Analytical Method for Lithium Isotopes Determination by Thermal Ionization Mass Spectrometry: A Useful Tool for Hydrogeochemical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Procedure Adopted in the Clean Chemistry Laboratory: Sample Preparation
2.2. Sample Dissolution
2.3. Chemical Separation of Lithium
2.4. Procedure Adopted in the Mass Spectrometry Laboratories: Loading of Lithium on Rhenium Filament.
- (1)
- Load 1 μL solution on a degassed filament and dry it at 1.5–max 2.0 A;
- (2)
- Turn down, before glowing.
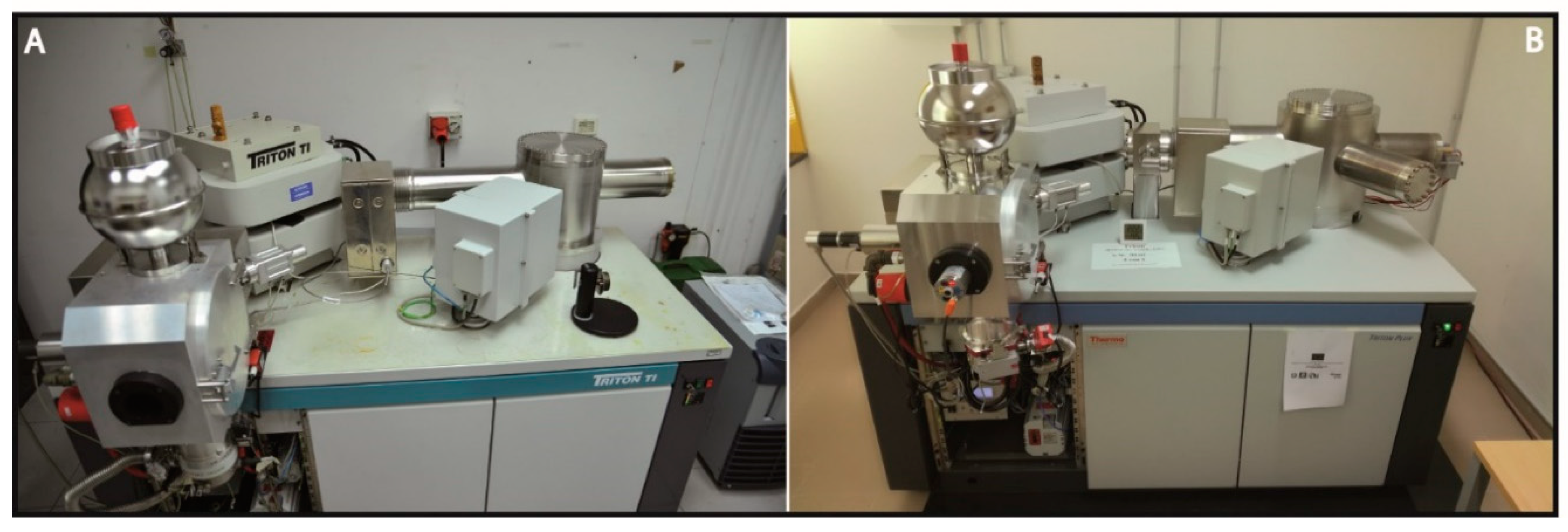
2.5. Lithium Isotopic Determinations by Thermal Ionization Mass Spectrometry
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berzelius, J.J. Ein neues mineralisches Alkali und ein neues Metall. J. Chem. Phys. 1817, 21, 44–48. [Google Scholar]
- Qi, H.P.; Taylor, P.D.P.; Berglund, M.; Bièvre, P. Calibrated measurement of the isotopic composition and atomic weight of the natural Li isotopic reference material IRMM-016. Int. J. Mass Spectrom. Ion Proc. 1997, 171, 263–268. [Google Scholar] [CrossRef]
- De Laeter, J.R.; Böhlke, J.K.; De Bièvre, P.; Hidaka, H.; Peiser, H.S.; Rosman, K.J.R.; Taylor, P.D.P. Atomic weights of the elements: Review 2000 (IUPAC Technical Report). Pure Appl. Chem. 2003, 75, 683–800. [Google Scholar] [CrossRef]
- Tomascak, P.B.; Magna, T.; Dohmen, R. Advances in Lithium Isotope Geochemistry; Springer: Cham, Switzerland, 2016; p. 120. [Google Scholar]
- Penniston-Dorland, S.; Liu, X.M.; Rudnick, R.L. Lithium isotope geochemistry. Rev. Mineral. Geochem. 2017, 82, 165–217. [Google Scholar] [CrossRef]
- Chan, L.H.; Edmond, J.M.; Thompson Gillis, K. Lithium isotopic composition of submarine basalts: Implications for the Lithium cycle in the oceans. Earth Planet. Sci. Lett. 1992, 108, 151–160. [Google Scholar] [CrossRef]
- Lynton, S.J.; Walker, R.J.; Candela, P.A. Lithium isotopes in the system Qz–Ms–fluid: An experimental study. Geochim. Cosmoch. Acta 2005, 69, 3337–3347. [Google Scholar] [CrossRef]
- Wunder, B.; Meixner, A.; Romer, R.L.; Heinrich, W. Temperature-dependent isotopic fractionation of lithium between clinopyroxene and high-pressure hydrous fluids. Contrib. Mineral. Petrol. 2006, 151, 112–120. [Google Scholar] [CrossRef]
- Wunder, B.; Meixner, A.; Romer, R.L.; Feenstra, A.; Schettler, G.; Heinrich, W. Lithium isotope fractionation between Li-bearing staurolite, Li-mica and aqueous fluids: An experimental study. Chem. Geol. 2007, 238, 277–290. [Google Scholar] [CrossRef]
- Richter, F.M.; Davis, A.M.; DePaolo, D.J.; Watson, E.B. Isotope fractionation by chemical diffusion between molten basalt and rhyolite. Geochim. Cosmochim. Acta 2003, 67, 3905–3923. [Google Scholar] [CrossRef]
- Richter, F.M.; Mendybaev, R.A.; Christensen, J.N.; Hutcheon, I.D.; Williams, R.W.; Sturchio, N.C.; Beloso, A.D. Kinetic isotopic fractionation during diffusion of ionic species in water. Geochim. Cosmochim. Acta 2006, 70, 277–289. [Google Scholar] [CrossRef]
