CO2 Degassing in Sicily (Central Mediterranean) as Inferred from Groundwater Composition
Abstract
1. Introduction
2. Geological Background
3. Materials and Methods
4. Results and Discussion
4.1. Physicochemical Composition of Groundwater
4.2. Distribution of Dissolved CO2 Partial Pressure
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Helgeson, H.C. Evaluation of irreversible reactions in geochemical processes involving minerals and aqueous solutions—I. Thermodynamic relations. Geochim. Cosmochim. Acta 1968, 32, 853–877. [Google Scholar] [CrossRef]
- Marini, L.; Ottonello, G.; Canepa, M.; Cipolli, F. Water-rock interaction in the Bisagno valley (Genoa, Italy): Application of an inverse approach to model spring water chemistry. Geochim. et Cosmochim. Acta 2000, 64, 2617–2635. [Google Scholar] [CrossRef]
- Marini, L.; Canepa, M.; Cipolli, F.; Ottonello, G.; Zuccolini, M. Use of stream sediment chemistry to predict trace element chemistry of groundwater. A case study from the Bisagno valley (Genoa, Italy). J. Hydrol. 2001, 241, 194–220. [Google Scholar] [CrossRef]
- Aiuppa, A.; Federico, C.; Allard, P.; Gurrieri, S.; Valenza, M. Trace metal modeling of groundwater–gas–rock interactions in a volcanic aquifer: Mount Vesuvius, Southern Italy. Chem. Geol. 2005, 216, 289–311. [Google Scholar] [CrossRef]
- Marini, L. Geological sequestration of carbon dioxide—Thermodynamics, kinetics, and reaction path modeling. Develop. Geochem. 2005, 11, 453. [Google Scholar]
- Federico, C.; Pizzino, L.; Cinti, D.; De Gregorio, S.; Favara, R.; Galli, G.; Giudice, G.; Gurrieri, S.; Quattrocchi, F.; Voltattorni, N. Inverse and forward modelling of groundwater circulation in a seismically active area (Monferrato, Piedmont, NW Italy): Insights into stress-induced variations in water chemistry. Chem. Geol. 2008, 248, 14–39. [Google Scholar] [CrossRef]
- Capaccioni, B.; Didero, M.; Paletta, C.; Salvadori, P. Hydrogeochemistry of groundwaters from carbonate formations with basal gypsiferous layers: An example from the Mt Catria Mt Nerone ridge (Northern Appennnines, Italy). J. Hydrol. 2011, 253, 14–26. [Google Scholar] [CrossRef]
- Morgantini, N.; Frondini, F.; Cardellini, C. Natural trace elements baselines and dissolved loads in groundwater from carbonate aquifers of central Italy. Phys. Chem. Earth, Parts A/B/C 2009, 34, 520–529. [Google Scholar] [CrossRef]
