Changes in Fungal Community Structure in Freshwater Canals across a Gradient of Urbanization
Abstract
1. Introduction
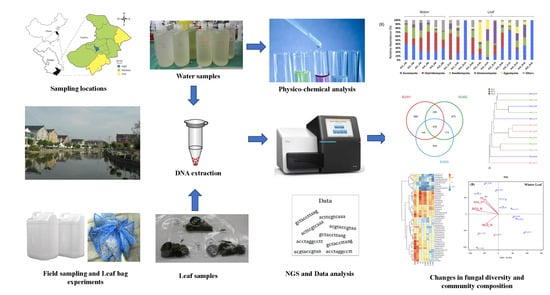
2. Materials and Methods
2.1. Sampling Sites
2.2. Field Sampling
2.3. Sample Processing and Laboratory Methods
2.3.1. DNA Extraction from Water and Leaf Samples
2.3.2. Fungal Community Analysis
2.3.3. Physico-Chemical Analyses of Water Samples
2.4. Statistical Analyses
3. Results
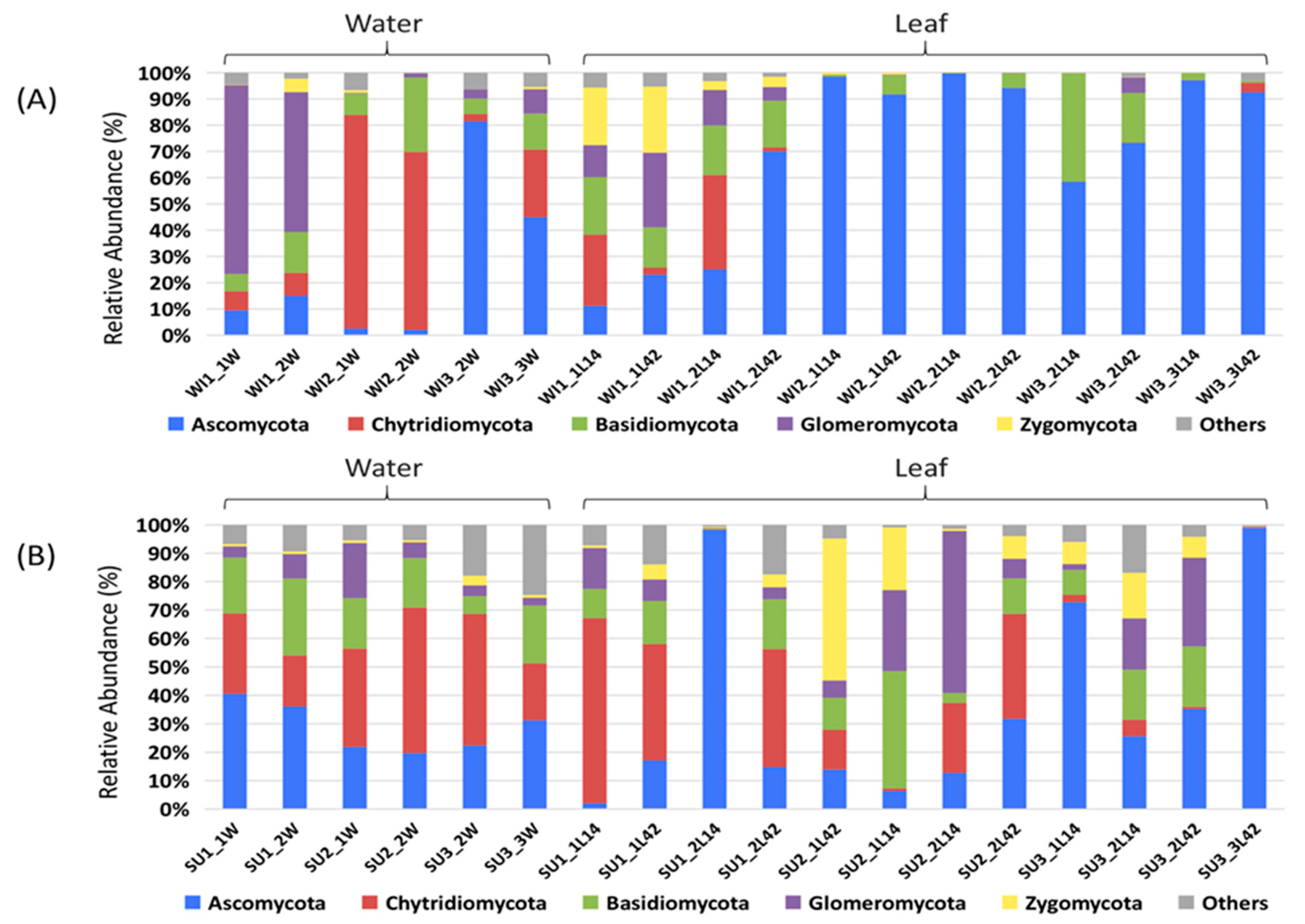
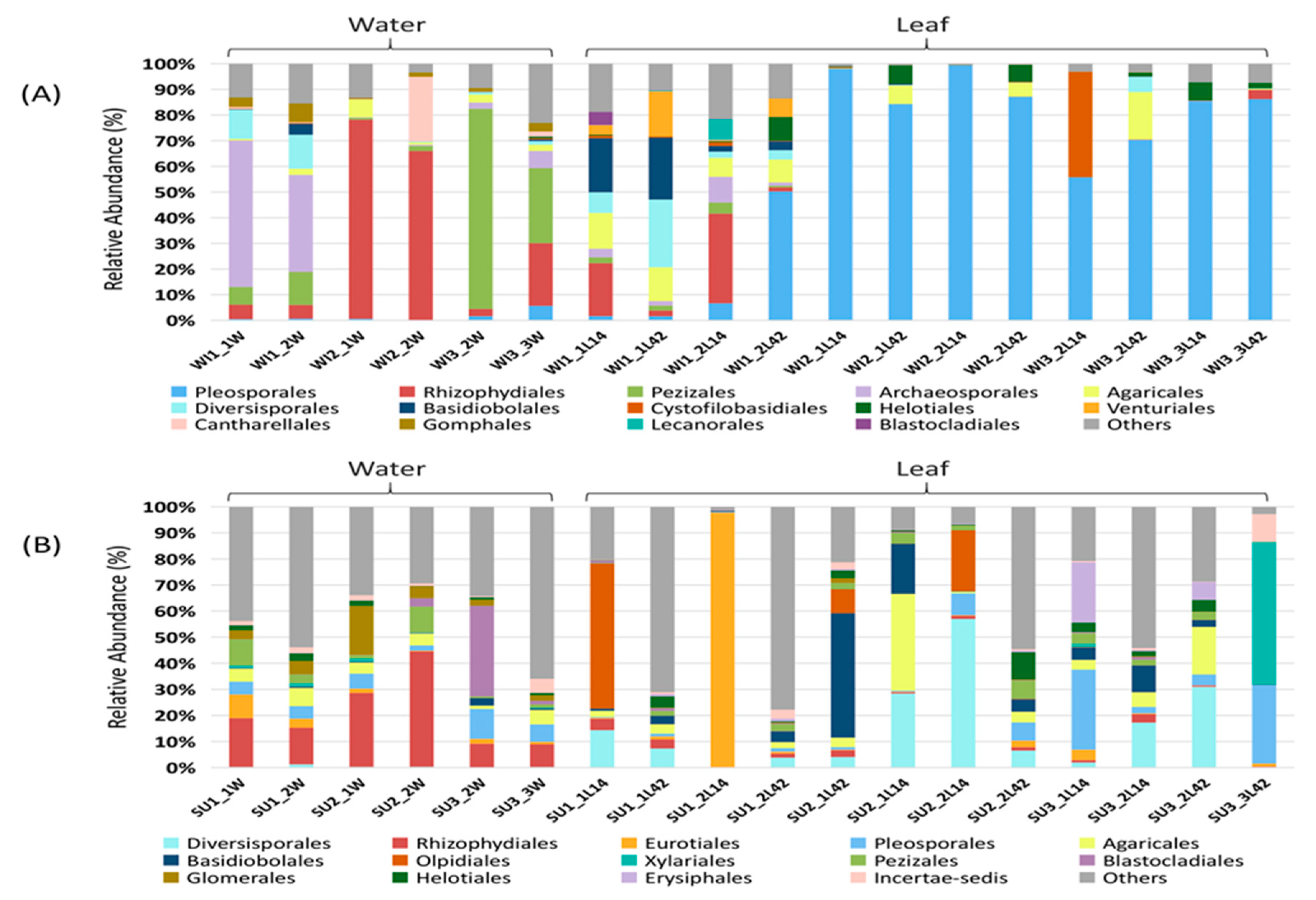
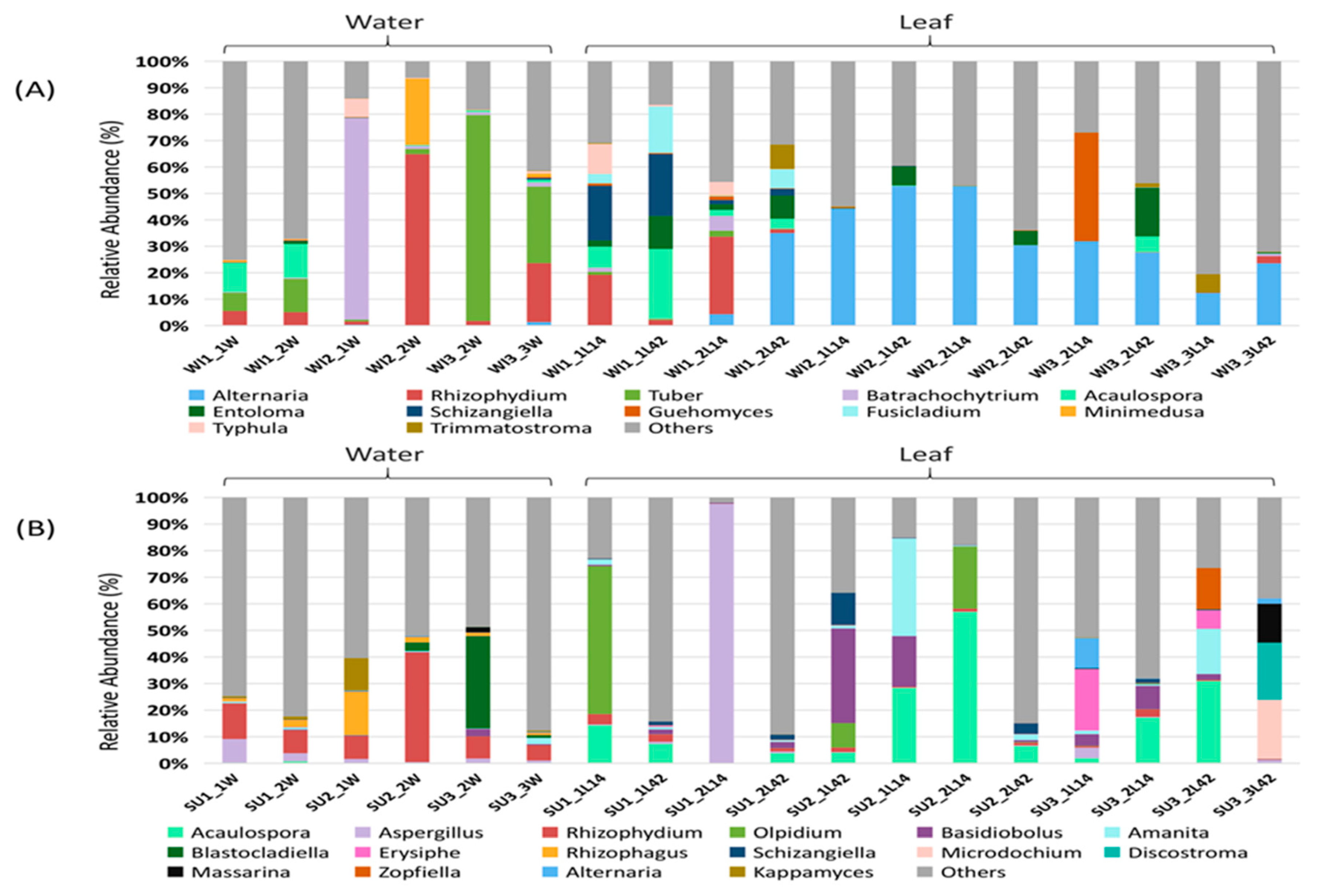
3.1. Fungal Diversity and Community Composition in Water and Leaf Samples
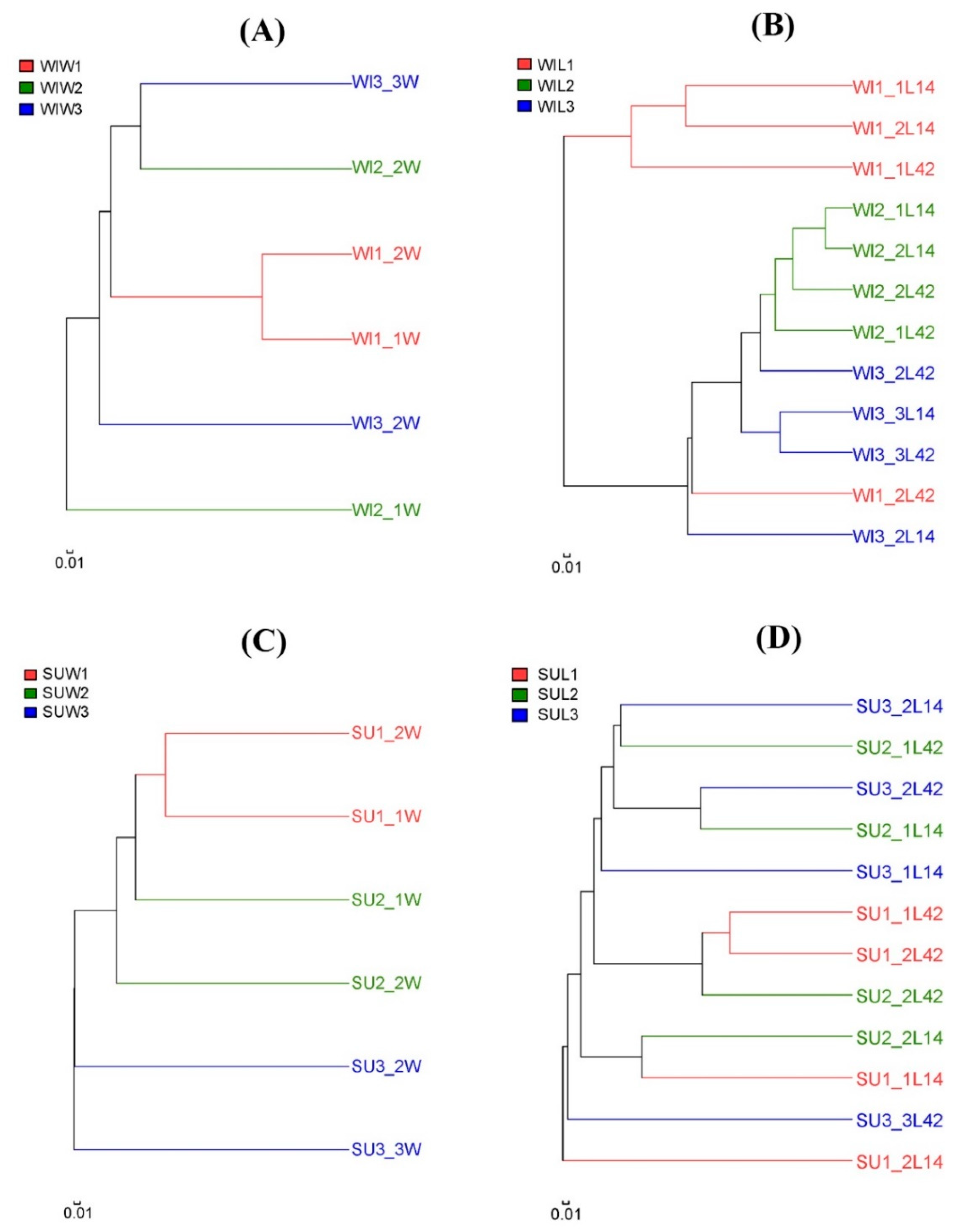
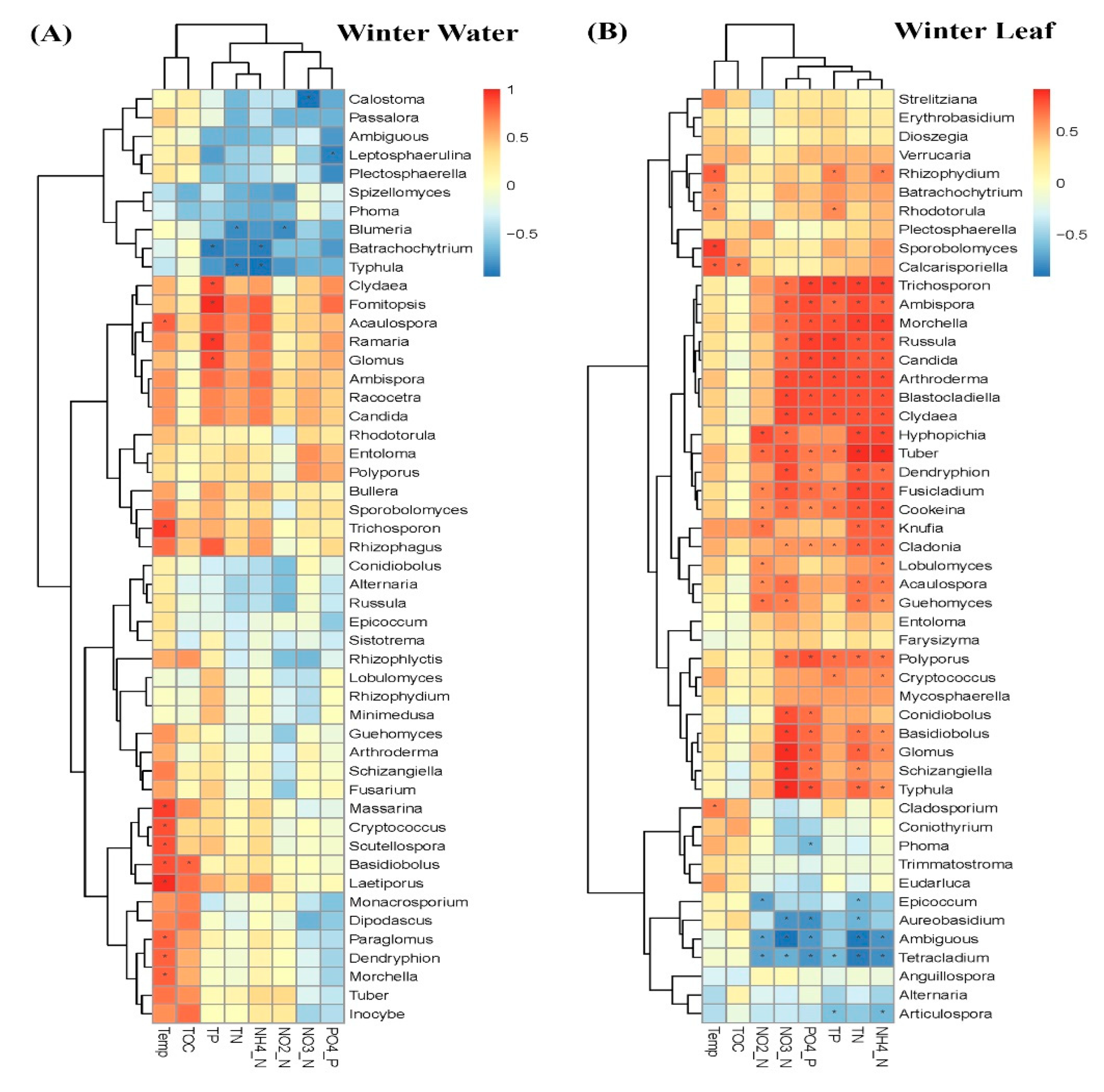
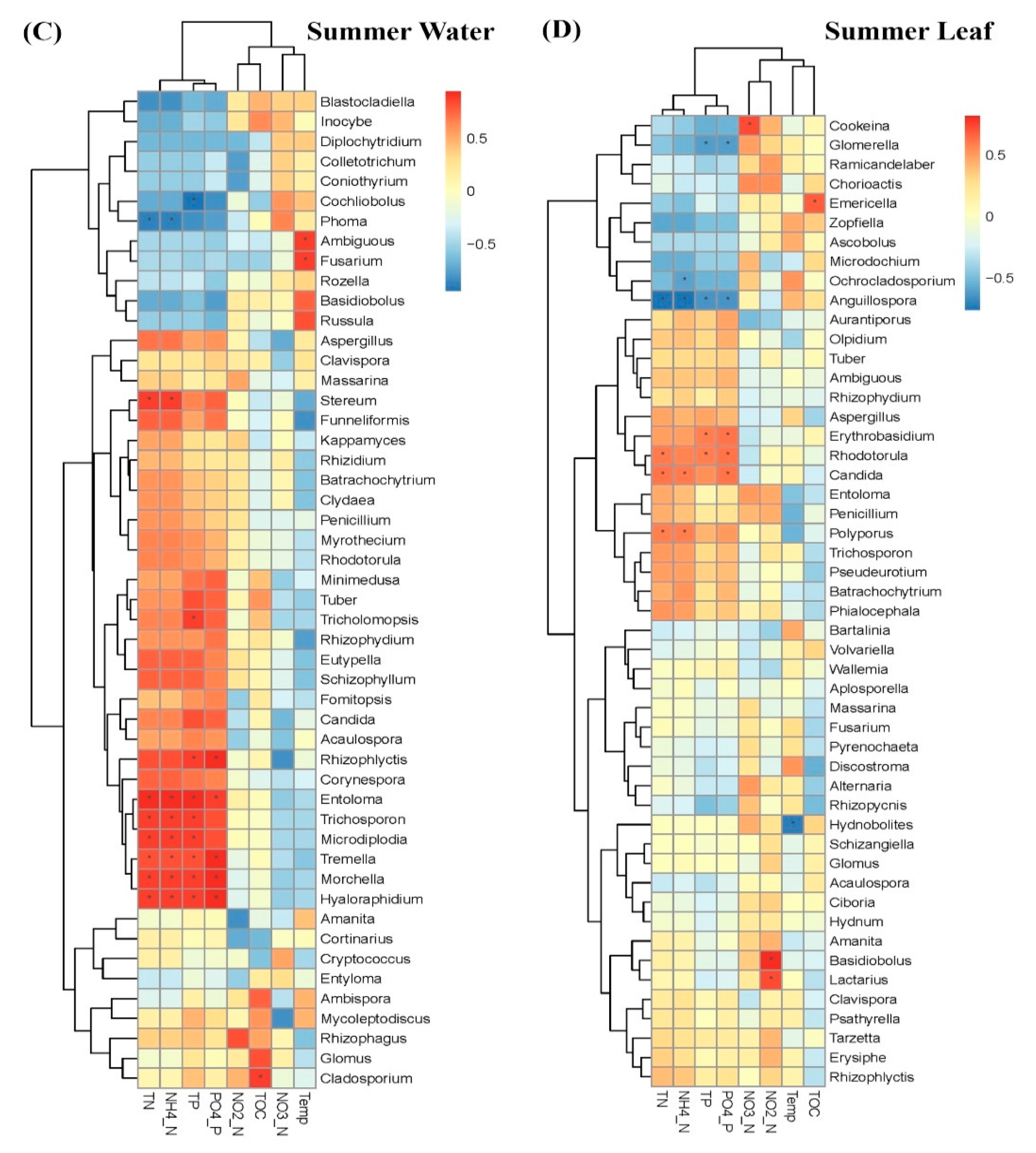
3.2. Comparison of Fungal Communities of Water and Colonized Leaves and the Impact of Urbanization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haines, J.R.; Herrmann, R.; Lee, K.; Cobanli, S.; Blaise, C. Microbial population analysis as a measure of ecosystem restoration. Bioremed. J. 2002, 6, 283–296. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Liang, C.; Hao, Z.; Zhou, L.; Ma, S.; Li, X.; Yang, S.; Yao, F.; Jiang, Y. Aboveground-belowground biodiversity linkages differ in early and late successional temperate forests. Sci. Rep. 2015, 5, 12234. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yan, M.; Huang, T.; Zhang, H.; Liu, K.; Huang, X.; Li, N.; Miao, Y.; Sekar, R. Disentangling the drivers of microcystis decomposition: Metabolic profile and co-occurrence of bacterial community. Sci. Total Environ. 2020, 739, 140062. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Dyble, J.; Moisander, P.H.; Noble, R.T.; Piehler, M.F.; Pinckney, J.L.; Steppe, T.F.; Twomey, L.; Valdes, L.M. Microbial indicators of aquatic ecosystem change: Current applications to eutrophication studies. FEMS Microbiol. Ecol. 2003, 46, 233–246. [Google Scholar] [CrossRef]
- Duarte, S.; Bärlocher, F.; Trabulo, J.; Cássio, F.; Pascoal, C. Stream-dwelling fungal decomposer communities along a gradient of eutrophication unraveled by 454 pyrosequencing. Fungal Divers. 2014, 70, 127–148. [Google Scholar] [CrossRef]
- Duarte, S.; Bärlocher, F.; Pascoal, C.; Cássio, F. Biogeography of aquatic hyphomycetes: Current knowledge and future perspectives. Fungal Ecol. 2016, 19, 169–181. [Google Scholar] [CrossRef]
- Ittner, L.D.; Junghans, M.; Werner, I. Aquatic fungi: A disregarded trophic level in ecological risk assessment of organic fungicides. Front. Environ. Sci. 2018, 6, 105. [Google Scholar] [CrossRef]
- Ingold, C.T. Aquatic hyphomycetes of decaying alder leaves. Trans. Br. Mycol. Soc. 1942, 25, 339–417. [Google Scholar] [CrossRef]
