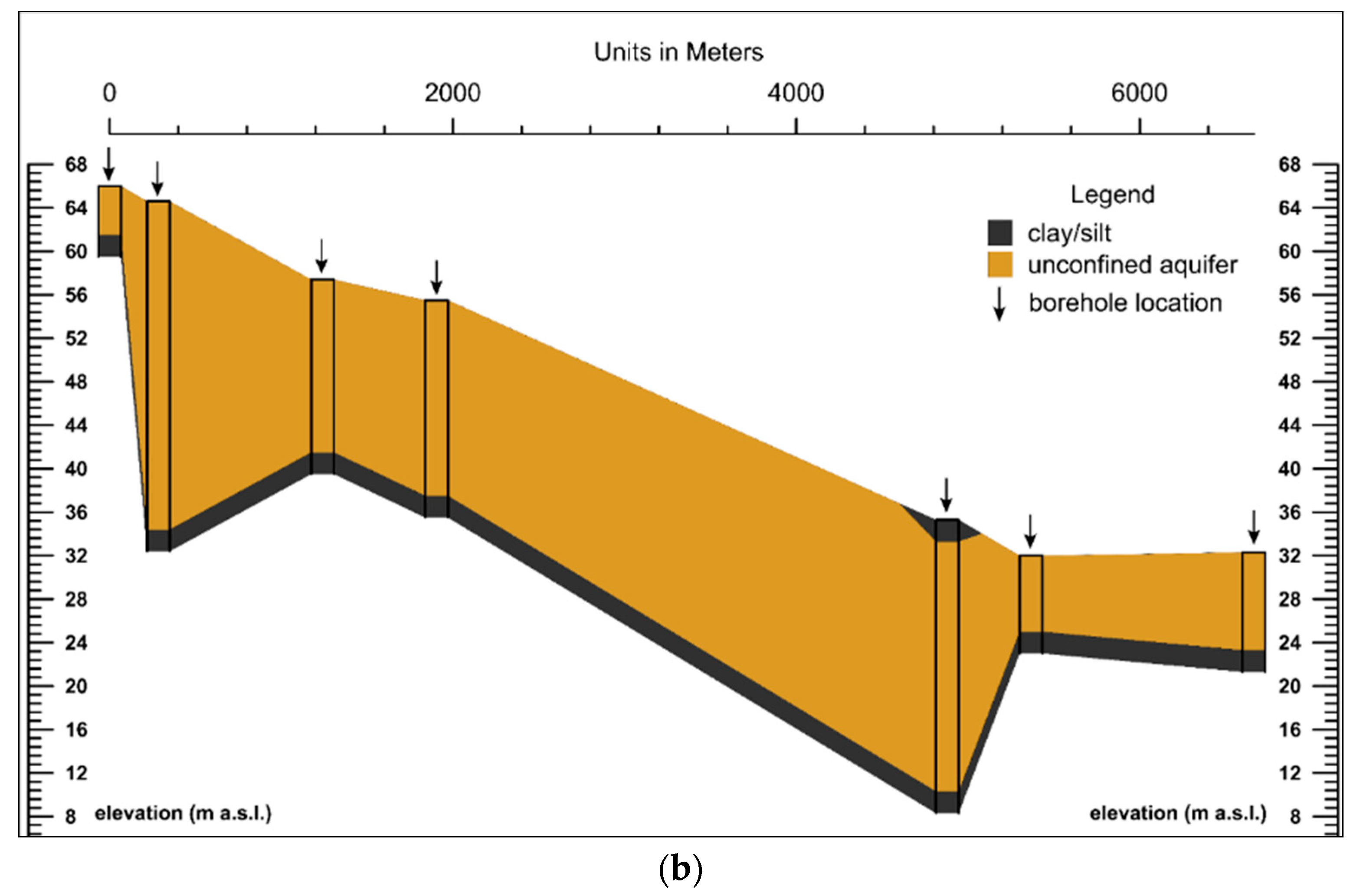
2.2. Hydrogeological Characterization
2.3. Sampling and Physico-Chemical Analyses
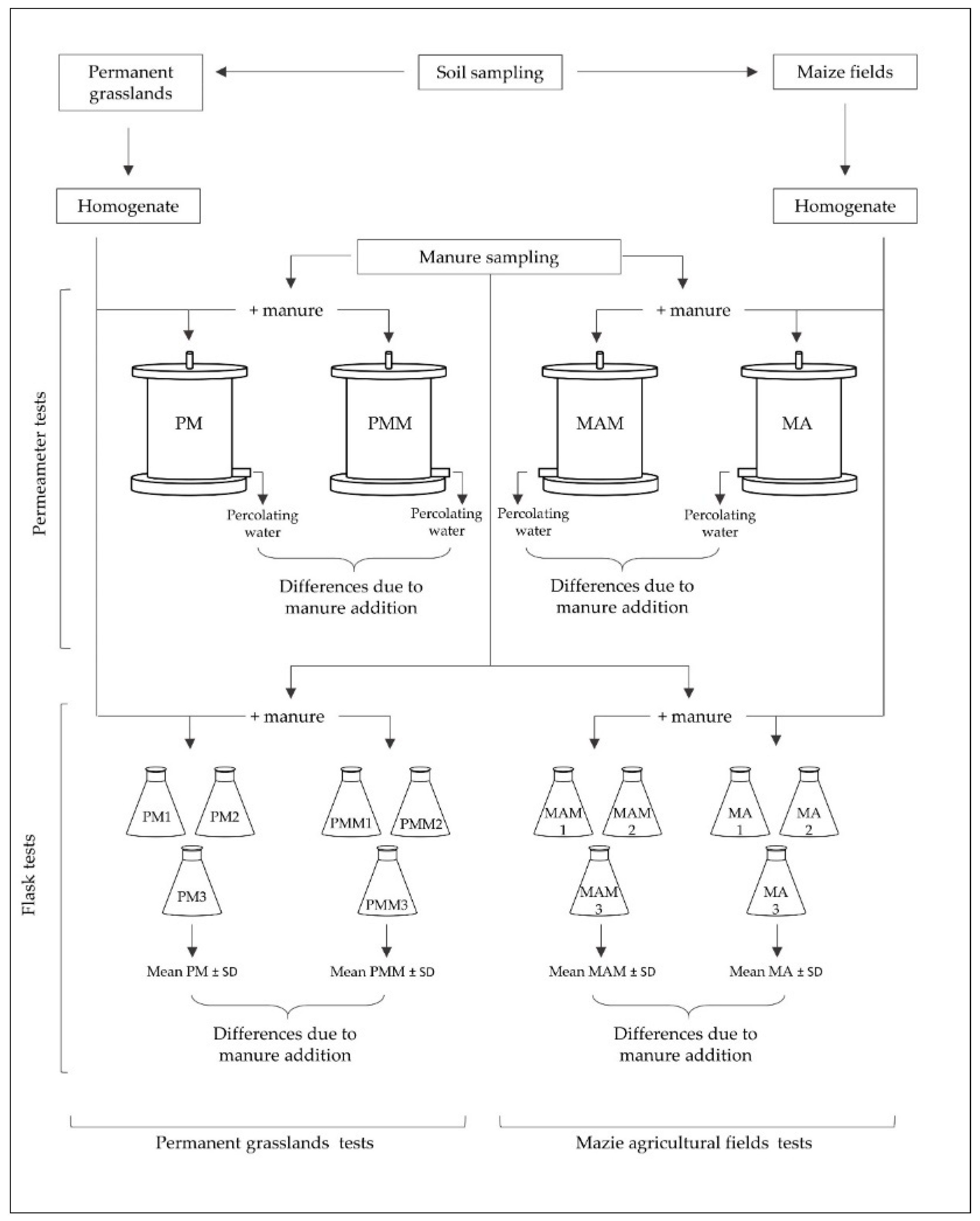
2.4. Soil Leaching Tests
3. Results
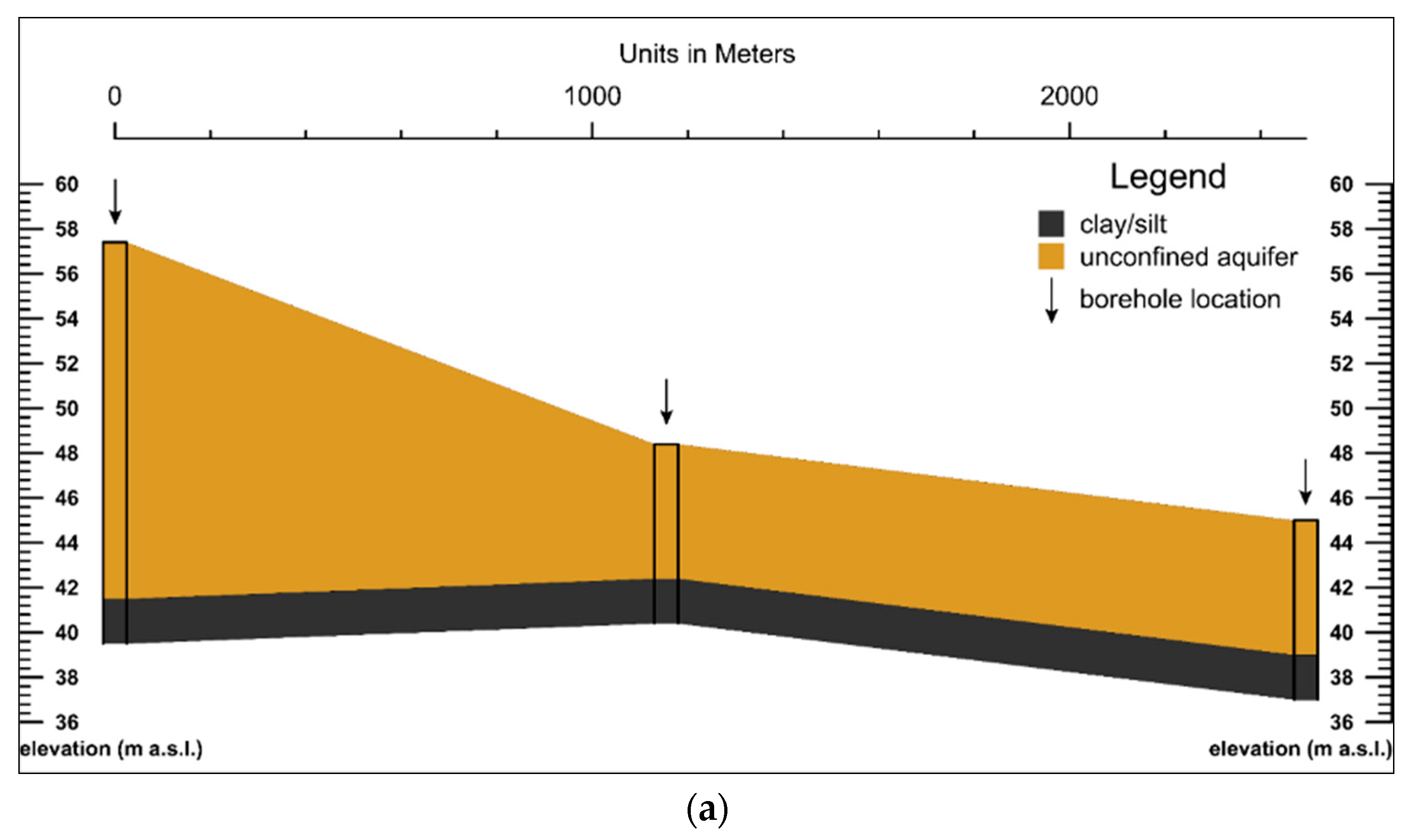
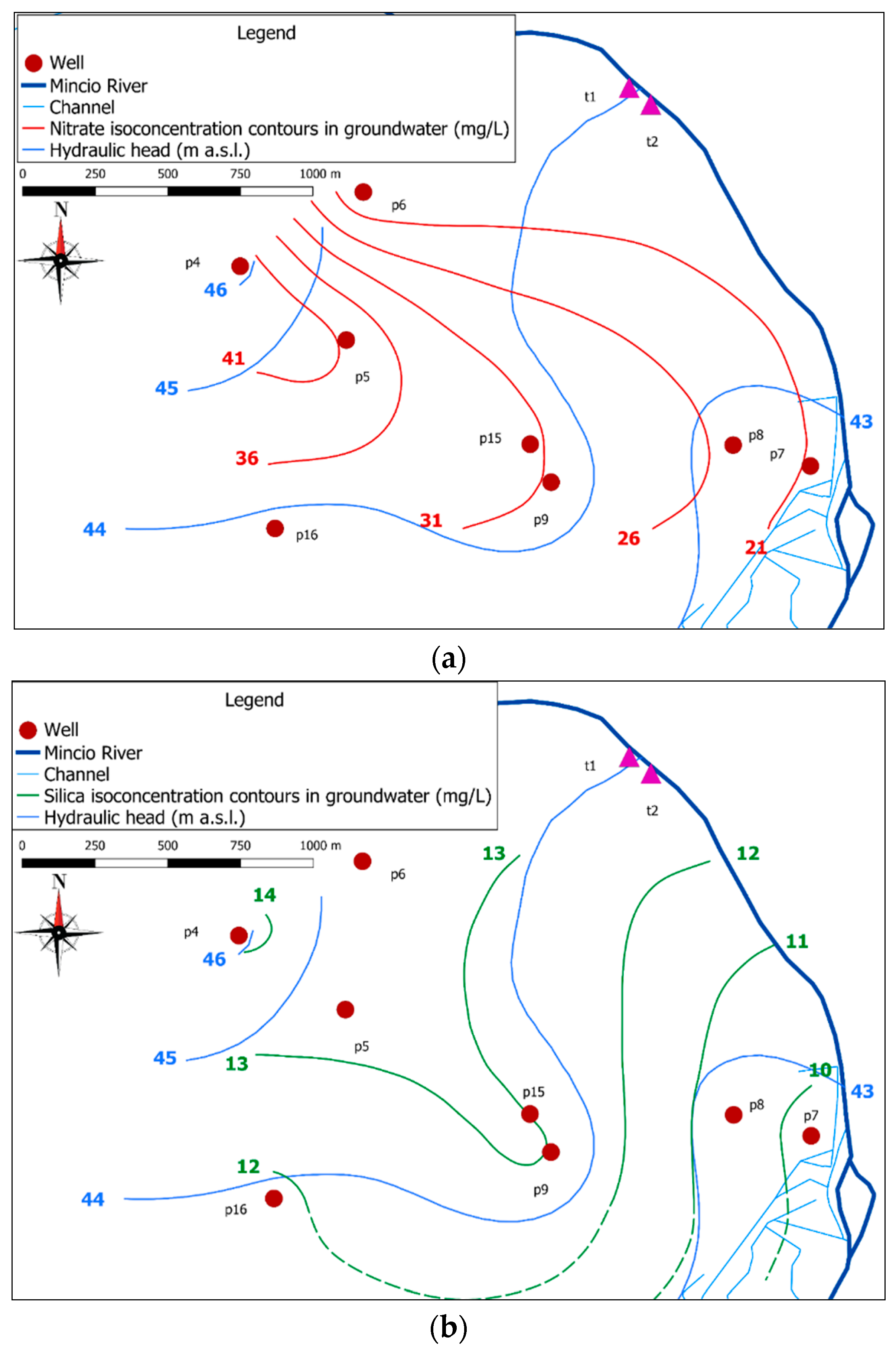
3.1. Geological and Hydrogeological Settings
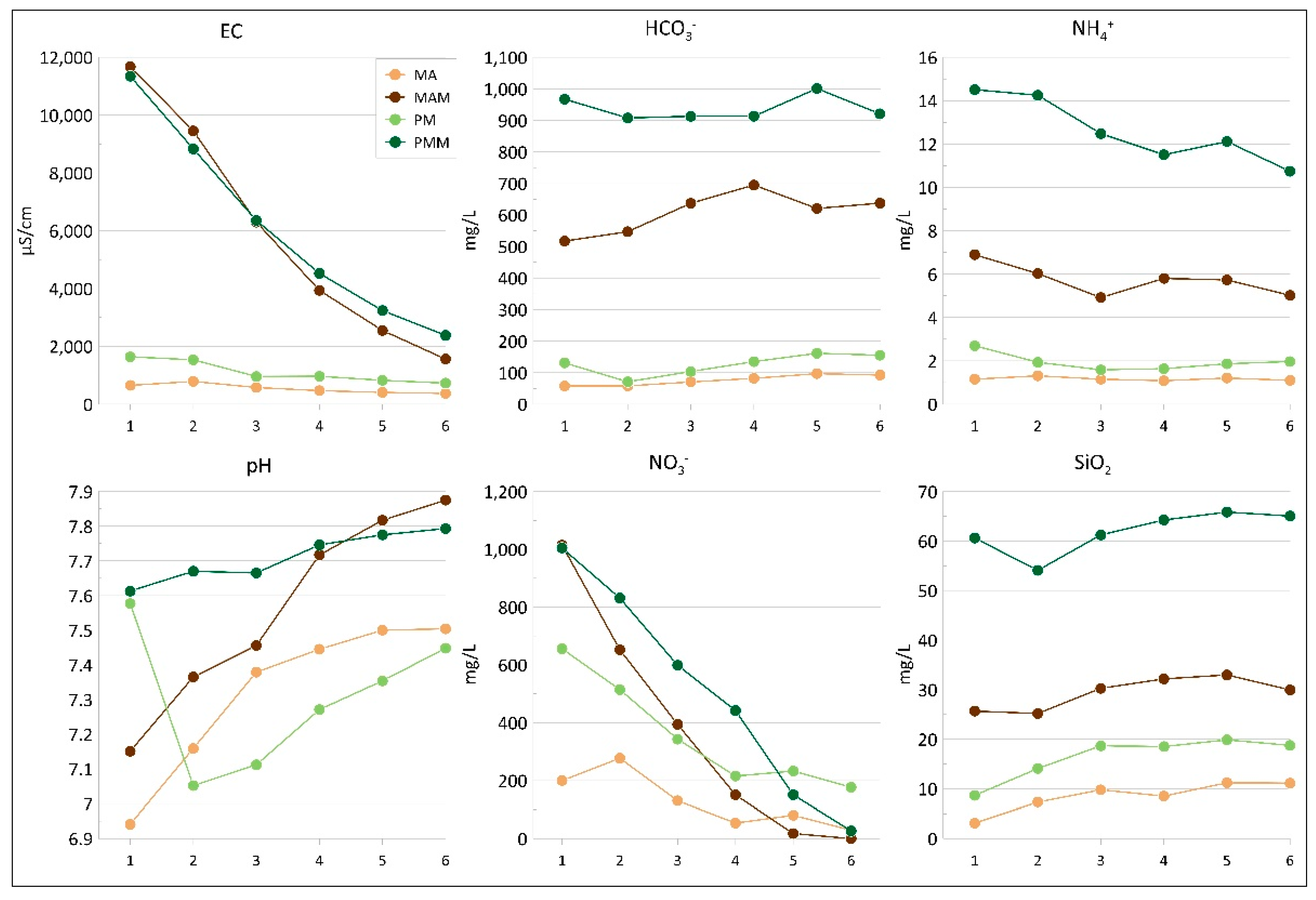
3.2. Column Tests
3.3. Flasks Tests
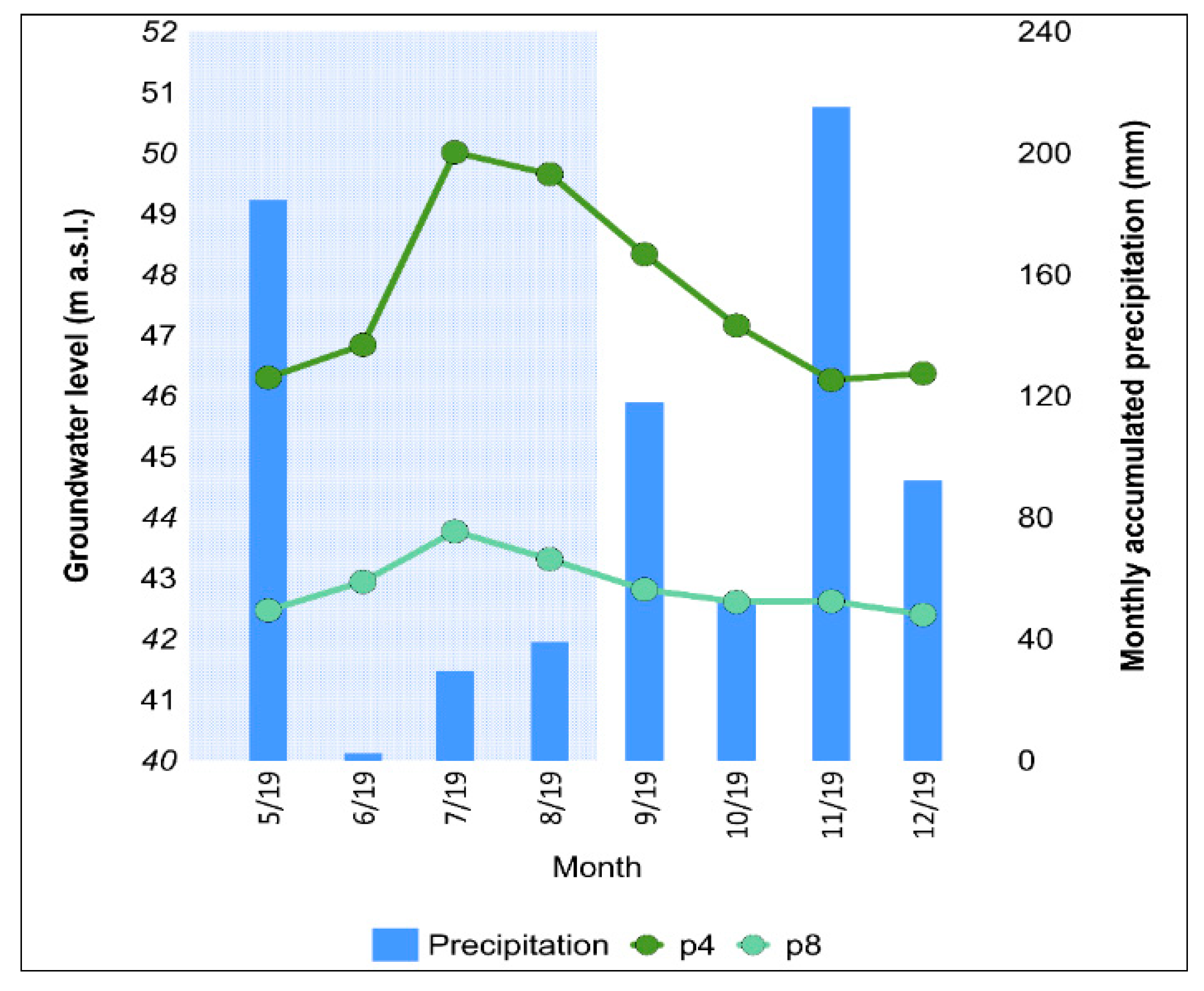
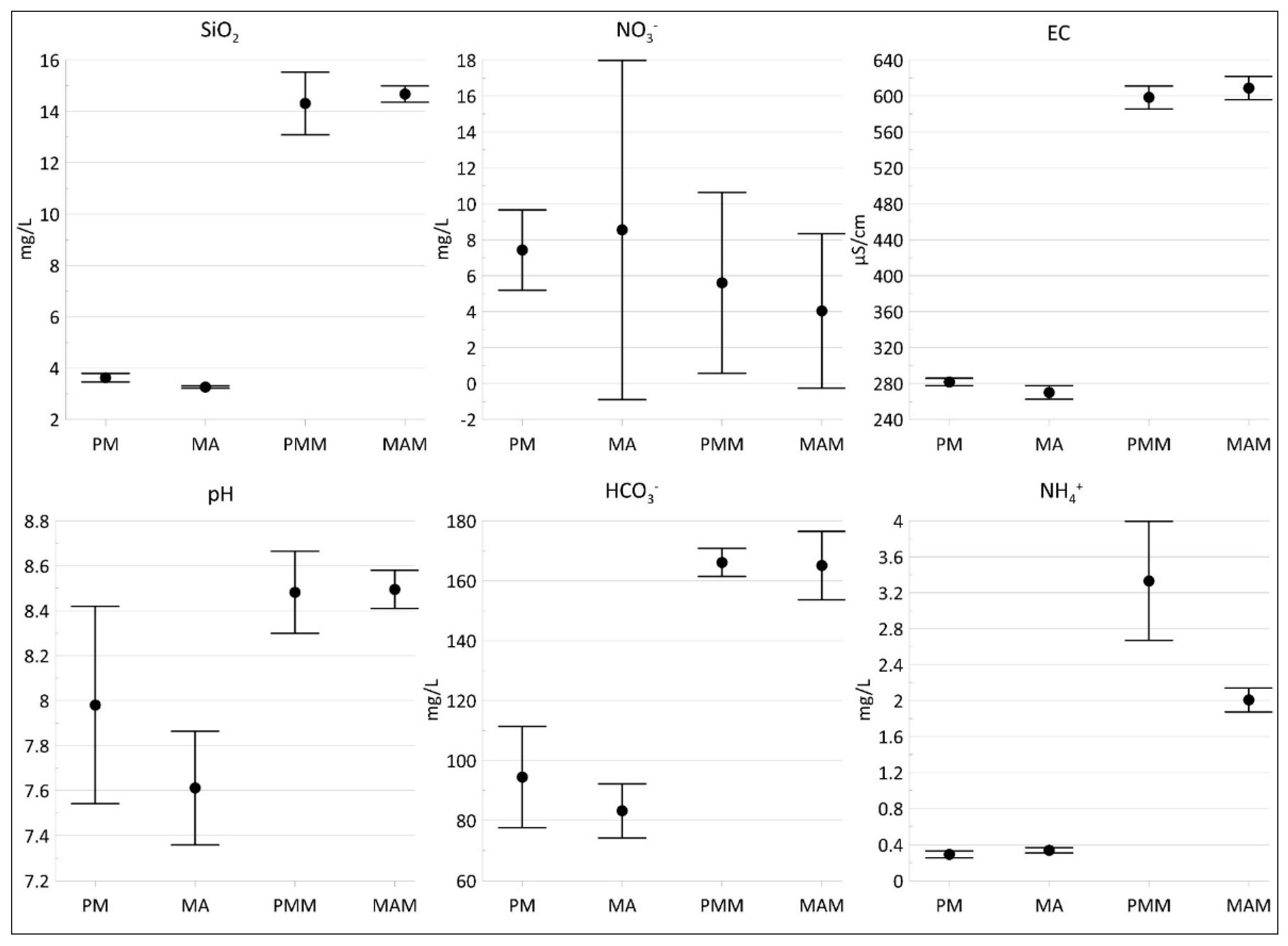
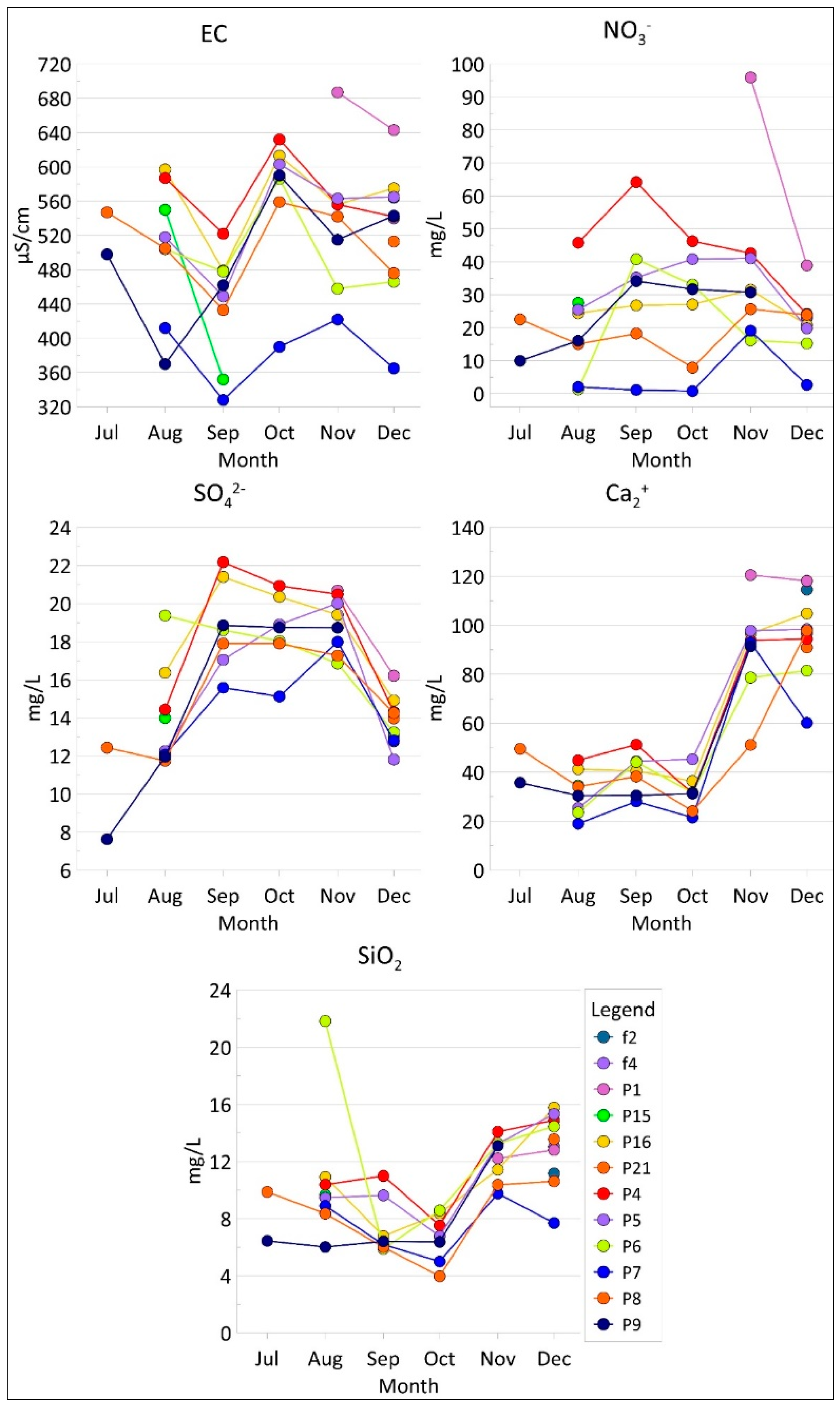
3.4. Groundwater Contamination
4. Discussion
4.1. Manure, Soil and Water Interactions
4.2. Groundwater Contamination in the Study Area: Insights from Laboratory Tests
5. Statements and Open Questions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ward, M.H.; Jones, R.R.; Brender, J.D.; de Kok, T.M.; Weyer, P.J.; Nolan, B.T.; Villanueva, C.M.; van Breda, S.G. Drinking water nitrate and human health: An updated review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, J. World Agriculture: Towards 2015/2030: An FAO Perspective; Earthscan: London, UK, 2003. [Google Scholar]
- Steinfeld, H.; Gerber, P.; Wassenaar, T.; Castel, V.; Rosales, M.; Rosales, M.; de Haan, C. Livestock’s Long Shadow: Environmental Issues and Options; Food & Agriculture Organization: Rome, Italy, 2006. [Google Scholar]
- Bernot, M.J.; Dodds, W.K. Nitrogen retention, removal, and saturation in lotic ecosystems. Ecosystems 2005, 8, 442–453. [Google Scholar] [CrossRef]
- Mulholland, P.J.; Helton, A.M.; Poole, G.C.; Hall, R.O., Jr.; Hamilton, S.K.; Peterson, B.J.; Tank, J.L.; Ashkenas, L.R.; Cooper, L.W.; Dahm, C.N.; et al. Stream denitrification across biomes and its response to anthropogenic nitrate loading. Nature 2008, 452, 202–205. [Google Scholar] [CrossRef]
- Paerl, H.W. Controlling eutrophication along the freshwater–marine continuum: Dual Nutrient (N and P) reductions are essential. Estuaries Coasts 2009, 32, 593–601. [Google Scholar] [CrossRef]
