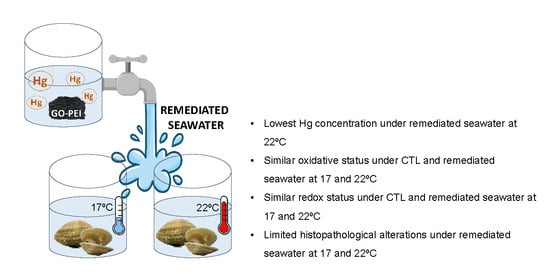
The Role of Temperature on the Impact of Remediated Water towards Marine Organisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory Conditions and Experimental Setup
2.2. Synthesis and Characterization of Graphene Oxide Functionalized with Polyethyleneimine
2.3. Mercury Quantification
2.4. Biochemical Markers
2.5. Histopathological Measurements
2.6. Integrated Biomarker Response
2.7. Statistical Analyses
3. Results
3.1. Mortality
3.2. Mercury Concentration in Seawater and Clams
3.3. Biochemical Markers
3.3.1. Metabolic Capacity
3.3.2. Antioxidant Enzymes Activity
3.3.3. Cellular Damage
3.3.4. Redox Balance
3.4. Histopathological Measurements
3.4.1. Gills
3.4.2. Digestive Tubules
3.5. Integrated Biomarker Response (IBR)
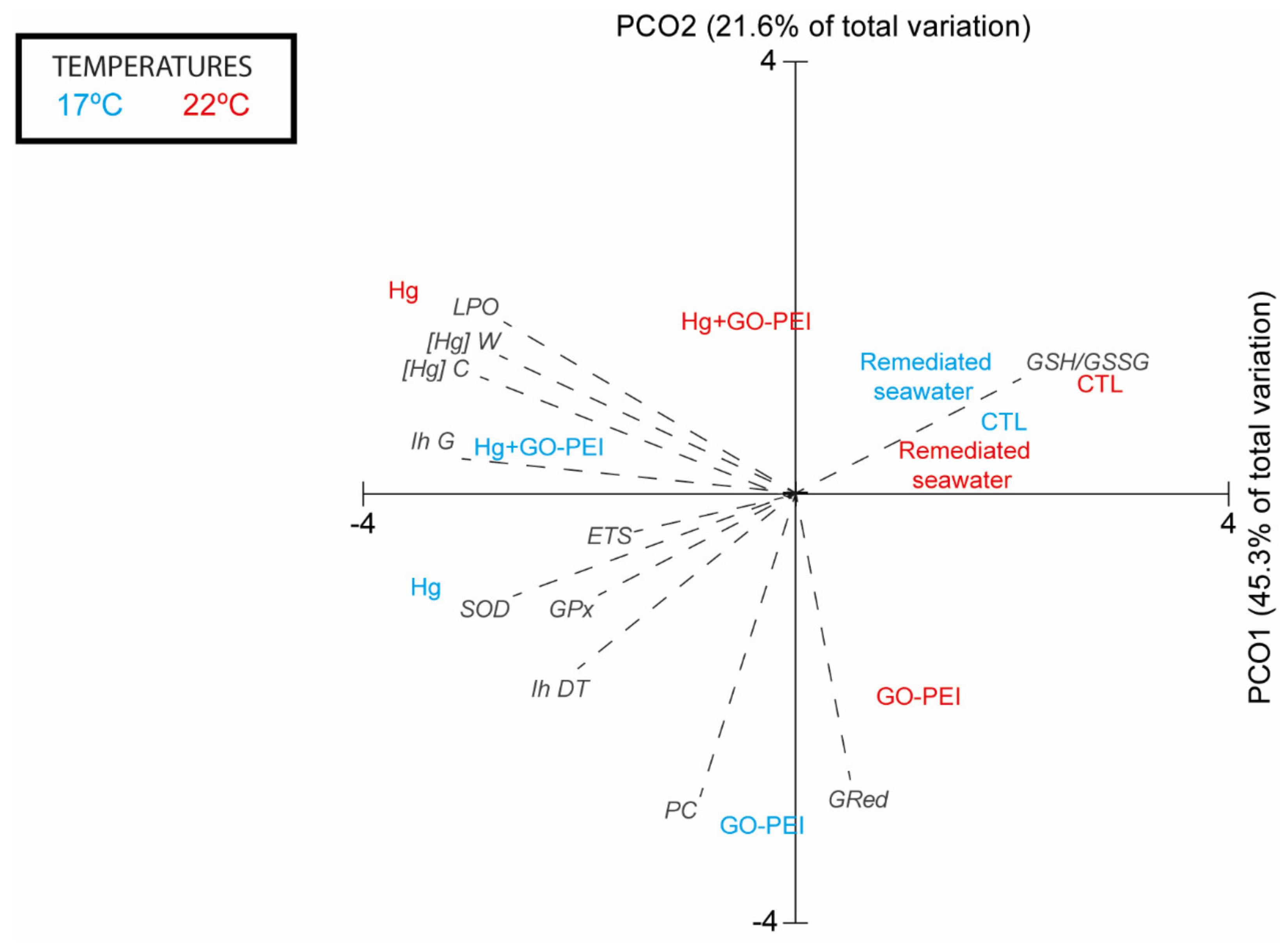
3.6. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dahlke, F.; Butzin, M.; Nahrgang, J.; Puvanendran, V.; Mortensen, A.; Pörtner, H.-O.; Storch, D. Northern cod species face spawning habitat losses if global warming exceeds 1.5 °C. Sci. Adv. 2018, 4, eaas8821. [Google Scholar] [CrossRef] [Green Version]
- IPCC. Climate Change 2007: The Physical Science Basis. Contribution of Work Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- IPCC. Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5°C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., Eds.; Springer: Geneva, Switzerland, 2018. [Google Scholar]
- Manciocco, A.; Calamandrei, G.; Alleva, E. Global warming and environmental contaminants in aquatic organisms: The need of the etho-toxicology approach. Chemosphere 2014, 100, 1–7. [Google Scholar] [CrossRef]
- Sokolova, I.M.; Lannig, G. Interactive effects of metal pollution and temperature on metabolism in aquatic ectotherms: Implications of global climate change. Clim. Res. 2008, 37, 181–201. [Google Scholar] [CrossRef] [Green Version]
- Newton, A.; Brito, A.C.; Icely, J.D.; Derolez, V.; Clara, I.; Angus, S.; Schernewski, G.; Inácio, M.; Lillebø, A.I.; Sousa, A.I.; et al. Assessing, quantifying and valuing the ecosystem services of coastal lagoons. J. Nat. Conserv. 2018, 44, 50–65. [Google Scholar] [CrossRef]
- Earp, H.S.; Prinz, N.; Cziesielski, M.J.; Andskog, M. For a World without Boundaries: Connectivity between Marine Tropical Ecosystems in Times of Change. In YOUMARES 8—Oceans across Boundaries: Learning from Each Other; Jungblut, S., Liebich, V., Bode, M., Eds.; Springer: Cham, Switzerland, 2018; pp. 125–144. [Google Scholar]
- Pan, K.; Wang, W.-X. Mercury accumulation in marine bivalves: Influences of biodynamics and feeding niche. Environ. Pollut. 2011, 159, 2500–2506. [Google Scholar] [CrossRef] [PubMed]
- Boukadida, K.; Banni, M.; Gourves, P.-Y.; Cachot, J. High sensitivity of embryo-larval stage of the Mediterranean mussel, Mytilus galloprovincialis to metal pollution in combination with temperature increase. Mar. Environ. Res. 2016, 122, 59–66. [Google Scholar] [CrossRef]
- Pörtner, H.-O.; Knust, R. Climate Change affects marine fishes through the oxygen limitation of thermal tolerance. Science 2007, 315, 95–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, R.; Pimentel, M.; Boavida-Portugal, J.; Teixeira, T.; Trübenbach, K.; Diniz, M.S. Ocean Warming enhances malformations, premature hatching, metabolic suppression and oxidative stress in the early life stages of a keystone squid. PLoS ONE 2012, 7, e38282. [Google Scholar] [CrossRef]
