Characteristics of Fluorescence Spectra, UV Spectra, and Specific Growth Rates during the Outbreak of Toxic Microcystis Aeruginosa FACHB-905 and Non-Toxic FACHB-469 under Different Nutrient Conditions in a Eutrophic Microcosmic Simulation Device
Abstract
1. Introduction
2. Materials and Methods
2.1. Algae Strains
2.2. Experimental Reagents and Instruments
2.3. Pretreatment
2.4. Experimental Method
2.4.1. Culture Conditions
2.4.2. Determination of the UV Spectra of Water Samples during Microcystis Aeruginosa Blooms
2.4.3. Determination of Three-Dimensional Fluorescence Spectra of Water Samples during Microcystis Aeruginosa Blooms
2.4.4. Calculation of Specific Growth Rate of Microcystis aeruginosa
- μ—specific growth rate of the micro-organism [d−1];
- μmax—the maximum specific growth rate that can theoretically be achieved by the micro-organism when the concentration of the substrate is limited [d−1];
- C—the concentration of substrate mass in a solution that restricts the proliferation of microbes [mg/L]; and
- KS—the half-saturation constant [mg/L], whose value is the mass concentration of a substrate at μ = μ/2.
3. Results and Discussion
3.1. Changes of Ultraviolet Indices in the Process of Algal Blooms
3.1.1. E2/E3
3.1.2. E2/E4
3.1.3. E4/E6
3.1.4. S275–295, S350–400
3.1.5. A2/A1, A3/A1, and A3/A2
3.2. Changes of Three-Dimensional Fluorescence Indices in the Process of Algal Blooms
3.3. Specific Growth Rate Analyses of Microcystis Aeruginosa
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Lefebvre, K.A.; Quakenbush, L.; Frame, E.; Huntington, K.B.; Sheffield, G.; Stimmelmayr, R.; Bryan, A.; Kendrick, P.; Ziel, H.; Goldstein, T.; et al. Prevalence of algal toxins in Alaskan marine mammals foraging in a changing arctic and subarctic environment. Harmful Algae 2016, 55, 13–24. [Google Scholar] [CrossRef]
- Paerl, H.W.; Gardner, W.S.; Havens, K.E.; Joyner, A.R.; McCarthy, M.J.; Newell, S.E.; Qin, B.Q.; Scott, J.T. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 2016, 54, 213–222. [Google Scholar] [CrossRef] [PubMed]
- International Joint Commission. A balanced diet for lake erie: Reducing phosphorus loadings and harmful algal blooms. In Proceedings of the Report of the Lake Erie Ecosystem Priority, International Joint Commission, Washington, DC, USA, 27 February 2014. [Google Scholar]
- Tang, C.Y.; Sun, B.; Yu, K.F.; Shi, J.T.; Liu, M.M.; Jiang, T.; Huo, Y.Z.; He, P.M. Environmental triggers of a Microcystis (Cyanophyceae) blooms in an artificial lagoon of Hangzhou Bay, China. Mar. Pollut. Bull. 2018, 135, 776–782. [Google Scholar] [CrossRef]