- Han, H.; Allan, J.D. Uneven rise in N inputs to the Lake Michigan Basin over the 20th century corresponds to agricultural and societal transitions. Biogeochemistry 2012, 109, 175–187. [Google Scholar] [CrossRef]
- Directive 2000/60/EC of the European Parliament and of the Council Establishing a Framework for the Community Action in the Field of Water Policy; European Union: Brussels, Belgium, 2000; pp. 1–73.
- Directive 2006/118/EC of the European Parliament and of the Council on the Protection of Groundwater against Pollution and Deterioration; European Union: Brussels, Belgium, 2006; pp. 19–31.
- WWAP (United Nations World Water Assessment Programme). The United Nations World Water Development Report 2015: Water for a Sustainable World; UNESCO: Paris, France, 2015. [Google Scholar]
- Winter, T.C. Relation of streams, lakes, and wetlands to groundwater flow systems. Hydrogeol. J. 1999, 7, 28–45. [Google Scholar] [CrossRef]
- Holman, I.P.; Whelan, M.J.; Howden, N.J.K.; Bellamy, P.H.; Willby, N.J.; Rivas-Casado, M.; McConvey, P. Phosphorus in groundwater-an overlooked contributor to eutrophication? Hydrol. Process. 2008, 22, 5121–5127. [Google Scholar] [CrossRef]
- Smolders, A.J.P.; Lucassen, E.C.H.E.T.; Bobbink, R.; Roelofs, J.G.M.; Lamers, L.P.M. How nitrate leaching from agricultural lands provokes phosphate eutrophication in groundwater fed wetlands: The sulphur bridge. Biogeochemistry 2010, 98, 1–7. [Google Scholar] [CrossRef]
- Hart, M.R.; Quin, B.F.; Nguyen, M.L. Phosphorus runoff from agricultural land and direct fertilizer effects: A review. J. Environ. Qual. 2004, 33, 1954–1972. [Google Scholar] [CrossRef]
- Cassman, K.G.; Dobermann, A.; Walters, D.T. Agroecosystems, nitrogen-use efficiency, and nitrogen management. Ambio 2002, 31, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, M.G. What impact might mitigation of diffuse nitrate pollution have on river water quality in a rural catchment? J. Environ. Manag. 2012, 109, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Ruehl, C.R.; Fisher, A.T.; Huertos, M.L.; Wankel, S.D.; Wheat, C.G.; Kendall, C.; Hatch, C.E.; Shennan, C. Nitrate dynamics within the Pajaro River, a nutrient-rich, losing stream. J. N. Am. Benthol. Soc. 2007, 26, 191–206. [Google Scholar] [CrossRef]