- Bodin, N.; Burgeot, T.; Stanisière, J.; Bocquené, G.; Menard, D.; Minier, C.; Boutet, I.; Amat, A.; Cherel, Y.; Budzinski, H. Seasonal variations of a battery of biomarkers and physiological indices for the mussel Mytilus galloprovincialis transplanted into the northwest Mediterranean Sea. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2004, 138, 411–427. [Google Scholar] [CrossRef]
- Hiebenthal, C.; Philipp, E.E.R.; Eisenhauer, A.; Wahl, M. Effects of seawater pCO2 and temperature on shell growth, shell stability, condition and cellular stress of Western Baltic Sea Mytilus edulis (L.) and Arctica islandica (L.). Mar. Biol. 2012, 160, 2073–2087. [Google Scholar] [CrossRef]
- MacKenzie, C.L.; Lynch, S.A.; Culloty, S.C.; Malham, S.K. Future Oceanic Warming and Acidification alter immune response and disease status in a commercial shellfish species, Mytilus edulis L. PLoS ONE 2014, 9, e99712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petes, L.E.; Menge, B.A.; Harris, A.L. Intertidal mussels exhibit energetic trade-offs between reproduction and stress resistance. Ecol. Monogr. 2008, 78, 387–402. [Google Scholar] [CrossRef]
- Jansen, J.M.; Hummel, H.; Bonga, S.W. The respiratory capacity of marine mussels (Mytilus galloprovincialis) in relation to the high temperature threshold. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 153, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Pörtner, H.-O. Oxygen- and capacity-limitation of thermal tolerance: A matrix for integrating climate-related stressor effects in marine ecosystems. J. Exp. Biol. 2010, 213, 881–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velez, C.; Figueira, E.; Soares, A.M.; Freitas, R. Effects of seawater temperature increase on economically relevant native and introduced clam species. Mar. Environ. Res. 2017, 123, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.; Silvestro, S.; Pagano, M.; Coppola, F.; Meucci, V.; Battaglia, F.; Intorre, L.; Soares, A.M.V.M.; Pretti, C.; Faggio, C. Impacts of salicylic acid in Mytilus galloprovincialis exposed to warming conditions. Environ. Toxicol. Pharmacol. 2020, 252, 103448. [Google Scholar] [CrossRef]
- Coppola, F.; Almeida, Â.; Henriques, B.; Soares, A.M.; Figueira, E.; Pereira, E.; Freitas, R. Biochemical responses and accumulation patterns of Mytilus galloprovincialis exposed to thermal stress and Arsenic contamination. Ecotoxicol. Environ. Saf. 2018, 147, 954–962. [Google Scholar] [CrossRef]
- Coppola, F.; Henriques, B.; Soares, A.M.; Figueira, E.; Pereira, E.; Freitas, R. Influence of temperature rise on the recovery capacity of Mytilus galloprovincialis exposed to mercury pollution. Ecol. Indic. 2018, 93, 1060–1069. [Google Scholar] [CrossRef]
- Kefaloyianni, E.; Gourgou, E.; Ferle, V.; Kotsakis, E.; Gaitanaki, C.; Beis, I. Acute thermal stress and various heavy metals induce tissue-specific pro- or anti-apoptotic events via the p38-MAPK signal transduction pathway in Mytilus galloprovincialis (Lam.). J. Exp. Biol. 2005, 208, 4427–4436. [Google Scholar] [CrossRef] [Green Version]
- Coppola, F.; Almeida, Â.; Henriques, B.; Soares, A.M.; Figueira, E.; Pereira, E.; Freitas, R. Biochemical impacts of Hg in Mytilus galloprovincialis under present and predicted warming scenarios. Sci. Total Environ. 2017, 601, 1129–1138. [Google Scholar] [CrossRef]
- Verlecar, X.; Jena, K.; Chainy, G. Biochemical markers of oxidative stress in Perna viridis exposed to mercury and temperature. Chem. Interact. 2007, 167, 219–226. [Google Scholar] [CrossRef]
