Quantification of Neonicotinoid Pesticides in Six Cultivable Fish Species from the River Owena in Nigeria and a Template for Food Safety Assessment
Abstract
1. Introduction
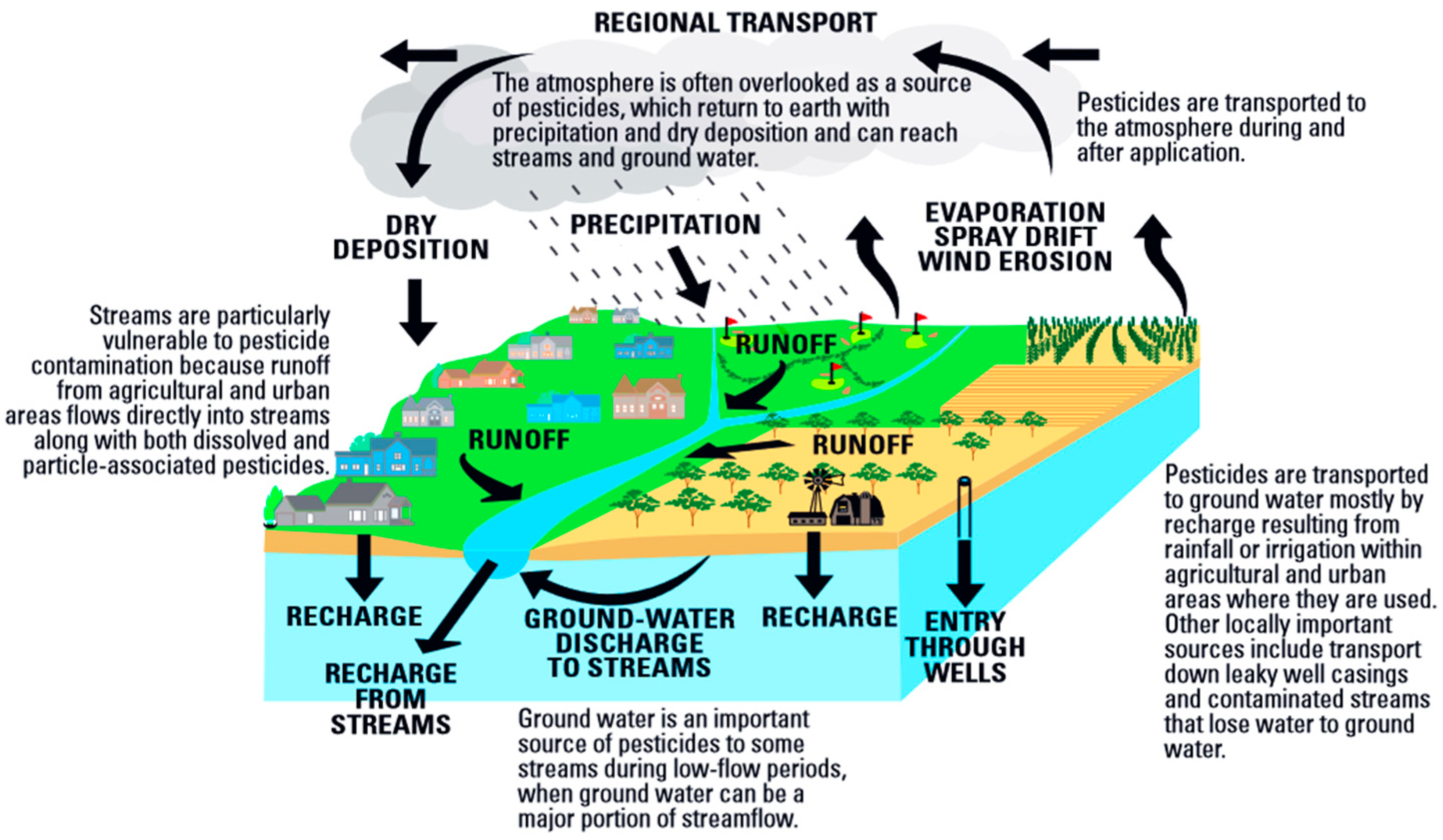
1.1. Neonicotinoid Insecticides as Potential Water Pollutants and Food Contaminants
1.2. Aims of the Present Work
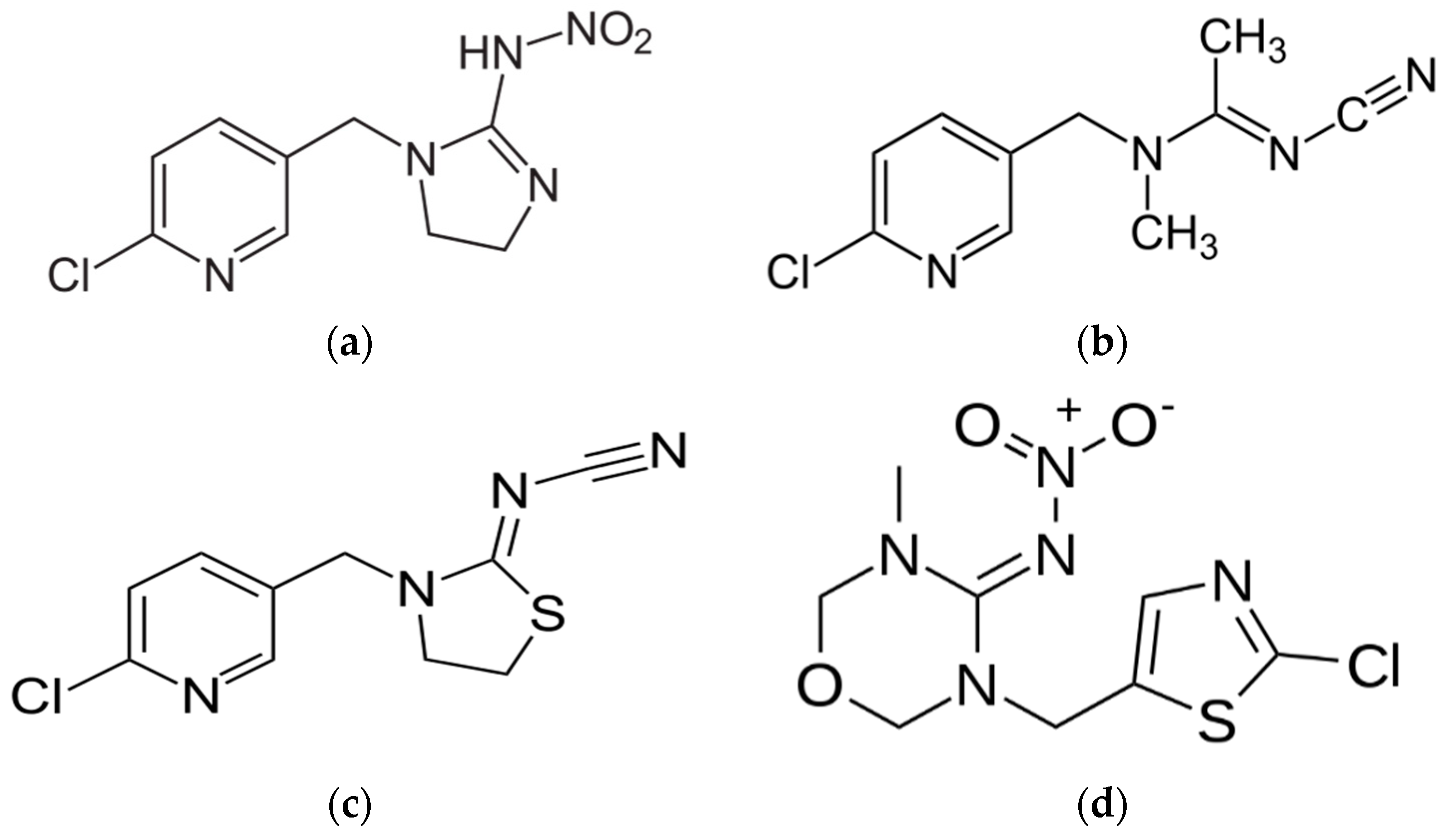
2. Neurophysiology, Solubilities and Photolysis of Neonics
2.1. Insecticidal Properties
2.2. Water Solubilities
2.3. Photolysis
3. Materials and Methods
3.1. The Study Area
3.2. Clarification of Two Technical Terms in Literature
3.3. Laboratory Chemicals, Reagents, Materials
3.4. Collection and Storage of Spiked and Unspiked Fish Samples
- Clarias gariepinus (Burchell, 1822), the African sharptooth catfish; Clariidae (airbreathing catfishes);
- Clarias anguillaris (Linnaeus, 1758), the mudfish; Clariidae (airbreathing catfishes);
- Sarotherodon galilaeus (Linnaeus, 1758), the mango tilapia; Cichlidae (cichlids);
- Parachanna obscura (Günther, 1861), the obscure snakehead fish; Channidae (snakeheads);
- Oreochromis niloticus (Linnaeus, 1758), the Nile tilapia; Cichlidae (cichlids);
- Gymnarchus niloticus (Cuvier, 1829), the African knifefish; Gymnarchidae (abas).
3.5. Extraction of Neonics from Fish Samples
- Stage 1: sample extraction.
- Solvent type. Two solvent systems were tested:
- (a)
- Non-aqueous: methanol, hexane and acetonitrile;
- (b)
- Mixed solvent: de-ionised water, methanol, and acetonitrile.
- Composition of solvent system in terms of volumetric ratios between components. Four ratios were tested:
- (a)
- 10:10:10;
- (b)
- 10:15:5;
- (c)
- 5:10:15;
- (d)
- 15:5:10.
- Mass concentration of the “spike” itself in the fish meat samples. Three such concentrations were tested:
- (a)
- 0.1 ppm;
- (b)
- 0.5 ppm;
- (c)
- 1.0 ppm.
- Stage 2: dSPE clean-up.
3.6. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS-MS) Operational Conditions
3.7. Operational Requirements: Linearity, LOD/LOQ and Extent of Analyte Recovery
- I.