- Frondini, F. Geochemistry of regional aquifer systems hosted by carbonate-evaporite formations in Umbria and southern Tuscany (central Italy). Appl. Geochem. 2008, 23, 2091–2104. [Google Scholar] [CrossRef]
- Grassa, F.; Capasso, G.; Favara, R.; Inguaggiato, S. Chemical and Isotopic Composition of Waters and Dissolved Gases in Some Thermal Springs of Sicily and Adjacent Volcanic Islands, Italy. Pure Appl. Geophys. PAGEOPH 2006, 163, 781–807. [Google Scholar] [CrossRef]
- Stolper, E.; Holloway, J.R. Experimental determination of the solubility of carbon dioxide in molten basalt at low pressure. Earth Planet. Sci. Lett. 1988, 87, 397–408. [Google Scholar] [CrossRef]
- De Gregorio, S.; Camarda, M.; Longo, M.; Cappuzzo, S.; Giudice, G.; Gurrieri, S. Long-term continuous monitoring of the dissolved CO2 performed by using a new device in groundwater of the Mt. Etna (southern Italy). Water Res. 2011, 45, 3005–3011. [Google Scholar] [CrossRef]
- Irwin, W.P.; Barnes, I. Tectonic relations of carbon dioxide discharges and earthquakes. J. Geophys. Res. Space Phys. 1980, 85, 3115. [Google Scholar] [CrossRef]
- Sugisaki, R.; Ido, M.; Takeda, H.; Isobe, Y.; Hayashi, Y.; Nakamura, N.; Satake, H.; Mizutani, Y. Origin of Hydrogen and Carbon Dioxide in Fault Gases and Its Relation to Fault Activity. J. Geol. 1983, 91, 239–258. [Google Scholar] [CrossRef]
- Klusman, R.W. Soil Gas and Related Methods for Natural Resource Exploration; John Wiley and Sons: New York. NY, USA, 1993; p. 483. [Google Scholar]
- Italiano, F.; Martinelli, G.; Rizzo, A.L. Geochemical evidence of seismogenic-induced anomalies in the dissolved gases of thermal waters: A case study of Umbria (Central Apennines, Italy) both during and after the 1997-1998 seismic swarm. Geochem. Geophys. Geosystems 2004, 5. [Google Scholar] [CrossRef]
- Ray, M.C.; Hilton, D.; Munoz, J.; Fischer, T.P.; Shaw, A.M. The effects of volatile recycling, degassing and crustal contamination on the helium and carbon geochemistry of hydrothermal fluids from the Southern Volcanic Zone of Chile. Chem. Geol. 2009, 266, 38–49. [Google Scholar] [CrossRef]
- Camarda, M.; De Gregorio, S.; Gurrieri, S. Magma-ascent processes during 2005–2009 at Mt Etna inferred by soil CO2 emissions in peripheral areas of the volcano. Chem. Geol. 2012, 330, 218–227. [Google Scholar] [CrossRef]
