Site Fidelity, Habitat Use, and Movement Patterns of the Common Carp during Its Breeding Season in the Pearl River as Determined by Acoustic Telemetry
Abstract
1. Introduction
2. Materials and Methods
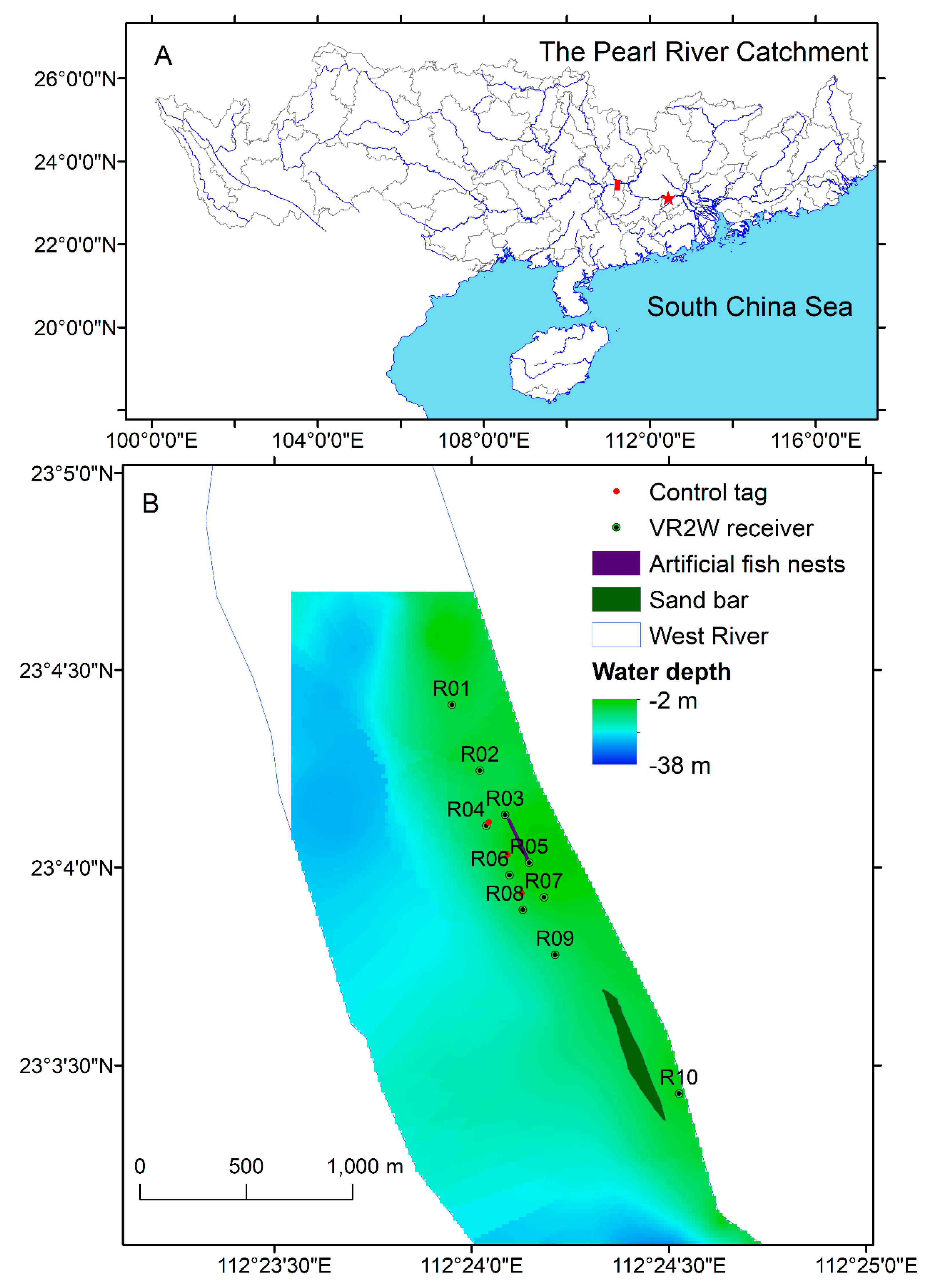
2.1. Study Area
2.2. Receiver Placement
2.3. Tag Attachment
2.4. Data Analysis
3. Results
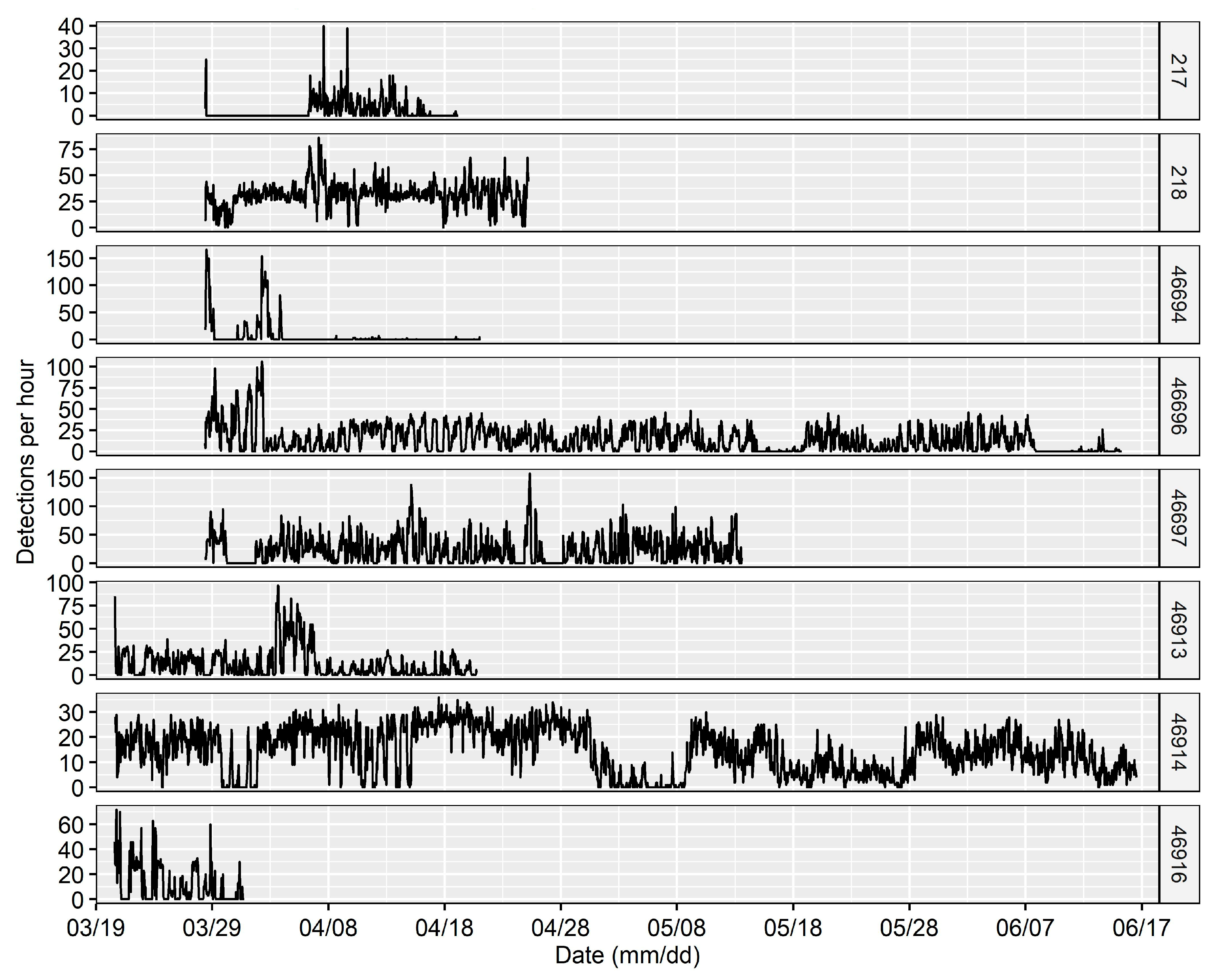
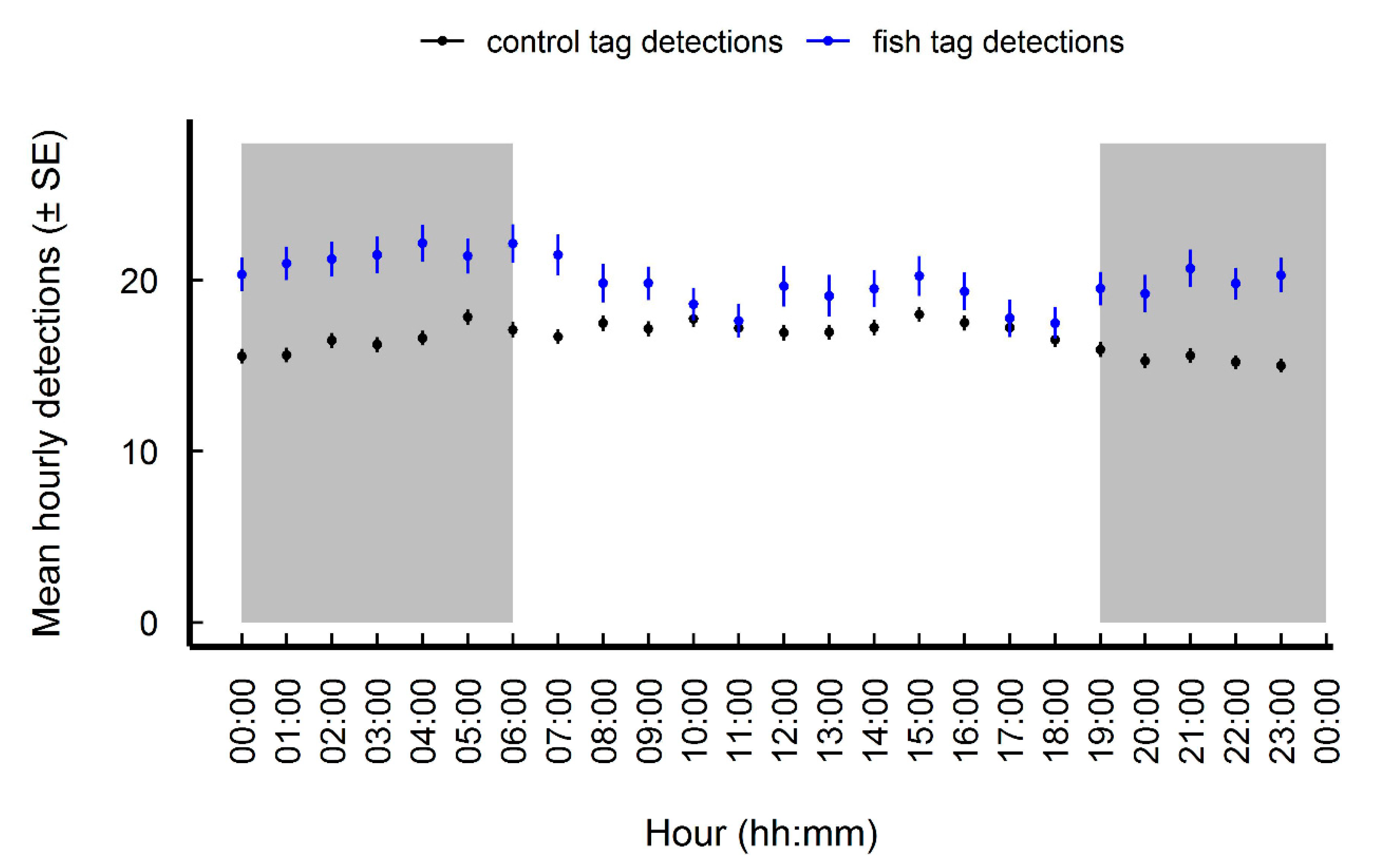
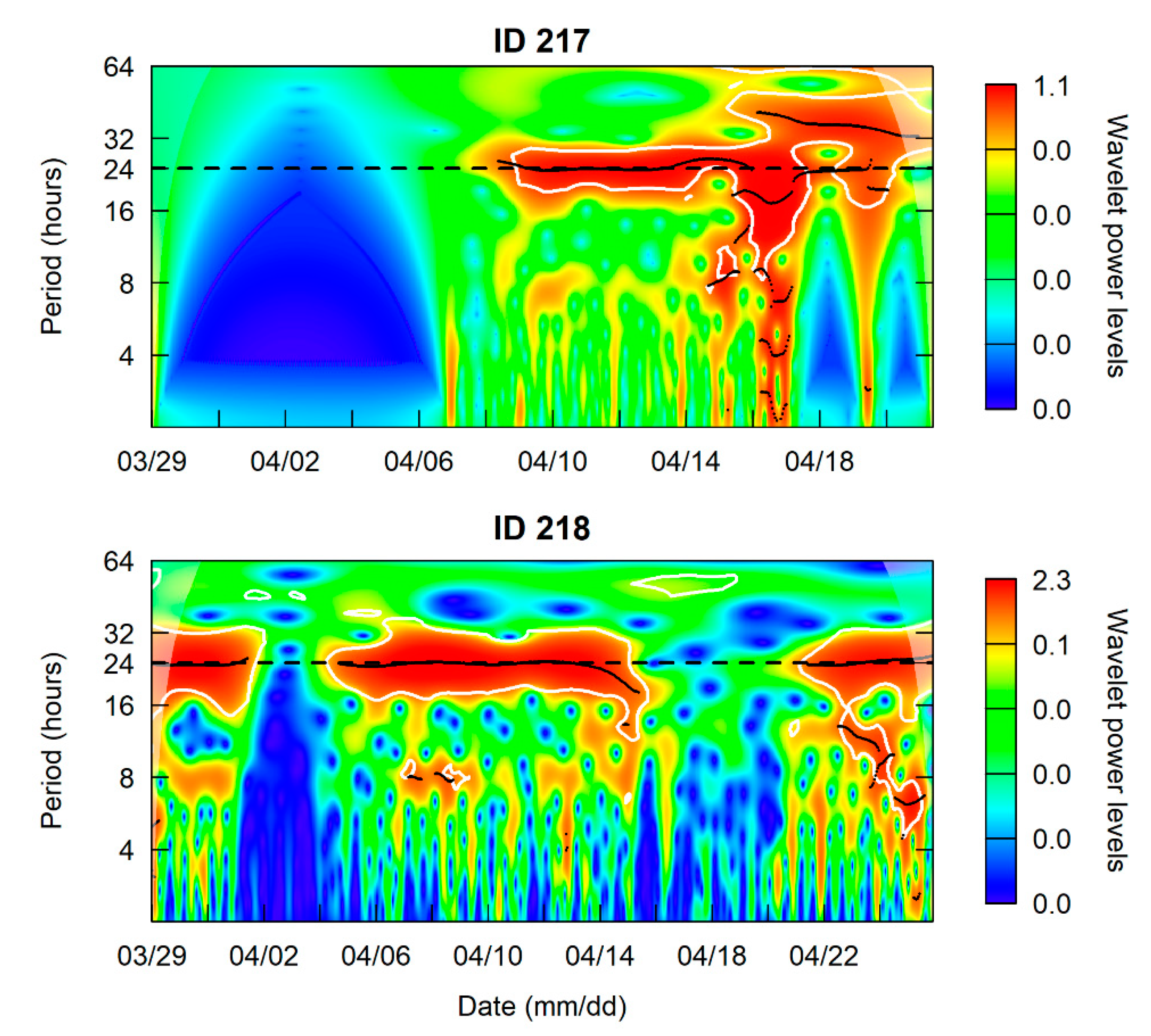
3.1. Site Fidelity and Diel Detection Pattern
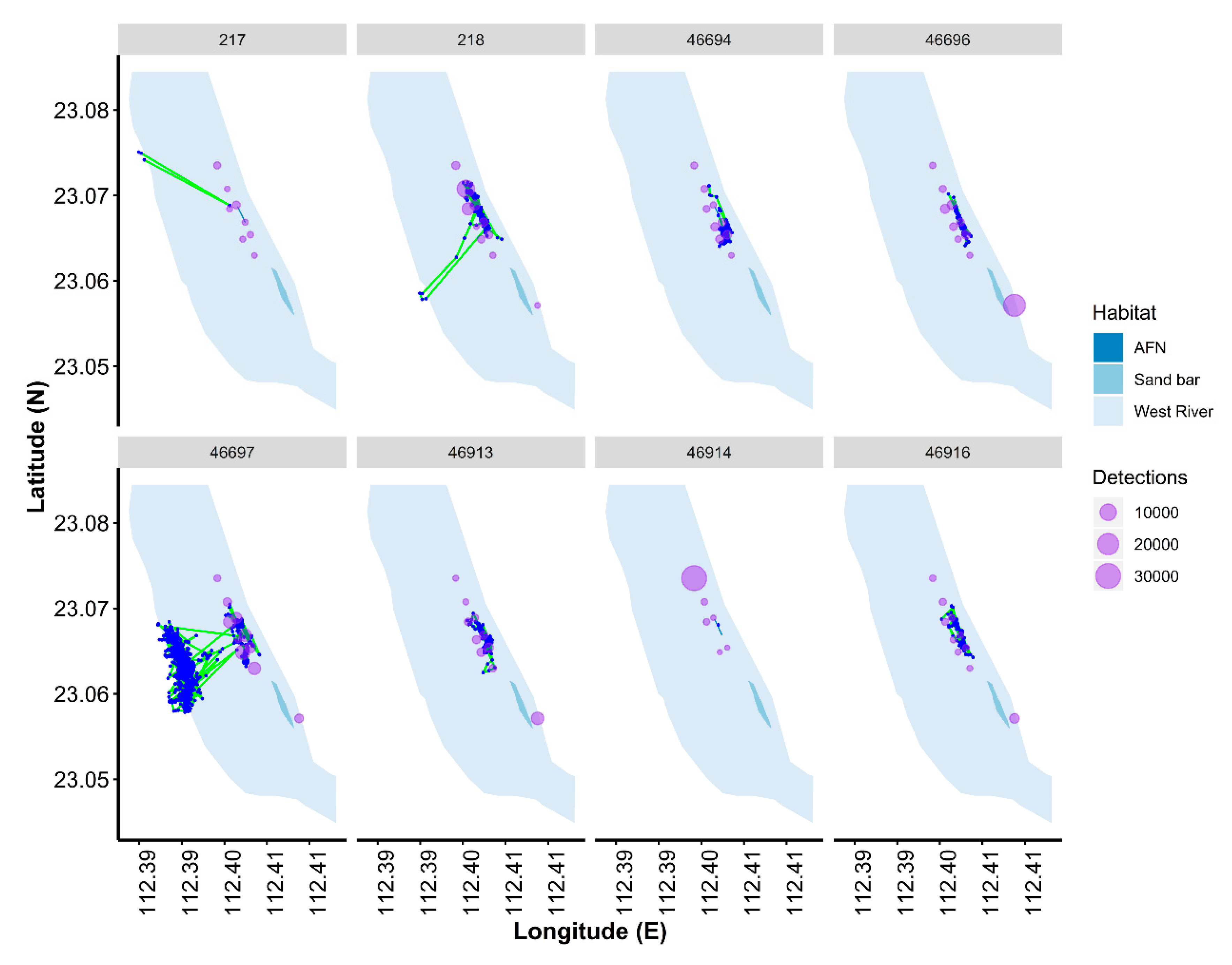
3.2. Habitat Use
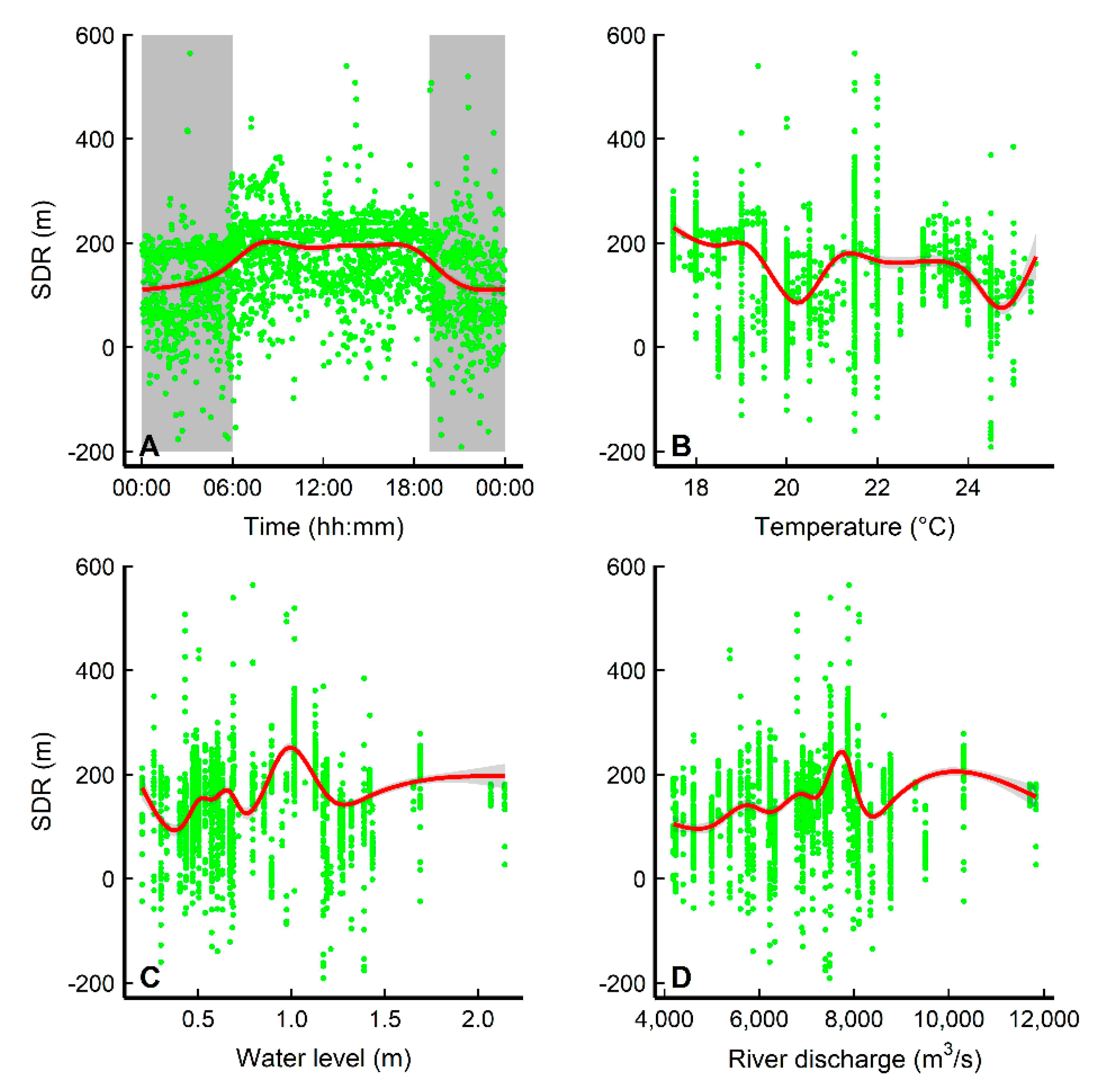
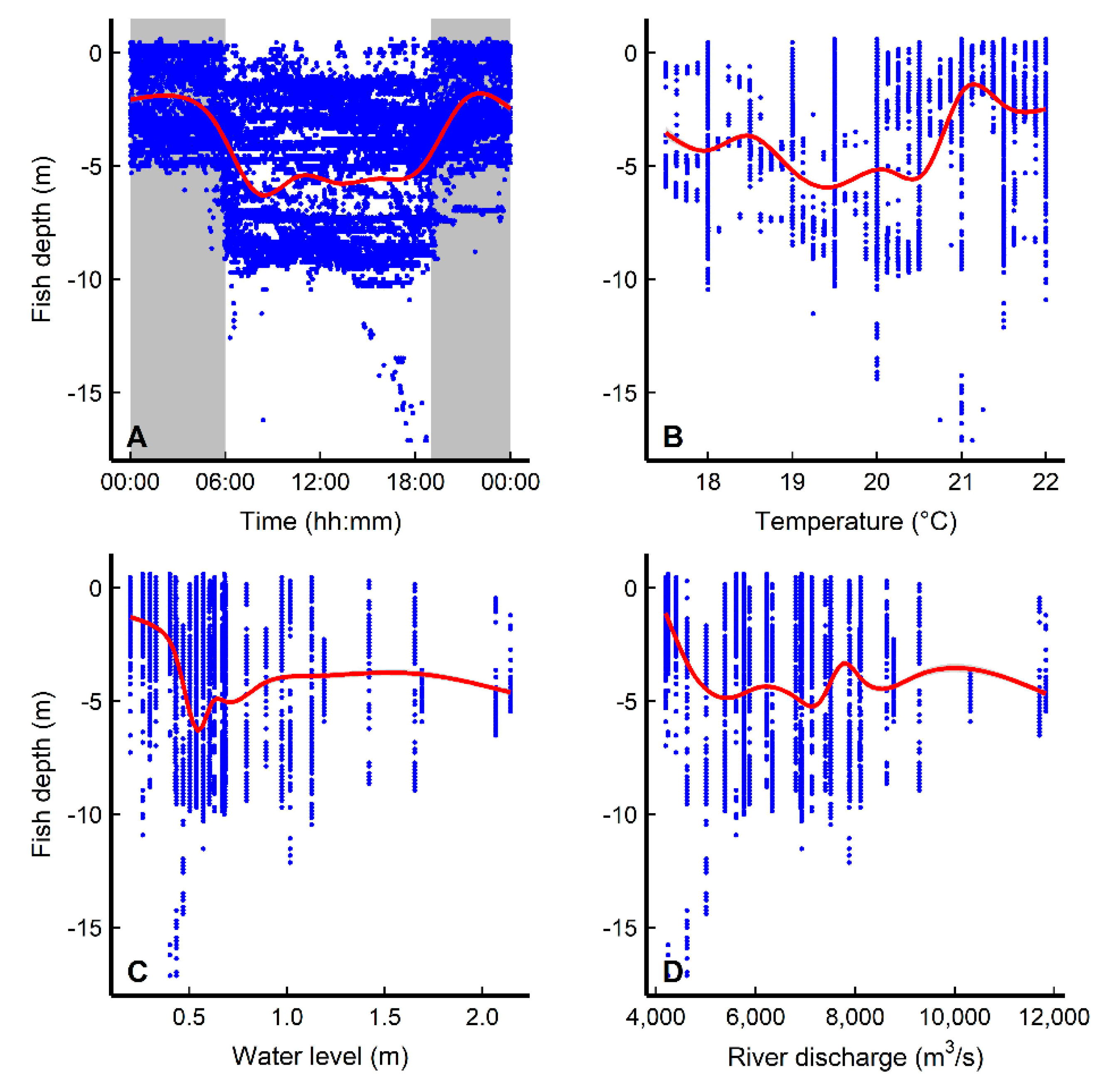
3.3. Diel Movement Behaviors and Environmental Effects
4. Discussion
4.1. Site Fidelity
4.2. Diel Pattern of Common Carp Movement and Distribution
4.3. Habitat Use