- Linearity. The linearity of a method is its ability to demonstrate responses that are directly proportional to the concentration of a known analyte. The usual format of presentation is one which includes the coefficient of determination, r2, the y-intercept, slope of the regression line “m” and the residual sum of the squares in addition to graphical plotting. For a regression line y = mx + c, calculation of the Pearson’s correlation coefficient (r) shall suffice. Statistically speaking, if a “perfect” linear relationship exists between × and y, then r2 = 1; however if R2 < 0.95, the linearity of the system is questionable and most analysts re-examine the purity of the analyte as the first step of inquiry, bearing in mind that there can be all sorts of issues such as pipetting or even instrumental drift. Often, the coefficient of determination r2 is used, and analysts aim for minimal values of r ≥ 0.98 ⇒ r2 ≥ 0.9604 as an acceptable standard of performance (see [38] as an example). In this work, linearity was evaluated by using seven different concentrations of each neonic between 0.001 and 1.0 ppm. The calibration curve for each neonic was carried out three times.
- II, III.
- Limit of Detection (LOD) and Limit of Quantification (LOQ). LOD is defined as the lowest concentration or quantity of a component or substance that can be reliably distinguished with a specific analytical method, but not necessarily quantified, under the stated conditions of the test. LOQ is defined as the lowest concentration of the analyte which can be detected and quantified within defined limits of certainty (i.e., acceptable precision and accuracy) simultaneously, after replicate measurements are made on the blank and known low concentration, under the stated conditions of the test. (Adapted and modified from [43].) Table 3 shows the R2, LOD and LOQ values in this work. The value of R2 exceeded 0.99 for all the neonics standards. The lowest LOD is 0.005 µg/g, and LOQ is 0.003 µg/g, both values reserved for thiocloprid. The entire LOD ranged from 0.0005 to 0.002 μg/g and the entire LOQ level ranged from 0.003 to 0.005 μg/g.
- IV.
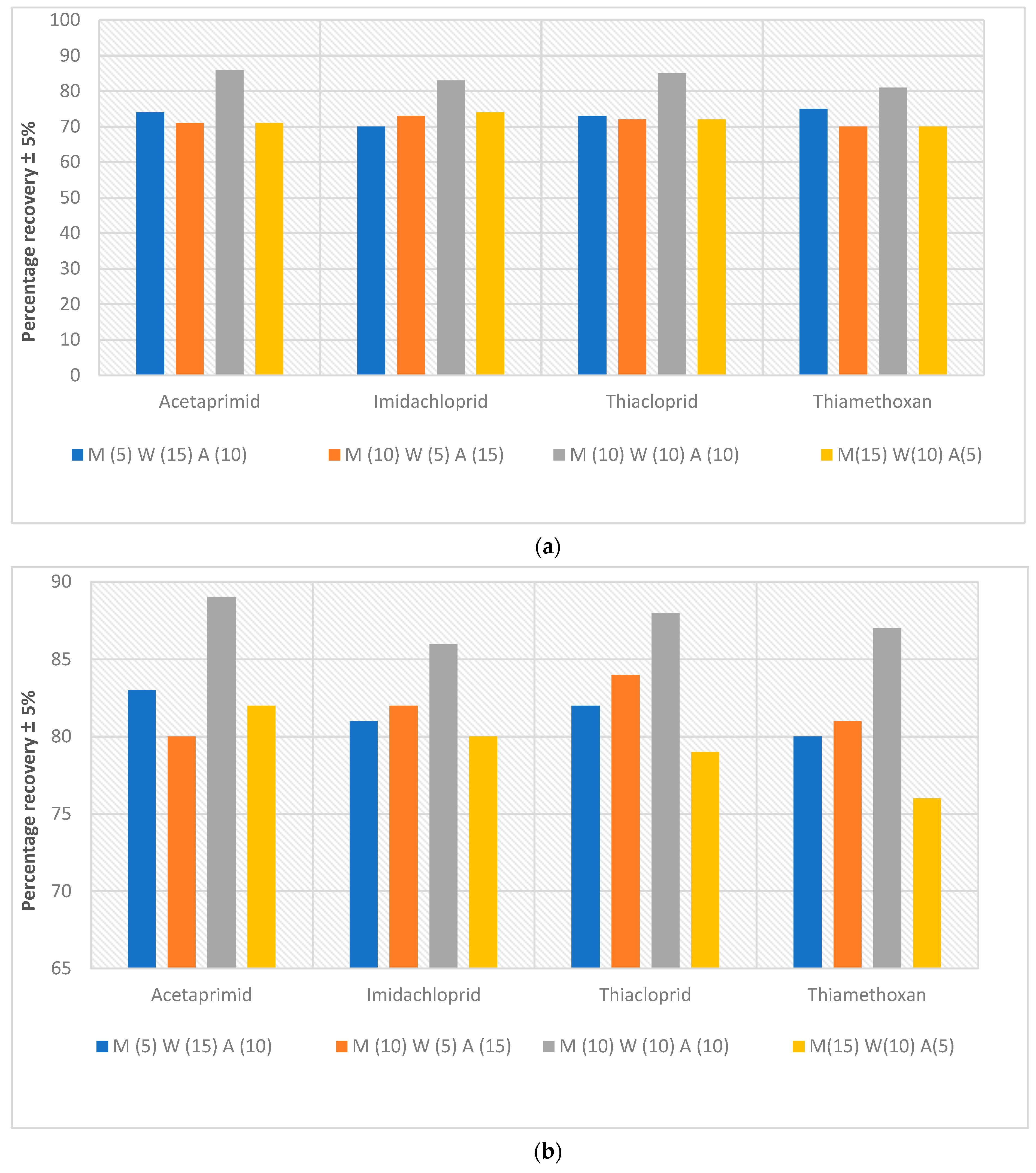
- Extent of analyte recovery. Experiments were conducted to discover how much of the neonics that had been spiked into the Kingston market fish can be retrieved from the meat. In these extractive-quantification endeavours, a high % recovery is desired; i.e., a low recovery will lead to gross underestimation of the amount of neonics contained in the Owena fish samples. The recoveries of all the spiked samples were within the range of recommended recovery limits of 70–120% with the coefficient of variation ≤15% between replicates [43]. The recoveries for the fish samples ranged between 70 and 86%, 76 and 89% and 88 and 104% (Figure 4a–c) at 1, 0.5 and 0.1 ppm, respectively. These recovery rates and their reproducibility are acceptable for this work. The recovery results of this study are in agreement with the recovery range reported in a previous study (see [41] (c.f. [42,44])).
4. Results and Discussion
4.1. Biological Data on Fish Collected from the Owena River
4.1.1. Estimation of Age
4.1.2. Condition Factor
4.1.3. Examples of Conditions of Fingerlings in Aquaculture
- C. gariepinus. Feed for fish farming is the most expensive item of all production costs, in the region of 50–70% [76]. It is strongly linked to the fluctuation of global energy prices, but fish farmers have attempted to discover ways to reduce the cost of feed materials. For example, an attempt was made to reduce the cost of feeding sharptooth fingerlings by partially substituting a formulated “Coppens” feed (45 wt.% protein in 2 mm pellets [77]) with Eichhornia crassipes (Mart.) Solms, 1883 (the water hyacinth) [78]. In the control experiment, the fish diet was not substituted with hyacinth, and the corresponding value of Kf at the commencement of the 70-day experiment was 0.6 which increased to 1.02 at the end. When 20% of the Coppens diet was substituted with hyacinth, Kf dropped to 0.96 (a decrease of 5.8%); when 40% of the diet was substituted, Kf dropped even further to 0.85 (a decrease of 16% from the control value of 1.02). The experimenters settled for a 20% substitution and did not compromise quality for the sake of some cost savings.
- C. anguillaris. Whether an aquatic environment happens to be natural or artificial, the transition from larvae to fingerling is always challenging. Successful laviculture depends on a diet of the smallest zooplankton (rotifers) [79], but growing monocultures of freshwater rotifers such as the much-studied Brachionus calyciflorus (Pallas, 1766) have their difficulties and the quest is for viable mixed cultures. In a set of experiments [80], three groups of larvae were fed with a different type of diet each: Diet 1 contained the freshwater rotifer only (the un-enriched diet), Diet 2 contained the same rotifer with cod liver oil added (the enriched diet) and Diet 3 was a mixture of rotifers, copepods and cladocerans (the mixed diet). At the beginning of the experiment, Kf = 0.6 for all three groups of larvae. The values of Kf peaked at t = 5 days simultaneously for the larvae fed with Diet 1 and Diet 2, at 1.3 and 1.8, respectively. Eventually, both of these Kf values fell to just below 1.10 at t = 24 days (end of the observation period). For the larvae on Diet 3, Kf reached a peak much later at t = 15 days with a value of 1.60, which fell to 0.8 at t = 24 days. Larvae fed with the mixed diet also had the lowest survival rate, of just 39%. Therefore, it seems that a diet containing monocultures of rotifers gave the best results and there may be no need to add cod liver oil.
- O. niloticus. The effect of increasing the weight percentage of maltose in a formulated diet of 11 ingredients (which includes maltose) on the growth of Nile tilapia fingerlings has been investigated [81]. The mean condition factor Kf was used to indicate the health of the fish at the end of an eight-week observation period. With no maltose in the control, Kf = 1.64. At 20% maltose, Kf hit a maximum value of 1.86. At maltose concentrations > 20%, Kf decreased; in fact, it dropped to 1.72 at 30% maltose. Overall, the Nile tilapia fingerlings were in good condition throughout the experiment. Occasionally, the growth patterns of the fingerlings of the Nile and mango tilapias are juxtaposed under similar rearing conditions [82]. A deep analysis into the feeding and growth behaviour of the mango tilapia (S. galilaeus) fingerlings also makes interesting reading [83].
- P. obscura. Snakehead fingerlings were fed with three types of diet to discover which affects the best growth [84]. The diets were: (a) live Nile tilapia fry; (b) trash fish (interesting choice of word because if it is a potential resource it may no longer be useless); and (c) compounded feed. After observing growth for 180 days, the best results were furnished by the diet which consisted of live tilapia fry, with the largest gain in weight of 64%, and the largest increase in Kf of 31% (an increment from 0.63 to 0.83). The corresponding survival rate was 84%.
- G. niloticus. From June to November 2018, aba juveniles in Epe Lagoon (Lagos, Nigeria) exhibited very low values of Kf in the range 0.21 ≤ Kf < 0.27. Conservation and management practices for the fish stocks were suggested [85].