- Camarda, M.; De Gregorio, S.; Di Martino, R.; Favara, R. Temporal and spatial correlations between soil CO2 flux and crustal stress. J. Geophys. Res. Solid Earth 2016, 121, 7071–7085. [Google Scholar] [CrossRef]
- Chiodini, G.; Frondini, F.; Kerrick, D.; Rogie, J.; Parello, F.; Peruzzi, L.; Zanzari, A. Quantification of deep CO2 fluxes from Central Italy. Examples of carbon balance for regional aquifers and of soil diffuse degassing. Chem. Geol. 1999, 159, 205–222. [Google Scholar] [CrossRef]
- De Paola, N.; Chiodini, G.; Hirose, T.; Cardellini, C.; Caliro, S.; Shimamoto, T. The geochemical signature caused by earthquake propagation in carbonate-hosted faults. Earth Planet. Sci. Lett. 2011, 310, 225–232. [Google Scholar] [CrossRef]
- Caracausi, A.; Favara, R.; Italiano, F.; Nuccio, P.M.; Paonita, A.; Rizzo, A.L. Active geodynamics of the central Mediterranean Sea: Tensional tectonic evidences in western Sicily from mantle-derived helium. Geophys. Res. Lett. 2005, 32. [Google Scholar] [CrossRef]
- Censi, P.; Ferla, P. I marmi dei Peloritani. Composizione isotopica dell’ossigeno e del carbonio e ricostruzione degli ambienti formazionali. Rend. Soc. lt. Min. Petr. 1982, 38, 1101–1117. [Google Scholar]
- Favara, R.; Grassa, F.; Inguaggiato, S.; D′amore, F. Geochemical and hydrogeological characterization of thermal springs in Western Sicily, Italy. J. Volcanol. Geotherm. Res. 1998, 84, 125–141. [Google Scholar] [CrossRef]
- Favara, R.; Grassa, F.; Inguaggiato, S.; Valenza, M. Hydrogeochemistry and stable isotopes of thermal springs: Earthquake-related chemical changes along Belice Fault (Western Sicily). Appl. Geochem. 2001, 16, 1–17. [Google Scholar] [CrossRef]
- Caracausi, A.; Favara, R.; Italiano, F.; Nuccio, P.M.; Paonita, A.; Rizzo, A. Evidence of mantle derived fluid contributions to the thermal basins of Western Sicily: Geotectonic and geodynamic implications. In Proceeding of the WRI-11 International Symposium; Wanty & Seal II, Ed.; Springs: Saratoga, NY, USA, 2004; pp. 91–94. [Google Scholar]
- Favara, R.; Grassa, F.; Madonia, P.; Valenza, M. Flow Changes and Geochemical Anomalies in Warm and Cold Springs Associated with the 1992–1994 Seismic Sequence at Pollina, Central Sicily, Italy. Pure Appl. Geophys. PAGEOPH 2007, 164, 2411–2430. [Google Scholar] [CrossRef]