- Bärlocher, F. Aquatic hyphomycetes in a changing environment. Fungal Ecol. 2016, 19, 14–27. [Google Scholar] [CrossRef]
- Grossart, H.-P.; Rojas-Jimenez, K. Aquatic fungi: Targeting the forgotten in microbial ecology. Curr. Opin. Microbiol. 2016, 31, 140–145. [Google Scholar] [CrossRef]
- Grossart, H.-P.; Van den Wyngaert, S.; Kagami, M.; Wurzbacher, C.; Cunliffe, M.; Rojas-Jimenez, K. Fungi in aquatic ecosystems. Nat. Rev. Microbiol. 2019, 17, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.; Pascoal, C.; Alves, A.; Correia, A.; Cassio, F. Assessing the dynamic of microbial communities during leaf decomposition in a low-order stream by microscopic and molecular techniques. Microbiol. Res. 2010, 165, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Giaramida, L.; Reich, P.B.; Khachane, A.N.; Hamonts, K.; Edwards, C.; Lawton, L.A.; Singh, B.K.; Brophy, C. Lack of functional redundancy in the relationship between microbial diversity and ecosystem functioning. J. Ecol. 2016, 104, 936–946. [Google Scholar] [CrossRef]
- Collier, K.J.; Clapcott, J.E.; Duggan, I.C.; Hamilton, D.P.; Hamer, M.; Young, R.G. Spatial variation of structural and functional indicators in a large new zealand river. River Res. Appl. 2013, 29, 1277–1290. [Google Scholar] [CrossRef]
- Thompson, M.S.A.; Bankier, C.; Bell, T.; Dumbrell, A.J.; Gray, C.; Ledger, M.E.; Lehmann, K.; McKew, B.A.; Sayer, C.D.; Shelley, F.; et al. Gene-to-ecosystem impacts of a catastrophic pesticide spill: Testing a multilevel bioassessment approach in a river ecosystem. Freshw. Biol. 2016, 61, 2037–2050. [Google Scholar] [CrossRef]
- Hill, B.H.; Perrotte, W.T. Microbial colonization, respiration, and breakdown of maple leaves along a stream-marsh continuum. Hydrobiologia 1995, 312, 11–16. [Google Scholar] [CrossRef]
- Cummins, K.W. Structure and function of stream ecosystems. BioScience 1974, 24, 631–641. [Google Scholar] [CrossRef]
- Suberkropp, K.; Klug, M.J. Fungi and bacteria associated with leaves during processing in a woodland steam. Ecology 1976, 57, 707–719. [Google Scholar] [CrossRef]
- Das, M.; Royer, T.V.; Leff, L.G. Diversity of fungi, bacteria, and actinomycetes on leaves decomposing in a stream. Appl. Environ. Microbiol. 2007, 73, 756–767. [Google Scholar] [CrossRef]
- Cuffney, T.F.; Wallace, J.B.; Lugthart, G.J. Experimental evidence quantifying the role of benthic invertebrates in organic matter dynamics of headwater streams. Freshw. Biol. 1990, 23, 281–299. [Google Scholar] [CrossRef]
- Findlay, S.E.G.; Dye, S.; Kuehn, K.A. Microbial growth and nitrogen retention in litter of phragmites australis compared to typha angustifolia. Wetlands 2002, 22, 616–625. [Google Scholar] [CrossRef]