- Wang, B.L.; Song, Q.Y.; Long, J.J.; Song, G.F.; Mi, W.J.; Bi, Y.H. Optimization method for Microcystis blooms mitigation by hydrogen peroxide and its stimulative effects on growth of chlorophytes. Chemosphere 2019, 228, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Bortoli, S.; Oliveira-Silva, D.; Krüger, T.; Dörr, F.A.; Colepicolo, P.; Volmer, D.A.; Pinto, E. Growth and microcystin production of a Brazilian Microcystis aeruginosa strain (LTPNA 02) under different nutrient conditions. Rev. Bras. Farm. 2014, 24, 389–398. [Google Scholar] [CrossRef]
- Wu, Y.L.; Li, L.; Gan, N.Q.; Zheng, L.L.; Ma, H.Y.; Shan, K.; Liu, J.; Xiao, B.D.; Song, L.R. Seasonal dynamics of water blooms-forming Microcystis morphospecies and the associated extracellular microcystin concentrations in large, shallow, eutrophic Dianchi Lake. J. Environ. Sci. 2014, 26, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.C.; Zhang, Y.J.; Huang, Q.; Gao, J.F. When and where to reduce nutrient for controlling harmful algal blooms in large eutrophic lake Chaohu, China? Ecol. Indic. 2018, 89, 808–817. [Google Scholar] [CrossRef]
- Zhang, R.; Qi, F.; Liu, C.; Zhang, Y.P.; Wang, Y.T.; Song, Z.L.; Kumirska, J.; Sun, D.Z. Cyanobacteria derived taste and odor characteristics in various lakes in China: Songhua Lake, Chaohu Lake and Taihu Lake. Ecotox. Environ. Safe 2019, 181, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Jiang, D.; Dai, H. Spatial-temporal hydrodynamic and algal blooms modelling analysis of a reservoir tributary embayment. J. Hydro-Environ. Res. 2015, 9, 200–215. [Google Scholar] [CrossRef]
- O’Boyle, S.; McDermott, G.; Silke, J.; Cusack, C. Potential impact of an exceptional blooms of Karenia Mikimotoi on dissolved oxygen levels in waters off western Ireland. Harmful Algae 2016, 53, 77–85. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Boyer, G.L. Health impacts from cyanobacteria harmful algae blooms: Implications for the North American Great Lakes. Harmful Algae 2016, 54, 194–212. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Georgic, W.; Klaiber, H.A. Reeling in the damages: Harmful algal blooms’ impact on Lake Erie’s recreational fishing industry. J. Environ. Manag. 