- Dewey, J.F.; Helman, M.L.; Knott, S.D.; Turco, E.; Hutton, D.H.W.; Knott, S.D. Kinematics of the western Mediterranean. Alpine Tectonics. Geol. Soc. Spec. Publ. 1989, 45, 265–283. [Google Scholar] [CrossRef]
- Serpelloni, E.; Vannucci, G.; Pondrelli, S.; Argnani, A.; Casula, G.; Anzidei, M.; Baldi, P.; Gasperini, P. Kinematics of the Western Africa-Eurasia plate boundary from focal mechanisms and GPS data. Geophys. J. Int. 2007, 169, 1180–1200. [Google Scholar] [CrossRef]
- Catalano, S.; De Guidi, G.; Romagnoli, G.; Torrisi, S.; Tortorici, G.; Tortorici, L. The migration of plate boundaries in SE Sicily: Influence on the large-scale kinematic model of the African promontory in southern Italy. Tectonophys. 2008, 449, 41–62. [Google Scholar] [CrossRef]
- Vallone, P.; Giammarinaro, M.; Crosetto, M.; Agudo, M.; Biescas, E. Ground motion phenomena in Caltanissetta (Italy) investigated by InSAR and geological data integration. Eng. Geol. 2008, 98, 144–155. [Google Scholar] [CrossRef]
- 32. Catalano, R.; D’Argenio, B. Schema geologico della Sicilia. In Guida Alla Geologia Della Sicilia Occidentale; Catalano, R., D’Argenio, B., Eds.; Guide Geologiche Regionali (I Serie), SOCIETA’ GEOLOGICA ITALIANA: Roma, Italy, 1982; pp. 9–41. [Google Scholar]
- Roure, F.; Howell, D.; Muller, C.; Moretti, I. Late cenozoic subduction complex of Sicily. J. Struct. Geol. 1990, 12, 259–266. [Google Scholar] [CrossRef]
- Lentini, F.; Carbone, S.; Catalano, S. Main structural domains of the central Mediterranean region and their Neogene tectonic evolution. Boll. Geofis. Teor. ed Appl. 1994, 36, 141–144. [Google Scholar]
- Catalano, R.; Franchino, A.; Merlini, S.; Sulli, A. A crustal section from North Algerian to the Ionian ocean (Central Mediterranean). Mem. Soc. Geol. It. 2000, 55, 71–85. [Google Scholar]
- Finetti, I.R. CROP project: deep seismic exploration of the central Mediterranean and Italy. In Atlases in Geoscience 1; Finetti, I.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 1–794. [Google Scholar]
- Catalano, R.; Franchino, A.; Merlini, S.; Sulli, A. Central Western Sicily structural setting interpreted from seismic reflection profiles. Mem. Soc. Geol. It. 2000, 55, 5–16. [Google Scholar]