- Kim, M.; Kim, W.-S.; Tripathi, B.M.; Adams, J. Distinct bacterial communities dominate tropical and temperate zone leaf litter. Microb. Ecol. 2014, 67, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Kominkova, D.; Kuehn, K.A.; Busing, N.; Steiner, D.; Gessner, M.O. Microbial biomass, growth, and respiration associated with submerged litter of phragmites australis decomposing in a littoral reed stand of a large lake. Aquat. Microb. Ecol. 2000, 22, 271–282. [Google Scholar] [CrossRef]
- Johnston, S.R.; Boddy, L.; Weightman, A.J. Bacteria in decomposing wood and their interactions with wood-decay fungi. FEMS Microbiol. Ecol. 2016, 92, fiw179. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.; Kritzberg, E.S.; Rousk, J. Labile carbon ‘primes’ fungal use of nitrogen from submerged leaf litter. FEMS Microbiol. Ecol. 2017, 93, fix110. [Google Scholar] [CrossRef] [PubMed]
- Gossiaux, A.; Rollin, M.; Guérold, F.; Felten, V.; Laviale, M.; Bachelet, Q.; Poupin, P.; Chauvet, E.; Bec, A.; Danger, M. Temperature and nutrient effects on the relative importance of brown and green pathways for stream ecosystem functioning: A mesocosm approach. Freshw. Biol. 2020, 65, 1239–1255. [Google Scholar] [CrossRef]
- Niyogi, D.K.; Hu, C.-Y.; Vessell, B.P. Response of stream fungi on decomposing leaves to experimental drying. Int. Rev. Hydrobiol. 2020, 105, 52–58. [Google Scholar] [CrossRef]
- Liu, Z.; Lv, Y.; Ding, R.; Chen, X.; Pu, G. Light pollution changes the toxicological effects of cadmium on microbial community structure and function associated with leaf litter decomposition. Int. J. Mol. Sci. 2020, 21, 422. [Google Scholar] [CrossRef]
- Seena, S.; Barlocher, F.; Sobral, O.; Gessner, M.O.; Dudgeon, D.; McKie, B.G.; Chauvet, E.; Boyero, L.; Ferreira, V.; Frainer, A.; et al. Biodiversity of leaf litter fungi in streams along a latitudinal gradient. Sci. Total Environ. 2019, 661, 306–315. [Google Scholar] [CrossRef]
- Geraldes, P.; Pascoal, C.; Cássio, F. Effects of increased temperature and aquatic fungal diversity on litter decomposition. Fungal Ecol. 2012, 5, 734–740. [Google Scholar] [CrossRef]
- Du, J.; Zhang, Y.; Yin, Y.; Zhang, J.; Ma, H.; Li, K.; Wan, N. Do environmental concentrations of zinc oxide nanoparticle pose ecotoxicological risk to aquatic fungi associated with leaf litter decomposition? Water Res. 2020, 178, 115840. [Google Scholar] [CrossRef] [PubMed]
- Juvigny-Khenafou, N.P.D.; Zhang, Y.; Piggott, J.J.; Atkinson, D.; Matthaei, C.D.; Van Bael, S.A.; Wu, N. Anthropogenic stressors affect fungal more than bacterial communities in decaying leaf litter: A stream mesocosm experiment. Sci. Total Environ. 2020, 716, 135053. [Google Scholar] [CrossRef] [PubMed]