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Block, B.A.; Dewar, H.; Blackwell, S.B.; Williams, T.D.; Prince, E.D.; Farwell, C.J.; Boustany, A.; Teo, S.L.H.; Seitz, A.; Walli, A.; et al. Migratory movements, depth preferences, and thermal biology of Atlantic bluefin tuna. Science 2001, 293, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Block, B.A.; Jonsen, I.D.; Jorgensen, S.J.; Winship, A.J.; Shaffer, S.A.; Bograd, S.J.; Hazen, E.L.; Foley, D.G.; Breed, G.A.; Harrison, A.L.; et al. Tracking apex marine predator movements in a dynamic ocean. Nature 2011, 475, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Heupel, M.R.; Knip, D.M.; de Lestang, P.; Allsop, Q.A.; Grace, B.S. Short-term movement of barramundi in a seasonally closed freshwater habitat. Aquat. Biol. 2011, 12, 147–155. [Google Scholar] [CrossRef]
- Branco, P.; Amaral, S.D.; Ferreira, M.T.; Santos, J.M. Do small barriers affect the movement of freshwater fish by increasing residency? Sci. Total Environ. 2017, 581, 486–494. [Google Scholar] [CrossRef]
- Boyce, M.S.; Johnson, C.J.; Merrill, E.H.; Nielsen, S.E.; Solberg, E.J.; van Moorter, B. Can habitat selection predict abundance? J. Anim. Ecol. 2016, 85, 11–20. [Google Scholar] [CrossRef] [PubMed]
- van Moorter, B.; Rolandsen, C.M.; Basille, M.; Gaillard, J.M. Movement is the glue connecting home ranges and habitat selection. J. Anim. Ecol. 2016, 85, 21–31. [Google Scholar] [CrossRef]
- King, A.J. Ontogenetic patterns of habitat use by fishes within the main channel of an Australian floodplain river. J. Fish Biol. 2004, 65, 1582–1603. [Google Scholar] [CrossRef]
- Rosenberger, A.; Angermeier, P.L. Ontogenetic shifts in habitat use by the endangered Roanoke logperch (Percina rex). Freshw. Biol. 2003, 48, 1563–1577. [Google Scholar] [CrossRef]
- Kattel, G.R.; Closs, G.P. Spatial and temporal variation in the fish community of a South Island, New Zealand coastal lake. N. Z. J. Mar. Freshw. Res. 2007, 41, 1–11. [Google Scholar] [CrossRef]