- Ghisetti, F.; Vezzani, L. Thin-skinned deformations of the Western Sicily thrust belt and relationships with crustal shortening: mesostructural data on the Mt. Kumeta Alcantara. Boll. Soc. Geol. It. 1984, 103, 129–157. [Google Scholar]
- Bianchi, F.; Carbone, S.; Grasso, M.; Invernizzi, G.; Lentini, F.; Longaretti, G.; Merlini, S.; Moscardini, F. Sicilia orientale: Profilo geologico Nebrodi-Iblei. Mem. Soc. Geol. It. 1989, 38, 429–458. [Google Scholar]
- Basilone, L. Litostratigrafia Della Sicilia; ARTA (Reg. Sicil.)-ORGS. Arti Grafiche Palermitane S.R.L: Palermo, Italy, 2012. [Google Scholar]
- Cirrincione, R.; Fiannacca, P.; Lustrino, M.; Romano, V.; Tranchina, A. Late Triassic tholeiitic magmatism in Western Sicily: A possible extension of the Central Atlantic Magmatic Province (CAMP) in the Central Mediterranean area? Lithos 2014, 188, 60–71. [Google Scholar] [CrossRef]
- Patacca, E.; Scandone, P.; Giunta, G.; Liguori, V. Mesozoic palaeotectonic evolution of the Ragusa Zone (Southern Sicily). Geol. Rom. 1979, 18, 331–369. [Google Scholar]
- Longaretti, G.; Rocchi, S. Il magmatismo dell’avampaese ibleo (Sicilia orientale) tra il Trias e il Quaternario: Dati stratigrafici e petrologici del sottosuolo. Mem Soc Geol Ital. 1990, 45, 911–925. [Google Scholar]
- Branca, S.; Coltelli, M.; De Beni, E.; Wijbrans, J. Geological evolution of Mount Etna volcano (Italy) from earliest products until the first central volcanism (between 500 and 100 ka ago) inferred from geochronological and stratigraphic data. Acta Diabetol. 2008, 98, 239. [Google Scholar] [CrossRef]
- Casero, P.; Roure, F. Neogene deformations at the Sicilian–North African plate boundary. In Peri-Tethian Platforms. Institut Francaise du Petrole Research Conference, Arles, Proceedings; Roure, F., Ed.; Editions Technip: Paris, France, 1994; pp. 27–50. [Google Scholar]
- Cernobori, L.; Hirn, H.; McBride, J.H.; Nicolich, R.; Petronio, M.; Romanelli, M.; Streamers Profiles, Working Group. Crustal image of the Ionian basin and its Calabrian margins. Tectonophysics 1996, 264, 175–189. [Google Scholar] [CrossRef]
- Pondrelli, S.; Piromallo, C.; Serpelloni, E. Convergence vs. retreat in Southern Tyrrhenian Sea: Insights from kinematics. Geophys. Res. Lett. 2004, 31, 1–4. [Google Scholar] [CrossRef]