- Bouwer, H. Effect of Irrigated Agriculture on Groundwater. J. Irrig. Drain. Eng. 1987, 113, 4–15. [Google Scholar] [CrossRef]
- Bouwer, H.; Dedrick, A.R.; Jaynes, D.B. Irrigation management for groundwater quality protection. Irrig. Drain. Syst. 1990, 4, 375–383. [Google Scholar] [CrossRef]
- Postma, D.; Boesen, C.; Kristiansen, H.; Larsen, F. Nitrate reduction in an unconfined sandy aquifer: Water chemistry, reduction processes, and geochemical modeling. Water Resour. Res. 1991, 27, 2027–2045. [Google Scholar] [CrossRef]
- Baker, L.A. Introduction to nonpoint source pollution in the United States and prospects for wetland use. Ecol. Eng. 1992, 1, 1–26. [Google Scholar] [CrossRef]
- Chowdary, M.; Rao, N.H.; Sarma, P.B.S. Decision support framework for assessment of non-point-source pollution of groundwater in large irrigation projects. Agric. Water Manag. 2005, 75, 194–225. [Google Scholar] [CrossRef]
- Liao, L.; Green, C.T.; Bekins, B.A.; Böhlke, J.K. Factors controlling nitrate fluxes in groundwater in agricultural areas. Water Resour. Res. 2012, 48. [Google Scholar] [CrossRef]
- Ascott, M.J.; Gooddy, D.C.; Wang, L.; Stuart, M.E.; Lewis, M.A.; Ward, R.S.; Binley, A.M. Global patterns of nitrate storage in the vadose zone. Nat. Commun. 2017, 8, 1416. [Google Scholar] [CrossRef]
- Leng, G.; Huang, M.; Tang, Q.; Gao, H.; Leung, L.R. Modeling the Effects of Groundwater-Fed Irrigation on Terrestrial Hydrology over the Conterminous United States. J. Hydrometeorol. 2014, 15, 957–972. [Google Scholar] [CrossRef]
- Rotiroti, M.; Bonomi, T.; Sacchi, E.; McArthur, J.M.; Stefania, G.A.; Zanotti, C.; Taviani, S.; Patelli, M.; Nava, V.; Soler, V.; et al. The effects of irrigation on groundwater quality and quantity in a human-modified hydro-system: The Oglio River basin, Po Plain, northern Italy. Sci. Total Environ. 2019, 672, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Fan, A.M.; Willhite, C.C.; Book, S.A. Evaluation of the nitrate drinking water standard with reference to infant methemoglobinemia and potential reproductive toxicity. Regul. Toxicol. Pharmacol. 1987, 7, 135–148. [Google Scholar] [CrossRef]
- Schullehner, J.; Hansen, B.; Thygesen, M.; Pedersen, C.B.; Sigsgaard, T. Nitrate in drinking water and colorectal cancer risk: A nationwide population-based cohort study. Int. J. Cancer 2018, 143, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Council Directive 91/676/EEC of 12 December 1991 concerning the protection of waters against pollution caused by nitrates from agricultural sources. 91/676/EEC; European Union: Brussels, Belgium, 1991; pp. 1–8.
- Commission Implementing Decision of 3 November 2011 on Granting a Derogation Requested by Italy with regard to the Regions of Emilia Romagna, Lombardia, Piemonte and Veneto Pursuant to Council Directive 91/676/EEC Concerning the Protection of Waters against Pollution Caused by Nitrates from Agricultural Sources (Notified under Document C(2011) 7770); European Union: Brussels, Belgium, 2011; pp. 36–41.
- Commission Implementing Decision (EU) 2016/1040 of 24 June 2016 on Granting a Derogation Requested by Italian Republic with regard to the Regions of Lombardia and Piemonte pursuant to Council Directive 91/676/EEC Concerning the Protection of Waters against Pollution Caused by Nitrates from Agricultural Sources (Notified under Document C(2016) 3820); European Union: Brussels, Belgium, 2016; pp. 6–13.
- Lasagna, M.; De Luca, D.A.; Franchino, E. Nitrate contamination of groundwater in the western Po Plain (Italy): The effects of groundwater and surface water interactions. Environ. Earth Sci. 2016, 75. [Google Scholar] [CrossRef]
- Zanini, A.; Petrella, E.; Sanangelantoni, A.M.; Angelo, L.; Ventosi, B.; Viani, L.; Rizzo, P.; Remelli, S.; Bartoli, M.; Bolpagni, R.; et al. Groundwater characterization from an ecological and human perspective: An interdisciplinary approach in the Functional Urban Area of Parma, Italy. Rend. Lincei Sci. Fis. Nat. 2018, 30, 93–108. [Google Scholar] [CrossRef]
- Sacchi, E.; Acutis, M.; Bartoli, M.; Brenna, S.; Delconte, C.A.; Laini, A.; Pennisi, M. Origin and fate of nitrates in groundwater from the central Po plain: Insights from isotopic investigations. Appl. Geochem. 2013, 34, 164–180. [Google Scholar] [CrossRef]
- Soana, E.; Racchetti, E.; Laini, A.; Bartoli, M.; Viaroli, P. Soil budget, net export, and potential sinks of nitrogen in the lower oglio river watershed (Northern Italy). CLEAN Soil Air Water 2011, 39, 956–965. [Google Scholar] [CrossRef]
- Martinelli, G.; Dadomo, A.; De Luca, D.A.; Mazzola, M.; Lasagna, M.; Pennisi, M.; Pilla, G.; Sacchi, E.; Saccon, P. Nitrate sources, accumulation and reduction in groundwater from Northern Italy: Insights provided by a nitrate and boron isotopic database. Appl. Geochem. 2018, 91, 23–35. [Google Scholar] [CrossRef]
- Fantoni, G. Water management in Milan and Lombardy in medieval times: An outline. J. Water Land Dev. 2008, 12, 15–25. [Google Scholar] [CrossRef]
- Pinardi, M.; Soana, E.; Laini, A.; Bresciani, M.; Bartoli, M. Soil system budgets of N, Si and P in an agricultural irrigated watershed: Surplus, differential export and underlying mechanisms. Biogeochemistry 2018, 140, 175–197. [Google Scholar] [CrossRef]
- Viaroli, P.; Soana, E.; Pecora, S.; Laini, A.; Naldi, M.; Fano, E.A.; Nizzoli, D. Space and time variations of watershed N and P budgets and their relationships with reactive N and P loadings in a heavily impacted river basin (Po river, Northern Italy). Sci. Total Environ. 2018, 639, 1574–1587. [Google Scholar] [CrossRef]
- Laini, A.; Bartoli, M.; Castaldi, S.; Viaroli, P.; Capri, E.; Trevisan, M. Greenhouse gases (CO2, CH4 and N2O) in lowland springs within an agricultural impacted watershed (Po River Plain, northern Italy). Chem. Ecol. 2011, 27, 177–187. [Google Scholar] [CrossRef]
- Bartoli, M.; Racchetti, E.; Delconte, C.A.; Sacchi, E.; Soana, E.; Laini, A.; Longhi, D.; Viaroli, P. Nitrogen balance and fate in a heavily impacted watershed (Oglio River, Northern Italy): In quest of the missing sources and sinks. Biogeosciences 2012, 9, 361–373. [Google Scholar] [CrossRef]
- Racchetti, E.; Salmaso, F.; Pinardi, M.; Quadroni, S.; Soana, E.; Sacchi, E.; Severini, E.; Celico, F.; Viaroli, P.; Bartoli, M. Is flood irrigation a potential driver of river-groundwater interactions and diffuse nitrate pollution in agricultural watersheds? Water 2019, 11, 2304. [Google Scholar] [CrossRef]
- Amorosi, A.; Pavesi, M.; Ricci Lucchi, M.; Sarti, G.; Piccin, A. Climatic signature of cyclic fluvial architecture from the Quaternary of the central Po Plain, Italy. Sediment. Geol. 2008, 209, 58–68. [Google Scholar] [CrossRef]
- Chelli, A.; Zanini, A.; Petrella, E.; Feo, A.; Celico, F. A multidisciplinary procedure to evaluate and optimize the efficacy of hydraulic barriers in contaminated sites: A case study in Northern Italy. Environ. Earth Sci. 2018, 77, 246. [Google Scholar] [CrossRef]
- Guzzetti, F.; Marchetti, M.; Reichenbach, P.J.G. Large alluvial fans in the north-central Po Plain (Northern Italy). Geomorphology 1997, 18, 119–136. [Google Scholar] [CrossRef]
- Marchetti, M. Environmental changes in the central Po Plain (northern Italy) due to fluvial modifications and anthropogenic activities. Geomorphology 2002, 44, 361–373. [Google Scholar] [CrossRef]
- Kløve, B.; Ala-Aho, P.; Bertrand, G.; Boukalova, Z.; Ertürk, A.; Goldscheider, N.; Ilmonen, J.; Karakaya, N.; Kupfersberger, H.; Kvœrner, J.J.E.S.; et al. Groundwater dependent ecosystems. Part I: Hydroecological status and trends. Environ. Sci. Policy 2011, 14, 770–781. [Google Scholar] [CrossRef]
- Italian National Institute of Statistics. 6th General Census of Agriculture. 2010. Available online: http://dati-censimentoagricoltura.istat.it/Index.aspx. (accessed on 15 July 2020).
- Perego, A.; Basile, A.; Bonfante, A.; De Mascellis, R.; Terribile, F.; Brenna, S.; Acutis, M. Nitrate leaching under maize cropping systems in Po Valley (Italy). Agric. Ecosyst. Environ. 2012, 147, 57–65. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Köppen, W. Das Geographische System der Klimate; Gebrüder Borntraeger: Berlin, Germany, 1936; Volume 1, p. 46. [Google Scholar]
- Regione Lombardia. Banca Dati Geologica Sottosuolo. 2020. Available online: http://www.geoportale.regione.lombardia.it/metadati?p_p_id=PublishedMetadata_WAR_geoportalemetadataportlet&p_p_lifecycle=0&p_p_state=maximized&p_p_mode=view&_PublishedMetadata_WAR_geoportalemetadataportlet_view=editPublishedMetadata&_PublishedMetadata_WAR_geoportalemetadataportlet_uuid=%7BDAF98B21-3257-4D23-9D53-5AECC966D872%7D&_PublishedMetadata_WAR_geoportalemetadataportlet_editType=view&_PublishedMetadata_WAR_geoportalemetadataportlet_fromAsset=true&rid=local (accessed on 16 June 2020).
- Jenkins, D.; Connors, J.J.; Greenberg, A.E. Standard Methods for the Examination of Water and Wastewater, 15th ed.; American Public Health Association: Washington, DC, USA, 1981. [Google Scholar]
- Anderson, L.G.; Hall, P.O.J.; Iverfeldt, A.; Rutgers van der Loejf, M.M.; Sundby, B.; Westerlund, S.F.G. Benthic respiration measured by total carbonate production. Limnol. Oceanogr. 1986, 31, 319–329. [Google Scholar] [CrossRef]
- Lienig, D.; Golterman, H.L.; Clymo, R.S.; Ohnstadt, M.A.M. Methods for Physical and Chemical Analysis of Freshwaters—1BP Handbook No 8, 2nd ed.; Blackwell Scientific Publications: Oxford, UK; Edinburgh, UK; London, UK; Melbourne, Australia, 1978. [Google Scholar]
- Rodier, J.; Geoffray, C.; Rodi, L. L’analyse de l’eau: Eaux Naturelles, Eaux Résiduaires, eau de mer: Chimie, Physico-Chimie, Bactériologie, Biologie; Dunod: Paris, France, 1975. [Google Scholar]
- Eghball, B.; Wienhold, B.; Gilley, J.E.; Eigenberg, R. Mineralization of manure nutrients. J. Soil Water Conserv. 2002, 57, 470–473. [Google Scholar]
- Mantovi, P.; Fumagalli, L.; Beretta, G.P.; Guermandi, M. Nitrate leaching through the unsaturated zone following pig slurry applications. J. Hydrol. 2006, 316, 195–212. [Google Scholar] [CrossRef]
- Boschetti, T.; Falasca, A.; Bucci, A.; De Felice, V.; Naclerio, G.; Celico, F. Influence of soil on groundwater geochemistry in a carbonate aquifer, southern Italy. Int. J. Speleol. 2014, 43, 79–94. [Google Scholar] [CrossRef]
- Borch, T.; Kretzschmar, R.; Kappler, A.; Cappellen, P.V.; Ginder-Vogel, M.; Voegelin, A.; Campbell, K. Biogeochemical redox processes and their impact on contaminant dynamics. Environ. Sci. Technol. 2010, 44, 15–23. [Google Scholar] [CrossRef]
- Christensen, T.H.; Bjerg, P.L.; Banwart, S.A.; Jakobsen, R.; Heron, G.; Albrechtsen, H.-J. Characterization of redox conditions in groundwater contaminant plumes. J. Contam. Hydrol. 2000, 45, 165–241. [Google Scholar] [CrossRef]
- Scheidegger, A.; Sparks, D.J.S.S. A critical assessment of sorption-desorption mechanisms at the soil mineral/water interface. Soil Sci. 1996, 161, 813–831. [Google Scholar] [CrossRef]
- Cornelis, J.T.; Delvaux, B.; Georg, R.B.; Lucas, Y.; Ranger, J.; Opfergelt, S. Tracing the origin of dissolved silicon transferred from various soil-plant systems towards rivers: A review. Biogeosciences 2011, 8, 89–112. [Google Scholar] [CrossRef]
- Song, Z.; Wang, H.; Strong, P.J.; Shan, S. Increase of available soil silicon by Si-rich manure for sustainable rice production. Agron. Sustain. Dev. 2014, 34, 813–819. [Google Scholar] [CrossRef]
- Wick, K.; Heumesser, C.; Schmid, E. Groundwater nitrate contamination: Factors and indicators. J. Environ. Manag. 2012, 111, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Kirchmann, H.; Hansen, J.; Henriksen, K. A 3-year N balance study with aerobic, anaerobic and fresh 15N-labelled poultry manure. Nitrogen Org. Wastes 1989, 113–125. [Google Scholar] [CrossRef]
- Finck, A.; Hagin, J.; Tucker, B. Fertilization of Dryland and Irrigated Soils (Advanced Series in Agriculture Science 12); Springer: Berlin, Germany, 1982. [Google Scholar]
- Estavillo, J.M.; Merino, P.; Pinto, M.; Yamulki, S.; Gebauer, G.; Sapek, A.; Corré, W. Short term effect of ploughing a permanent pasture on N2O production from nitrification and denitrification. Plant Soil 2002, 239, 253–265. [Google Scholar] [CrossRef]
- Jabloun, M.; Schelde, K.; Tao, F.; Olesen, J.E. Effect of temperature and precipitation on nitrate leaching from organic cereal cropping systems in Denmark. Eur. J. Agron. 2015, 62, 55–64. [Google Scholar] [CrossRef]
- Hess, L.J.T.; Hinckley, E.-L.S.; Robertson, G.P.; Matson, P.A. Rainfall intensification increases nitrate leaching from tilled but not no-till cropping systems in the U.S. Midwest. Agric. Ecosyst. Environ. 2020, 290, 106747. [Google Scholar] [CrossRef]
- Zilio, M.; Motta, S.; Tambone, F.; Scaglia, B.; Boccasile, G.; Squartini, A.; Adani, F. The distribution of functional N-cycle related genes and ammonia and nitrate nitrogen in soil profiles fertilized with mineral and organic N fertilizer. PLoS ONE 2020, 15, e0228364. [Google Scholar] [CrossRef]