2017, 199, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.C.; Zhang, Y.J.; Arhonditsis, G.B.; Gao, J.F.; Chen, Q.W.; Wu, N.C.; Dong, F.F.; Shi, W.Q. How successful are the restoration efforts of China’s lakes and reservoirs? Environ. Int. 2019, 123, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, H.; Mladenov, N.; Datta, S. Effects of acidification on the optical properties of dissolved organic matter from high and low arsenic groundwater and surface water. Sci. Total Environ. 2018, 653, 1326–1332. [Google Scholar] [CrossRef]
- Helms, J.R.; Stubbins, A.; Ritchie, J.D.; Minor, E.C.; Kieber, D.J.; Mopper, K. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef]
- Zhang, H.W.; Zhang, F.; Song, J. Analysis of Hydrochemical Characteristics and Three-Dimensional Fluorescence Spectra in the Semi-Arid Ebinur Lake Watershed, Xinjiang, China. Water 2018, 10, 426. [Google Scholar] [CrossRef]
- Baker, A.; Inverarity, R.; Charlton, M.; Richmond, S. Detecting river pollution using fluorescence spectrophotometry: Case studies from the Ouseburn, NE England. Environ. Pollut. 2003, 124, 57–70. [Google Scholar] [CrossRef]
- Goldman, J.H.; Rounds, S.A.; Needoba, J.A. Applications of Fluorescence Spectroscopy for Predicting Percent Wastewater in an Urban Stream. Environ. Sci. Technol. 2012, 46, 4374–4381. [Google Scholar] [CrossRef]
- Yu, H.; Song, Y.; Tu, X.; Du, E.; Liu, R.; Peng, J. Assessing removal efficiency of dissolved organic matter in wastewater treatment using fluorescence excitation emission matrices with parallel factor analysis and second derivative synchronous fluorescence. Bioresour. Technol. 2013, 144, 595–601. [Google Scholar] [CrossRef]
- Qu, J.Q.; Shen, L.P.; Zhao, M.; Li, W.T.; Jia, C.X.; Zhu, H.; Zhang, Q.J. Determination of the Role of Microcystis aeruginosa in Toxin Generation Based on Phosphoproteomic Profiles. Toxins 2018, 10, 304. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Fang, T.; Thring, R.W.; Li, Y.; Yu, H.; Zhou, Q.; Zhao, M. Toxic and non-toxic strains of Microcystis aeruginosa induce temperature dependent allelopathy toward growth and photosynthesis of Chlorella vulgaris. Harmful Algae 2015, 48, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Pang, W.J.; Mao, Y.D.; Ge, S.J.; Yu, H.G.; Dai, C.J.; Zhao, M. Changes of Extracellular Polymeric Substance (EPS) during Microcystis aeruginosa blooms at Different Levels of Nutrients in a Eutrophic Microcosmic Simulation Device. Pol. J. Environ. Stud. 2020, 29, 349–360. [Google Scholar] [CrossRef]