- Barreca, G.; Bruno, V.; Cocorullo, C.; Cultrera, F.; Ferranti, L.; Guglielmino, F.; Guzzetta, L.; Mattia, M.; Monaco, C.L.; Pepe, F. Geodetic and geological evidence of active tectonic in south-westerm Sicily (Italy). J. Geodynamic. 2014, 82, 138–149. [Google Scholar] [CrossRef]
- Regione Sicilia. Available online: http://www.osservatorioacque.it/?cmd=section&id=9&tpl=default (accessed on 20 May 2020).
- Liotta, M. Geochemical Processes Governing Groundwater Composition in North-Western Sicily:isotopic Model and Water Rock Interaction. Ph.D. Thesis, University of Palermo, Palermo PA, Italy, 2004; p. 97. [Google Scholar]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters; Wiley: New York, NY, USA, 1996; p. 1024. [Google Scholar]
- Parkhurst, D.L.; Appelo, C.A.J. User’s guide to PHREEQC (version 2)—A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical modelling calculations. In U.S. Geological Survey Water—Resources Investigations Report; United States Geological Survey: Reston, VA, USA, 1999; pp. 99–4259. 312p. [Google Scholar]
- Rovida, A.; Locati, M.; Camassi, R.; Lolli, B.; Gasperini, P. (Eds.) CPTI15, the 2015 Version of the Parametric Catalogue of Italian; Earthquakes. Istituto Nazionale di Geofisica e Vulcanologia: Rome, Italy, 2016. [Google Scholar] [CrossRef]
- Madonia, P.; Liotta, M. Chemical composition of precipitation at Mt. Vesuvius and Vulcano Island, Italy: Volcanological and environmental implications. Environ. Earth Sci. 2009, 61, 159–171. [Google Scholar] [CrossRef]
- Liotta, M.; D’Alessandro, W.; Bellomo, S.; Brusca, L. Volcanic plume fingerprint in the groundwater of a persistently degassing basaltic volcano: Mt. Etna. Chem. Geol. 2016, 433, 68–80. [Google Scholar] [CrossRef]
- Dongarraa, G.; Manno, E.; Sabatino, G.; Varrica, D. Geochemical char- acteristics of waters in mineralised area of Peloritani Mountains (Sicily, Italy). Appl. Geochem. 2009, 24, 900–914. [Google Scholar] [CrossRef]
- Di Stefano, P.; Favara, R.; Luzio, D.; Renda, P.; Cacciatore, M.S.; Calo’, M.; Napoli, G.; Parisi, L.; Todaro, S.; Zarcone, G. A regional-scale discontinuity in western Sicily revealed by a multidisciplinary approach: A new piece for understanding the geodynamic puzzle of the southern Mediterranean. Tectonics 2015, 34, 2067–2085. [Google Scholar] [CrossRef]