4.1.4. Vulnerability of Juvenile and Young Fish
Population Level
Trophic Level
Gaps in Knowledge
4.2. Neonic Content in fish Collected from the Owena River
4.2.1. Determination of Neonics in Fish Muscle
4.2.2. Ecotoxicological Models
4.2.3. Cytochrome P450 Enzymes and Chemical Species Specificity
- (a)
- For single neonics, no. of experiments = 4 (i.e., 1 for each neonic);
- (b)
- For combinations of two neonics, no. of experiments = 4!/{2!(4 − 2)!} = 6;
- (c)
- For combinations of three neonics, no. of experiments = 4!/{3!(4 − 3)!} = 4;
- (d)
- For all four neonics present in aqueous solution, no. of experiments = 1.
4.3. Fish as an Ecological Compartment
4.3.1. Preparative Work for Pilot-Scale Study
- Plantation soil in cocoa farms;
- Surface water of the Owena River;
- Sediments;
- Cultivable fish species (this work).
4.3.2. Diet and Habitat
4.4. Illustration of a Risk Assessment of Hypothetical Consumption of Contaminated Fish
4.4.1. Methodology
- (a)
- An estimation of the daily intake of fish from databases. From a database called FAOSTAT, the Dutch Embassy in Lagos (Nigeria) extracted the first important piece of information for this calculation: fish consumption reached 13.3 kg/person/year in 2017 in Nigeria [143], or 36.4 g/person/day. (Note that the reported value of 13.3 kg is below the world’s average of 20.5 kg for the same year, 2017 [143]; no update of this numerical value of 13.3 kg was given by UN’s FAO between 2013 and 2019. In 1991, the FAO recommended a minimum dietary requirement of 35 g of animal protein/person/day for human health, but also reported that the consumption in Nigeria at the time was only 7 g [144]).
- (b)
- Experimental data on the amount of neonics in fish obtained in this work.
- (c)
- Combination of the fish consumption and fish contaminant information, from (a) and (b), for the estimation of daily exposures from consumption.
- (d)
- Comparison of exposures thus estimated to benchmark values set by international authorities such as the Fish and Agriculture Organization (FAO) of the United Nations (UN) with the aim of the determination of potential public health impacts.
- (e)
- Three underpinning assumptions:
- Any other type of food in the daily diet of an adult does not contain any pesticide;
- Other types of pesticides other than neonics are absent in the fish examined;
- If two or more neonics are present in an adult human, they do not nullify nor augment each other’s toxicity. Their biochemistry is independent of each other.
- (f)
- A safety criterion. If just one of the many neonic components exceeds an “acceptable daily intake” (ADI) limit while others do not, then the fish is deemed unsafe to eat.
= M1/Ave. wt. of adult = M1/61.6
4.4.2. Calculation and Results
4.4.3. Gaps in Knowledge
4.4.4. Toxicity of Pesticides in Perspective
5. Further Work
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Population Review. Cocoa Producing Countries 2020. 2019. Available online: http://worldpopulationreview.com/countries/cocoa-producing-countries/ (accessed on 10 August 2020).
- Adesoji, B.S. Nigeria’s Top 10 Agricultural Exports Hit N152 Billion in Half-Year 2019. Nairametrics. 25 September 2019. Available online: https://nairametrics.com/2019/09/25/nigerias-top-10-agricultural-exports-hit-n152-billion-in-half-year-2019/ (accessed on 10 August 2020).
- U.S. Environmental Protection Agency. National Management Measures to Control Nonpoint Source Pollution from Agriculture (EPA 841-B-03-004), Chapter 4B: Pesticide Management. 2016. Available online: https://www.epa.gov/sites/production/files/2015-10/documents/chap4b.pdf (accessed on 10 August 2020).
- National Pesticide Information Centre (NPIC). Pesticide Drift. (NPIC Is a Cooperative Agreement between Oregon State University and the U.S. Environmental Protection Agency, cooperative agreement #X8-83560101). 2017. Available online: http://npic.orst.edu/reg/drift.html (accessed on 10 August 2020).
- U.S. Environmental Protection Agency. Introduction to Pesticide Drift. 2016. Available online: https://www.epa.gov/reducing-pesticide-drift/introduction-pesticide-drift (accessed on 10 August 2020).
- Government of Canada. Pesticide Spray Drift near Homes. 2019. Available online: https://www.canada.ca/en/health-canada/services/about-pesticides/pesticide-spray-drift-near-homes.html (accessed on 10 August 2020).
- Stokstad, E. France’s Decade-Old Effort to Slash Pesticide Use Failed. Will a New Attempt Succeed? 2018. Available online: https://www.sciencemag.org/news/2018/10/france-s-decade-old-effort-slash-pesticide-use-failed-will-new-attempt-succeed (accessed on 10 August 2020).
- Struger, J.; Grabuski, J.; Cagampan, S.; Sverko, E.; McGoldrick, D.; Marvin, C.H. Factors influencing the occurrence and distribution of neonicotinoid insecticides in surface waters of southern Ontario, Canada. Chemosphere 2017, 169, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.S.; Capel, P.D. Pesticides in The Atmosphere-Distribution, Trend, and Governing Factors; Ann Arbor Press Inc.: Chelsea, MI, USA, 1995; p. 118. [Google Scholar]
- U.S. Geological Survey. Pesticides in Groundwater. 2020 Current. Available online: https://www.usgs.gov/special-topic/water-science-school/science/pesticides-groundwater?qt-science_center_objects=0#qt-science_center_objects (accessed on 10 August 2020).
- Main, A.R.; Headley, J.V.; Peru, K.M.; Michel, N.L.; Cessna, A.J.; Morrissey, C.A. Widespread Use and Frequent Detection of Neonicotinoid Insecticides in Wetlands of Canada’s Prairie Pothole Region. PLoS ONE 2014, 9, e92821. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, R.L.; Nehring, R.F.; Grube, A.; Goss, D.W.; Plotkin, S. Environmental Indicators of Pesticide Leaching and Runoff from Farm Fields; U.S. Department of Agriculture, Natural Resources Conservation Service (Utah); Springer: Boston, MA, USA, 2002. Available online: https://www.nrcs.usda.gov/wps/portal/nrcs/detail/ut/home/?cid=nrcs143_014053 (accessed on 10 August 2020).
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide Exposure, Safety Issues, and Risk Assessment Indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef] [PubMed]
- Stanfill, S.B.; da Silva, A.L.O.; Lisko, J.G.; Lawler, T.S.; Kuklenyik, P.; Tyx, R.E.; Peuchen, E.H.; Richter, P.; Watson, C.H. Comprehensive chemical characterization of Rapé tobacco products: Nicotine, un-ionized nicotine, tobacco-specific N′-nitrosamines, polycyclic aromatic hydrocarbons, and flavor constituents. Food Chem. Toxicol. 2015, 82, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Banyasz, J.L. Analytical Determination of Nicotine and Related Compounds and Their Metabolites, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 1999; pp. 149–190. [Google Scholar]
- Griffin, E.L.; Philips, G.; Claffey, J.; Skalamera, J. Nicotine sulfate from Nicotiana rustica. Ind. Eng. Chem. 1952, 44, 274–279. [Google Scholar] [CrossRef]
- Worthing, C.R. (Ed.) Pesticide Manual, 6th ed.; British Crop Protection Council: Worcestershire, UK, 1979; p. 382. [Google Scholar]
- U.S. Environmental Protection Agency. List of “Extremely Hazardous Substances” defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002). The List can be Found as an Appendix to 40 C.F.R. 355. 2008. Available online: https://web.archive.org/web/20120225051612/http://edocket.access.gpo.gov/cfr_2008/julqtr/pdf/40cfr355AppA.pdf (accessed on 10 August 2020).
- U.S. Environmental Protection Agency. Nicotine: Product Cancellation Order (U.S. EPA Order: EPA-HQ-OPP-2007-1019; FRL-8414-5). U.S. Fed. Regist. 2009, 74, 26695. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/frn_PC-056702_3-Jun-09.pdf (accessed on 10 August 2020).
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the Status and Global Strategy for Neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908. [Google Scholar] [CrossRef]
- Jeschke, P.; Nauen, R. Neonicotinoids-from zero to hero in insecticide chemistry. Pest Manag. Sci. 2008, 64, 1084–1098. [Google Scholar] [CrossRef]
- Moffat, C.; Buckland, S.T.; Samson, A.J.; McArthur, R.; Pino, V.C.; Bollan, K.A.; Huang, J.T.-J.; Connolly, C.N. Neonicotinoids target distinct nicotinic acetylcholine receptors and neurons, leading to differential risks to bumblebees. Sci. Rep. 2016, 6, 24764. [Google Scholar] [CrossRef]
- Lundin, O.; Rundlöf, M.; Smith, H.G.; Fries, I.; Bommarco, R. Neonicotinoid Insecticides and Their Impacts on Bees: A Systematic Review of Research Approaches and Identification of Knowledge Gaps. PLoS ONE 2015, 10, e0136928. [Google Scholar] [CrossRef]
- Cornell University. Neonicotinoids. College of Agriculture and Life Sciences, a publication by Pollinator Network @ Cornell. 2020. Available online: https://pollinator.cals.cornell.edu/threats-wild-and-managed-bees/pesticides/neonicotinoids/ (accessed on 10 August 2020).
- Morrision, S. “Bayer Wants You to Know That It Does Not Kill Bees. It Loves Bees”, the Atlantic. 12 December 2013. Available online: https://www.theatlantic.com/international/archive/2013/12/bayer-wants-you-know-it-does-not-kill-bees-bayer-loves-bees/356104/ (accessed on 10 August 2020).
- U.S. Environmental Protection Agency. EPA Releases Proposed Interim Decisions for Neonicotinoids for Release. 30 January 2020. Available online: https://www.epa.gov/pesticides/epa-releases-proposed-interim-decisions-neonicotinoids (accessed on 10 August 2020).
- National Collaborating Centre for Environmental Health. Neonicotinoid Pesticides. Available online: http://www.ncceh.ca/environmental-health-in-canada/health-agency-projects/neonicotinoid-pesticides (accessed on 10 August 2020).