- Chan, L.H.; Leeman, W.P.; You, C.F. Lithium isotopic composition of Central American Volcanic Arc, lavas: Implications for modification of subarc mantle by slab-derived fluids. Chem. Geol. 1999, 160, 255–280. [Google Scholar] [CrossRef]
- Chan, L.H.; Leeman, W.P.; You, C.F. Lithium isotopic composition of Central American volcanic arc lavas: Implications for modification of subarc mantle by slab-derived fluids: Correction. Chem. Geol. 2002, 182, 293–300. [Google Scholar] [CrossRef]
- Chan, L.H.; Alt, J.C.; Teagle, D.A.H. Lithium and lithium isotope profiles through the upper oceanic crust: A study of seawater-basalt exchange at ODP Sites 504B and 896A. Earth Planet. Sci. Lett. 2002, 201, 187–201. [Google Scholar] [CrossRef]
- Maffre, P.; Goddéris, Y.; Vigier, N.; Moquet, J.S.; Carretier, S. Modelling the riverine δ7Li variability throughout the Amazon Basin. Chem. Geol. 2019, 532, 119–336. [Google Scholar] [CrossRef]
- Marschall, H.R.; Wanless, V.D.; Shimizu, N.; Pogge von Strandmann, P.A.E.; Elliott, T.; Monteleone, B.D. The boron and lithium isotopic composition of mid-ocean ridge basalts and the mantle. Geoch. Cosmoch. Acta 2017, 207, 102–138. [Google Scholar] [CrossRef]
- Moriguti, T.; Nakamura, E. Across-arc variation of Li isotopes in lavas and implications for crust/mantle recycling at subduction zones. Earth Planet. Sci. Lett. 1998, 163, 167–174. [Google Scholar] [CrossRef]
- Chan, L.H.; Frey, F.A. Lithium isotope geochemistry of the Hawaiian plume: Results from the Hawaii Scientific Drilling Project and Koolau Volcano. Geochem. Geophys. Geosyst. 2003, 4, 1–20. [Google Scholar] [CrossRef]
- Ryan, J.G.; Kyle, P.R. Lithium abundance and lithium isotope variations in mantle sources: Insights from intraplate volcanic rocks from Ross Island and Marie Byrd Land (Antarctica) and other oceanic islands. Chem. Geol. 2004, 212, 125–142. [Google Scholar] [CrossRef]
- Elliott, T.; Thomas, A.; Jeffcoate, A.; Niu, Y. Lithium isotope evidence for subduction-enriched mantle in the source of mid-ocean-ridge basalts. Nature 2006, 443, 565–568. [Google Scholar] [CrossRef]
- Vlastélic, I.; Koga, K.; Chauvel, C.; Jacques, G.; Télouk, P. Survival of lithium isotopic heterogeneities in the mantle supported by HIMU-lavas from Rurutu Island, Austral Chain. Earth Planet. Sci. Lett. 2009, 286, 456–466. [Google Scholar] [CrossRef]
- Schiavi, F.; Kobayashi, K.; Nakamura, E.; Tiepolo, M.; Vannucci, R. Trace element and Pb–B–Li isotope systematics of olivine-hosted melt inclusions: Insights into source metasomatism beneath Stromboli (southern Italy). Contrib. Mineral. Petrol. 2012, 163, 1011–1031. [Google Scholar] [CrossRef]
- Schiavi, F.; Kobayashi, K.; Moriguti, T.; Nakamura, E.; Pompilio, M.; Tiepolo, M.; Vannucci, R. Degassing, crystallization and eruption dynamics at Stromboli: Trace element and lithium isotopic evidence from 2003 ashes. Contrib. Mineral. Petrol. 2010, 159, 541–561. [Google Scholar] [CrossRef]