- De Sherbinin, A.; Schiller, A.; Pulsipher, A. The vulnerability of global cities to climate hazards. Environ. Urban 2007, 19, 39–64. [Google Scholar] [CrossRef]
- Du, N.; Ottens, H.; Sliuzas, R. Spatial impact of urban expansion on surface water bodies—A case study of wuhan, china. Landsc. Urban Plan. 2010, 94, 175–185. [Google Scholar] [CrossRef]
- Yuan, T.; Vadde, K.K.; Tonkin, J.D.; Wang, J.; Lu, J.; Zhang, Z.; Zhang, Y.; McCarthy, A.J.; Sekar, R. Urbanization impacts the physicochemical characteristics and abundance of fecal markers and bacterial pathogens in surface water. Int. J. Environ. Res. Public Health 2019, 16, 1739. [Google Scholar] [CrossRef]
- Hale, R.L.; Turnbull, L.; Earl, S.R.; Childers, D.L.; Grimm, N.B. Stormwater infrastructure controls runoff and dissolved material export from arid urban watersheds. Ecosystems 2014, 18, 62–75. [Google Scholar] [CrossRef]
- Carrino-Kyker, S.R.; Swanson, A.K.; Burke, D.J. Changes in eukaryotic microbial communities of vernal pools along an urban–rural land use gradient. Aquat. Microb. Ecol. 2011, 62, 13–24. [Google Scholar] [CrossRef]
- Iniguez-Armijos, C.; Rausche, S.; Cueva, A.; Sanchez-Rodriguez, A.; Espinosa, C.; Breuer, L. Shifts in leaf litter breakdown along a forest-pasture-urban gradient in andean streams. Ecol. Evol. 2016, 6, 4849–4865. [Google Scholar] [CrossRef]
- Cunningham, S.C.; Mac Nally, R.; Baker, P.J.; Cavagnaro, T.R.; Beringer, J.; Thomson, J.R.; Thompson, R.M. Balancing the environmental benefits of reforestation in agricultural regions. Perspect. Plant Ecol. Evol. Syst. 2015, 17, 301–317. [Google Scholar] [CrossRef]
- Emilson, C.E.; Kreutzweiser, D.P.; Gunn, J.M.; Mykytczuk, N.C.S. Effects of land use on the structure and function of leaf-litter microbial communities in boreal streams. Freshw. Biol. 2016, 61, 1049–1061. [Google Scholar] [CrossRef]
- Rossi, F.; Mallet, C.; Portelli, C.; Donnadieu, F.; Bonnemoy, F.; Artigas, J. Stimulation or inhibition: Leaf microbial decomposition in streams subjected to complex chemical contamination. Sci. Total Environ. 2019, 648, 1371–1383. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Pesce, S.; Mallet, C.; Margoum, C.; Chaumot, A.; Masson, M.; Artigas, J. Interactive effects of pesticides and nutrients on microbial communities responsible of litter decomposition in streams. Front. Microbiol. 2018, 9, 2437. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Barros, J.; Gonçalves, A.L.; Canhoto, C. Salinisation effects on leaf litter decomposition in fresh waters: Does the ionic composition of salt matter? Freshw. Biol. 2020. [Google Scholar] [CrossRef]