- Greco, L.; Pellerin, J.; Capri, E.; Garnerot, F.; Louis, S.; Fournier, M.; Sacchi, A.; Fusi, M.; Lapointe, D.; Couture, P. Physiological effects of temperature and a herbicide mixture on the soft-shell clam Mya arenaria (Mollusca, Bivalvia). Environ. Toxicol. Chem. 2010, 30, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Maulvault, A.L.; Camacho, C.; Barbosa, V.; Alves, R.; Anacleto, P.; Fogaça, F.H.D.S.; Kwadijk, C.; Kotterman, M.; Cunha, S.C.; Fernandes, J.; et al. Assessing the effects of seawater temperature and pH on the bioaccumulation of emerging chemical contaminants in marine bivalves. Environ. Res. 2018, 161, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Matozzo, V.; Chinellato, A.; Munari, M.; Bressan, M.; Marin, M.G. Can the combination of decreased pH and increased temperature values induce oxidative stress in the clam Chamelea gallina and the mussel Mytilus galloprovincialis? Mar. Pollut. Bull. 2013, 72, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Freitas, R.; Figueira, E.; Ghirardini, A.V.; Soares, A.M.; Radaelli, M.; Guida, M.; Libralato, G. Combined effects of arsenic, salinity and temperature on Crassostrea gigas embryotoxicity. Ecotoxicol. Environ. Saf. 2018, 147, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Van Colen, C.; Jansson, A.; Saunier, A.; Lacoue-Labathe, T.; Vincx, M. Biogeographic vulnerability to ocean acidification and warming in a marine bivalve. Mar. Pollut. Bull. 2018, 126, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Tankoua, O.F.; Buffet, P.; Amiard-Triquet, C.; Amiard-Triquet, C.; Méléder, V.; Gillet, P.; Mouneyrac, C.; Berthet, B. Intersite variations of a battery of biomarkers at different levels of biological organisation in the estuarine endobenthic worm Nereis diversicolor (Polychaeta, Nereididae). Aquat. Toxicol. 2012, 114, 96–103. [Google Scholar] [CrossRef]
- Giani, M.; Djakovac, T.; Degobbis, D.; Cozzi, S.; Solidoro, C.; Umani, S.F. Recent changes in the marine ecosystems of the northern Adriatic Sea. Estuarine Coast. Shelf Sci. 2012, 115, 1–13. [Google Scholar] [CrossRef]
- Pereira, E.; Abreu, S.; Coelho, J.P.; Pato, P.; Pardal, M.; Duarte, A.C. Influence of tidal resuspension on seston lithogenic and biogenic partitioning in shallow estuarine systems: Implications for sampling. Mar. Pollut. Bull. 2008, 56, 348–354. [Google Scholar] [CrossRef]
- Randall, P.M.; Chattopadhyay, S. Mercury contaminated sediment sites—An evaluation of remedial options. Environ. Res. 2013, 125, 131–149. [Google Scholar] [CrossRef]
- Suriya, J.; Bharathiraja, S.; Sekar, V.; Rajasekaran, R. Metallothionein induction and antioxidative responses in the estuarine polychaeta Capitella capitata (Capitellidae). Asian Pac. J. Trop. Biomed. 2012, 2, S1052–S1059. [Google Scholar] [CrossRef]
- Al Naggar, Y.; Khalil, M.S.; Ghorab, M.A. Environmental Pollution by Heavy Metals in the Aquatic Ecosystems of Egypt. Open Access J. Toxicol. 2018, 3, 555603. [Google Scholar] [CrossRef]
- Bielen, A.; Bošnjak, I.; Sepčić, K.; Jaklič, M.; Cvitanić, M.; Lušić, J.; Lajtner, J.; Simčič, T.; Hudina, S. Differences in tolerance to anthropogenic stress between invasive and native bivalves. Sci. Total Environ. 2016, 543, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.; Leite, C.; Pinto, J.; Costa, M.; Monteiro, R.; Henriques, B.; Di Martino, F.; Coppola, F.; Soares, A.M.; Solé, M.; et al. The influence of temperature and salinity on the impacts of lead in Mytilus galloprovincialis. Chemosphere 2019, 235, 403–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velez, C.; Freitas, R.; Antunes, S.C.; Soares, A.M.; Figueira, E. Clams sensitivity towards As and Hg: A comprehensive assessment of native and exotic species. Ecotoxicol. Environ. Saf. 2016, 125, 43–54. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Priority List of Hazardous Substances. 2015. Available online: https://www.atsdr.cdc.gov/spl/index.html (accessed on 16 January 2007).