- Bonmatin, J.-M.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; Long, E.; Marzaro, M.; Mitchell, E.A.D.; et al. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. 2014, 22, 35–67. [Google Scholar] [CrossRef] [PubMed]
- Martins, O. Water Resources Management and Development in Nigeria: Issues and Challenges in a New Millennium; An Inaugural Lecture Delivered at the University of Agriculture; University of Agriculture: Abeokuta, Nigeria, 22 August 2001. [Google Scholar]
- Idu, A.M. Threats to Water Resources Development in Nigeria. J. Geol. Geophys. 2015, 4, 1000205. [Google Scholar] [CrossRef]
- Adegun, A.O. Determination of Neonicotinoids Insecticide Residues in Cocoa-Producing Soil, Sediment and Surface Water of Owena River Basin, Nigeria. Interim Report on Doctoral Thesis, Department of Chemical Sciences, Adekunle Ajasin University, Akungba Akoko, Nigeria, 2019; p. 20. [Google Scholar]
- Codex Alimentarius Commission. Codex Maximum Residue Limits for Pesticides. Codex Alimentarius Commission, Joint FAO/WHO Food Standards Programme; FAO: Rome, Italy, 1997; Available online: http://www.fao.org/waicent/faostat/Pest-Residue/pest-e.htm#E10E1 (accessed on 10 August 2020).
- Tyner, T.; Francis, J. (Eds.) American Chemical Society Reagent Chemicals: Specifications and Procedures for Reagents and Standard Grade Reference Materials. 2017. Available online: https://pubs.acs.org/isbn/9780841230460 (accessed on 10 August 2020).
- Dalia, M.S.; Salemy, A.; Sikaily, A.E. Organochlorines and their Risk in Marine Shellfish Collected from the Mediterranean Coast. Egypt. J. Aquatic Res. 2014, 40, 198–212. [Google Scholar]
- Enbaia, S.; Ahamad, M.; Absur, A. Determination of Organochlorine Pesticide Residues in Libyan Fish. Int. J. Microbiol. Appl. Sci. 2014, 10, 198–212. [Google Scholar]
- Association of Official Agricultural Chemists (AOAC) International. AOAC Official Method 2007.01, Pesticide Residues in Foods by Acetonitrile Extraction and Partitioning with Magnesium Sulfate. 2007. Available online: https://nucleus.iaea.org/sites/fcris/Shared%20Documents/SOP/AOAC_2007_01.pdf (accessed on 10 August 2020).
- LabX. Information Sheet on the Agilent 1260 Infinity HPLC. 2020. Available online: https://www.labx.com/product/agilent-1260-infinity-hplc (accessed on 10 August 2020).
- Agilent Technologies. Hardware Manual of the “5973 Network Mass Selective Detector”. 1999. Available online: https://www.agilent.com/cs/library/usermanuals/public/73nhw_030751.pdf (accessed on 10 August 2020).
- Agilent Technologies. Agilent 6400 Series Triple Quadrupole LC/MS System: Concepts Guide, the Big Picture. 2012. Available online: https://www.agilent.com/cs/library/usermanuals/public/G3335-90135_QQQ_Concepts.pdf (accessed on 10 August 2020).
- Agilent Technologies. Agilent Jet Stream Thermal Gradient Focusing Technology (Technical Note). 2009. Available online: https://www.agilent.com/cs/library/technicaloverviews/Public/5990-3494en_lo%20CMS.pdf (accessed on 10 August 2020).
- Valverde, S.; Ares, A.M.; Bernal, J.L.; Del Nozal, M. Fast determination of neonicotinoid insecticides in beeswax by ultra-high performance liquid chromatography-tandem mass spectrometry using an enhanced matrix removal-lipid sorbent for clean-up. Microchem. J. 2018, 142, 70–77. [Google Scholar] [CrossRef]
- Suganthi, A.; Bhuvaneswari, K.; Ramya, M. Determination of neonicotinoid insecticide residues in sugarcane juice using LCMSMS. Food Chem. 2018, 241, 275–280. [Google Scholar] [CrossRef]
- Lu, L.; Seenivasan, R.; Wang, Y.-C.; Yu, J.-H.; Gunasekaran, S. An Electrochemical Immunosensor for Rapid and Sensitive Detection of Mycotoxins Fumonisin B1 and Deoxynivalenol. Electrochim. Acta 2016, 213, 89–97. [Google Scholar] [CrossRef]
- Barbieri, M.V.; Postigo, C.; Guillem-Argiles, N.; Monllor-Alcaraz, L.S.; Simionato, J.I.; Stella, E.; Barceló, D.; De Alda, M.L. Analysis of 52 pesticides in fresh fish muscle by QuEChERS extraction followed by LC-MS/MS determination. Sci. Total Environ. 2019, 653, 958–967. [Google Scholar] [CrossRef]
- FishBase. Species Profile: Clarius gariepinus (Burchell, 1822), North African catfish NAGA. WorldFish Center Q. 2003, 26, 7. Available online: http://pubs.iclarm.net/Naga/na_451.pdf (accessed on 10 August 2020).
- Froese, R.; Pauly, D. (Eds.) FishBase. Clarius gariepinus (Burchell, 1822), North African Catfish. 2019. Available online: https://www.fishbase.se/summary/1934 (accessed on 10 August 2020).
- Kurbanov, A.; Kamilov, B. Maturation of African catfish, Clarias gariepinus, in condition of seasonal climate of Uzbekistan. J. Fish. Aquat. Stud. 2017, 5, 236–239. [Google Scholar]
- Okogwu, O.I. Age, growth and mortality of Clarias gariepinus (Siluriformes: Clariidae) in the Mid-Cross River-Floodplain ecosystem, Nigeria. Rev. Biol. Trop. 2011, 59, 1707–1716. [Google Scholar] [PubMed]
- Weyl, O.L.F.; Booth, A.J. Validation of annulus formation in otoliths of a temperate population of adult African sharptooth catfish Clarias gariepinus using fluorochrome marking of wild fish. J. Fish Biol. 2008, 73, 1033–1038. [Google Scholar] [CrossRef]
- Offem, B.O.; Yemi Akegbejo-Samsons, Y.; Omoniyi, I.T. Aspects of Ecology of Clarias anguillaris (Teleostei: Clariidae) in the Cross River, Nigeria. Turk. J. Fish. Aquatic Sci. 2010, 10, 101–110. [Google Scholar]
- Teugels, G.G. Clariidae. In Faune des Poissons d’eaux Douce et Saumâtres de l’Afrique de l’Ouest, Tome 2. Coll. Faune et Flore Tropicales 40; Lévêque, C., Paugy, D., Teugels, G.G., Eds.; Musée Royal de l’Afrique Centrale: Tervuren, Belgique; Museum National d’Histoire Naturalle: Paris, France; Institut de Recherche pour le Développement: Paris, France, 2003; pp. 144–173, 815. [Google Scholar]
- Teugels, G.G.; Adriaens, D.; Devaere, S.; Musschoot, T. Clariidae. In The Fresh and Brackish Water Fishes of Lower Guinea, West-Central Africa. Volume I. Collection Faune et Flore tropicales 42; Stiassny, M.L.J., Teugels, G.G., Hopkins, C.D., Eds.; Institut de Recherche pour le Développement: Paris, France; Muséum National d’Histoire Naturelle: Paris, France; Musée Royal de l’Afrique Centrale: Tervuren, Belgium, 2007; pp. 653–691, 800. [Google Scholar]
- Hyslop, E. The growth and feeding habits of Clarias anguillaris during their first season in the floodplain pools of the Sokoto–Rima River Basin, Nigeria. J. Fish Biol. 2006, 30, 183–192. [Google Scholar] [CrossRef]
- World Life Expectancy. Lifespan of the African Lungfish (WHO Data, 2018). Available online: https://www.worldlifeexpectancy.com/fish-life-expectancy-african-lungfish (accessed on 10 August 2020).
- Food and Agriculture Organization. Synopsis of Biological Data on Sarotherodon galilaeus. FAO Fisheries Synopsis No. 80, Rome. 1974. Available online: http://www.fao.org/3/f1176e/f1176e.pdf (accessed on 10 August 2020).
- Kpogue, D.N.S.; Mensah, G.A.; Fiogbe, E.D. A review of biology, ecology and prospect for aquaculture of Parachanna obscura. Rev. Fish. Biol. Fish. 2013, 23, 41–50. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. (Eds.) FishBase. Parachanna obscura. (Günther, 1861). 2019. Available online: http://fishbase.mnhn.fr/summary/SpeciesSummary.php?id=5467 (accessed on 10 August 2020).
- Bonou, C.A.; Teugels, G.G. Révision systématique du genre Parachanna Teugels et Daget 1984 (Pisces: Channidae). Rev. Hydrobiol. Trop. 1985, 18, 2G7-280. [Google Scholar]
- CABI. Datasheet: Channa argus argus (northern snakehead). In Invasive Species Compendium; CAB International: Wallingford, UK, 2016; Available online: https://www.cabi.org/isc/datasheet/89026 (accessed on 10 August 2020).
- Froese, R.; Pauly, D. (Eds.) FishBase. Oreochromis niloticus. (Linnaeus, 1758). 2019. Available online: https://www.fishbase.se/summary/Oreochromis-niloticus.html (accessed on 10 August 2020).
- Towers, L. Farming tilapia: Life history and biology. The Fish Site 2005. Available online: https://thefishsite.com/articles/tilapia-life-history-and-biology (accessed on 10 August 2020).
- Agbugui, M. Gymnarchus niloticus (Cuvier, 1830), a treathened fish species in the lower river Niger at Agenebode, Nigeria; the need for its conservation. Int. J. Fish. Aquat. Res. 2020, 5, 44–48. [Google Scholar]
- Animal World. Aba Knife Fish. 2020 Current. Available online: https://animal-world.com/encyclo/fresh/Knifefish/AbaKnifefish.php (accessed on 10 August 2020).
- Fulton, T.W. The Rate of Growth of Fishes; 20th Annual Report of the Fishery Board of Scotland; Fishery Board of Scotland: Edinburgh, UK, 1902; pp. 326–446. [Google Scholar]
- Fulton, T.W. The Rate of Growth of Fishes, 22nd ed.; Annual Report of the Fishery Board of Scotland; Fishery Board of Scotland: Edinburgh, UK, 1904; pp. 141–241. [Google Scholar]
- Meek, A. A Contribution to Our Knowledge of the Growth of Plaice; Scientific Investigations of the Northumberland Sea Fisheries Commission; The Northumberland Sea Fisheries Commission: Richmond, UK, 1903; p. 40. [Google Scholar]
- Johnstone, J. Report on measurements of plaice made during the year 1911. Trans. Liverp. Biol. Soc. 1912, 26, 85–102. Available online: https://www.biodiversitylibrary.org/ia/proceedingstr26191112live/#page/114/mode/1up (accessed on 10 August 2020).