- Palano, M.; Schiavone, D.; Loddo, M.; Neri, M.; Presti, D.; Quarto, R.; Totaro, C.; Neri, G. Active upper crust deformation pattern along the southern edge of the Tyrrhenian subduction zone (NE Sicily): Insights from a multidisciplinary approach. Tectonophysics 2015, 657, 205–218. [Google Scholar] [CrossRef]
- Bonforte, A.; Catalano, S.; Maniscalco, R.; Pavano, F.; Romagnoli, G.; Sturiale, G.; Tortorici, G. Geological and geodetic constraints on the active deformation along the northern margin of the Hyblean Plateau (SE Sicily). Tectonophysics 2015, 640, 80–89. [Google Scholar] [CrossRef]
- Caracausi, A.; Italiano, F.; Paonita, A.; Rizzo, A.; Nuccio, P.M. Evidence of deep magma degassing and ascent by geochemistry of peripheral gas emissions at Mount Etna (Italy): Assessment of the magmatic reservoir pressure. J. Geophys. Res. Space Phys. 2003, 108, 2463. [Google Scholar] [CrossRef]
- Rizzo, A.L.; Caracausi, A.; Favara, R.; Martelli, M.; Paonita, A.; Paternoster, M.; Nuccio, P.M.; Rosciglione, A. New insights into magma dynamics during last two eruptions of Mount Etna as inferred by geochemical monitoring from 2002 to 2005. Geochem. Geophys. Geosystems 2006, 7. [Google Scholar] [CrossRef]
- DISS Working Group. Database of Individual Seismogenic Sources (DISS), Version 3.2.1: A Compilation of Potential Sources for Earthquakes Larger than M 5.5 in Italy and Surrounding Areas. 2018. Available online: http://diss.rm.ingv.it/diss/ (accessed on 16 April 2019). [CrossRef]
- Lentini, F.; Carbone, S. Geologia della Sicilia. In Memorie Descrittive della Carta Geologica d’Italia; XCV. ISPRA—Servizio Geologico d’Italia: Roma, Italy, 2014. [Google Scholar]
- Liotta, M.; Martelli, M. Dissolved gases in brackish thermal waters: An improved analytical method. Geofluids 2012, 12, 236–244. [Google Scholar] [CrossRef]
- Liotta, M.; D’Alessandro, W.; Arienzo, I.; Longo, M. Tracing the circulation of groundwater in volcanic systems using the 87Sr/86Sr ratio: Application to Mt. Etna. J. Volcanol. Geotherm. Res. 2017, 331, 102–107. [Google Scholar] [CrossRef]



| pH | Alk | Na | K | Ca | Mg | Cl | NO3 | SO4− | PCO2 | SI | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| meq·L−1 | mmol·L−1 | mmol·L−1 | mmol·L−1 | mmol·L−1 | mmol·L−1 | mmol·L−1 | mmol·L−1 | Atm | Calcite | ||
| Number of values | 618 | 618 | 618 | 618 | 618 | 618 | 618 | 618 | 618 | 618 | 618 |
| Minimum | 5.68 | 1.20 × 10−1 | 1.80 × 10−1 | 0.00 | 5.85 × 10−2 | 1.70 × 10−2 | 3.01 × 10−2 | 0.00 | 2.00 × 10−2 | 2.50 × 10−4 | −4.47 |