- Freitas, R.; Coppola, F.; De Marchi, L.; Codella, V.; Pretti, C.; Chiellini, F.; Morelli, A.; Polese, G.; Soares, A.M.; Figueira, E.; et al. The influence of Arsenic on the toxicity of carbon nanoparticles in bivalves. J. Hazard. Mater. 2018, 358, 484–493. [Google Scholar] [CrossRef]
- Nardi, A.; Mincarelli, L.F.; Benedetti, M.; Fattorini, D.; D’Errico, G.; Regoli, F. Indirect effects of climate changes on cadmium bioavailability and biological effects in the Mediterranean mussel Mytilus galloprovincialis. Chemosphere 2017, 169, 493–502. [Google Scholar] [CrossRef]
- Samuel, S.; Kathirvel, R.; Jayavelu, T.; Chinnakkannu, P. Protein oxidative damage in arsenic induced rat brain: Influence of dl-α-lipoic acid. Toxicol. Lett. 2005, 155, 27–34. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, J.; Yuan, H.; Xu, Y.; He, Z.; Duan, L. Biomarker responses in the bivalve (Chlamys farreri) to exposure of the environmentally relevant concentrations of lead, mercury, copper. Environ. Toxicol. Pharmacol. 2010, 30, 19–25. [Google Scholar] [CrossRef]
- Velez, C.; Galvao, P.; Longo, R.; Malm, O.; Soares, A.M.; Figueira, E.; Freitas, R. Ruditapes philippinarum and Ruditapes decussatus under Hg environmental contamination. Environ. Sci. Pollut. Res. 2015, 22, 11890–11904. [Google Scholar] [CrossRef]
- Gagnaire, B.; Thomas-Guyon, H.; Renault, T. In vitro effects of cadmium and mercury on Pacific oyster, Crassostrea gigas (Thunberg), haemocytes. Fish Shellfish Immunol. 2004, 16, 501–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, P.; Almeida, Â.; Calisto, V.; Esteves, V.I.; Schneider, R.J.; Wrona, F.; Soares, A.M.; Figueira, E.; Freitas, R. Physiological and biochemical alterations induced in the mussel Mytilus galloprovincialis after short and long-term exposure to carbamazepine. Water Res. 2017, 117, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Attig, H.; Kamel, N.; Sforzini, S.; Dagnino, A.; Jamel, J.; Boussetta, H.; Viarengo, A.; Banni, M. Effects of thermal stress and nickel exposure on biomarkers responses in Mytilus galloprovincialis (Lam). Mar. Environ. Res. 2014, 94, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Mubiana, V.K.; Blust, R. Effects of temperature on scope for growth and accumulation of Cd, Co, Cu and Pb by the marine bivalve Mytilus edulis. Mar. Environ. Res. 2007, 63, 219–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas, R.; Pinto, L.R.; Sampaio, M.; Costa, A.; Silva, M.; Rodrigues, A.M.; Quintino, V.; Figueira, E. Effects of depuration on the element concentration in bivalves: Comparison between sympatric Ruditapes decussatus and Ruditapes philippinarum. Estuarine Coast. Shelf Sci. 2012, 110, 43–53. [Google Scholar] [CrossRef]
- Donnici, S.; Serandrei-Barbero, R.; Canali, G. Evidence of climatic changes in the Venetian Coastal Plain (Northern Italy) during the last 40,000 years. Sediment. Geol. 2012, 281, 139–150. [Google Scholar] [CrossRef]
- Briant, L.J.; Salehi, A.A.; Vergari, E.; Zhang, Q.; Rorsman, P. Glucagon secretion from pancreatic α-cells. Upsala J. Med. Sci. 2016, 121, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Faganeli, J.; Hines, M.E.; Covelli, S.; Emili, A.; Giani, M. Mercury in lagoons: An overview of the importance of the link between geochemistry and biology. Estuarine Coast. Shelf Sci. 2012, 113, 126–132. [Google Scholar] [CrossRef]
