Multi-Biomarker Assessment in Common Carp (Cyprinus carpio, Linnaeus 1758) Liver after Acute Chlorpyrifos Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals and Treatments
2.3. Histological Procedures
2.4. Semi-Quantitative Screening
2.5. Biochemical Analysis
2.6. Statistical Tests
3. Results
3.1. Water Quality Parameters
3.2. Liver Histological Structure
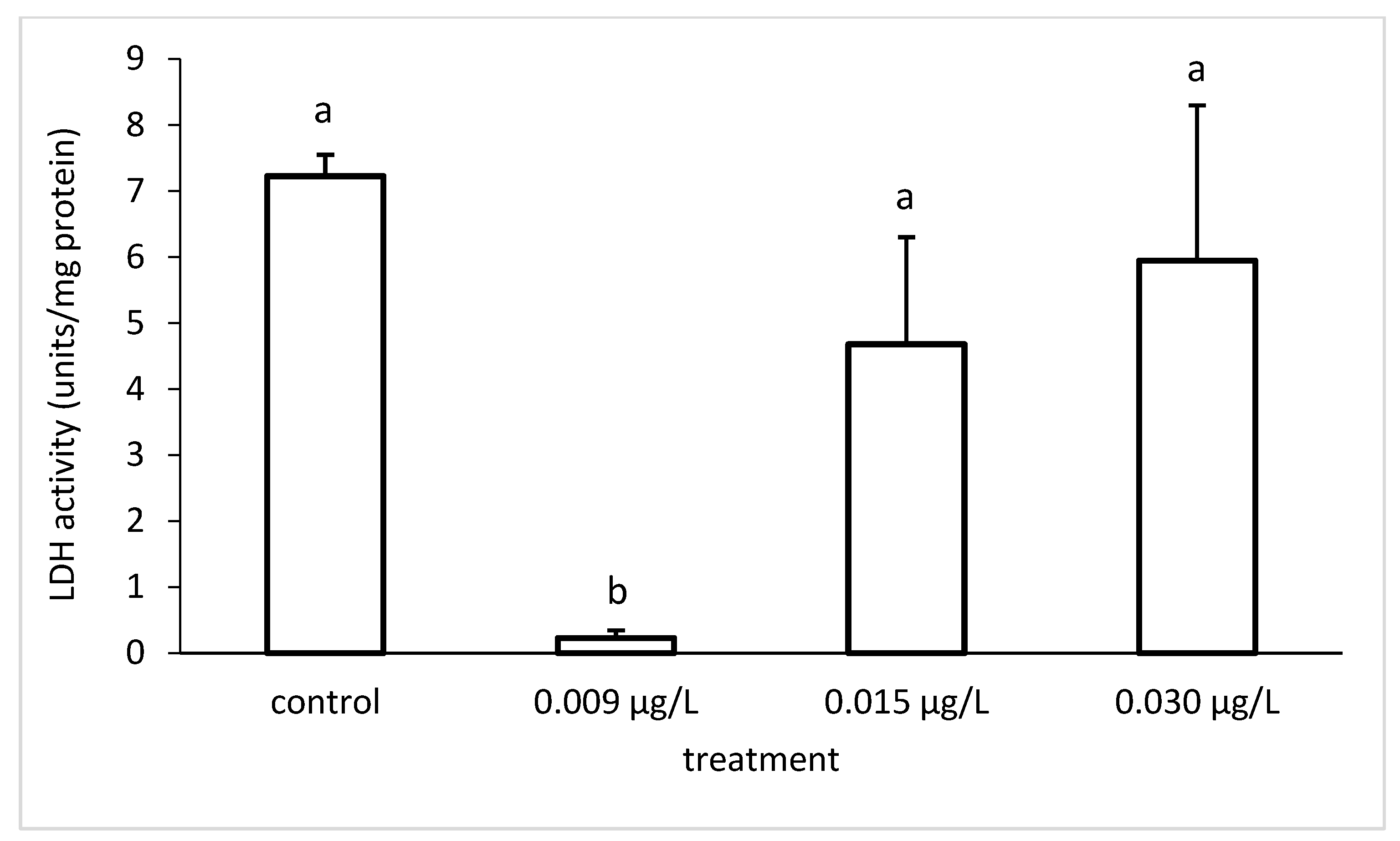
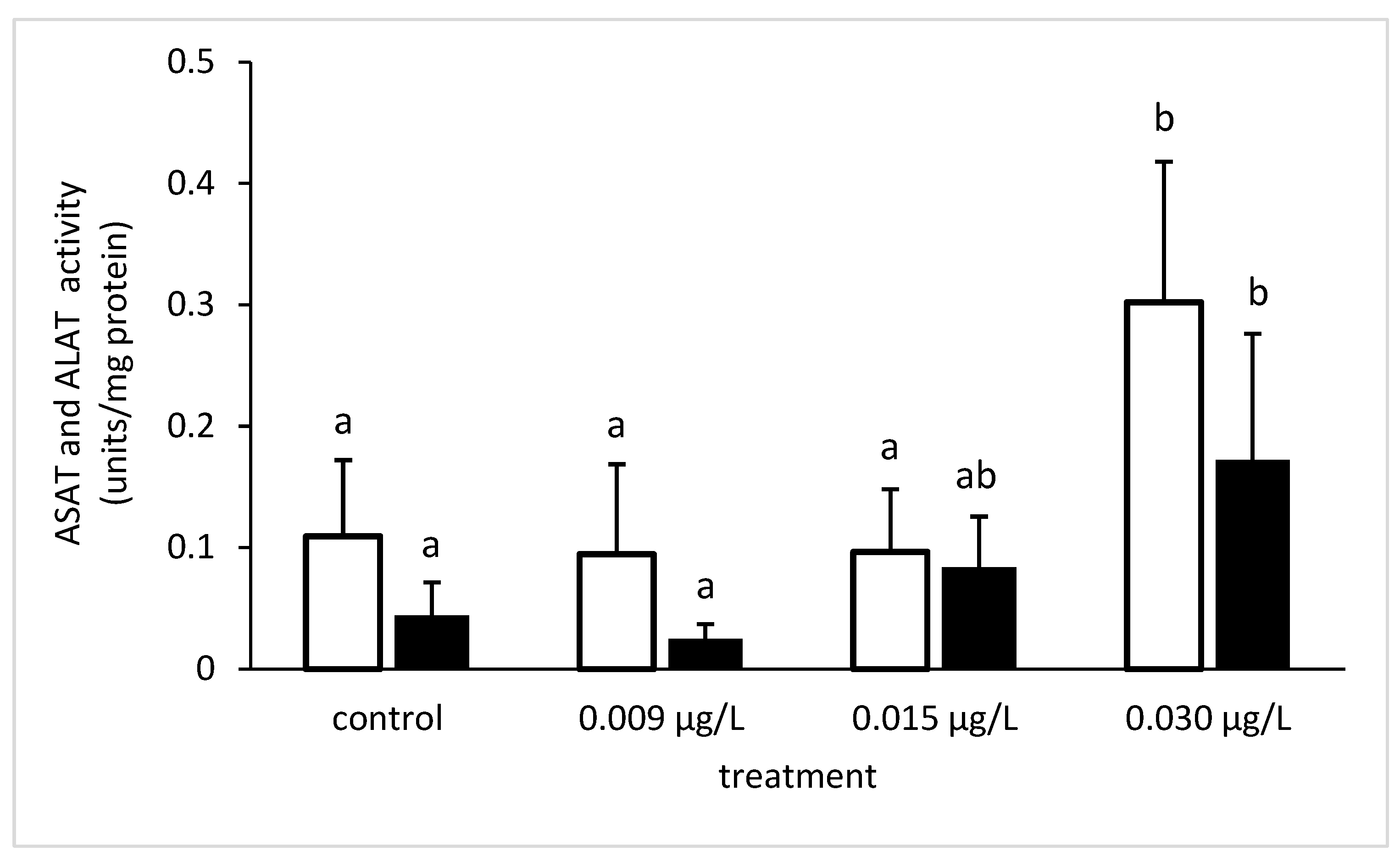
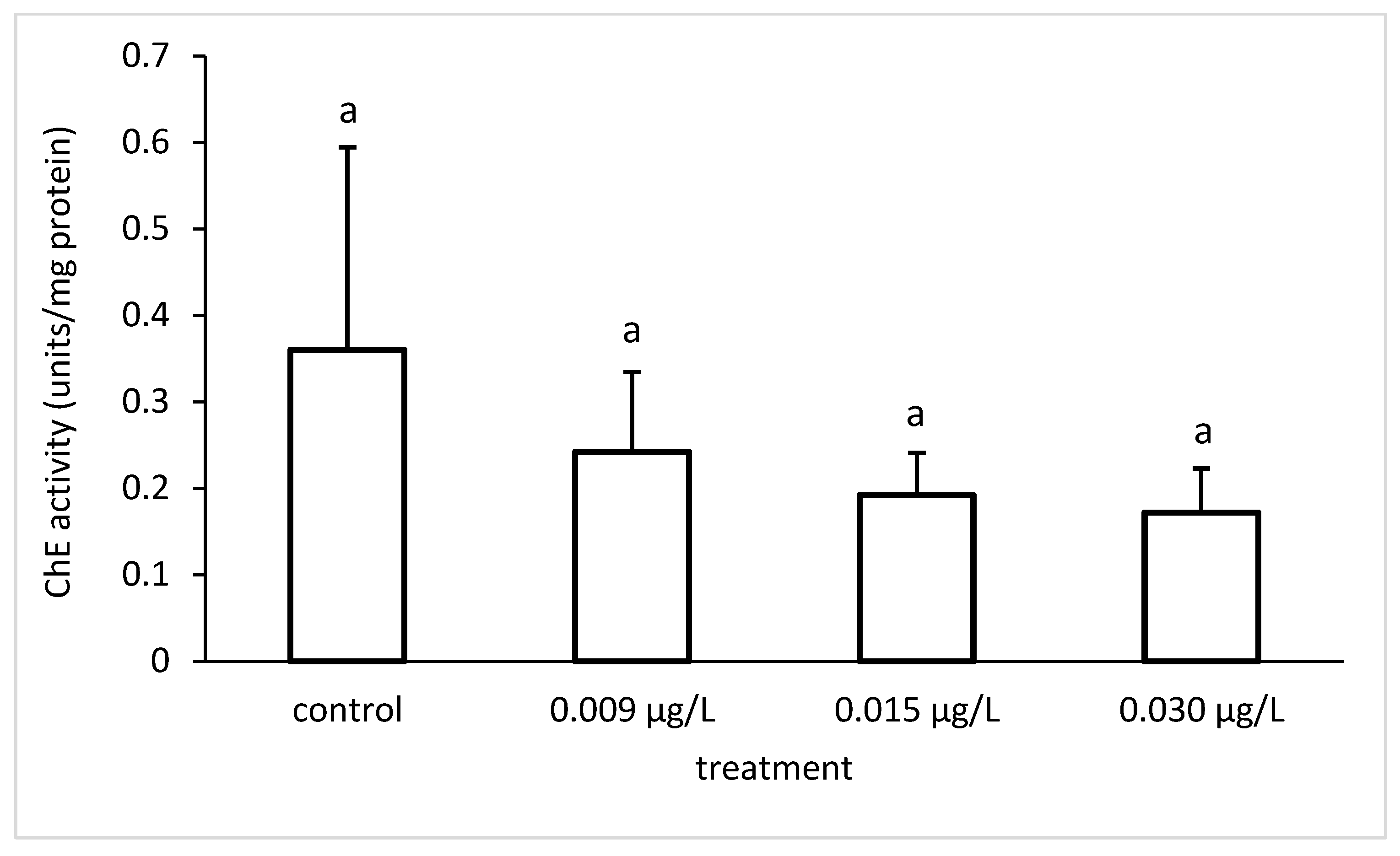
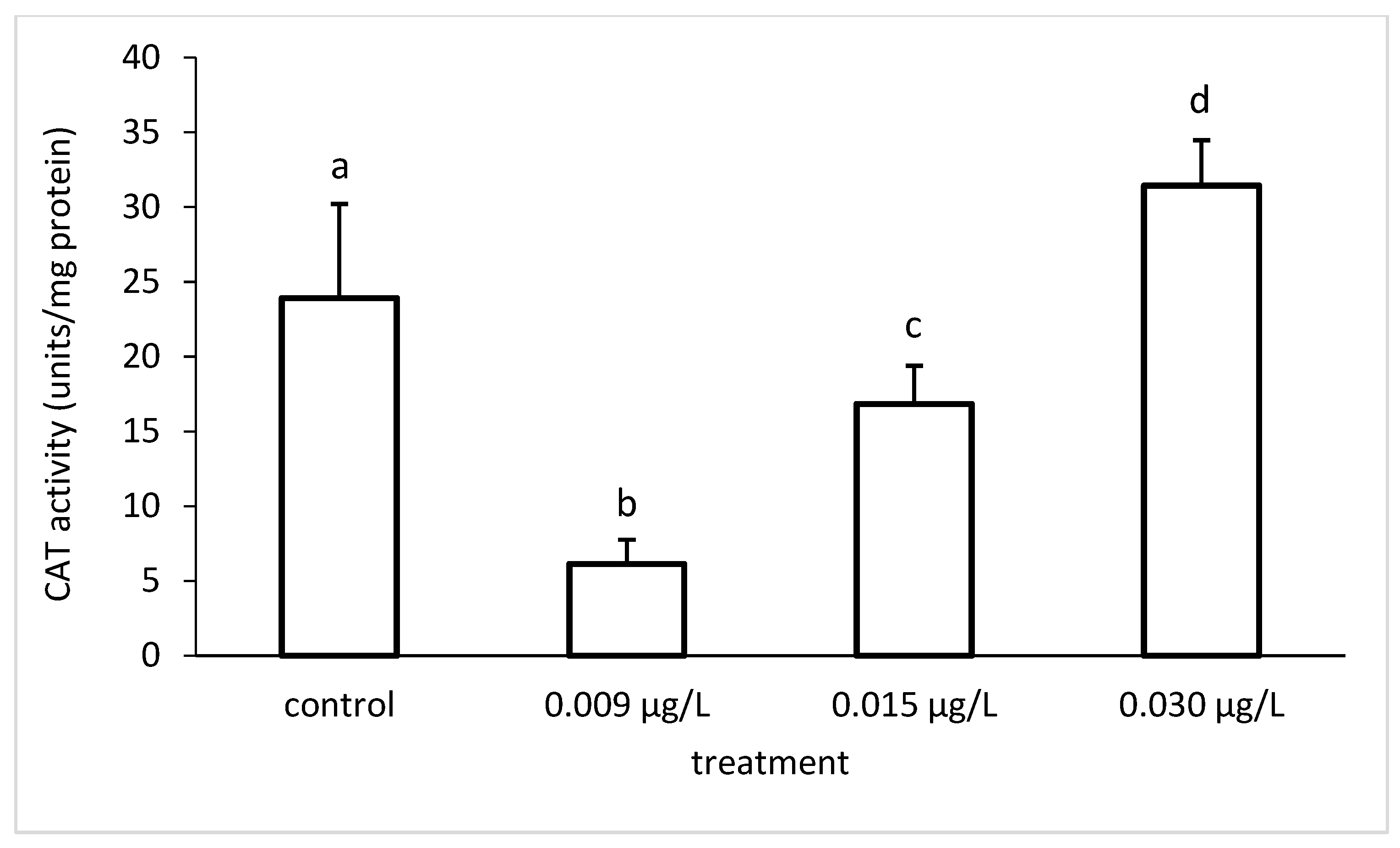