- Pépino, M.; Rodríguez, M.A.; Magnan, P. Shifts in movement behavior of spawning fish under risk of predation by land-based consumers. Behav. Ecol. 2015, 26, 996–1004. [Google Scholar] [CrossRef]
- Reebs, S.G. Plasticity of diel and circadian activity rhythms in fishes. Rev. Fish Biol. Fish. 2002, 12, 349–371. [Google Scholar] [CrossRef]
- Winemiller, K.O.; Jepsen, D.B. Effects of seasonality and fish movement on tropical river food webs. J. Fish Biol. 1998, 53, 267–296. [Google Scholar] [CrossRef]
- Meyer, C.G. Electronic tags reveal the hidden lives of fishes. Bull. Mar. Sci. 2017, 93, 301–318. [Google Scholar] [CrossRef]
- Taylor, M.D.; van der Meulen, D.E.; Brodie, S.; Cadiou, G.; Knott, N.A. Applying acoustic telemetry to understand contaminant exposure and bioaccumulation patterns in mobile fishes. Sci. Total Environ. 2018, 625, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes; Kottelat, Cornol, Switzerland and Freyhof, Berlin, Germany. 2007, p. 646. Available online: https://www.nhbs.com/handbook-of-european-freshwater-fishes-book (accessed on 10 February 2017).
- Le, P. Fauna Sinica Actinopterygii Cypriniformes III; Science Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Stuart, I.G.; Conallin, A.J. Control of globally invasive common carp: An 11-year commercial trial of the Williams’ cage. N. Am. J. Fish. Manag. 2018, 38, 1160–1169. [Google Scholar] [CrossRef]
- Lu, K. The Fisheries Resource in the Pearl River; Guangdong Science and Technology Press: Guangzhou, China, 1990. (In Chinese) [Google Scholar]
- Tan, X.; Li, X.; Lin, J.; Li, Y.; Bi, Y.; Li, J.; Wang, C. Early morphogenesis and laval resources of common carp at Zhaoqing section in the Pearl River. J. Dalian Fish. Univ. 2009, 24, 125–129. (In Chinese) [Google Scholar]
- Freyhof, J.; Kottelat, M. Cyprinus Carpio, Wild Common Carp. In The IUCN Red List of Threatened Species 2008; IUCN: Gland, Switzerland, 2008. [Google Scholar]
- Pan, P.; Li, Y.; Li, X. Effect evaluation of artificial fishnest on common carp (Cyprinus carpio) in Xijiang River. Freshw. Fish. 2016, 46, 45–49. (In Chinese) [Google Scholar]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. Modeling zero-inflated count data with glmmTMB. BioRxiv 2017, 132753. [Google Scholar] [CrossRef]
- Lindén, A.; Mäntyniemi, S. Using the negative binomial distribution to model overdispersion in ecological count data. Ecology 2011, 92, 1414–1421. [Google Scholar] [CrossRef]
- Aho, K.; Derryberry, D.; Peterson, T. Model selection for ecologists: The worldviews of AIC and BIC. Ecology 2014, 95, 631–636. [Google Scholar] [CrossRef]
- Roesch, A.; Schmidbauer, H. WaveletComp 1.1: A Guided Tour through the R Package. 2018. Available online: https://CRAN.R-project.org/package=WaveletComp (accessed on 1 June 2019).