- Vlastélic, I.; Staudacher, T.; Bachèlery, P.; Télouk, P.; Neuville, D.; Benbakkar, M. Lithium isotope fractionation during magma degassing: Constraints from silicic differentiates and natural gas condensates from Piton de la Fournaise volcano (Réunion Island). Chem. Geol. 2011, 284, 26–34. [Google Scholar] [CrossRef]
- Weyer, S.; Seitz, H.M. Coupled lithium- and iron isotope fractionation during magmatic differentiation. Chem. Geol. 2012, 294, 42–50. [Google Scholar] [CrossRef]
- Chan, L.H.; Gieskes, J.M.; You, C.F.; Edmond, J.M. Lithium isotope geochemistry of sediments and hydrothermal fluids of the Guaymas Basin, Gulf of California. Geochim. Cosmochim. Acta 1994, 58, 4443–4454. [Google Scholar]
- Bullen, T.D.; Kharaka, Y.K. Isotopic composition of Sr, Nd and Li in thermal waters from the Norris-Mammoth corridor, Yellowstone National Park and surrounding region. In Proceedings of the 7th International Symposium on Water-Rock Interaction, Park City, UT, USA, 13 July 1992; Balkema Publishers: Rotterdam, The Netherlands, 1992; p. 897. [Google Scholar]
- Millot, R.; Petelet-Giraud, E.; Guerrot, C.; Négrel, P. Multi-isotopic composition (d7Li-d11B-dD-d18O) of rainwaters in France: Origin and spatio-temporal characterization. Appl. Geochem. 2010, 25, 1510–1524. [Google Scholar] [CrossRef]
- Kloppmann, W.; Chikurel, H.; Picot, G.; Guttman, J.; Pettani, M.; Aharoni, A.; Guerrot, C.; Millot, R.; Gaus, I.; Wintgens, T. B and Li isotopes as intrinsic tracers for injection tests in aquifer storage and recovery systems. Appl. Geochem. 2009, 24, 1214–1223. [Google Scholar] [CrossRef]
- Meredith, K.; Moriguti, T.; Tomascak, P.; Hollins, S.; Nakamura, E. The lithium, boron and strontium isotopic systematics of groundwaters from an arid aquifer system: Implication for recharge and weathering processes. Geochim. Cosmochim. Acta 2013, 112, 20–31. [Google Scholar] [CrossRef]
- Pogge von Strandmann, P.A.E.; Porcelli, D.; James, R.H.; van Calsteren, P.; Schaefer, B.; Cartwright, I.; Reynolds, B.C.; Burton, K.W. Chemical weathering processes in the Great Artesian Basin: Evidence from lithium and silicon isotopes. Earth Planet. Sci. Lett. 2014, 406, 24–36. [Google Scholar] [CrossRef]
- Négrel, P.; Millot, R.; Guerrot, C.; Petelet-Giraud, E.; Brenot, A.; Malcuit, E. Heterogeneities and interconnections in groundwaters: Coupled B, Li andstable-isotope variations in a large aquifer system (Eocene Sand aquifer, Southwestern France). Chem. Geol. 2012, 296, 83–95. [Google Scholar]
- Millot, R.; Négrel, P. Multi-isotopic tracing (d7Li, d11B, 87Sr/86Sr) and chemical geothermometry: Evidence from hydro-geothermal systems in France. Chem. Geol. 2007, 244, 664–678. [Google Scholar] [CrossRef]