- Pereira, P.; Korbas, M.; Pereira, V.; Cappello, T.; Maisano, M.; Canário, J.; Almeida, A.; Pacheco, M. A multidimensional concept for Mercury neuronal and sensory toxicity in fish—From toxicokinetics and biochemistry to morphometry and behavior. BBA Gen. Subj. 2019, 1863, 129298. [Google Scholar] [CrossRef]
- Matlock, M.M.; Henke, K.R.; Atwood, D.A.; Robertson, D. Aqueous leaching properties and environmental implications of cadmium, lead and zinc trimercaptotriazine (TMT) compounds. Water Res. 2001, 35, 3649–3655. [Google Scholar] [CrossRef]
- Henke, K.R.; Hutchison, A.R.; Krepps, M.K.; Parkin, S.; Atwood, D.A. Chemistry of 2,4,6-trimercapto-1,3,5-triazine (TMT): Acid dissociation constants and group 2 complexes. Inorg. Chem. 2001, 40, 4443–4447. [Google Scholar] [CrossRef] [PubMed]
- Aroua, M.K.; Zuki, F.M.; Sulaiman, N.M. Removal of chromium ions from aqueous solutions by polymer-enhanced ultrafiltration. J. Hazard. Mater. 2007, 147, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, M.; Guha, B. Effect of pH on rejection of hexavalent chromium by nanofiltration. Desalination 2008, 219, 171–178. [Google Scholar] [CrossRef]
- Pugazhenthi, G.; Sachan, S.; Kishore, N.; Kumar, A. Separation of chromium (VI) using modified ultrafiltration charged carbon membrane and its mathematical modeling. J. Membr. Sci. 2005, 254, 229–239. [Google Scholar] [CrossRef]
- Ali, I.; Khan, T.A.; Asim, M. Removal of arsenate from groundwater by electrocoagulation method. Environ. Sci. Pollut. Res. 2011, 19, 1668–1676. [Google Scholar] [CrossRef]
- Anjum, M.; Miandad, R.; Waqas, M.; Gehany, F.; Barakat, M. Remediation of wastewater using various nano-materials. Arab. J. Chem. 2019, 12, 4897–4919. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Su, Y.; Conway, J.R.; Keller, A.A.; Adeleye, A.S.; Garner, K. Engineered nanomaterials for water treatment and remediation: Costs, benefits, and applicability. Chem. Eng. J. 2015, 286, 640–662. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Feng, C.; Zhang, Z.; Yang, S.; Sugiura, N. Treatment of nitrate contaminated water using an electrochemical method. Bioresour. Technol. 2010, 101, 6553–6557. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Gehrke, I.; Geiser, A.; Somborn-Schulz, A. Innovations in nanotechnology for water treatment. Nanotechnol. Sci. Appl. 2015, 8, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Mohan, D.J.; Pittman, C.U., Jr. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Henriques, B.; Gonçalves, G.; Emami, N.; Pereira, E.; Vila, M.; Marques, P.A. Optimized graphene oxide foam with enhanced performance and high selectivity for mercury removal from water. J. Hazard. Mater. 2016, 301, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G.; Marques, P.A.; Granadeiro, C.M.; Nogueira, H.I.; Singh, M.K.; Grácio, J. Surface modification of graphene nanosheets with gold nanoparticles: The role of oxygen moieties at graphene surface on gold nucleation and growth. Chem. Mater. 2009, 21, 4796–4802. [Google Scholar] [CrossRef]
- Cohen-Tanugi, D.; Grossman, J.C. Water Desalination across Nanoporous Graphene. Nano Lett. 2012, 12, 3602–3608. [Google Scholar] [CrossRef]
- Fang, Q.; Zhou, X.; Deng, W.; Liu, Z. Hydroxyl-containing organic molecule induced self-assembly of porous graphene monoliths with high structural stability and recycle performance for heavy metal removal. Chem. Eng. J. 2017, 308, 1001–1009. [Google Scholar] [CrossRef]