- Derrien, M.; Yang, L.; Hur, J. Lipid biomarkers and spectroscopic indices for identifying organic matter sources in aquatic environments: A review. Water Res. 2017, 112, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Moyo, W.; Chaukura, N.; Msagati, T.A.M.; Mamba, B.B.; Heijman, S.G.J.; Nkambule, T.T.I. The properties and removal efficacies of natural organic matter fractions by South African drinking water treatment plants. J. Environ. Chem. Eng. 2019, 7, 103101. [Google Scholar] [CrossRef]
- Zhou, Y.P.; Zhang, X.F.; Li, X.; Jia, P.L.; Dai, R.H. Evaluation of changes in Microcystis aeruginosa growth and microcystin production by urea via transcriptomic surveys. Sci. Total Environ. 2019, 655, 181–187. [Google Scholar] [CrossRef]
- Stein, E.J.R.; Stein Janet, R. Handbook of Phycological Methods, Culture Methods and Growth Measurements; Cambridge University Press: Cambridge, UK, 1973. [Google Scholar]
- Caperon, J.; Meyer, J. Nitrogen-limited growth of marine phytoplankton-I. Changes in population characteristics with steady-state growth rate. Deep Sea Res. Oceanogr. Abstr. 1972, 19, 601–618. [Google Scholar] [CrossRef]
- Caperon, J.; Meyer, J. Nitrogen-limited growth of marine phytoplankton-II. Uptake kinetics and their role in nutrient limited growth of phytoplankton*. Deep Sea Res. Oceanogr. Abstr. 1972, 19, 619–632. [Google Scholar] [CrossRef]
- Artinger, R.; Buckau, G.; Geyer, S.; Fritz, P.; Wolf, M.; Kim, J.I. Characterization of groundwater humic substances: Influence of sedimentary organic carbon. Appl. Geochem. 2000, 15, 97–116. [Google Scholar] [CrossRef]
- Chin, Y.P.; Aiken, G.; O’Loughlin, E. Molecular weight, polydispersity and spect roscopic properties of aquatic humic substances. Environ. Sci. Technol. 1994, 28, 1853–1858. [Google Scholar] [CrossRef]
- Giancoli Barreto, S.R.; Nozaki, J.; Barreto, W.J. Origin of dissolved organic carbon studied by UV-Vis spectroscopy. Acta. Hydroch. Hydrob. 2003, 31, 513–518. [Google Scholar] [CrossRef]
- Minero, C.; Lauri, V.; Falletti, G.; Maurino, V.; Pelizzetti, E.; Vione, D. Spectrophotometric characterization of surface lakewater samples: Implications for the quantification of nitrate and the properties of dissolved organic matter. Ann. Di Chem. 2007, 97, 1007–1116. [Google Scholar] [CrossRef]
- Peuravuori, J.; Pihlaja, K. Molecular size distribution and spectroscopic properties of aquatic humic substances. Anal. Chim. Acta. 1997, 337, 133–149. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Li, Z.W.; Nie, X.D.; Huang, M.; Wang, D.Y.; Xiao, H.B.; Liu, C.; Peng, H.; Jiang, J.Y.; Zeng, G.M. The role of dissolved organic matter in soil organic carbon stability under water erosion. Ecol. Indic. 2019, 102, 724–733. [Google Scholar] [CrossRef]