- Heincke, F.; Henking, H. Über Schollen und Schollenfischerei in der südöstlichen Nordsee. Die Beteiligung Deutschlands an der Internationalen Meeresforschung 1907, 4, 1–90. [Google Scholar]
- Thompson, D.W. On Growth and Form; Cambridge University Press: Cambridge, UK, 1917. [Google Scholar]
- Nash, R.; Valencia, A.H.; Geffen, A. The origin of Fulton’s condition factor-Setting the record straight. Fisheries. 2006, 31, 236–238. [Google Scholar]
- Wootton, R.J. Ecology of Teleost Fishes, 1st ed.; Chapman and Hall: London, UK, 1990; p. 404. [Google Scholar]
- Atalitsa, J.L.; Obegi, B.; Waithaka, E. Length–weight relationship and condition factor of Clarias gariepinus in Lake Naivasha, Kenya. J. Fish. Aquat. Stud. 2015, 2, 382–385. [Google Scholar]
- Ayo-Olalusi, C.I. Length-weight Relationship, Condition Factor and Sex Ratio of African Mud Catfish (Clarias gariepinus) Reared in Flow-through System Tanks. J. Fish. Aquat. Sci. 2014, 9, 430–434. [Google Scholar] [CrossRef]
- Charles Barnham PSM & Alan Baxter. Condition Factor, K., for Salmonid Fish. Fisheries Notes. March 1998. Available online: http://bamboorods.ca/Trout%20condition%20factor.pdf (accessed on 10 August 2020).
- Pauly, D. Some Simple Methods for the Assessment of Tropical Fish Stocks; FAO Fisheries Technical Paper; FAO: Rome, Italy, 1983; p. 234. Available online: http://www.fao.org/3/X6845E/X6845E00.htm (accessed on 10 August 2020).
- Partos, L. FAO: Fish Feed costs to remain high. Seafood Source. 2010. Available online: https://www.seafoodsource.com/news/aquaculture/fao-fish-feed-costs-to-remain-high (accessed on 10 August 2020).
- Emmanuel, C.A.; Solomon, R.J. The Growth Rate and Survival of Clarias Gariepinus Fingerlings in Tap, Borehole and Stream Waters. Academic Arena. 2013, p. 5, (Table 2). Available online: https://www.researchgate.net/publication/319163125_The_Growth_Rate_And_Survival_Of_Clarias_Gariepinus_Fingerlings_In_Tap_Borehole_And_Stream_Waters/figures?lo=1 (accessed on 10 August 2020).
- Fola-Matthews, O.; Kusemiju, K. Growth pattern and proximate composition of the African catfish clarias gariepinus, L. fed on water hyacinth substituted diets. Adv Plants Agric Res. 2018, 8, 617–622. [Google Scholar]
- Arimoro, F.; Ofojekwu, P.C. Incidence of feeding, growth and survival of the toothed carp, Aphyosemion gardneri larvae reared on the freshwater rotifer, Brachionus calyciflorus. Trop. Freshw. Biol. 2006, 12, 35–43. [Google Scholar] [CrossRef]
- Arimoro, F. First feeding in the African catfish Clarias anguillaris fry in tanks with the freshwater rotifer brachionus calyciflorus cultured in a continuous feedback mechanism in comparison with a mixed zooplankton diet. J. Fish. Aquat. Sci. 2007, 2, 2275–2284. [Google Scholar]
- Ighwela, K.A.; Ahmed, A.B.; Abol-Munafi, A. Condition factor as an indicator of growth and feeding intensity of Nile Tilapia Fingerlings (Oreochromis niloticus) feed on different levels of maltose. JAES 2011, 11, 559–563. [Google Scholar]
- Goda, A.M.A.; Wafa, M.E.; Chowdhury, M.A.K.; El-Haroun, E. Growth performance and feed utilization of Nile tilapia Oreochromis niloticus (Linnaeus, 1758) and tilapia galilae Sarotherodon galilaeus (Linnaeus, 1758) fingerlings fed plant protein-based diets. Aquac. Res. 2007, 38, 827–837. [Google Scholar] [CrossRef]
- Gophen, M. Food sources, feeding behaviour and growth rates of Sarotherodon galilaeum (Linnaeus) fingerlings. Aquaculture 1980, 20, 101–115. [Google Scholar] [CrossRef]
- Bassey, A.U.; Ajah, P.O. Effect of three Feeding Regimes on Growth, Condition Factor and Food Conversion rate of Pond Cultured Parachanna obscura (Gunther, 1861) (Channidae) in Calabar, Nigeria. Turk. J. Fish. Aquat. Sci. 2010, 10, 195–202. [Google Scholar] [CrossRef]
- Oluwale, F.V.; Ugwumba, A.A.A.; Ugwumba, O.A. Aspects of the biology of juvenile Aba, Gymnarchus niloticus (Curvier 1829) from Epe Lagoon, Lagos, Nigeria. J. Fish. Aquat. Stud. 2019, 7, 267–274. [Google Scholar]
- Tyor, A. Effects of imidacloprid on viability and hatchability of embryos of the common carp (Cyprinus carpio L.). J. Fish. Aquat. Stud. 2019, 4, 385–389. [Google Scholar]
- Finnegan, M.C.; Baxter, L.R.; Maul, J.D.; Hanson, M.L.; Hoekstra, P.F. Comprehensive characterization of the acute and chronic toxicity of the neonicotinoid insecticide thiamethoxam to a suite of aquatic primary producers, invertebrates, and fish. Environ. Toxicol. Chem. 2017, 36, 2838–2848. [Google Scholar] [CrossRef]
- Islam, A.; Hossen, S.; Sumon, K.A.; Rahman, M.M. Acute Toxicity of Imidacloprid on the Developmental Stages of Common Carp Cyprinus carpio. Toxicol. Environ. Heal. Sci. 2019, 11, 244–251. [Google Scholar] [CrossRef]
- Özdemir, S.; Altun, S.; Arslan, H. Imidacloprid exposure cause the histopathological changes, activation of TNF-α, iNOS, 8-OHdG biomarkers, and alteration of caspase 3, iNOS, CYP1A, MT1 gene expression levels in common carp (Cyprinus carpio L.). Toxicol. Rep. 2017, 5, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bayo, F.; Goka, K. Unexpected effects of zonc pyrithione and imidacloprid on Japanese medaka fish (Oryzias latipes). Aquat Toxicol. 2005, 74, 285–293. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. Ecological Effects Test Guidelines. OCSPP 850.1075. Freshwater and Saltwater Fish Acute Toxicity Test. 2016. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi/P100SH65.PDF?Dockey=P100SH65.PDF (accessed on 10 August 2020).
- Food and Agriculture Organization (FAO/UN). Inventory of Evaluations Performed by the Joint Meeting on Pesticides Residues: Thiacloprid. 2006. Available online: https://apps.who.int/pesticide-residues-jmpr-database/pesticide?name=THIACLOPRID (accessed on 10 August 2020).
- Food and Agriculture Organization (FAO/UN). Inventory of Evaluations Performed by the Joint Meeting on Pesticides Residues: Acetamiprid. 2011. Available online: https://apps.who.int/pesticide-residues-jmpr-database/pesticide?name=ACETAMIPRID (accessed on 10 August 2020).
- Food and Agriculture Organization (FAO/UN). List of Pesticides Evaluated by JMPR and JMPS. 2020. Available online: http://www.fao.org/agriculture/crops/thematic-sitemap/theme/pests/lpe/en/ (accessed on 10 August 2020).
- Food and Agriculture Organization (FAO/UN). Inventory of Evaluations Performed by the Joint Meeting on Pesticides Residues: Thiamethoxam. 2010. Available online: https://apps.who.int/pesticide-residues-jmpr-database/pesticide?name=THIAMETHOXAM (accessed on 10 August 2020).
- U.S. EPA. KABAM Version 1.0 User’s Guide and Technical Documentation to Kow Based Aquatic BioAccumulation Model. 2009. Available online: https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/kabam-version-10-users-guide-and-technical (accessed on 10 August 2020).
- Smit, C.E. Water Quality Standards For Imidacloprid: Proposal for an Update According to the Water Framework Directive; RIVM Letter report 270006001/2014; National Institute for Public Health and the Environment, Ministry of Health, Welfare and Sport: Bilthoven, The Netherlands, 2014.