| Maximum | 8.92 | 6.68 × 10 | 3.06 × 102 | 8.62 | 3.12 × 10 | 2.79 × 10 | 3.60 × 102 | 2.05 | 3.71 × 10 | 3.37 | 1.47 |
| Mean | 7.34 | 5.38 | 6.54 | 2.50 × 10−1 | 2.95 | 1.60 | 6.77 | 1.29 × 10−1 | 1.56 | 3.21 × 10−2 | 0.11 |
| Median | 7.34 | 4.85 | 1.51 | 1.00 × 10−1 | 2.18 | 9.34 × 10−1 | 1.21 | 3.57 × 10−2 | 5.60 × 10−1 | 1.17 × 10−2 | 0.16 |
| Average deviation | 0.28 | 1.80 | 8.08 | 2.57 × 10−1 | 1.66 | 1.28 | 8.84 | 1.45 × 10−1 | 1.60 | 3.60 × 10−2 | 0.27 |
| Standard deviation | 0.39 | 3.65 | 2.38 × 10 | 6.30 × 10−1 | 2.85 | 2.45 | 2.75 × 10 | 2.39 × 10−1 | 3.39 | 1.66 × 10−1 | 0.51 |
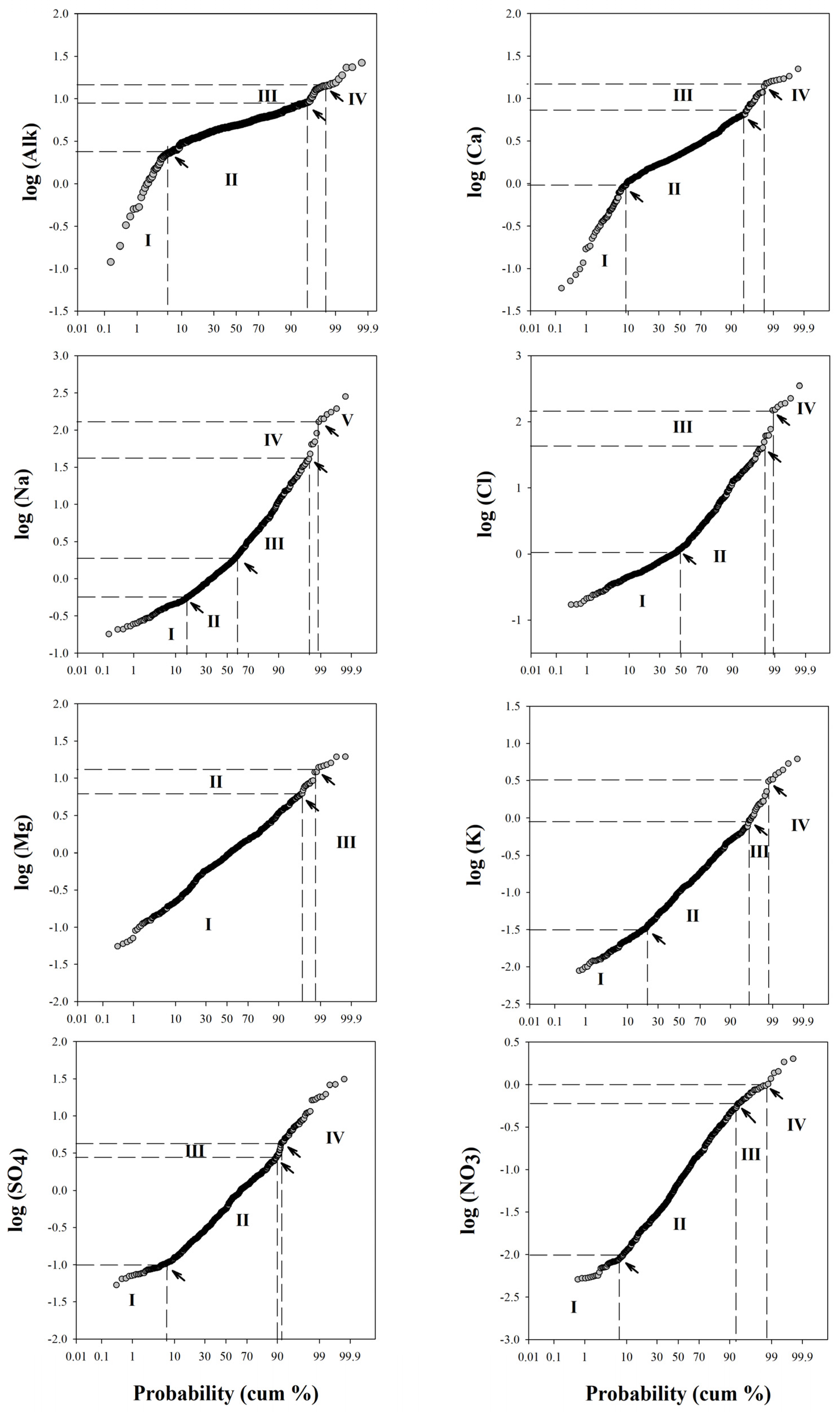
| I % | Threshold Value | II % | Threshold Value | III % | Threshold Value | IV % | Threshold Value | V % | |
|---|---|---|---|---|---|---|---|---|---|
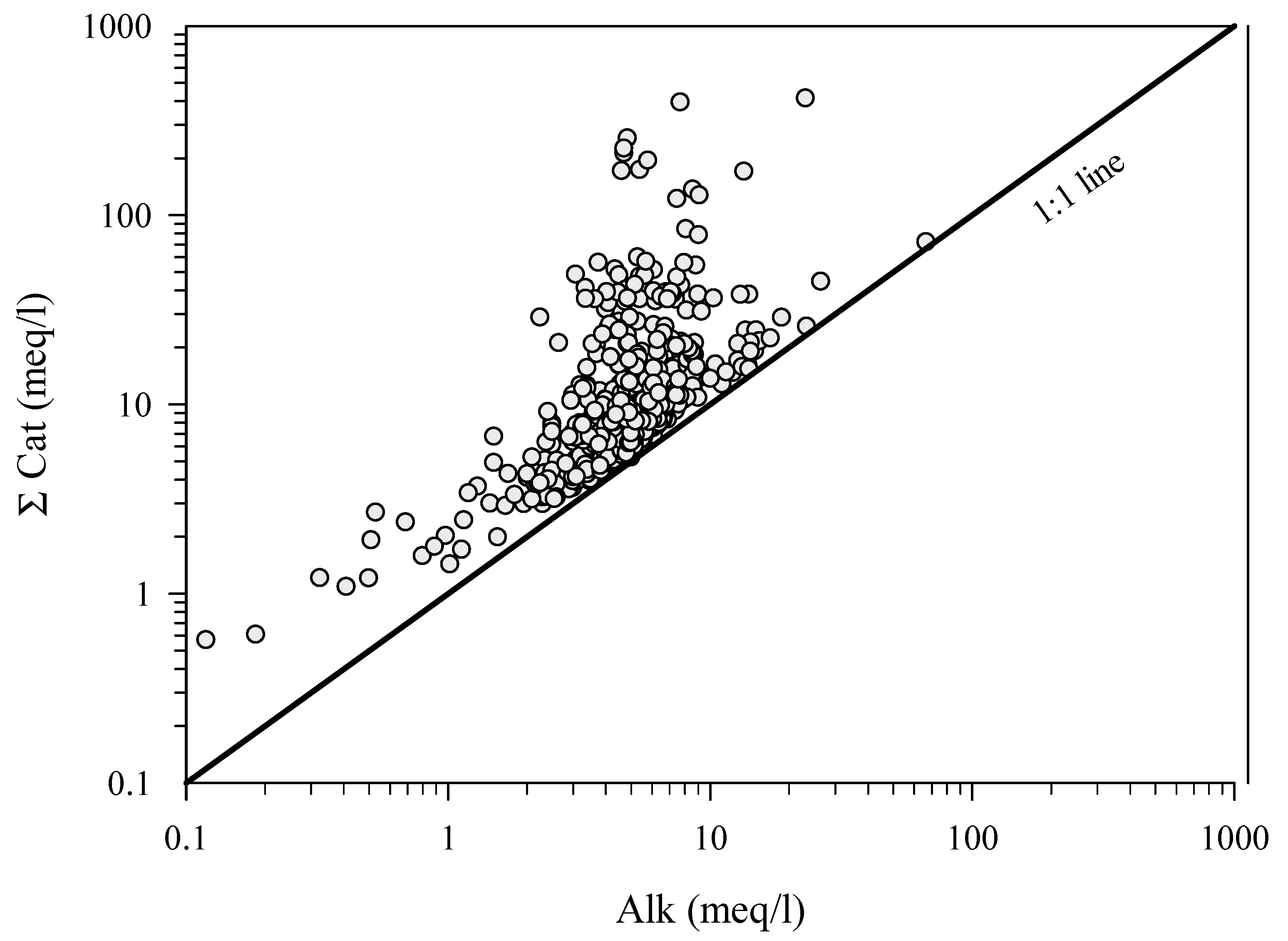
| Alk meq·L−1 | 5.5 | 2.69 | 89.5 | 8.9 | 2 | 14.8 | 3 | ||
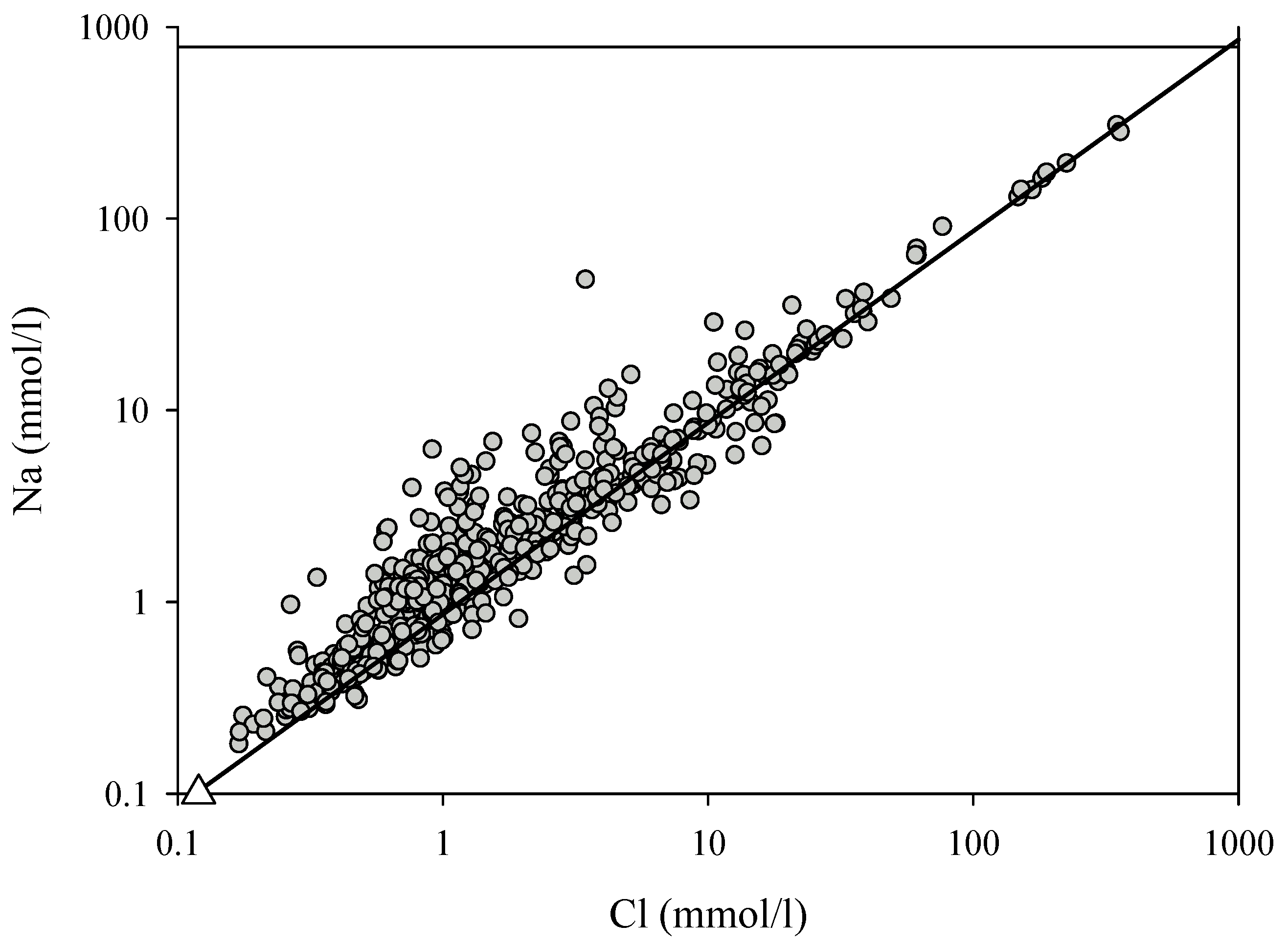
| Na mmol·L−1 | 16 | 0.56 | 43 | 2 | 39 | 40 | 0.8 | 126 | 1.2 |
| K mmol·L−1 | 21 | 0.03 | 75 | 0.83 | 2.8 | 3.24 | 1.2 | ||
| Ca mmol·L−1 | 9 | 0.85 | 85 | 7.1 | 4 | 14.8 | 2 | ||
| Mg mmol·L−1 | 96.8 | 6.02 | 2 | 13.1 | 1.2 | ||||
| Cl mmol·L−1 | 50 | 1.12 | 47 | 39.8 | 1.8 | 147.9 | 1.2 | ||
| NO3 mmol·L−1 | 7.4 | 0.01 | 85.6 | 0.61 | 5.6 | 1.02 | 1.4 | ||
| SO4 mmol·L−1 | 7.1 | 0.1 | 82.9 | 2.8 | 1.7 | 4.2 | 8.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cangemi, M.; Di Figlia, M.G.; Favara, R.; Liotta, M. CO2 Degassing in Sicily (Central Mediterranean) as Inferred from Groundwater Composition. Water 2020, 12, 1959. https://doi.org/10.3390/w12071959
Cangemi M, Di Figlia MG, Favara R, Liotta M. CO2 Degassing in Sicily (Central Mediterranean) as Inferred from Groundwater Composition. Water. 2020; 12(7):1959. https://doi.org/10.3390/w12071959
Chicago/Turabian StyleCangemi, Marianna, Maria Grazia Di Figlia, Rocco Favara, and Marcello Liotta. 2020. "CO2 Degassing in Sicily (Central Mediterranean) as Inferred from Groundwater Composition" Water 12, no. 7: 1959. https://doi.org/10.3390/w12071959
APA StyleCangemi, M., Di Figlia, M. G., Favara, R., & Liotta, M. (2020). CO2 Degassing in Sicily (Central Mediterranean) as Inferred from Groundwater Composition. Water, 12(7), 1959. https://doi.org/10.3390/w12071959