- Strobel, B.W.; Hansen, H.C.B.; Borggaard, O.K.; Andersen, M.K.; Raulund-Rasmussen, K. Composition and reactivity of DOC in forest floor soil solutions in relation to tree species and soil type. Biogeochemistry 2001, 56, 1–26. [Google Scholar] [CrossRef]
- Guo, C.X.; Zhang, C.J.; Sun, Z.Y.; Zhao, X.Y.; Zhou, Q.; Hoffmann, M.R. Synergistic impact of humic acid on the photo-reductive decomposition of perfluorooctanoic acid. Chem. Eng. J. 2019, 360, 1101–1110. [Google Scholar] [CrossRef]
- Baes, A.U.; Blooms, P.R. Fulvic acid ultraviolet-visible spectra: Influence of solvent and pH. Soil. Sci. Soc. Am. J. 1990, 54, 1248–1254. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S.; Kaas, H. Optical Properties and Signatures of Chromophoric Dissolved Organic Matter (CDOM) in Danish Coastal Waters. Estuar. Coast. Shelf Sci. 2000, 51, 267–278. [Google Scholar] [CrossRef]
- Carder, K.L.; Steward, R.G.; Harvey, G.R.; Ortner, P.B. Marine humic and fulvic acids: Their effects on remote sensing of ocean chlorophyll. Limnol. Oceanogr. 1989, 34, 68–81. [Google Scholar] [CrossRef]
- Albrecht, R.; Le Petit, J.; Terrom, G.; Périssol, C. Comparison between UV spectroscopy and Nirs to assess humification process during sewage sludge and green wastes co-composting. Bioresour. Technol. 2011, 102, 4495. [Google Scholar] [CrossRef]
- Porcal, P.; Kopáček, J. Photochemical degradation of dissolved organic matter reduces the availability of phosphorus for aquatic primary producers. Chemosphere 2018, 193, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.F.; Xenopoulos, M.A. Effects of agricultural land use on the composition of fluvial dissolved organic matter. Nat. Geosci. 2008, 2, 37–41. [Google Scholar] [CrossRef]
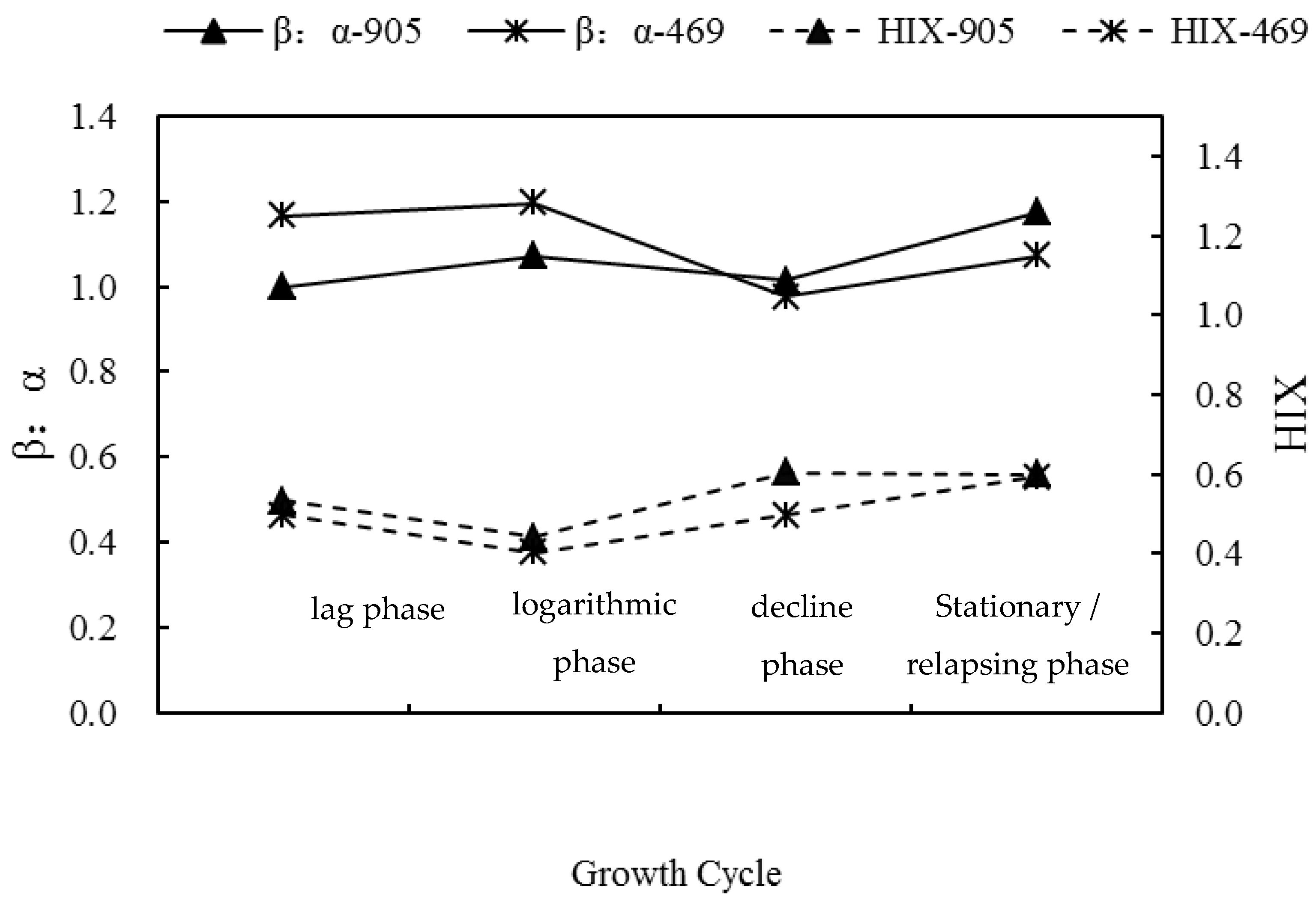
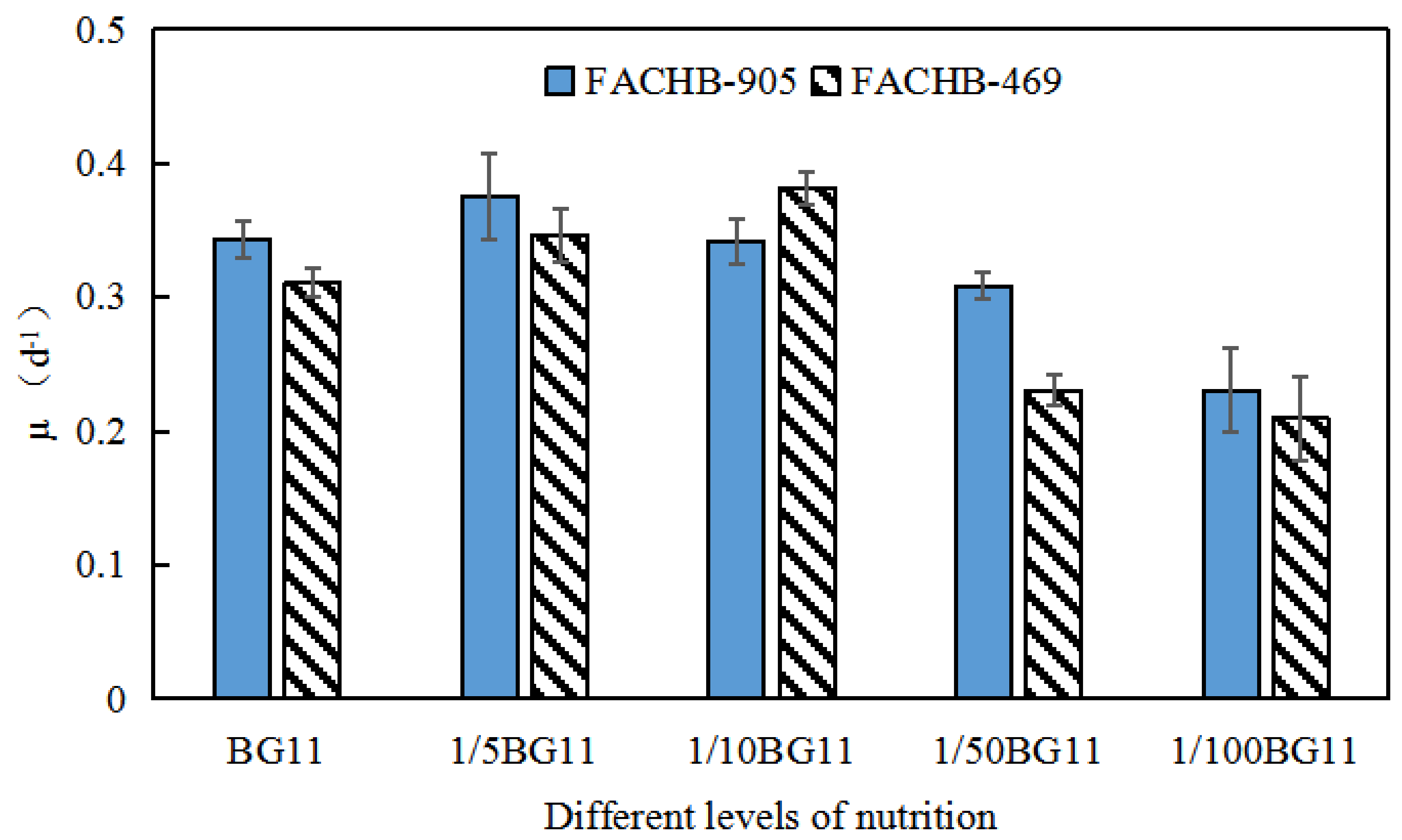

| Microcystis Aeruginosa | UV Spectral Index | Lag Phase | Logarithmic Phase | Decline Phase | Stationary/Relapsing Phase |
|---|---|---|---|---|---|
| FACHB-905 | E2/E3 | 15.999 ± 2.202 | 8.478 ± 1.291 | 7.041 ± 1.192 | 10.176 ± 1.17 |
| E2/E4 | 119.343 ± 8.175 | 82.975 ± 9.927 | 47.556 ± 3.648 | 73.339 ± 8.486 | |
| E4/E6 | 0.9 ± 0.102 | 2.004 ± 0.626 | 2.065 ± 0.563 | 1.555 ± 0.585 | |
| S275–295 | 0.025 ± 0.006 | 0.007 ± 0.003 | −0.004 ± 0.001 | −0.007 ± 0.001 | |
| S350–400 | 0.005 ± 0.002 | −0.012 ± 0.004 | −0.014 ± 0.003 | −0.015 ± 0.003 | |