- Organization for Economic Cooperation and Development (OECD). Guidance Document on the Validation of Quantitative Structure-Activity Relationship (QSAR) Model; OECD Environment Health and Safety Publication, Series of Testing and Assessment No. 69; OECD Environment Directorate: Paris, France, 2007. [Google Scholar]
- Arimoro, F.; Muller, W.J. Mayfly (Insecta: Ephemeroptera) community structure as an indicator of the ecological status of a stream in the Niger Delta area of Nigeria. Environ. Monit. Assess. 2009, 166, 581–594. [Google Scholar] [CrossRef]
- Basant, N.; Gupta, S.; Singh, K.P. Predicting aquatic toxicities of chemical pesticides in multiple test species using non-linear QSTR modelling approaches. Chemosphere 2015, 139, 246–255. [Google Scholar] [CrossRef]
- Fahmy, G.H. Malathion Toxicity: Effect on Some Metabolic Activities in Oreochromis Niloticus, the Tilapia Fish. IJBBB 2012, 2, 52–55. [Google Scholar] [CrossRef]
- Nebert, D.W.; Russel, D.W. Clinical importance of the cytochrome P450. The Lancet 2002, 360, 1155–1162. [Google Scholar] [CrossRef]
- Goksøyr, A. Use of Cytochrome p450 1a (cyp1a) in Fish as a Biomarker of Aquatic Pollution. In Toxicology in Transition; Degen, G.H., Seiler, J.P., Bentley, P., Eds.; Archives of Toxicology (Supplement), vol. 17; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar] [CrossRef]
- Shailaja, M.S.; D’Silva, C. Evaluation of impact of PAH on a tropical fish, Oreochromis mossambicus using multiple biomarkers. Chemosphere 2003, 53, 835–841. [Google Scholar] [CrossRef]
- Parente, T.E.; De-Oliveira, A.C.; Paumgartten, F.J. Induced cytochrome P450 1A activity in cichlid fishes from Guandu River and Jacarepagua Lake, Rio de Janeiro, Brazil. Environ. Pollut. 2008, 152, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Stegeman, J.J.; Livingstone, D.R. Forms and functions of cytochrome P450. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1998, 121, 1–3. [Google Scholar]
- Whyte, J.J.; Jung, R.E.; Schmitt, C.J.; Tillitt, D.E. Ethoxyresorufin-O-deethylase (EROD) Activity in Fish as a Biomarker of Chemical Exposure. Crit. Rev. Toxicol. 2000, 30, 347–570. [Google Scholar] [CrossRef] [PubMed]
- Abbas, W.T.; Hassanain, M.; Abdel-Rahman, E.H.; Abo-Hegab, S.; Tawfik, M.A.; Tawfik, M.A. Induction of cytochrome P450 1A1 as a biomarker of Benzo-a-pyrene pollution in Egyptian fresh water fish. Pak. J. Boil. Sci. 2007, 10, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Pérez, O.; Gold-Bouchot, G.; Ortega, A.; Lopez, T.; Albores, A. Effect of Pyrene on Hepatic Cytochrome P450 1A (CYP1A) Expression in Nile Tilapia (Oreochromis niloticus). Arch. Environ. Contam. Toxicol. 2002, 42, 477–485. [Google Scholar] [CrossRef]
- Mdegela, R.H.; Braathen, M.; Correia, D.; Mosha, R.D.; Skaare, J.U.; Sandvik, M. Influence of 17α-ethynylestradiol on CYP1A, GST and biliary FACs responses in male African sharptooth catfish (Clarias gariepinus) exposed to waterborne Benzo[a]Pyrene. Ecotoxicol. 2006, 15, 629–637. [Google Scholar] [CrossRef]
- Gadagbui, B.K.-M.; Goksrøyr, A. CYP1A andother biomarker responses to effluents from a textile mill in the Volta River (Ghana) using caged tilapia (Oreochromis niloticus) andsediment-exposed mudfish (Clarias anguillaris). Biomarkers 1996, 1, 252–261. [Google Scholar] [CrossRef]
- El Euony, O.I.; Elblehi, S.S.; Abdel-Latif, H.M.; Abdel-Daim, M.M.; El-Sayed, Y.S. Modulatory role of dietary Thymus vulgaris essential oil and Bacillus subtilis against thiamethoxam-induced hepatorenal damage, oxidative stress, and immunotoxicity in African catfish (Clarias garipenus). Environ. Sci. Pollut. Res. 2020, 27, 23108–23128. [Google Scholar] [CrossRef]
- Gadagbui, B.K.-M.; Addy, M.; Goksøyr, A. Species characteristics of hepatic biotransformation enzymes in two tropical freshwater teleosts, tilapia (Oreochromis niloticus) and mudfish (Clarias anguillaris). Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1996, 114, 201–211. [Google Scholar] [CrossRef]
- Mariottini, M.; Corsi, I.; Bonacci, S.; Focardi, S.; Regoli, F. PCB Muscle Content and Liver EROD Activity in the European EEL (Anguilla Anguilla) Treated with Aroclor 1254. Chem. Ecol. 2003, 19, 91–98. [Google Scholar] [CrossRef]
- Schlussel, A.; Leininger, E. Neonicotinoid insecticides and selective serotonin reuptake inhibitors interact antagonistically in Daphnia magna. BIOS 2019, 90, 245–256. [Google Scholar] [CrossRef]
- Ayinla, O.A. The Food and Feeding Habits of African Mud Catfish C. Gariepinus (Burchell, 1822) Caught from the Wild. Nigerian Institute of Oceanography & Marine Research (Lagos), Technical Paper No. 34. 1988. Available online: https://www.oceandocs.org/bitstream/handle/1834/2215/NIOMR?sequence=1 (accessed on 10 August 2020).
- Uys, W.; Hecht, T. Assays on the digestive enzymes of sharptooth catfish, Clarias gariepinus (Pisces: Clariidae). Aquaculture 1987, 63, 301–313. [Google Scholar] [CrossRef]
- Uys, W.; Hecht, T.; Walters, M. Changes in digestive enzyme activities of Clarias gariepinus (Pisces: Clariidae) after feeding. Aquaculture 1987, 63, 243–250. [Google Scholar] [CrossRef]
- Witte, F.; de Winter, W. Appendix II. Biology of the Major Fish Species of Lake Victoria. In Fish stocks and fisheries of Lake Victoria; Witte, F., Van Densen, W.L.T., Eds.; A handbook for field observations; Samara Publishing Limited: Dyfed, UK, 1995; pp. 301–320. [Google Scholar]
- Madu , C.T.; Ufodike, E.D.C.; Ita, E.O. Food and feeding habits of hatchlings of the mudfish Clarius Anguillaris L. J. Aquat. Sci. 1990, 5, 27–31. [Google Scholar]
- Ben-Tuvia, A. The biology of the cichlid fishes of Lakes Tiberias and Huleh. Bull. Res. Counc. Isr. 1959, 8B, 153–188. [Google Scholar]
- Ita, E.O. Food and Feeding Relationships of Fish in a Tropical Fish Pond. Master’s Thesis, University of Ibadan, Ibadan, Nigeria, 1971; p. 137. [Google Scholar]
- Gosse, J.P. Dispositions specials de l’appareil branchial des tilapia et citharinus. Ann.Soc.R.Zool. Belg. 1956, 86, 303–306. [Google Scholar]
- Evans, W.A. Ghana-Stock Assessment and Fish Biology; FI:SF/CHA 10/9:32p; FAO: Rome, Italy, 1971. [Google Scholar]
- Adriana, N.A.; Noll, M.S.M.C.; Valenti, W.C. Zooplankton capturing by Nile Tilapia, Oreochromis niloticus (Teleostei: Cichlidae) throughout post-larval development. Zoologia (Curitiba) 2015, 32. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Oreochromis niloticus (Linnaeus, 1758). Cultured Aquatic Species Information Programme. 2020. Available online: http://www.fao.org/fishery/culturedspecies/Oreochromis_niloticus/en (accessed on 10 August 2020).
- Moriarty, D.J.W.; Moriarty, C.M. The assimilation of carbon from phytoplankton by two herbivourous fishes: Tilapia nilotica and Haplochromis nigripinnis. J. Zool. 1973, 171, 41–55. [Google Scholar]
- Moriarty, D.; Darlington, J.P.E.C.; Dunn, I.G.; Moriarty, C.M.; Tevlin, M.P. Feeding and grazing in Lake George, Uganda. Proc. R. Soc. London Series B. Biol. Sci. R. Soc. 1973, 184, 299–319. [Google Scholar]
- Riede, K. Global Register of Migratory Species—From Global to Regional Scales. Final Report of the R&D—Projekt 808 05 081; Federal Agency for Nature Conservation: Bonn, Germany, 2004; p. 329. [Google Scholar]
- Bailey, R.G. Guide to the fishes of the River Nile in the Republic of the Sudan. J. Nat. Hist. 1994, 28, 937–970. [Google Scholar] [CrossRef]
- Murray, A.M. Relationships and Biogeography of the Fossil and living African snakehead fishes (Percomorpha, Channidae, Parachanna). J. Vertebr. Paleontol. 2012, 32, 820–835. [Google Scholar] [CrossRef]
- Courtenay, W.R.; Williams, J.D. Snakeheads (Pisces, Channidae): A Biological Synopsis and Risk Assessment; Dept. of the Interior, U.S. Geological Survey: Gainesville, FL, USA, 2004.
- George, U.U.; Albert, P.E.; George, E. Food and feeding habits of Ophiocephalus obscura (African snakehead) in the Cross River estuary, Cross River State, Nigeria. Int. J. Fish. Aquac. 2011, 3, 231–238. [Google Scholar]
- Oladosun, O.O.; Oladosun, G.A.; Hart, A.I. Some ecological factors of the tropical floodplain influencing the breeding and conservation of Gymnarchus niloticus (Cuvier 1829): A review. In Proceedings of the 26th Annual Conference of the Fisheries Society of Nigeria (FISON), Minna, Nigeria, 28 November–2 December 2011; pp. 193–200. Available online: http://aquaticcommons.org/24096/2/30_opt.pdf (accessed on 10 August 2020).
- Daget, J. Données récentes sur la biologie des poissons dans le delta central du Niger. Hydrobiology 1957, 9, 321–347. [Google Scholar] [CrossRef]
- McCabe, W.L.; Smith, J.C.; Harriott, P. Unit Operations of Chemical Engineering, 5th ed.; McGraw-Hill: New York, NY, USA, 1993; pp. 614–623. [Google Scholar]
- McCabe, W.L.; Smith, J.C.; Harriott, P. Unit Operations of Chemical Engineering, 6th ed.; McGraw-Hill: New York, NJ, USA, 2001; pp. 742–747. [Google Scholar]
- Seader, J.D.; Henley, E.J. Separation Process Principles; John Wiley: Hoboken, NJ, USA, 1998; pp. 198–201. [Google Scholar]
- Treybal, R.E. Mass-Transfer Operations, 3rd ed.; Reissue; McGraw-Hill: New York, NY, USA, 1987; pp. 717–761. [Google Scholar]
- Anatra-Cordone, M.; Durkin, P. Imidacloprid: Human Health and Ecological Risk Assessment. Final Report (SERA TR 05-43-24-03a) prepared for the U.S. Department of Agriculture (Forest Service). 2005. Available online: http://cues.cfans.umn.edu/old/pollinators/pdf-pesticides/ForestServiceImidacloprid.pdf (accessed on 10 August 2020).
- Food and Agriculture Organization (FAO/UN). List of Pesticides Evaluated by JMPR and JMPS. 2020. Available online: http://www.fao.org/agriculture/crops/thematic-sitemap/theme/pests/jmpr/en/ (accessed on 10 August 2020).
- Ibitayo, O. Agricultural pesticide contamination. The Encyclopedia of Earth. National Council for Science and the Environment. 2011. Available online: http://editors.eol.org/eoearth/wiki/Agricultural_pesticide_contamination (accessed on 10 August 2020).
- The Embassy of the Kingdom of the Netherlands, Lagos Office. Aquaculture in Nigeria (fact sheet). 2019. Available online: https://www.agroberichtenbuitenland.nl/landeninformatie/nigeria (accessed on 10 August 2020).
- Food and Agriculture Organization (FAO/UN). Protein Quality Evaluation: Report of the Joint FAO/WHO Expert Consultation; FAO Food and Nutrition Paper 51; FAO: Rome, Italy, 1991; Available online: https://aquatichubafrique.com/2019/03/29/state-of-aquaculture-in-nigeria/ (accessed on 10 August 2020).
- Dougherty, C.P.; Holtz, S.H.; Reinert, J.C.; Panyacosit, L.; Axelrad, D.A.; Woodruff, T.J. Dietary exposures to food contaminants across the United States. Environ. Res. 2000, 84, 170–185. [Google Scholar] [CrossRef]
- Akoto, O.; Azuure, A.A.; Adotey, K.D. Pesticide residues in water, sediment and fish from Tono Reservoir and their health risk implications. SpringerPlus 2016, 5, 1849. [Google Scholar] [CrossRef] [PubMed]
- Oyewole, O.; Akinpelu, A.O.; Adekanla, B.A. Body size perceptions and weight status of adults in a nigerian rural community. Ann. Med. Health Sci. Res. 2015, 5, 358–364. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Principles and Methods for the Risk Assessment of Chemicals in Food. International Programme on Chemical Safety. Environmental Health Criteria 240. Annex 1: Glossary of Terms. 2009. Available online: http://www.inchem.org/documents/ehc/ehc/ehc240_annex1.pdf (accessed on 10 August 2020).
- European Union. EU Pesticides Databse. Acetamiprid. 2016. Available online: https://e[c.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=activesubstance.detail&language=EN&selectedID=911 (accessed on 10 August 2020).
- European Union. EU Pesticides Databse. Thiamethoxam. 2016. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=activesubstance.detail&language=EN&selectedID=1937 (accessed on 10 August 2020).
- U.S. Department of Health and Human Services. Protocol for Scoping Review of Health Effects of Neonicotinoid Pesticides. National Toxicology Programme. 2017. Available online: https://ntp.niehs.nih.gov/ntp/ohat/neonicotinoid/nachrs_protocol_508.pdf (accessed on 10 August 2020).
- U.S. Department of Health and Human Services. Neonicotinoid Pesticides & Adverse Health Outcomes. National Toxicology Programme. 2019. Available online: https://ntp.niehs.nih.gov/whatwestudy/assessments/noncancer/ongoing/neonicotinoid/index.html (accessed on 10 August 2020).
- Cimino, A.M.; Boyles, A.L.; Thayer, K.A.; Perry, M.J. Effects of Neonicotinoid Pesticide Exposure on Human Health: A Systematic Review. Environ. Health Perspect. 2016, 125, 155–162. [Google Scholar] [CrossRef]
- Okoffo, E.D.; Fosu-Mensah, B.Y.; Gordon, C. Persistent organochlorine pesticide residues in cocoa beans from Ghana, a concern for public health. Int. J. Food Contam. 2016, 3, 160. [Google Scholar] [CrossRef]
- World Health Organization. Aldrin & Dieldrin. Inventory of Evaluations Performed by the Joint Meeting on Pesticide Residues (JMPR). 2012. Available online: https://apps.who.int/pesticide-residues-jmpr-database/pesticide?name=Aldrin%20and%20Dieldrin (accessed on 10 August 2020).
- World Health Organization (WHO/IPSC) Aldrin and Dieldrin. International Programme on Chemical Safety (INCHEM) Database. Environmental Health Criteria 91, Section 1.5.3. 1989. Available online: http://www.inchem.org/documents/ehc/ehc/ehc91.htm#SubSectionNumber:1.5.3 (accessed on 10 August 2020).
- U.S. Environmental Protection Agency (EPA). Aquatic Life Benchmarks and Ecological Risk Assessments for Registered Pesticides. 2020. Available online: https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/aquatic-life-benchmarks-and-ecological-risk (accessed on 10 August 2020).
- Wang, H.-S.; Sthiannopkao, S.; Du, J.; Chen, Z.-J.; Kim, K.-W.; Yasin, M.S.M.; Hashim, J.H.; Wong, C.K.-C.; Wong, M.H. Daily intake and human risk assessment of organochlorine pesticides (OCPs) based on Cambodian market basket data. J. Hazard. Mater. 2011, 192, 1441–1449. [Google Scholar] [CrossRef]
- Smit, E. Review of the DRAFT Water Quality Criteria Report for Imidacloprid. Phase III: Application of the Pesticide Water Quality Criteria Methodology; Prepared for the Central Coast Regional Water Quality Control Board. Version October 2018; National Institute of Public Health and the Environment Centre for Safety of Substances and Products: Bilthoven, The Netherlands, 2019. Available online: https://www.waterboards.ca.gov/centralcoast/water_issues/programs/tmdl/docs/pesticide_criteria/peer_rev_els_smit.pdf (accessed on 10 August 2020).
- U.S. Environmental Protection Agency (EPA). Pesticide Fact Sheet: Thiacloprid. 2003. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/fs_PC-014019_26-Sep-03.pdf (accessed on 10 August 2020).
- MacBean, C. (Ed.) e-Pesticide Manual, 15th ed.; ver. 5.1; Acetamiprid. (135410-20-7) (2008–2010); British Crop Protection Council: Alton, UK, 2012. [Google Scholar]
- University of Hertfordshire (UK). Thiamethoxam. Pesticide Properties Data Base (PPDB). 2019. Available online: https://sitem.herts.ac.uk/aeru/ppdb/en/Reports/631.htm (accessed on 10 August 2020).
- World Health Organization. Aldrin and Dieldrin in Drinking-Water. WHO/SDE/WSH/03.04/73. 2003. Available online: https://www.who.int/water_sanitation_health/dwq/chemicals/adrindieldrin.pdf (accessed on 10 August 2020).
- U.S. National Research Council. Estimating Exposures. In Pesticides in the Diets of Infants and Children; National Academies Press: Washington, DC, USA, 1993. Available online: https://www.ncbi.nlm.nih.gov/books/NBK236273/ (accessed on 10 August 2020).


| Neonic | Molar Mass (M.W.) | Solubility (ppm), 20 °C, pH = 7 | Solubility (mol/dm−3), 20 °C, pH = 7 | Direct Photolysis, DT50 (days), pH = 7 |
|---|---|---|---|---|
| Imidacloprid | 255.66 | 610 | 0.002 | 0.2 |
| Acetamiprid | 222.67 | 2950 | 0.013 | 34 |
| Thiacloprid | 252.72 | 184 | 0.0007 | (recalcitrant) |
| Thiamethoxam | 291.71 | 4100 | 0.014 | 3.7 |
| Neonics | Precursor Ion Masses | MS 1 Resolution (Quadrupole) | Product Ions Masses | MS 2 Resolution (Quadrupole) | Dwell Time (10−3 s) | Fragmentor Masses | Collision Energy (eV) | Cell Accelerator Voltage (V) | Polarity |
|---|---|---|---|---|---|---|---|---|---|
| IMI | 256.1 | Unit | 209 | Unit | 100 | 96 | 14 | 7 | + ve |