- Sahraei, R.; Ghaemy, M. Synthesis of modified gum tragacanth/graphene oxide composite hydrogel for heavy metal ions removal and preparation of silver nanocomposite for antibacterial activity. Carbohydr. Polym. 2017, 157, 823–833. [Google Scholar] [CrossRef]
- Bessa, A.; Henriques, B.; Gonçalves, G.; Irurueta, G.; Pereira, E.; Marques, P.A.A.P. Graphene oxide/polyethyleneimine aerogel for high-performance mercury sorption from natural waters. Chem. Eng. J. 2020, 398, 125587. [Google Scholar] [CrossRef]
- Banni, M.; Hajer, A.; Sforzini, S.; Oliveri, C.; Boussetta, H.; Viarengo, A. Transcriptional expression levels and biochemical markers of oxidative stress in Mytilus galloprovincialis exposed to nickel and heat stress. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2014, 160, 23–29. [Google Scholar] [CrossRef]
- Banni, M.; Hajer, A.; Sforzini, S.; Oliveri, C.; Mignone, F.; Boussetta, H.; Viarengo, A. Transcriptomic responses to heat stress and nickel in the mussel Mytilus galloprovincialis. Aquat. Toxicol. 2014, 148, 104–112. [Google Scholar] [CrossRef]
- Izagirre, U.; Errasti, A.; Bilbao, E.; Múgica, M.; Marigómez, I. Combined effects of thermal stress and Cd on lysosomal biomarkers and transcription of genes encoding lysosomal enzymes and HSP70 in mussels, Mytilus galloprovincialis. Aquat. Toxicol. 2014, 149, 145–156. [Google Scholar] [CrossRef]
- Marques, A.; Piló, D.; Carvalho, S.; Araújo, O.; Guilherme, S.; Santos, M.A.; Vale, C.; Pereira, F.; Pacheco, M.; Pereira, P. Metal bioaccumulation and oxidative stress profiles in Ruditapes philippinarum—Insights towards its suitability as bioindicator of estuarine metal contamination. Ecol. Indic. 2018, 95, 1087–1099. [Google Scholar] [CrossRef]
- Almeida, A.; Freitas, R.; Calisto, V.; Esteves, V.I.; Schneider, R.J.; Soares, A.M.; Figueira, E.M.D.A.P. Chronic toxicity of the antiepileptic carbamazepine on the clam Ruditapes philippinarum. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2015, 172–173, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Coppola, F.; Pretti, C.; Intorre, L.; Meucci, V.; Soares, A.M.; Freitas, R.; Solé, M. The influence of climate change related factors on the response of two clam species to diclofenac. Ecotoxicol. Environ. Saf. 2019, 189, 109899. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.D.; Almeida, Â.; Calisto, V.; Esteves, V.I.; Schneider, R.J.; Wrona, F.; Soares, A.M.; Figueira, E.; Freitas, R. Caffeine impacts in the clam Ruditapes philippinarum: Alterations on energy reserves, metabolic activity and oxidative stress biomarkers. Chemosphere 2016, 160, 95–103. [Google Scholar] [CrossRef]
- Coppola, F.; Tavares, D.; Henriques, B.; Monteiro, R.; Trindade, T.; Soares, A.M.; Figueira, E.; Polese, G.; Pereira, E.; Freitas, R. Remediation of arsenic from contaminated seawater using manganese spinel ferrite nanoparticles: Ecotoxicological evaluation in Mytilus galloprovincialis. Environ. Res. 2019, 175, 200–212. [Google Scholar] [CrossRef]
- De Marchi, L.; Neto, V.; Pretti, C.; Figueira, E.; Chiellini, F.; Morelli, A.; Soares, A.M.; Freitas, R. The impacts of seawater acidification on Ruditapes philippinarum sensitivity to carbon nanoparticles. Environ. Sci. Nano 2017, 4, 1692–1704. [Google Scholar] [CrossRef]
- IPMA. Portuguese Institute for Sea and Atmosphere (IPMA). Available online: http://www.ipma.pt/pt/maritima/sst/ (accessed on 20 August 2019).