- Booth, G.D.; Niccolucci, M.J.; Schuster, E.G. Identifying Proxy Sets in Multiple Linear Regression [Microform]: An Aid to Better Coefficient Interpretation; U.S. Dept. of Agriculture, Forest Service, Intermountain Research Station: Ogden, UT, USA, 1993.
- Koehn, J.D.; Nicol, S.J. Comparative movements of four large fish species in a lowland river. J. Fish Biol. 2016, 88, 1350–1368. [Google Scholar] [CrossRef] [PubMed]
- Benito, J.; Benejam, L.; Zamora, L.; Garcia-Berthou, E. Diel cycle and effects of water flow on activity and use of depth by Common Carp. Trans. Am. Fish. Soc. 2015, 144, 491–501. [Google Scholar] [CrossRef]
- Rahman, M.M.; Meyer, C.G. Effects of food type on diel behaviours of common carp Cyprinus carpio in simulated aquaculture pond conditions. J. Fish Biol. 2009, 74, 2269–2278. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.J.; Stuart, I.G. Lateral movement of common carp (Cyprinus carpio L.) in a large lowland river and floodplain. Ecol. Freshw. Fish 2009, 18, 72–82. [Google Scholar] [CrossRef]
- Conallin, A.J.; Smith, B.B.; Thwaites, L.A.; Walker, K.F.; Gillanders, B.M. Exploiting the innate behaviour of common carp, Cyprinus carpio, to limit invasion and spawning in wetlands of the River Murray, Australia. Fish. Manag. Ecol. 2016, 23, 431–449. [Google Scholar] [CrossRef]
- Penne, C.R.; Pierce, C.L. Seasonal distribution, aggregation, and habitat selection of common carp in Clear Lake, Iowa. Trans. Am. Fish. Soc. 2008, 137, 1050–1062. [Google Scholar] [CrossRef]
- Chizinski, C.J.; Bajer, P.G.; Headrick, M.E.; Sorensen, P.W. Different migratory strategies of invasive Common Carp and native northern pike in the American Midwest suggest an opportunity for selective management strategies. N. Am. J. Fish. Manag. 2016, 36, 769–779. [Google Scholar] [CrossRef]
- Taylor, A.H.; Tracey, S.R.; Hartmann, K.; Patil, J.G. Exploiting seasonal habitat use of the common carp, Cyprinus carpio, in a lacustrine system for management and eradication. Mar. Freshw. Res. 2012, 63, 587–597. [Google Scholar] [CrossRef]
- Hennen, M.J.; Brown, M.L. Movement and spatial distribution of Common Carp in a South Dakota Glacial Lake System: Implications for management and removal. N. Am. J. Fish. Manag. 2014, 34, 1270–1281. [Google Scholar] [CrossRef]
- Wang, J.Y.; Kuo, T.C.; Hsieh, C.H. Causal effects of population dynamics and environmental changes on spatial variability of marine fishes. Nat. Commun. 2020, 11, 2635. [Google Scholar] [CrossRef]
- Parsapour-Moghaddam, P.; Brennan, C.P.; Rennie, C.D.; Elvidge, C.K.; Cooke, S.J. Impacts of channel morphodynamics on fish habitat utilization. Environ. Manag. 2019, 64, 272–286. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, W.; Xia, Y.; Yang, J.; Zhu, S.; Li, X. Selection of artificial fish nest material and influencing factors of implementation effects. South China Fish. Sci. 2020, 16, 21–28. (In Chinese) [Google Scholar] [CrossRef]
| ID | Tag Life (d) | Burst Interval (s) | Sex | SL (mm) | TL (mm) | Tag Attaching Time | Releasing Time |
|---|---|---|---|---|---|---|---|
| 46912 | 188 | 60–180 | undetermined | 390 | 500 | 19 March 2017 14:00 | 21 March 2017 14:00 |
| 46913 | 188 | 60–180 | Female | 340 | 440 | 19 March 2017 14:00 | 21 March 2017 14:00 |
| 46914 | 188 | 60–180 | undetermined | 345 | 440 | 19 March 2017 14:00 | 21 March 2017 14:00 |
| 46916 | 188 | 60–180 | Female | 305 | 370 | 19 March 2017 14:00 | 21 March 2017 14:00 |
| 46694 | 124 | 45–105 | Male | 250 | 290 | 22 March 2017 14:00 | 29 March 2017 11:00 |
| 46696 | 124 | 45–105 | Male | 400 | 450 | 22 March 2017 14:00 | 29 March 2017 11:00 |