| 256.1 | Unit | 175.1 | Unit | 100 | 96 | 18 | 7 | + ve | |
| THA | 253 | Unit | 126 | Unit | 100 | 103 | 22 | 7 | + ve |
| 253 | Unit | 90 | Unit | 100 | 103 | 42 | 7 | + ve | |
| ACE | 223.1 | Unit | 126 | Unit | 100 | 103 | 18 | 7 | + ve |
| 223.1 | Unit | 90.1 | Unit | 100 | 103 | 38 | 7 | + ve | |
| THX | 292 | Unit | 211.1 | Unit | 100 | 103 | 8 | 7 | +ve |
| 292 | Unit | 131 | Unit | 100 | 104 | 20 | 7 | +ve | |
| 3-CA | 128 | Unit | 92.1 | Unit | 100 | 96 | 25 | 7 | + ve |
| Neonics | Retention Time (min) | Coefficient of Determination, r2 | LOD (µg/g) | LOQ (µg/g) | Regression Equation y = m(x) + c |
|---|---|---|---|---|---|
| IMI | 5.909 | 0.9992 | 0.002 | 0.005 | y = 1E6(x) + 7317.3 |
| THA | 8.379 | 0.9995 | 0.0005 | 0.003 | y = 0.39E6(x) + 1683.2 |
| ACE | 6.859 | 0.9994 | 0.003 | 0.004 | y = 2E6(x) + 8944.8 |
| THX | 5.149 | 0.9985 | 0.004 | 0.005 | y = 0.25E(x) + 2024.8 |
| I | II | III | IV | V | VI | VII | VIII |
|---|---|---|---|---|---|---|---|
| Fish Species | Range of Lengths (TL) | Range of Weights | Lm (TL at Maturity) | tm (Age at Maturity) | Longevity | Estimated Age | Lower Limit of Kf |
| Clarias gariepinus (sharptooth catfish) | 10–13 cm | 40–45 g | 30.5–37.5 cm (adults, 90 cm) [45,46] | 2 years [47] | 6.12 years (up to 15 years [48,49]) | <2 years, fingerlings. | 1.8 (>1) |
| Clarias anguillaris (mudfish) | 9–12 cm | 35–40 g | Male, 14.8 cm Female, 15.7 cm (adults, 65 cm) [50,51,52] | Male, 3.1 years Female, 3.0 years [53] | 10–18 years [54] | <3 years, fingerlings. | 2.0 (>1) |
| Sarotherodon galilaeus (mango tilapia) | 13–15 cm | 60–85 g | 18–22 cm (adults, 39.5 cm) [55] | 2 years [55] | 7 years [55] | <2 years, fingerlings. | 1.7 (>1) |
| Parachanna obscura (snakehead) | 14–16 cm | 50–70 g | 24.5 cm (adults, 45.5–50 cm) [56,57] | 2 years (for larger fish) [58] | 8–12 years [58,59] | <2 years, fingerlings. | 1.2 (>1) |
| Oreochromis niloticus (Nile tilapia) | 13–16 cm | 56–72 g | 18.6 cm; (adults, 28 cm) [60] | 10–12 months [61] | 9 years [62] | <10 months, fingerlings. | 1.3 (>1) |
| Gymnarchus niloticus (aba) | 15–17 cm | 55–74 g | Maximum length observed in adults, 167 cm [63] | At least 6 months [64] | 4–14 years (aquarium); up to 40 years (in the wild) [63] | <6 months based on length alone, possibly fingerlings. | 1.1 (>1) |
| I | II | III | IV | V | VI | VII | VIII |
|---|---|---|---|---|---|---|---|
| Fish Species | Neonics | Range (µg/g) | Mean ± σ (µg/g) | EDI (µg/kg Body Mass/day) | Maximum ADI (µg/kg Body Mass/Day) [91,92,93,94,95] | Risk Index (EDI/Max. ADI) | Risk Assessment |
| C. gariepinus | THA | 0.08–0.10 | 0.09 ± 0.07 | 0.054 | 10 | 5.4 × 10−3 < 1 | No risk |
| ACE | 0.04–0.09 | 0.07 ± 0.05 | 0.041 | 70 | 5.8 × 10−4 < 1 | No risk | |
| C. anguillaris | ACE | 0.12–0.16 | 0.14 ± 0.03 | 0.082 | 70 | 1.2 × 10−3 < 1 | No risk |
| THX | 0.05–0.10 | 0.08 ± 0.02 | 0.047 | 80 | 5.9 × 10−4 < 1 | No risk | |
| S. galilaeus | THA | 0.02–0.06 | 0.04 ± 0.01 | 0.023 | 10 | 2.3 × 10−3 < 1 | No risk |
| THX | 0.04–0.09 | 0.06 ± 0.02 | 0.035 | 80 | 4.4 × 10−4 < 1 | No risk | |
| P. obscura | THA | 0.02–0.04 | 0.03 ± 0.01 | 0.017 | 10 | 1.7 × 10−3 < 1 | No risk |
| ACE | 0.01–0.03 | 0.02 ± 0.01 | 0.012 | 70 | 1.7 × 10−4 < 1 | No risk | |
| THX | 0.03–0.05 | 0.04 ± 0.03 | 0.023 | 80 | 2.8 × 10−4 < 1 | No risk | |
| O. niloticus | ACE | 0.02–0.08 | 0.05 ± 0.02 | 0.029 | 70 | 4.1 × 10−4 < 1 | No risk |
| THX | 0.12–0.14 | 0.12 ± 0.10 | 0.070 | 80 | 8.7 × 10−4 < 1 | No risk | |
| G. niloticus | THA | 0.06–0.10 | 0.08 ± 0.05 | 0.047 | 10 | 4.7 × 10−3 < 1 | No risk |
| ACE | 0.02–0.06 | 0.04 ± 0.03 | 0.023 | 70 | 3.3 × 10−4 < 1 | No risk | |
| THX | 0.05–0.09 | 0.07 ± 0.02 | 0.041 | 80 | 5.1 × 10−4 < 1 | No risk |
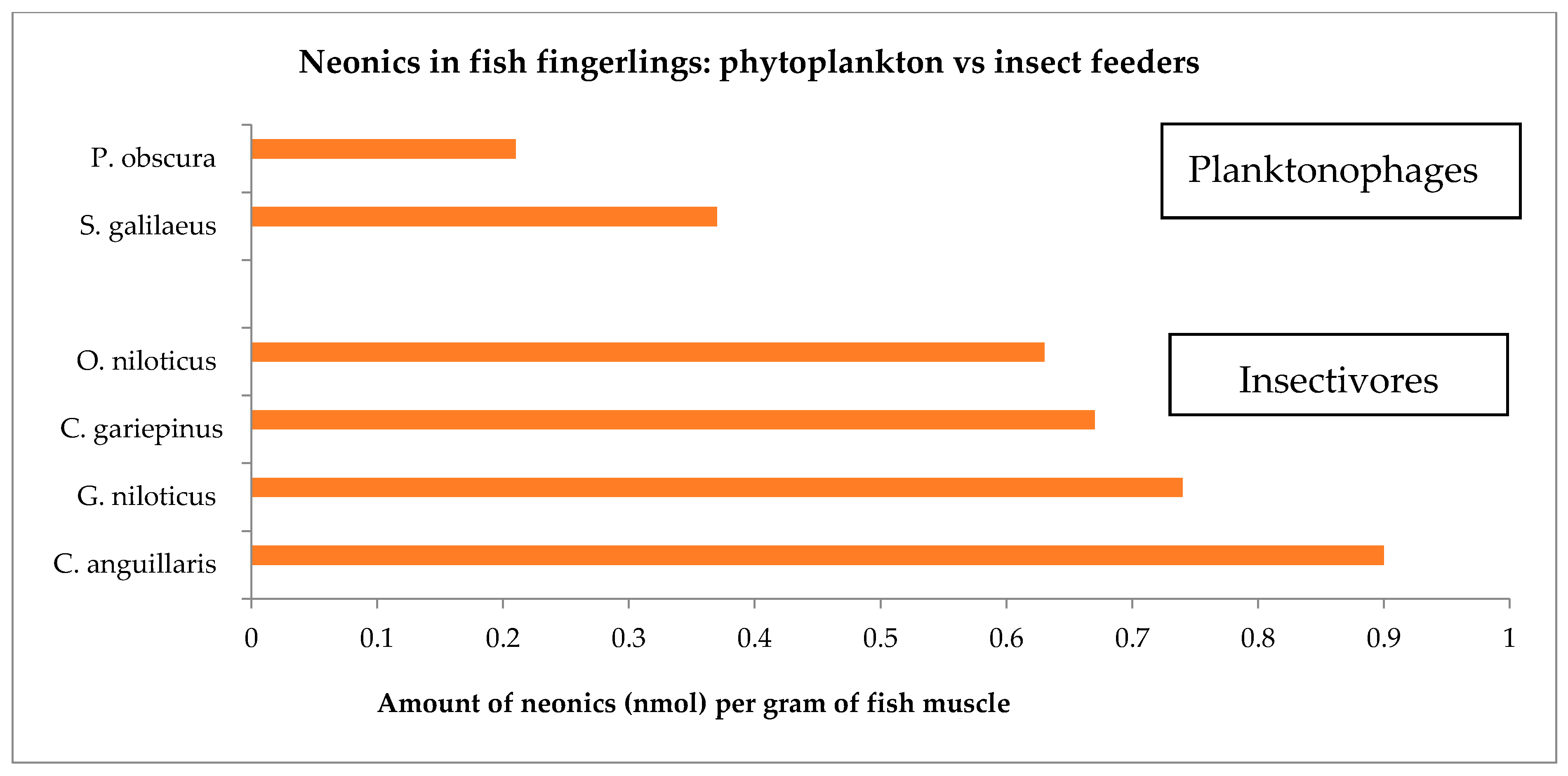
| Fish Species | Neonics | Molar Concentration Mean ± σ (nmol/g) |
|---|---|---|
| C. gariepinus | THA | 0.36 ± 0.29 |
| ACE | 0.31 ± 0.22 | |
| C. anguillaris | ACE | 0.63 ± 0.13 |
| THX | 0.27 ± 0.07 | |
| S. galilaeus | THA | 0.16 ± 0.04 |
| THX | 0.21 ± 0.07 | |
| P. obscura | THA | 0.12 ± 0.04 |
| ACE | 0.09 ± 0.04 | |
| THX | 0.14 ± 0.10 | |
| O. niloticus | ACE | 0.22 ± 0.09 |
| THX | 0.41 ± 0.34 | |
| G. niloticus | THA | 0.32 ± 0.20 |
| ACE | 0.18 ± 0.13 | |
| THX | 0.24 ± 0.07 |
| Ecological Compartment | Sum of Mass Concentrations of all Neonics | |
|---|---|---|
| I | Plantation soil in cocoa farms | <2000 ppb (i.e., <2 μg/g dried soil) |
| II | Surface water of Owena River | <0.3 ppb (i.e., <0.3 μg/liter) |
| III | Sediments | <300 ppb (i.e., <0.3 μg/g dried sediment) |
| Neonics | log10 Kow | AEBV (Ec50, ppb) |
|---|---|---|
| Imidacloprid (IMI) | 0.57 [159] | 9 × 103 [157] |
| Thiacloprid (THA) | 1.26 [160] | 918.0 [157] |
| Acetamiprid (ACE) | 0.8 [161] | 1.92 × 104 [157] |
| Thiamethoxam (THX) | −0.13 [162] | 2.0 × 104 [157] |
| Aldrin | 3.0 [163] | <53.0 (highest Lc50 reported) [155,156] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adegun, A.O.; Akinnifesi, T.A.; Ololade, I.A.; Busquets, R.; Hooda, P.S.; Cheung, P.C.W.; Aseperi, A.K.; Barker, J. Quantification of Neonicotinoid Pesticides in Six Cultivable Fish Species from the River Owena in Nigeria and a Template for Food Safety Assessment. Water 2020, 12, 2422. https://doi.org/10.3390/w12092422
Adegun AO, Akinnifesi TA, Ololade IA, Busquets R, Hooda PS, Cheung PCW, Aseperi AK, Barker J. Quantification of Neonicotinoid Pesticides in Six Cultivable Fish Species from the River Owena in Nigeria and a Template for Food Safety Assessment. Water. 2020; 12(9):2422. https://doi.org/10.3390/w12092422
Chicago/Turabian StyleAdegun, Ayodeji O., Thompson A. Akinnifesi, Isaac A. Ololade, Rosa Busquets, Peter S. Hooda, Philip C.W. Cheung, Adeniyi K. Aseperi, and James Barker. 2020. "Quantification of Neonicotinoid Pesticides in Six Cultivable Fish Species from the River Owena in Nigeria and a Template for Food Safety Assessment" Water 12, no. 9: 2422. https://doi.org/10.3390/w12092422
APA StyleAdegun, A. O., Akinnifesi, T. A., Ololade, I. A., Busquets, R., Hooda, P. S., Cheung, P. C. W., Aseperi, A. K., & Barker, J. (2020). Quantification of Neonicotinoid Pesticides in Six Cultivable Fish Species from the River Owena in Nigeria and a Template for Food Safety Assessment. Water, 12(9), 2422. https://doi.org/10.3390/w12092422