3.3. Liver Enzymatic Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adedeji, O.B.; Okocha, R.O. Overview of Pesticide Toxicity in Fish. Adv. Environ. Biol. 2012, 6, 2344–2351. Available online: http://www.aensiweb.com/old/aeb/2012/2344-2351.pdf (accessed on 1 July 2012).
- Rusyniak, D.; Nañagas, K. Organophosphate Poisoning. Semin. Neurol. 2004, 24, 197–204. [Google Scholar] [CrossRef]
- Sharbidre, A.A.; Metkari, V.; Patode, P. Effect of methyl parathion and chlorpyrifos on certain biomarkers in various tissues of guppy fish, Poecilia reticulata. Pestic. Biochem. Physiol. 2011, 101, 132–141. [Google Scholar] [CrossRef]
- Asselborn, V.; Fernández, C.; Zalocar, Y.; Parodi, E.R. Effects of chlorpyrifos on the growth and ultrastructure of green algae, Ankistrodesmus gracilis. Ecotoxicol. Environ. Saf. 2015, 120, 334–341. [Google Scholar] [CrossRef]
- Kumar, U.; Berliner, J.; Adak, T.; Rath, P.C.; Dey, A.; Pokhare, S.S.; Jambhulkar, N.N.; Panneerselvam, P.; Kumar, A.; Mohapatra, S.D. Non-target effect of continuous application of chlorpyrifos on soil microbes, nematodes and its persistence under sub-humid tropical rice-rice cropping system. Ecotoxicol. Environ. Saf. 2017, 135, 225–235. [Google Scholar] [CrossRef]
- Wood, B.; Stark, J.D. Acute toxicity of drainage ditch water from a Washington State cranberry-growing region to Daphnia pulex in laboratory bioassays. Ecotoxicol. Environ. Saf. 2002, 53, 273–280. [Google Scholar] [CrossRef]
- Marino, D.J.; Ronco, A. Cypermethrin and Chlorpyrifos Concentration Levels in Surface Water Bodies of the Pampa Ondulada, Argentina. Bull. Environ. Contam. Toxicol. 2005, 75, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Li, S.; Wang, Z.; Gao, X.; Xu, S.; Wang, X. Oxidative stress response and histopathological changes due to atrazine and chlorpyrifos exposure in common carp. Pestic. