Influence of Salinity Gradient Changes on Phytoplankton Growth Caused by Sluice Construction in Yongjiang River Estuary Area
Abstract
1. Introduction
2. Materials and Methods
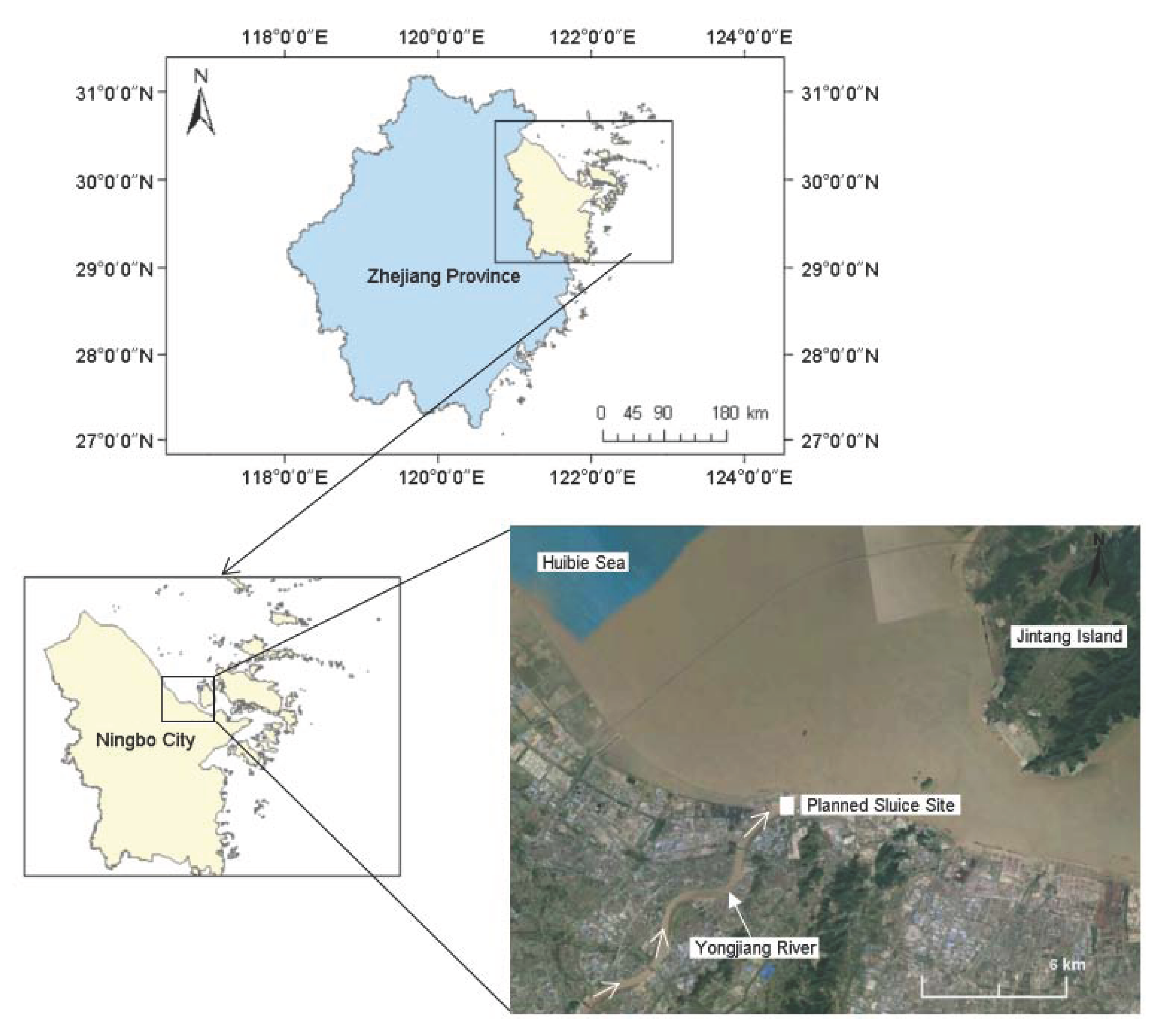
2.1. Description of Study Area
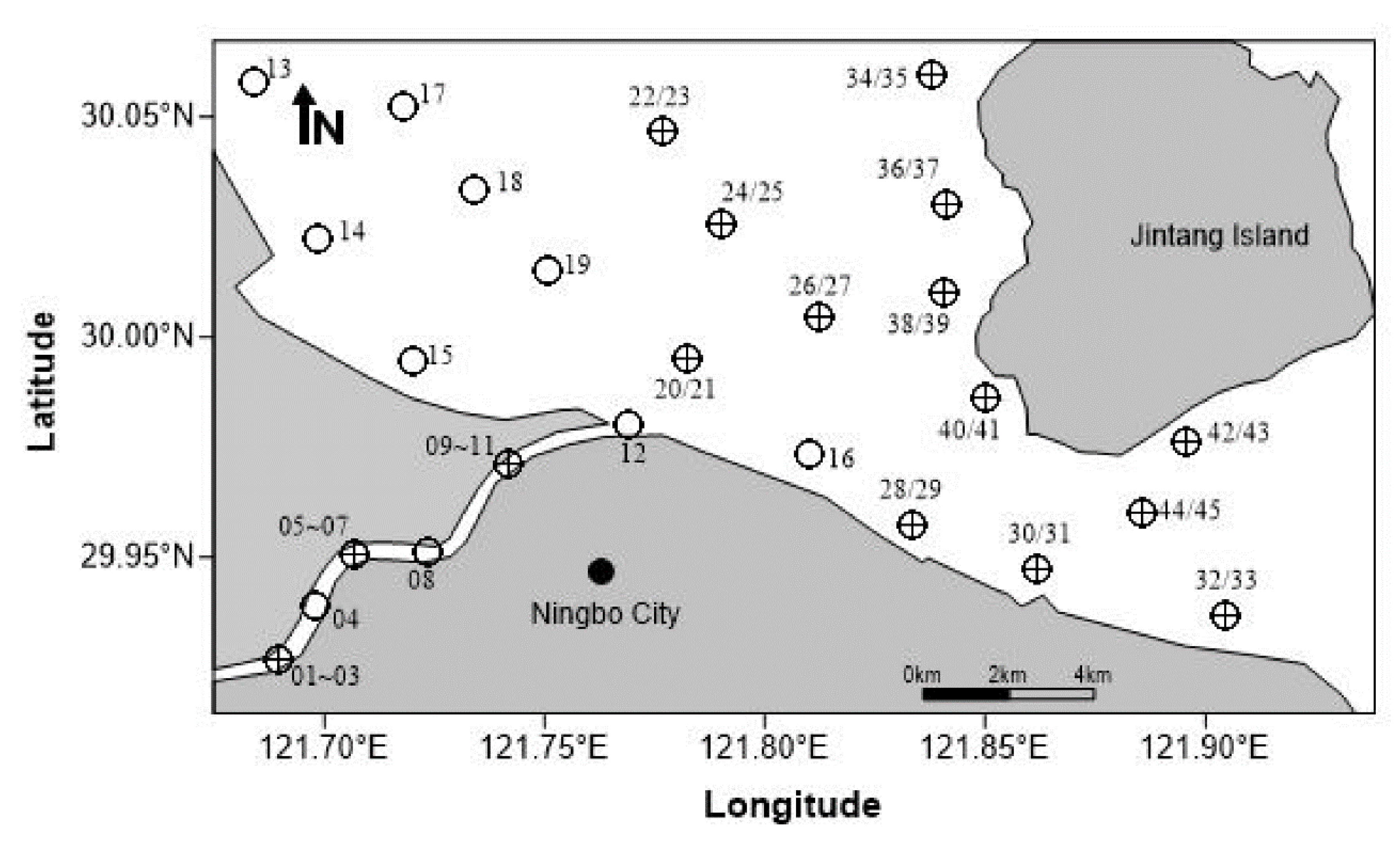
2.2. Data Collection
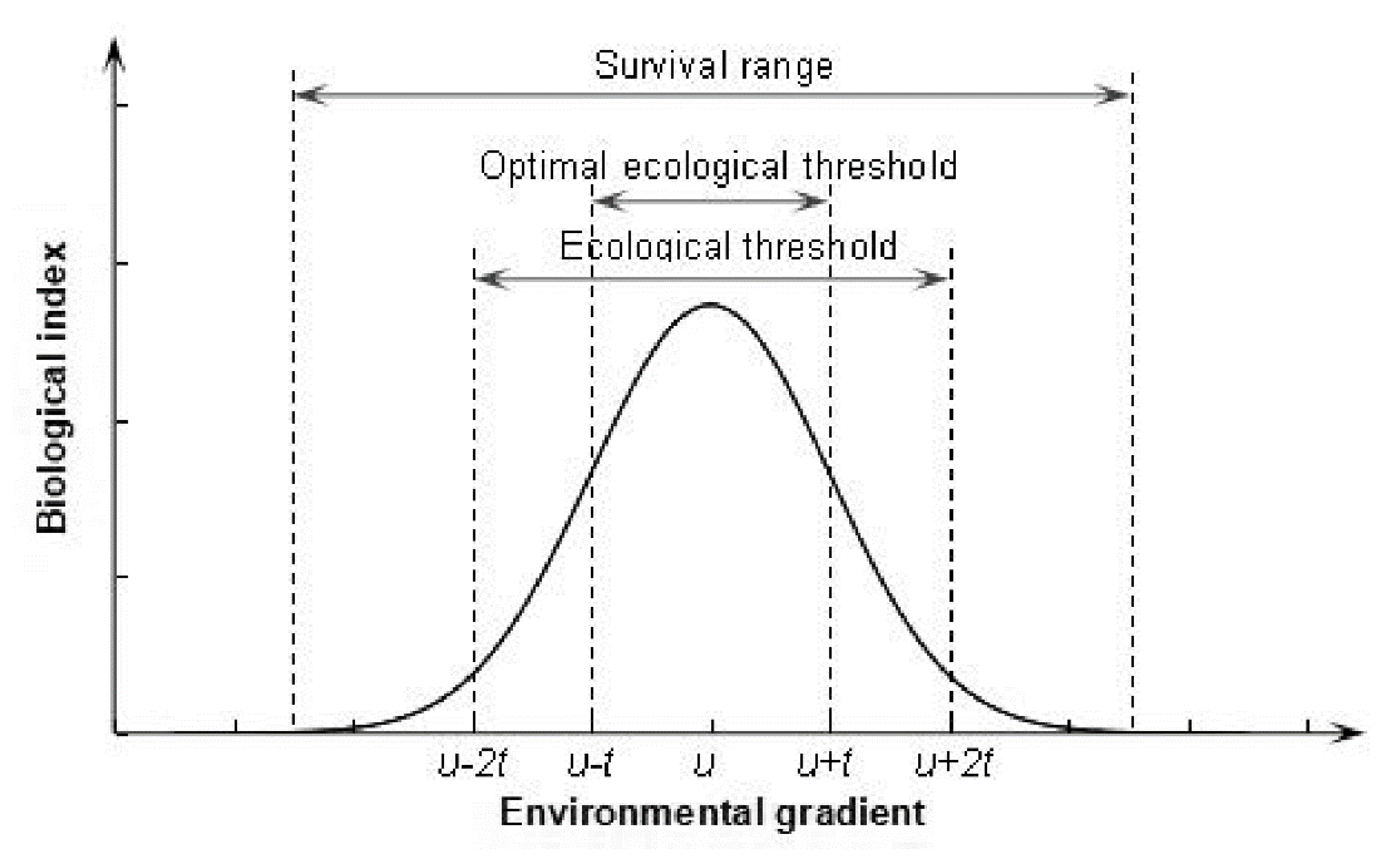
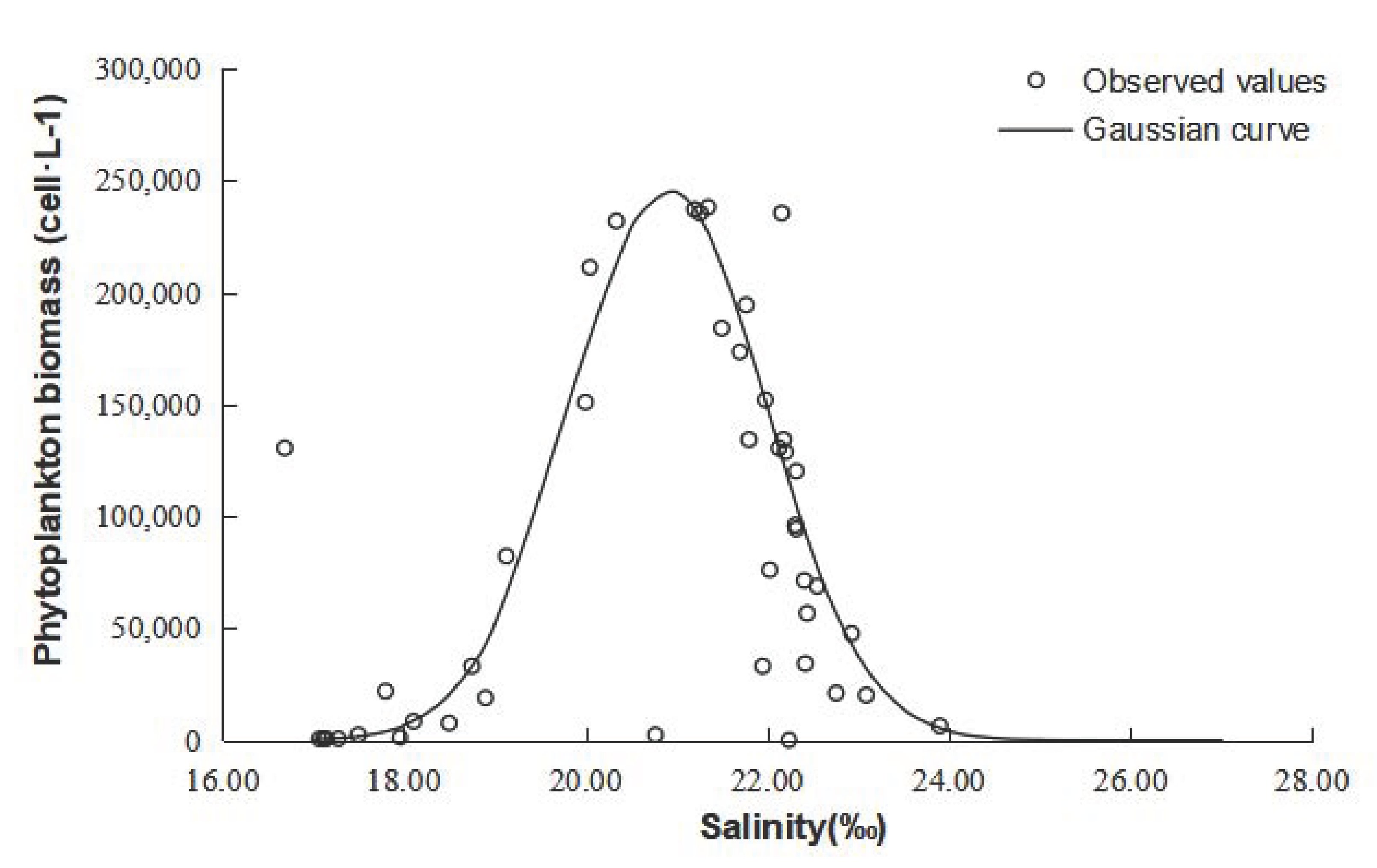
2.3. ET and Gaussian Curve Theory
2.4. Ecological Factor Selection
2.5. Numerical Simulation by MIKE 21 Software (DHI, Denmark)
- (1)
- Continuity Equation of water flow:
- (2)
- Momentum Equations pf water flow:
- , and are the space and time coordinates respectively; is water level;
- and are the components of vertical mean velocity in the direction of and respectively;
- and are the components of discharge per unit width in the directions of and ;
- , ; is the Manning roughness coefficient;
- is the Chezy coefficient, ;
- t is the turbulent viscosity coefficient; is the gravitational acceleration.
2.5.1. Model Establishment and Parameter Calibration
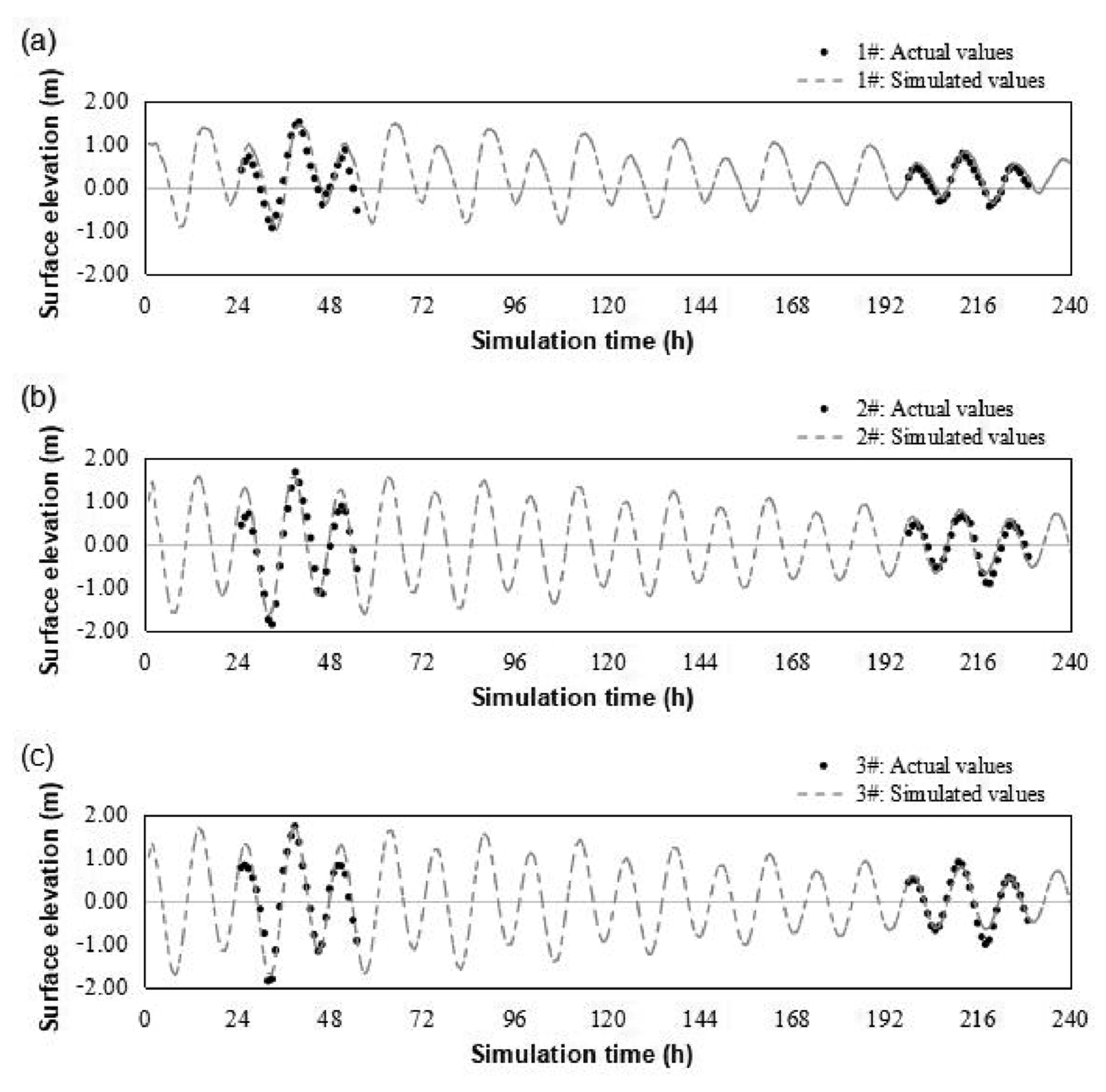
2.5.2. Tide Level Verification
2.5.3. Current Velocity Verification
2.5.4. Sluice Construction Simulation
3. Results
3.1. Results of Sample Analysis
3.2. Results of Water Level and Velocity Verification
3.2.1. Results of Water Level Verification
3.2.2. Results of Velocity Verification
3.3. Results of Salinity Simulation
3.4. ET Calculation
3.5. Descriptive Analysis of Salinity Values
4. Discussion and Conclusions
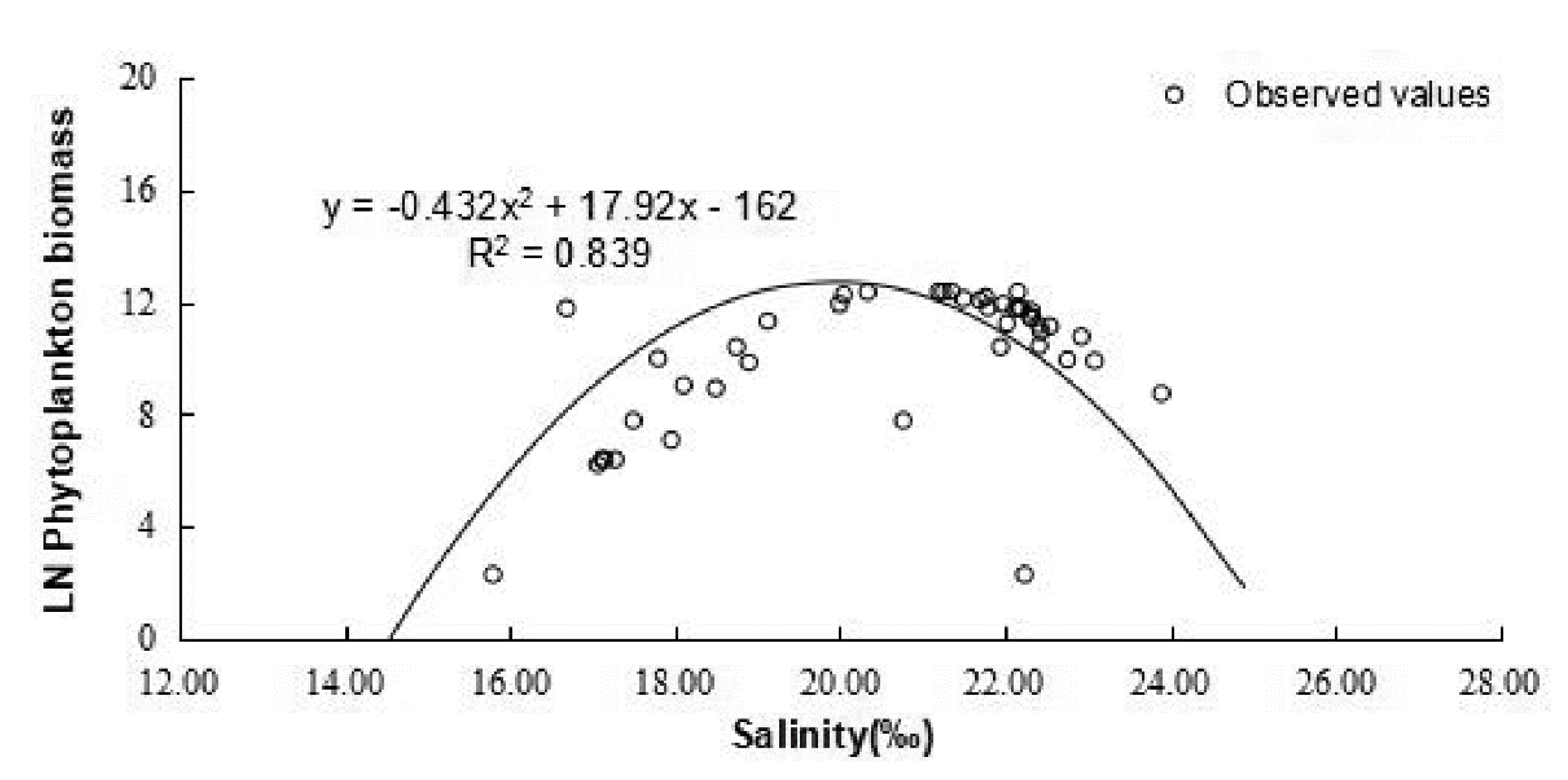
4.1. Checking of Gaussian Curve
4.2. Prediction of Phytoplankton Density
4.3. Limitation and Prospect
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Litchman, E.; Pinto, P.D.; Edwards, K.F.; Klausmeier, C.A.; Kremer, C.T.; Thomas, M.K. Global biogeochemical impacts of phytoplankton: A trait-based perspective. J. Ecol. 2015, 103, 1384–1396. [Google Scholar] [CrossRef]
- Goldyn, H. Changes in plant species diversity of aquatic ecosystems in the agricultural landscape in West Poland in the last 30 years. Biodivers. Conserv. 2010, 19, 61–80. [Google Scholar] [CrossRef]
- Caroppo, C. Ecology and biodiversity of picoplanktonic cyanobacteria in coastal and brackish environments. Biodivers. Conserv. 2015, 24, 949–971. [Google Scholar] [CrossRef]
- Peter, P.O.; Rashid, A.; Hou, L.; Nkinahamira, F.; Kiki, C.; Sun, Q.; Yu, C.-P.; Hu, A. Elemental contaminants in surface sediments from jiulong river estuary, china: Pollution level and ecotoxicological risk assessment. Water 2020, 12, 1640. [Google Scholar] [CrossRef]
- Lepisto, L.; Holopainen, L.L.; Vuoristo, H. Type-specific and indicator taxa of phytoplankton as a quality criterion for assessing the ecological status of Finnish boreal lakes. Limnologica 2004, 34, 236–248. [Google Scholar] [CrossRef]
- Rojo, C.; Alvarez-Cobelas, M.; Benavent-Corai, J.; Baron-Rodriguez, M.M.; Rodrigo, M.A. Trade-offs in plankton species richness arising from drought: Insights from long-term data of a National Park wetland (central Spain). Biodivers. Conserv. 2012, 21, 2453–2476. [Google Scholar] [CrossRef]
- Rath, A.R.; Mitbavkar, S.; Anil, A.C. Phytoplankton community structure in relation to environmental factors from the New Mangalore Port waters along the southwest coast of India. Environ. Monit. Assess. 2018, 190. [Google Scholar] [CrossRef]
- Thackeray, S.J.; Jones, I.D.; Maberly, S.C. Long-term change in the phenology of spring phytoplankton: Species-specific responses to nutrient enrichment and climatic change. J. Ecol. 2008, 96, 523–535. [Google Scholar] [CrossRef]
- Whitehead, P.G.; Bussi, G.; Bowes, M.J.; Read, D.S.; Hutchins, M.G.; Elliott, J.A.; Dadson, S.J. Dynamic modelling of multiple phytoplankton groups in rivers with an application to the Thames river system in the UK. Environ. Model. Softw. 2015, 74, 75–91. [Google Scholar] [CrossRef]
- Mousing, E.A.; Richardson, K.; Bendtsen, J.; Cetinic, I.; Perry, M.J. Evidence of small-scale spatial structuring of phytoplankton alpha- and beta-diversity in the open ocean. J. Ecol. 2016, 104, 1682–1695. [Google Scholar] [CrossRef]
- Santos, J.M.; Ferreira, M.T. Use of aquatic biota to detect ecological changes in freshwater: Current status and future directions. Water 2020, 12, 1611. [Google Scholar] [CrossRef]
- Zhao, G.; Pan, B.; Li, Y.; Zheng, X.; Zhu, P.; Zhang, L.; He, H. Phytoplankton in the heavy sediment-laden Weihe River and its tributaries from the northern foot of the Qinling Mountains: Community structure and environmental drivers. Environ. Sci. Pollut. Res. Int. 2020, 27, 8359–8370. [Google Scholar] [CrossRef] [PubMed]
- Huggett, A.J. The concept and utility of ’ecological thresholds’ in biodiversity conservation. Biol. Conserv. 2005, 124, 301–310. [Google Scholar] [CrossRef]
- Baker, M.E.; King, R.S. A new method for detecting and interpreting biodiversity and ecological community thresholds. Methods Ecol. Evol. 2010, 1, 25–37. [Google Scholar] [CrossRef]
- Roubeix, V.; Danis, P.A.; Feret, T.; Baudoin, J.M. Identification of ecological thresholds from variations in phytoplankton communities among lakes: Contribution to the definition of environmental standards. Environ. Monit. Assess. 2016, 188. [Google Scholar] [CrossRef]
- Groffman, P.; Baron, J.; Blett, T.; Gold, A.; Goodman, I.; Gunderson, L.; Levinson, B.; Palmer, M.; Paerl, H.; Peterson, G.; et al. Ecological thresholds: The key to successful environmental management or an important concept with no practical application? Ecosystems 2006, 9, 1–13. [Google Scholar] [CrossRef]
- Muradian, R. Ecological thresholds: A survey. Ecol. Econ. 2001, 38, 7–24. [Google Scholar] [CrossRef]
- With, K.A.; King, A.W. Extinction thresholds for species in fractal landscapes. Conserv. Biol. 1999, 13, 314–326. [Google Scholar] [CrossRef]
- Fahrig, L. How much habitat is enough? Biol. Conserv. 2001, 100, 65–74. [Google Scholar] [CrossRef]
- Soranno, P.A.; Cheruvelil, K.S.; Stevenson, R.J.; Rollins, S.L.; Holden, S.W.; Heaton, S.; Torng, E. A framework for developing ecosystem-specific nutrient criteria: Integrating biological thresholds with predictive modeling. Limnol. Oceanogr. 2008, 53, 773–787. [Google Scholar] [CrossRef]
- Andersen, T.; Carstensen, J.; Hernandez-Garcia, E.; Duarte, C.M. Ecological thresholds and regime shifts: Approaches to identification. Trends Ecol. Evol. 2009, 24, 49–57. [Google Scholar] [CrossRef]
- Catalan, J.; Barbieri, M.G.; Bartumeus, F.; Bitusik, P.; Botev, I.; Brancelj, A.; Cogalniceanu, D.; Manca, M.; Marchetto, A.; Ognjanova-Rumenova, N.; et al. Ecological thresholds in European alpine lakes. Freshwater Biol. 2009, 54, 2494–2517. [Google Scholar] [CrossRef]
- Basang, W.D.; Zhu, Y.B. Whole-genome analysis identifying candidate genes of altitude adaptive ecological thresholds in yak populations. J. Anim. Breed. Genet. 2019, 136, 371–377. [Google Scholar] [CrossRef]
- Wolski, K.; Tymiński, T. Studies on the threshold density of Phragmites australis plant concentration as a factor of hydraulic interactions in the riverbed. Ecol. Eng. 2020, 151. [Google Scholar] [CrossRef]
- Kanaya, G.; Nakamura, Y.; Koizumi, T. Ecological thresholds of hypoxia and sedimentary H2S in coastal soft-bottom habitats: A macroinvertebrate-based assessment. Mar. Environ. Res. 2018, 136, 27–37. [Google Scholar] [CrossRef]
- Daily, J.P.; Hitt, N.P.; Smith, D.R.; Snyder, C.D. Experimental and environmental factors affect spurious detection of ecological thresholds. Ecology 2012, 93, 17–23. [Google Scholar] [CrossRef]
- Cui, Y.P. Preliminary estimation of the realistic optimum temperature for vegetation growth in China. Environ. Manag. 2013. [Google Scholar] [CrossRef]
- Wen, S.Y.; Song, L.L.; Long, H.; Yu, J.; Gao, S.G.; Zhao, D.Z. Nutrient-based method for assessing the hazard degree of red tide: A case study in the zhejiang coastal waters, east china sea. Environ. Earth Sci. 2013, 70, 2671–2678. [Google Scholar] [CrossRef]
- McDonald, K.S.; Tighe, M.; Ryder, D.S. An ecological risk assessment for managing and predicting trophic shifts in estuarine ecosystems using a Bayesian network. Environ. Model. Softw. 2016, 85, 202–216. [Google Scholar] [CrossRef]
- Shelford, V.E. Physiological animal geography. J. Morphol. 1911, 22, 551–618. [Google Scholar] [CrossRef]
- Thompson, P.A. Temporal variability of phytoplankton in a salt wedge estuary, the Swan-Canning Estuary, Western Australia. Hydrol. Process. 2001, 15, 2617–2630. [Google Scholar] [CrossRef]
- Waylett, A.J.; Hutchins, M.G.; Johnson, A.C.; Bowes, M.J.; Loewenthal, M. Physico-chemical factors alone cannot simulate phytoplankton behaviour in a lowland river. J. Hydrol. 2013, 497, 223–233. [Google Scholar] [CrossRef]
- Kouhanestani, Z.M.; Roelke, D.L.; Ghorbani, R.; Fujiwara, M. Assessment of spatiotemporal phytoplankton composition in relation to environmental Conditions of Gorgan Bay, Iran. Estuaries Coasts 2019, 42, 173–189. [Google Scholar] [CrossRef]
- Li, X.Y.; Yu, H.X.; Wang, H.B.; Ma, C.X. Phytoplankton community structure in relation to environmental factors and ecological assessment of water quality in the upper reaches of the Genhe River in the Greater Hinggan Mountains. Environ. Sci. Pollut. Res. 2019, 26, 17512–17519. [Google Scholar] [CrossRef] [PubMed]
- Van Meerssche, E.; Pinckney, J.L. Nutrient Loading Impacts on Estuarine Phytoplankton Size and Community Composition: Community-Based Indicators of Eutrophication. Estuaries Coasts 2019, 42, 504–512. [Google Scholar] [CrossRef]
- Quinlan, E.L.; Phlips, E.J. Phytoplankton assemblages across the marine to low-salinity transition zone in a blackwater dominated estuary. J. Plankton Res. 2007, 29, 401–416. [Google Scholar] [CrossRef]
- Zhang, D.; Han, D.; Song, X. Impacts of the Sanmenxia Dam on the Interaction between Surface Water and Groundwater in the Lower Weihe River of Yellow River Watershed. Water 2020, 12, 1671. [Google Scholar] [CrossRef]
- Remya, P.G.; Kumar, R.; Basu, S.; Sarkar, A. Wave hindcast experiments in the Indian Ocean using MIKE 21 SW model. J. Earth Syst. Sci. 2012, 121, 385–392. [Google Scholar] [CrossRef]
- VishnuRadhan, R.; Vethamony, P.; Zainudin, Z.; Kumar, K.V. Waste Assimilative Capacity of Coastal Waters along Mumbai Mega City, West Coast of India Using MIKE-21 and WASP Simulation Models. Clean Soil Air Water 2014, 42, 295–305. [Google Scholar] [CrossRef]
- Kim, J.; Lee, T.; Seo, D. Algal bloom prediction of the lower Han River, Korea using the EFDC hydrodynamic and water quality model. Ecol. Model. 2017, 366, 27–36. [Google Scholar] [CrossRef]
- Gao, Q.F.; He, G.J.; Fang, H.W.; Bai, S.; Huang, L. Numerical simulation of water age and its potential effects on the water quality in Xiangxi bay of three gorges reservoir. J. Hydrol. 2018, 566, 484–499. [Google Scholar] [CrossRef]
- Waldman, S.; Baston, S.; Nemalidinne, R.; Chatzirodou, A.; Venugopal, V.; Side, J. Implementation of tidal turbines in MIKE 3 and Delft3D models of Pentland Firth & Orkney waters (vol 147, pg 21, 2017). Ocean Coast. Manag. 2018, 163, 535–536. [Google Scholar] [CrossRef]
- Gou, H.; Luo, F.; Li, R.; Dong, X.; Zhang, Y. Modeling Study on the Hydrodynamic Environmental Impact Caused by the Sea for Regional Construction near the Yanwo Island in Zhoushan, China. Water 2019, 11, 1674. [Google Scholar] [CrossRef]
- Pawlowicz, R.; Beardsley, B.; Lentz, S. Classical tidal harmonic analysis including error estimates in MATLAB using T-TIDE. Comput. Geosci. 2002, 28, 929–937. [Google Scholar] [CrossRef]
- Lan, X.; Zhang, H.; Wang, J.; Xu, J. Environmental impacts of building sluice on river estuary of Zhejiang Province. Water Resour. Prot. 2015, 2, 83–87. (In Chinese) [Google Scholar]
- Cui, B.; He, Q.; Zhao, X. Researches on the ecological thresholds of Suaeda salsa to the environmental gradients of water table depth and soil salinity. Acta Ecol. Sin. 2008, 28, 1408–1418. (In Chinese) [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Ye, S. Studies on the ecological threshold of phytoplankton to the environmental gradient of salinity in the Yueqing bay, Zhejiang province, China. Mar. Environ. Sci. 2018, 37, 499–504. (In Chinese) [Google Scholar]
- Li, Y.; Yang, Y.; Ye, S. Ecological thresholds of Labidocera euchaeta to the gradient of salinity in Yueqing Bay, Zhejiang. J. Appl. Oceanogr. 2019, 3, 393–398. (In Chinese) [Google Scholar]


| Statistical Indicator | No Gate | Estuary Gate |
|---|---|---|
| Minimum | 13.776 | 15.239 |
| Maximum | 23.643 | 24.021 |
| Mean | 22.137 | 23.146 |
| Median | 22.256 | 23.179 |
| Geometric Mean | 21.970 | 23.102 |
| Harmonic Mean | 21.590 | 23.046 |
| Root Mean Square | 22.221 | 23.181 |
| Trim Mean (10%) | 22.351 | 23.292 |
| Variance | 3.744 | 1.622 |
| Standard Deviation | 1.935 | 1.273 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, M.; Jiang, C.; Weng, X.; Zhang, M. Influence of Salinity Gradient Changes on Phytoplankton Growth Caused by Sluice Construction in Yongjiang River Estuary Area. Water 2020, 12, 2492. https://doi.org/10.3390/w12092492
Yuan M, Jiang C, Weng X, Zhang M. Influence of Salinity Gradient Changes on Phytoplankton Growth Caused by Sluice Construction in Yongjiang River Estuary Area. Water. 2020; 12(9):2492. https://doi.org/10.3390/w12092492
Chicago/Turabian StyleYuan, Menglin, Cuiling Jiang, Xi Weng, and Manxue Zhang. 2020. "Influence of Salinity Gradient Changes on Phytoplankton Growth Caused by Sluice Construction in Yongjiang River Estuary Area" Water 12, no. 9: 2492. https://doi.org/10.3390/w12092492
APA StyleYuan, M., Jiang, C., Weng, X., & Zhang, M. (2020). Influence of Salinity Gradient Changes on Phytoplankton Growth Caused by Sluice Construction in Yongjiang River Estuary Area. Water, 12(9), 2492. https://doi.org/10.3390/w12092492