- Pu, G.; Zeng, D.; Mo, L.; Liao, J.; Chen, X.; Qiu, S.; Lv, Y. Artificial light at night alter the impact of arsenic on microbial decomposers and leaf litter decomposition in streams. Ecotoxicol. Environ. Saf. 2020, 191, 110014. [Google Scholar] [CrossRef]
- Wang, L.; Shen, J.; Chung, C.K.L. City profile: Suzhou—A chinese city under transformation. Cities 2015, 44, 60–72. [Google Scholar] [CrossRef]
- Cao, Y.-l.; Wang, X.; Yin, C.-q.; Xu, W.-w.; Shi, W.; Qian, G.-r.; Xun, Z.-m. Inland vessels emission inventory and the emission characteristics of the beijing-hangzhou grand canal in jiangsu province. Process Saf. Environ. Prot. 2018, 113, 498–506. [Google Scholar] [CrossRef]
- Zhuang, W.; Chen, Q.; Gao, X.; Zhou, F.; Wang, M.; Liu, Y. Characterization of surface sediments from the beijing-hangzhou grand canal (zaozhuang section), china: Assessment of beryllium enrichment, biological effect, and mobility. Environ. Sci. Pollut. Res. Int. 2016, 23, 13560–13568. [Google Scholar] [CrossRef]
- Zhuang, W.; Liu, Y.; Chen, Q.; Wang, Q.; Zhou, F. A new index for assessing heavy metal contamination in sediments of the beijing-hangzhou grand canal (zaozhuang segment): A case study. Ecol. Indic. 2016, 69, 252–260. [Google Scholar] [CrossRef]
- Reazin, C.; Morris, S.; Smith, J.E.; Cowan, A.D.; Jumpponen, A. Fires of differing intensities rapidly select distinct soil fungal communities in a northwest us ponderosa pine forest ecosystem. For. Ecol. Manag. 2016, 377, 118–127. [Google Scholar] [CrossRef]
- French, K.E.; Tkacz, A.; Turnbull, L.A. Conversion of grassland to arable decreases microbial diversity and alters community composition. Appl. Soil Ecol. 2017, 110, 43–52. [Google Scholar] [CrossRef]
- Dannemiller, K.C.; Reeves, D.; Bibby, K.; Yamamoto, N.; Peccia, J. Fungal high-throughput taxonomic identification tool for use with next-generation sequencing (fhitings). J. Basic Microbiol. 2014, 54, 315–321. [Google Scholar] [CrossRef] [PubMed]
- McTaggart, L.R.; Copeland, J.K.; Surendra, A.; Wang, P.W.; Husain, S.; Coburn, B.; Guttman, D.S.; Kus, J.V. Mycobiome sequencing and analysis applied to fungal community profiling of the lower respiratory tract during fungal pathogenesis. Front. Microbiol. 2019, 10, 512. [Google Scholar] [CrossRef] [PubMed]
- Vadde, K.; Wang, J.; Cao, L.; Yuan, T.; McCarthy, A.; Sekar, R. Assessment of water quality and identification of pollution risk locations in tiaoxi river (taihu watershed), China. Water 2018, 10, 183. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, J.; Chen, S.; Zhao, Z.; Li, B.; Wang, Y.; Jia, J.; Li, S.; Wang, Y.; Yan, M.; et al. Geographical patterns of nirs gene abundance and nirs-type denitrifying bacterial community associated with activated sludge from different wastewater treatment plants. Microb. Ecol. 2019, 77, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Vadde, K.K.; Feng, Q.; Wang, J.; McCarthy, A.J.; Sekar, R. Next-generation sequencing reveals fecal contamination and potentially pathogenic bacteria in a major inflow river of taihu lake. Environ. Pollut. 2019, 254, 113108. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Song, X.; Li, X.; Fu, S.; Li, M.; Liu, Y. Characterization of microbial communities in pilot-scale constructed wetlands with salicornia for treatment of marine aquaculture effluents. Archaea 2018, 2018, 7819840. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.H.D.; Thomas, T. Diversity, host-specificity and stability of sponge-associated fungal communities of co-occurring sponges. PeerJ 2018, 6, e4965. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Vera, M.P.; Olchanheski, L.R.; Da Silva, E.G.; De Lima, F.R.; Martinez, L.R.D.P.R.; Sato, M.I.Z.; Jaffe, R.; Alves, R.; Ichiwaki, S.; Padilla, G.; et al. Influence of water quality on diversity and composition of fungal communities in a tropical river. Sci. Rep. 2018, 8, 14799. [Google Scholar] [CrossRef]
- Miura, A.; Urabe, J. Spatial and seasonal changes in species diversity of epilithic fungi along environmental gradients of a river. Freshw. Biol. 2015, 60, 673–685. [Google Scholar] [CrossRef]
- Bergfur, J.; Sundberg, C. Leaf-litter-associated fungi and bacteria along temporal and environmental gradients in boreal streams. Aquat. Microb. Ecol. 2014, 73, 225–234. [Google Scholar] [CrossRef]
- Ferreira, V.; Canhoto, C. Effect of experimental and seasonal warming on litter decomposition in a temperate stream. Aquat. Sci. 2013, 76, 155–163. [Google Scholar] [CrossRef]
- Ferreira, V.; Canhoto, C. Future increase in temperature may stimulate litter decomposition in temperate mountain streams: Evidence from a stream manipulation experiment. Freshw. Biol. 2015, 60, 881–892. [Google Scholar] [CrossRef]
- Jabiol, J.; Gossiaux, A.; Lecerf, A.; Rota, T.; Guérold, F.; Danger, M.; Poupin, P.; Gilbert, F.; Chauvet, E. Variable temperature effects between heterotrophic stream processes and organisms. Freshw. Biol. 2020, 00, 1–20. [Google Scholar] [CrossRef]
- Bärlocher, F.; Boddy, L. Aquatic fungal ecology—How does it differ from terrestrial? Fungal Ecol. 2016, 19, 5–13. [Google Scholar] [CrossRef]
- Tolkkinen, M.; Mykra, H.; Annala, M.; Markkola, A.M.; Vuori, K.M.; Muotka, T. Multi-stressor impacts on fungal diversity and ecosystem functions in streams: Natural vs. Anthropogenic stress. Ecology 2015, 96, 672–683. [Google Scholar] [CrossRef]