- European Parliament; Council of the European Union. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 Amending Directives 2000/60/EC and 2008/105/EC as Regards Priority Substances in the Field of Water Policy; Publications Office of the EU: Brussels, Belgium, 2013. [Google Scholar]
- Henriques, B.; Teixeira, A.; Figueira, P.; Reis, A.T.; Almeida, J.; Vale, C.; Pereira, E. Simultaneous removal of trace elements from contaminated waters by living Ulva lactuca. Sci. Total Environ. 2019, 652, 880–888. [Google Scholar] [CrossRef]
- Costley, C.T.; Mossop, K.F.; Dean, J.R.; Garden, L.M.; Marshall, J.; Carroll, J. Determination of mercury in environmental and biological samples using pyrolysis atomic absorption spectrometry with gold amalgamation. Anal. Chim. Acta 2000, 405, 179–183. [Google Scholar] [CrossRef]
- Pinto, J.; Costa, M.; Leite, C.; Borges, C.; Coppola, F.; Henriques, B.; Monteiro, R.; Russo, T.; Di Cosmo, A.; Soares, A.M.; et al. Ecotoxicological effects of lanthanum in Mytilus galloprovincialis: Biochemical and histopathological impacts. Aquat. Toxicol. 2019, 211, 181–192. [Google Scholar] [CrossRef]
- Leite, C.; Coppola, F.; Monteiro, R.; Russo, T.; Polese, G.; Lourenço, M.A.O.; Ferreira, P.; Soares, A.M.; Pereira, E.; Freitas, R. Toxic impacts of Rutile (TiO2 nanoparticles) in Mytilus galloprovincialis exposed to warming conditions. Chemosphere 2020, in press. [Google Scholar] [CrossRef]
- Coppola, F.; Bessa, A.; Henriques, B.; Russo, T.; Soares, A.M.; Figueira, E.; Marques, P.A.; Polese, G.; Di Cosmo, A.; Pereira, E.; et al. Oxidative stress, metabolic and histopathological alterations in mussels exposed to remediated seawater by GO-PEI after contamination with mercury. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2020, 243, 110674. [Google Scholar] [CrossRef] [PubMed]
- Beliaeff, B.; Burgeot, T. Integrated biomarker response: A useful tool for ecological risk assessment. Environ. Toxicol. Chem. 2020, 21, 1316–1322. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVAþ for PRIMER. In Guide to Software and Statistical Methods; University of Auckland and PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Sanni, B.; Williams, K.; Sokolov, E.P.; Sokolova, I. Effects of acclimation temperature and cadmium exposure on mitochondrial aconitase and LON protease from a model marine ectotherm, Crassostrea virginica. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2008, 147, 101–112. [Google Scholar] [CrossRef] [PubMed]


| Treatments | Description |
|---|---|
| CTL | Artificial seawater (Hg 0.0 µg/L + GO-PEI 0.0 mg/L) |
| GO-PEI | Artificial seawater with GO-PEI 10 mg/L |
| Hg+ GO-PEI | Artificial seawater with Hg 50 µg/L and GO-PEI 10 mg/L |
| Hg | Artificial seawater with Hg 50 µg/L |
| Remediated seawater | Artificial seawater previously contaminated with Hg (50 µg/L), and remediated by GO-PEI (10 mg/L) during 24 h. |
| CTL | GO-PEI | Hg+GO-PEI | Hg | Remediated Seawater | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 17 °C | 22 °C | 17 °C | 22 °C | 17 °C | 22 °C | 17 °C | 22 °C | 17 °C | 22 °C | |||||||
| Hg Quantification | [Hg]W | <LOQ | <LOQ | <LOQ | <LOQ | 50.0 ± 3.90 A | 49.6 ± 3.26 a | 50.4 ± 2.95 A | 49.4 ± 5.09 a | 11.5 ± 3.71 B | 11.5 ± 3.71 b | |||||