- Falkner, K.K.; Church, M.; Measures, C.I.; LeBaron, G.; Thouron, D.; Jeandel, C.; Stordal, M.C.; Gill, G.A.; Mortlock, R.; Froelich, P.; et al. Minor and trace element chemistry of lake Baikal, its tributaries, and surrounding hot springs. Limnol. Oceanogr. 1997, 42, 329–345. [Google Scholar] [CrossRef]
- Brant, C.; Coogan, L.A.; Gillis, K.M.; Seyfried, W.E.; Pester, N.J.; Spence, J. Lithium and Li-isotopes in young altered upper oceanic crust from the East PacificRise. Geochim. Cosmochim. Acta 2012, 96, 272–293. [Google Scholar] [CrossRef]
- Pogge von Strandmann, P.A.E.; Burton, K.W.; James, R.H.; van Calsteren, P.; Gislason, S.R. Assessing the role of climate on uranium and lithium isotope behaviour in rivers draining a basaltic terrain. Chem. Geol. 2010, 270, 227–239. [Google Scholar] [CrossRef]
- Tomascak, P.B.; Hemming, N.G.; Hemming, S.R. The lithium isotopic composition of waters from the Mono Basin. California. Geochim. Cosmochim. Acta 2003, 67, 601–611. [Google Scholar] [CrossRef]
- Henchiri, S.; Clergue, C.; Dellinger, M.; Gaillardet, J.; Louvat, P.; Bouchez, J. The influence of hydrothermal activityon the Li isotopic signature of rivers draining volcanic areas. Procedia Earth Planet. Sci. 2014, 10, 223–230. [Google Scholar] [CrossRef]
- James, R.H.; Palmer, M.R. The lithium isotope composition of international rock standards. Chem. Geol. 2000, 166, 319–326. [Google Scholar] [CrossRef]
- Arienzo, I.; Carandente, A.; Di Renzo, V.; Belviso, P.; Civetta, L.; D’Antonio, M.; Orsi, G. Sr and Nd isotope analysis at the Radiogenic Isotope Laboratory of the Istituto Nazionale di Geofisica e Vulcanologia, Sezione di Napoli—Osservatorio Vesuviano. Rapp. Tec. INGV 2013, 260, 1–18. [Google Scholar]
- Goldstein, S.L.; Deines, P.; Oelkers, E.H.; Rudnick, R.L.; Walter, L.M. Standards for publication of isotope ratio and chemical data in Chemical Geology. Chem. Geol. 2003, 202, 1–4. [Google Scholar] [CrossRef]
- Misra, S.; Froelich, P.N. Measurements of Lithium isotope ratios by quadrupole-ICP-MS: Application to seawater and natural carbonates. J. Anal. At. Spectrom. 2009, 24, 1524–1533. [Google Scholar] [CrossRef]
- Paonita, A.; Caracausi, A.; Martelli, M.; Rizzo, A.L. Temporal variations of helium isotopes in volcanic gases quantify pre-eruptive refill and pressurization in magma reservoirs: The Mount Etna case. Geology 2016, 44, 499–502. [Google Scholar] [CrossRef]
- Rizzo, A.L.; Federico, C.; Inguaggiato, S.; Sollami, S.; Tantillo, M.; Vita, F.; Bellomo, S.; Longo, M.; Grassa, F.; Liuzzo, M. The 2014 effusive eruption at Stromboli volcano (Italy): Inferences from soil CO2 flux and 3He/4He ratio in thermal waters. Geophys. Res. Lett. 2015, 42, 2235–2243. [Google Scholar] [CrossRef]
- Cardellini, C.; Chiodini, G.; Frondini, F.; Avino, R.; Bagnato, E.; Caliro, S.; Lelli, M.; Rosiello, A. Monitoring diffuse volcanic degassing during volcanic unrests: The case of Campi Flegrei (Italy). Sci. Rep. 2017, 7, 6757. [Google Scholar] [CrossRef] [PubMed]