| A2/A1 | 0.284 ± 0.093 | 0.124 ± 0.027 | 0.071 ± 0.031 | 0.062 ± 0.027 | |
| A3/A1 | 1.449 ± 0.178 | 0.477 ± 0.107 | 0.203 ± 0.05 | 0.256 ± 0.042 | |
| A3/A2 | 5.099 ± 0.307 | 2.982 ± 1.031 | 2.538 ± 0.868 | 3.63 ± 1.255 | |
| FACHB-469 | E2/E3 | 11.473 ± 1.159 | 6.955 ± 1.965 | 3.972 ± 0.073 | 6.384 ± 1.035 |
| E2/E4 | 88.93 ± 5.715 | 49.857 ± 4.823 | 17.997 ± 3.95 | 32.087 ± 4.323 | |
| E4/E6 | 0.963 ± 0.149 | 1.411 ± 0.394 | 1.897 ± 0.072 | 1.486 ± 0.292 | |
| S275–295 | 0.012 ± 0.005 | −0.002 ± 0.002 | −0.006 ± 0.001 | −0.01 ± 0.001 | |
| S350–400 | −0.003 ± 0.001 | −0.01 ± 0.002 | −0.011 ±0.002 | −0.016 ± 0.003 | |
| A2/A1 | 0.305 ± 0.12 | 0.14 ± 0.029 | 0.194 ± 0.043 | 0.074 ± 0.035 | |
| A3/A1 | 1.567 ± 0.006 | 0.565 ± 0.28 | 0.784 ± 0.368 | 0.282 ± 0.072 | |
| A3/A2 | 5.24 ± 0.152 | 3.685 ± 0.684 | 3.462 ± 1.382 | 3.632 ± 0.663 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Pang, W.; Ge, S.; Yu, H.; Dai, C.; Huang, X.; Li, J.; Zhao, M. Characteristics of Fluorescence Spectra, UV Spectra, and Specific Growth Rates during the Outbreak of Toxic Microcystis Aeruginosa FACHB-905 and Non-Toxic FACHB-469 under Different Nutrient Conditions in a Eutrophic Microcosmic Simulation Device. Water 2020, 12, 2305. https://doi.org/10.3390/w12082305
Wang Q, Pang W, Ge S, Yu H, Dai C, Huang X, Li J, Zhao M. Characteristics of Fluorescence Spectra, UV Spectra, and Specific Growth Rates during the Outbreak of Toxic Microcystis Aeruginosa FACHB-905 and Non-Toxic FACHB-469 under Different Nutrient Conditions in a Eutrophic Microcosmic Simulation Device. Water. 2020; 12(8):2305. https://doi.org/10.3390/w12082305
Chicago/Turabian StyleWang, Qi, Wenjing Pang, ShuJie Ge, Hengguo Yu, Chuanjun Dai, Xianfeng Huang, Jun Li, and Min Zhao. 2020. "Characteristics of Fluorescence Spectra, UV Spectra, and Specific Growth Rates during the Outbreak of Toxic Microcystis Aeruginosa FACHB-905 and Non-Toxic FACHB-469 under Different Nutrient Conditions in a Eutrophic Microcosmic Simulation Device" Water 12, no. 8: 2305. https://doi.org/10.3390/w12082305
APA StyleWang, Q., Pang, W., Ge, S., Yu, H., Dai, C., Huang, X., Li, J., & Zhao, M. (2020). Characteristics of Fluorescence Spectra, UV Spectra, and Specific Growth Rates during the Outbreak of Toxic Microcystis Aeruginosa FACHB-905 and Non-Toxic FACHB-469 under Different Nutrient Conditions in a Eutrophic Microcosmic Simulation Device. Water, 12(8), 2305. https://doi.org/10.3390/w12082305