- Emerson, K.; Russo, R.C.; Lund, R.E.; Thurston, R.V. Aqueous ammonia equilibrium calculations: Effect of pH and temperature. J. Fish. Res. Board Can. 1975, 32, 2379–2383. [Google Scholar] [CrossRef]
- Spalding, R.F.; Exner, M.E. Occurrence of nitrate in groundwater—A review. J. Environ. Qual. 1993, 22, 392–402. [Google Scholar] [CrossRef]
- Strayer, R.F.; Lin, C.J.; Alexander, M. Effect of simulated acid rain on nitrification and nitrogen mineralization in forest soils. J. Environ. Qual. 1981, 10, 547–551. [Google Scholar] [CrossRef]
- Curtin, D.; Campbell, C.A.; Jalil, A. Effects of acidity on mineralization: pH-dependence of organic matter mineralization in weakly acidic soils. Soil Biol. Biochem. 1998, 30, 57–64. [Google Scholar] [CrossRef]
- Paul, E. Soil Microbiology, Ecology and Biochemistry; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Lozovik, P.A. Contribution of organic acid anions to the alkalinity of natural humic water. J. Anal. Chem. 2005, 60, 1000–1004. [Google Scholar] [CrossRef]
- Guenzi, W.D.; Beard, W.E.; Watanabe, F.S.; Olsen, S.R.; Porter, L.K. Nitrification and denitrification in cattle manure-amended soil. J. Environ. Qual. 1978, 7, 196–202. [Google Scholar] [CrossRef]
- Chang, C.; Sommerfeldt, T.G.; Entz, T. Soil Chemistry after Eleven Annual Applications of Cattle Feedlot Manure. J. Environ. Qual. 1991, 20, 475–480. [Google Scholar] [CrossRef]
- Perego, A.; Sanna, M.; Giussani, A.; Chiodini, M.E.; Fumagalli, M.; Pilu, S.R.; Bindi, M.; Moriondo, M.; Acutis, M. Designing a high-yielding maize ideotype for a changing climate in Lombardy plain (northern Italy). Sci. Total Environ. 2014, 499, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Reithmaier, G.-M.S.; Knorr, K.-H.; Arnhold, S.; Planer-Friedrich, B.; Schaller, J. Enhanced silicon availability leads to increased methane production, nutrient and toxicant mobility in peatlands. Sci. Rep. 2017, 7, 8728. [Google Scholar] [CrossRef]
- Schaller, J.; Frei, S.; Rohn, L.; Gilfedder, B.S. Amorphous silica controls water storage capacity and phosphorus mobility in soils. Front. Environ. Sci. 2020, 8. [Google Scholar] [CrossRef]
- Petrella, E.; Naclerio, G.; Falasca, A.; Bucci, A.; Capuano, P.; Felice, V.; Celico, F. Non-permanent shallow halocline in a fractured carbonate aquifer, southern Italy. J. Hydrol. 2009, 373, 267–272. [Google Scholar] [CrossRef]
- Petrella, E.; Celico, F. Mixing of water in a carbonate aquifer, Southern Italy, analysed through stable isotope investigations. Int. J. Speleol. 2013, 42, 25–33. [Google Scholar] [CrossRef]
- Bucci, A.; Naclerio, G.; Allocca, V.; Celico, P.; Celico, F. Potential use of microbial community investigations to analyse hydrothermal systems behaviour: The case of Ischia Island, Southern Italy. Hydrol. Process. 2011, 25, 1866–1873. [Google Scholar] [CrossRef]
- Rizzo, P.; Petrella, E.; Bucci, A.; Salvioli-Mariani, E.; Chelli, A.; Sanangelantoni, A.; Raimondo, M.; Quagliarini, A.; Celico, F. Studying hydraulic interconnections in low-permeability media by using bacterial communities as natural tracers. Water 2020, 12, 1795. [Google Scholar] [CrossRef]
- Crescenzo, R.; Mazzoli, A.; Cancelliere, R.; Bucci, A.; Naclerio, G.; Baccigalupi, L.; Cutting, S.M.; Ricca, E.; Iossa, S. Beneficial effects of carotenoid-producing cells of Bacillus indicus HU16 in a rat model of diet-induced metabolic syndrome. Benef. Microbes 2017, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mancabelli, L.; Milani, C.; Lugli, G.A.; Fontana, F.; Turroni, F.; van Sinderen, D.; Ventura, M. The impact of primer design on amplicon-based metagenomic profiling accuracy: Detailed insights into bifidobacterial community structure. Microorganisms 2020, 8, 131. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Severini, E.; Bartoli, M.; Pinardi, M.; Celico, F. Reactive Silica Traces Manure Spreading in Alluvial Aquifers Affected by Nitrate Contamination: A Case Study in a High Plain of Northern Italy. Water 2020, 12, 2511. https://doi.org/10.3390/w12092511
Severini E, Bartoli M, Pinardi M, Celico F. Reactive Silica Traces Manure Spreading in Alluvial Aquifers Affected by Nitrate Contamination: A Case Study in a High Plain of Northern Italy. Water. 2020; 12(9):2511. https://doi.org/10.3390/w12092511
Chicago/Turabian StyleSeverini, Edoardo, Marco Bartoli, Monica Pinardi, and Fulvio Celico. 2020. "Reactive Silica Traces Manure Spreading in Alluvial Aquifers Affected by Nitrate Contamination: A Case Study in a High Plain of Northern Italy" Water 12, no. 9: 2511. https://doi.org/10.3390/w12092511
APA StyleSeverini, E., Bartoli, M., Pinardi, M., & Celico, F. (2020). Reactive Silica Traces Manure Spreading in Alluvial Aquifers Affected by Nitrate Contamination: A Case Study in a High Plain of Northern Italy. Water, 12(9), 2511. https://doi.org/10.3390/w12092511