| N. of Analyses | 6Li/7Li NIST L-SVEC INGV | 6Li/7Li * | BHVO-2 | NA01 600 m | 6Li/7Li NIST L-SVEC DiSTAR |
|---|---|---|---|---|---|
| 1 | 0.082522 | 0.082522 | 0.082017 | 0.07982 | 0.082732 |
| 2 | 0.082493 | 0.082493 | 0.07997 | 0.082668 | |
| 3 | 0.082602 | 0.082602 | 0.08014 | 0.082682 | |
| 4 | 0.082600 | 0.082356 | 0.082685 | ||
| 5 | 0.082598 | 0.082509 | 0.082643 | ||
| 6 | 0.082401 | 0.082471 | 0.082634 | ||
| 7 | 0.082365 | 0.082374 | 0.082617 | ||
| 8 | 0.082725 | 0.082618 | |||
| 9 | 0.082802 | 0.082614 | |||
| 10 | 0.082840 | 0.082601 | |||
| 11 | 0.082434 | 0.082476 | |||
| 12 | 0.082585 | 0.082577 | |||
| 13 | 0.082675 | 0.082544 | |||
| 14 | 0.082287 | 0.082212 | |||
| 15 | 0.082456 | 0.082245 | |||
| 16 | 0.082525 | 0.082261 | |||
| 17 | 0.082454 | 0.082869 | |||
| 18 | 0.082753 | 0.082672 | |||
| 19 | 0.082775 | 0.082586 | |||
| 20 | 0.082567 | 0.082769 | |||
| 21 | 0.082761 | 0.082683 | |||
| 22 | 0.082743 | ||||
| 23 | 0.082933 | ||||
| 24 | 0.082569 | ||||
| 25 | 0.082637 | ||||
| 26 | 0.082704 | ||||
| 27 | 0.082559 | ||||
| 28 | 0.082356 | ||||
| 29 | 0.082509 | ||||
| 30 | 0.082471 | ||||
| 31 | 0.082374 | ||||
| 32 | 0.082373 | ||||
| 33 | 0.082340 | ||||
| 34 | 0.082327 | ||||
| 35 | 0.082510 | ||||
| 36 | 0.082416 | ||||
| 37 | 0.082673 | ||||
| 38 | 0.082658 | ||||
| 39 | 0.082673 | ||||
| 40 | 0.082629 | ||||
| 41 | 0.082641 | ||||
| 42 | 0.082459 | ||||
| 43 | 0.082398 | ||||
| 6Li/7Limean | 0.082562 | 0.082475 | 0.079977 | 0.082590 | |
| 1sigma | 0.000156 | 0.000086 | 0.000157 | 0.000168 | |
| 2sigma | 0.000312 | 0.000172 | 0.000313 | 0.000335 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arienzo, I.; Liotta, M.; Brusca, L.; D’Antonio, M.; Lupone, F.; Cucciniello, C. Analytical Method for Lithium Isotopes Determination by Thermal Ionization Mass Spectrometry: A Useful Tool for Hydrogeochemical Applications. Water 2020, 12, 2182. https://doi.org/10.3390/w12082182
Arienzo I, Liotta M, Brusca L, D’Antonio M, Lupone F, Cucciniello C. Analytical Method for Lithium Isotopes Determination by Thermal Ionization Mass Spectrometry: A Useful Tool for Hydrogeochemical Applications. Water. 2020; 12(8):2182. https://doi.org/10.3390/w12082182
Chicago/Turabian StyleArienzo, Ilenia, Marcello Liotta, Lorenzo Brusca, Massimo D’Antonio, Federica Lupone, and Ciro Cucciniello. 2020. "Analytical Method for Lithium Isotopes Determination by Thermal Ionization Mass Spectrometry: A Useful Tool for Hydrogeochemical Applications" Water 12, no. 8: 2182. https://doi.org/10.3390/w12082182
APA StyleArienzo, I., Liotta, M., Brusca, L., D’Antonio, M., Lupone, F., & Cucciniello, C. (2020). Analytical Method for Lithium Isotopes Determination by Thermal Ionization Mass Spectrometry: A Useful Tool for Hydrogeochemical Applications. Water, 12(8), 2182. https://doi.org/10.3390/w12082182