| [Hg]C | 0.18 ± 0.02 A | * | 0.29 ± 0.01 a | 0.14 ± 0.03 B | 0.16 ± 0.0056 b | 7.3 ± 0.63 C | * | 3.6 ± 0.29 c | 9.1 ± 1.9 C | * | 12 ± 2.5 d | 4.7 ± 0.34 D | * | 2.9 ± 0.98 c | ||
| Biochemical Markers | ETS | 31.7 ± 4.86 A | * | 25.5 ± 5.44 a | 35.5 ± 5.99 A | * | 15.5 ± 3.42 b | 13.2 ± 0.76 B | 20.2 ± 7.09 a,b | 30.1 ± 4.65 A | 39.0 ± 2.82 c | 11.1 ± 0.60 C | 11.1 ± 1.34 d | |||
| SOD | 0.43 ± 0.030 A | * | 0.24 ± 0.040 a | 0.46 ± 0.050 A | 0.34 ± 0.03 a | 0.41 ± 0.05 A | 0.33 ± 0.02 a | 0.77 ± 0.07 B | * | 0.54 ± 0.13 b | 0.28 ± 0.03 A | 0.29 ± 0.01 a | ||||
| GPx | 0.03 ±0.005 A | 0.03 ± 0.004 a | 0.04 ± 0.004 B | 0.05 ± 0.01 b | 0.06 ± 0.008 C | * | 0.04 ± 0.004 a,b | 0.04 ± 0.009 B,C | 0.04 ± 0.006 b,c | 0.04 ± 0.01 A,B | 0.04 ± 0.006 a,b | |||||
| GRed | 0.030 ± 0.0040 A,D | * | 0.060 ± 0.010 a | 0.14 ± 0.020 B | 0.12 ± 0.010 b | 0.060 ± 0.010 C,D | 0.070 ± 0.0070 c | 0.060 ± 0.010 C | * | 0.030 ± 0.0070 d | 0.035 ± 0.014 D | 0.041 ± 0.013 d | ||||
| LPO | 15.4 ± 0.75 A | 14.9 ± 1.09 a,d | 16.2 ± 0.64 A | * | 13.5 ± 0.48 a | 20.6 ± 0.58 B,C | 22.6 ± 3.67 b | 22.0 ± 0.25 B | * | 28.2 ± 0.384 c | 17.3 ± 3.049 A,C | * | 14.9 ± 0.645 d | |||
| PC | 0.90 ± 0.13 A | 0.89 ± 0.06 a | 0.99 ± 0.19 A | 0.97 ± 0.11 a | 0.95 ± 0.08 A | 0.88 ± 0.04 a | 1.03 ± 0.13 A | 0.87 ± 0.06 a | 0.89 ± 0.007 A | 0.95 ± 0.12 a | ||||||
| GSH/GSSG | 0.49±0.05 A | * | 0.75 ± 0.04 a | 0.13 ± 0.02 B | * | 0.23 ± 0.04 b | 0.13 ± 0.01 B | * | 0.21 ± 0.03 b | 0.12 ± 0.008 B | * | 0.22 ± 0.04 b | 0.11 ± 0.02 B | * | 0.23 ± 0.04 b | |
| Histopathological Index | Ih G | 0.05 ± 0.02 A | * | 0.08 ± 0.03 a | 0.15 ± 0.02 B | 0.13 ± 0.05 b | 0.17 ± 0.08 B | 0.18 ± 0.07 c | 0.27 ± 0.05 C | * | 0.33 ± 0.05 d | 0.12 ± 0.06 B | 0.16 ± 0.06 c,b | |||
| Ih DT | 0.23 ± 0.09 A | 0.16 ± 0.07 a,b | 0.38 ± 0.001 B | * | 0.21 ± 0.19 a | 0.23 ± 0.07 A | * | 0.09 ± 0.001 b | 0.31 ± 0.001 C | * | 0.37 ± 0.05 c | 0.21 ± 0.11 A | 0.19± 0.13 a | |||
| IBR | 2.81 | 2.01 | 4.16 | 3.44 | 2.39 | 3.38 | 4.27 | 2.20 | 2.09 | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coppola, F.; Bessa, A.; Henriques, B.; Russo, T.; Soares, A.M.V.M.; Figueira, E.; Pereira, E.; Marques, P.; Polese, G.; Freitas, R. The Role of Temperature on the Impact of Remediated Water towards Marine Organisms. Water 2020, 12, 2148. https://doi.org/10.3390/w12082148
Coppola F, Bessa A, Henriques B, Russo T, Soares AMVM, Figueira E, Pereira E, Marques P, Polese G, Freitas R. The Role of Temperature on the Impact of Remediated Water towards Marine Organisms. Water. 2020; 12(8):2148. https://doi.org/10.3390/w12082148
Chicago/Turabian StyleCoppola, Francesca, Ana Bessa, Bruno Henriques, Tania Russo, Amadeu M. V. M. Soares, Etelvina Figueira, Eduarda Pereira, Paula Marques, Gianluca Polese, and Rosa Freitas. 2020. "The Role of Temperature on the Impact of Remediated Water towards Marine Organisms" Water 12, no. 8: 2148. https://doi.org/10.3390/w12082148
APA StyleCoppola, F., Bessa, A., Henriques, B., Russo, T., Soares, A. M. V. M., Figueira, E., Pereira, E., Marques, P., Polese, G., & Freitas, R. (2020). The Role of Temperature on the Impact of Remediated Water towards Marine Organisms. Water, 12(8), 2148. https://doi.org/10.3390/w12082148