- Reese, A.T.; Savage, A.; Youngsteadt, E.; McGuire, K.L.; Koling, A.; Watkins, O.; Frank, S.D.; Dunn, R.R. Urban stress is associated with variation in microbial species composition—But not richness—In manhattan. ISME J. 2015, 10, 751–760. [Google Scholar] [CrossRef]
- Nikolcheva, L.G.; Bärlocher, F. Taxon-specific fungal primers reveal unexpectedly high diversity during leaf decomposition in a stream. Mycol. Prog. 2004, 3, 41–49. [Google Scholar] [CrossRef]
- Seena, S.; Wynberg, N.; Bärlocher, F. Fungal diversity during leaf decomposition in a stream assessed through clone libraries. Fungal Divers. 2008, 30, 1–14. [Google Scholar]
- Gleason, F.H.; Kagami, M.; Lefevre, E.; Sime-Ngando, T. The ecology of chytrids in aquatic ecosystems: Roles in food web dynamics. Fungal Biol. Rev. 2008, 22, 17–25. [Google Scholar] [CrossRef]
- Wurzbacher, C.; Warthmann, N.; Bourne, E.; Attermeyer, K.; Allgaier, M.; Powell, J.R.; Detering, H.; Mbedi, S.; Grossart, H.-P.; Monaghan, M. High habitat-specificity in fungal communities in oligo-mesotrophic, temperate lake stechlin (north-east germany). MycoKeys 2016, 16, 17–44. [Google Scholar] [CrossRef]
- Shearer, C.A.; Raja, H.A.; Miller, A.N.; Nelson, P.; Tanaka, K.; Hirayama, K.; Marvanova, L.; Hyde, K.D.; Zhang, Y. The molecular phylogeny of freshwater dothideomycetes. Stud. Mycol. 2009, 64, 145–153S144. [Google Scholar] [CrossRef] [PubMed]
- Khomich, M.; Davey, M.L.; Kauserud, H.; Rasconi, S.; Andersen, T. Fungal communities in scandinavian lakes along a longitudinal gradient. Fungal Ecol. 2017, 27, 36–46. [Google Scholar] [CrossRef]
- Kirk, P.M.; Cannon, P.F.; Minter, D.W.; Stalpers, J.A. Dictionary of the Fungi, 10th ed.; CABI: Wallingford, UK, 2008. [Google Scholar]
- Nowicki, M.; Nowakowska, M.; Niezgoda, A.; Kozik, E. Alternaria black spot of crucifers: Symptoms, importance of disease, and perspectives of resistance breeding. Veg. Crops Res. Bull. 2012, 76, 5–19. [Google Scholar] [CrossRef]
- Patriarca, A.; Fernández Pinto, V. Alternaria. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Gardeström, J.; Ermold, M.; Goedkoop, W.; McKie, B.G. Disturbance history influences stressor impacts: Effects of a fungicide and nutrients on microbial diversity and litter decomposition. Freshw. Biol. 2016, 61, 2171–2184. [Google Scholar] [CrossRef]
- Cudowski, A.; Pietryczuk, A.; Hauschild, T. Aquatic fungi in relation to the physical and chemical parameters of water quality in the augustów canal. Fungal Ecol. 2015, 13, 193–204. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Q.; Liao, K.; Jian, Z.; Zhao, C.; Qu, J. Fungal community as a bioindicator to reflect anthropogenic activities in a river ecosystem. Front. Microbiol. 2018, 9, 3152. [Google Scholar] [CrossRef]