| 46697 | 124 | 45–105 | Female | 350 | 420 | 22 March 2017 14:00 | 29 March 2017 11:00 |
| 217 | 251 | 60–180 | Male | 350 | 420 | 28 March 2017 11:00 | 29 March 2017 11:00 |
| 218 | 251 | 60–180 | Female | 440 | 530 | 28 March 2017 11:00 | 29 March 2017 11:00 |
| ID | RD (d) | TP (d) | Residence Index |
|---|---|---|---|
| 217 | 14 | 23 | 0.61 |
| 218 | 29 | 29 | 1 |
| 46694 | 18 | 25 | 0.72 |
| 46696 | 78 | 80 | 0.98 |
| 46697 | 45 | 47 | 0.96 |
| 46913 | 32 | 32 | 1 |
| 46914 | 89 | 89 | 1 |
| 46916 | 12 | 12 | 1 |
| Estimate | Std. Error | z Value | Pr (>|z|) | |
|---|---|---|---|---|
| diel phase effects on hourly fish tag detections | ||||
| (Intercept) | 2.844 | 0.133 | 21.41 | <2.0 × 10−16 |
| Day vs. night | 0.111 | 0.0234 | 4.744 | 2.1 × 10−6 |
| No significant diel pattern for hourly control tag detections | ||||
| (Intercept) | 2.747 | 0.118 | 23.191 | <2.0 × 10−16 |
| Day vs. night | 0.0132 | 0.00879 | 1.504 | 0.133 |
| Fish ID | HSI Around Each Receiver | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R01 | R02 | R03 | R04 | R05 | R06 | R07 | R08 | R09 | R10 | |
| 217 | 2.74 | 0.13 | 4.19 | 0.81 | 0.60 | 0.00 | 0.93 | 0.51 | 0.10 | 0.00 |
| 218 | 0.34 | 5.76 | 0.92 | 1.89 | 0.49 | 0.01 | 0.35 | 0.21 | 0.03 | 0.01 |
| 46694 | 0.58 | 0.63 | 0.29 | 0.61 | 1.70 | 1.91 | 2.47 | 1.75 | 0.06 | 0.00 |
| 46696 | 0.05 | 0.10 | 0.50 | 0.47 | 0.19 | 0.19 | 0.39 | 0.04 | 0.02 | 8.05 |
| 46697 | 0.09 | 0.30 | 1.00 | 1.55 | 1.64 | 0.03 | 0.86 | 2.60 | 1.55 | 0.36 |
| 46913 | 0.05 | 0.08 | 0.41 | 0.41 | 0.86 | 0.81 | 1.42 | 0.98 | 0.42 | 4.57 |
| 46914 | 9.88 | 0.06 | 0.00 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 46916 | 0.61 | 0.94 | 0.79 | 0.91 | 0.39 | 0.43 | 0.62 | 0.24 | 0.16 | 4.90 |
| Mean | 1.79 | 1.00 | 1.01 | 0.84 | 0.73 | 0.42 | 0.88 | 0.79 | 0.29 | 2.24 |
| Model | Explanatory Variables | Df | AIC | R2adj | Model Compared | LRT | p |
|---|---|---|---|---|---|---|---|
| SDR~ | |||||||
| S1 | f(hour) + (1|ID) | 5 | 32762 | 0.149 | |||
| S2 | f(hour) + f(temperature) + (1|ID) | 7 | 32388 | 0.00612 | S1 vs. S2 | 377.7732 | <0.0001 |
| S3 | f(hour) + f(water level) + (1|ID) | 7 | 32324 | 0.245 | |||
| S4 | f(hour) + f(river discharge) + (1|ID) | 7 | 32568 | 0.2 | |||
| Depth~ | |||||||
| D1 | f(hour) | 5 | 103728 | 0.296 | |||
| D2 | f(hour) + f(temperature) + (1|ID) | 7 | 97055 | 0.489 | D1 vs. D2 | 6676.919 | <0.0001 |
| D3 | f(hour) + f(water level) + (1|ID) | 7 | 96622 | 0.499 | |||
| D4 | f(hour) + f(river discharge) + (1|ID) | 7 | 99658 | 0.421 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Li, Y.; Zhang, L.; Wu, Z.; Zhu, S.; Li, J.; Li, X. Site Fidelity, Habitat Use, and Movement Patterns of the Common Carp during Its Breeding Season in the Pearl River as Determined by Acoustic Telemetry. Water 2020, 12, 2233. https://doi.org/10.3390/w12082233
Zhang Y, Li Y, Zhang L, Wu Z, Zhu S, Li J, Li X. Site Fidelity, Habitat Use, and Movement Patterns of the Common Carp during Its Breeding Season in the Pearl River as Determined by Acoustic Telemetry. Water. 2020; 12(8):2233. https://doi.org/10.3390/w12082233
Chicago/Turabian StyleZhang, Yingqiu, Yuefei Li, Lili Zhang, Zhi Wu, Shuli Zhu, Jie Li, and Xinhui Li. 2020. "Site Fidelity, Habitat Use, and Movement Patterns of the Common Carp during Its Breeding Season in the Pearl River as Determined by Acoustic Telemetry" Water 12, no. 8: 2233. https://doi.org/10.3390/w12082233
APA StyleZhang, Y., Li, Y., Zhang, L., Wu, Z., Zhu, S., Li, J., & Li, X. (2020). Site Fidelity, Habitat Use, and Movement Patterns of the Common Carp during Its Breeding Season in the Pearl River as Determined by Acoustic Telemetry. Water, 12(8), 2233. https://doi.org/10.3390/w12082233