- Duarte, S.; Cássio, F.; Pascoal, C. Environmental drivers are more important for structuring fungal decomposer communities than the geographic distance between streams. Limnetica 2017, 36, 491–506. [Google Scholar]



| Sample Group | OTUs | ACE 1 | Chao1 2 | Shannon 3 | Simpson 4 | Coverage 5 |
|---|---|---|---|---|---|---|
| WI, W, HU | 439 ± 81 | 572 ± 33 | 560 ± 54 | 3.66 ± 0.41 | 0.060 ± 0.025 | 0.996 ± 0.000 |
| WI, W, MU | 206 ± 52 | 286 ± 83 | 269 ± 70 | 1.26 ± 0.01 | 0.519 ± 0.057 | 0.998 ± 0.001 |
| WI, W, LU | 539 ± 286 | 663 ± 296 | 664 ± 280 | 2.94 ± 1.11 | 0.230 ± 0.168 | 0.995 ± 0.001 |
| SU, W, HU | 1063 ± 69 | 1128 ± 54 | 1128 ± 64 | 5.29 ± 0.25 | 0.014 ± 0.005 | 0.995 ± 0.001 |
| SU, W, MU | 919 ± 142 | 999 ± 122 | 991 ± 127 | 4.60 ± 0.32 | 0.050 ± 0.020 | 0.995 ± 0.000 |
| SU, W, LU | 798 ± 215 | 917 ± 144 | 905 ± 141 | 4.00 ± 0.92 | 0.093 ± 0.067 | 0.995 ± 0.001 |
| WI, L, HU | 482 ± 123 | 638 ± 147 | 616 ± 149 | 3.41 ± 0.43 | 0.097 ± 0.044 | 0.995 ± 0.001 |
| WI, L, MU | 69 ± 15 | 125 ± 55 | 109 ± 37 | 1.02 ± 0.26 | 0.438 ± 0.059 | 0.999 ± 0.000 |
| WI, L, LU | 99 ± 27 | 223 ± 84 | 158 ± 44 | 1.45 ± 0.22 | 0.374 ± 0.109 | 0.999 ± 0.000 |
| SU, L, HU | 621 ± 339 | 870 ± 316 | 797 ± 324 | 2.65 ± 1.29 | 0.265 ± 0.148 | 0.994 ± 0.003 |
| SU, L, MU | 628 ± 323 | 933 ± 204 | 814 ± 272 | 2.85 ± 1.07 | 0.209 ± 0.122 | 0.993 ± 0.002 |
| SU, L, LU | 544 ± 323 | 765 ± 348 | 723 ± 373 | 3.27 ± 0.95 | 0.104 ± 0.055 | 0.994 ± 0.003 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, T.; Zhang, H.; Feng, Q.; Wu, X.; Zhang, Y.; McCarthy, A.J.; Sekar, R. Changes in Fungal Community Structure in Freshwater Canals across a Gradient of Urbanization. Water 2020, 12, 1917. https://doi.org/10.3390/w12071917
Yuan T, Zhang H, Feng Q, Wu X, Zhang Y, McCarthy AJ, Sekar R. Changes in Fungal Community Structure in Freshwater Canals across a Gradient of Urbanization. Water. 2020; 12(7):1917. https://doi.org/10.3390/w12071917
Chicago/Turabian StyleYuan, Tianma, Haihan Zhang, Qiaoli Feng, Xiangyu Wu, Yixin Zhang, Alan J. McCarthy, and Raju Sekar. 2020. "Changes in Fungal Community Structure in Freshwater Canals across a Gradient of Urbanization" Water 12, no. 7: 1917. https://doi.org/10.3390/w12071917
APA StyleYuan, T., Zhang, H., Feng, Q., Wu, X., Zhang, Y., McCarthy, A. J., & Sekar, R. (2020). Changes in Fungal Community Structure in Freshwater Canals across a Gradient of Urbanization. Water, 12(7), 1917. https://doi.org/10.3390/w12071917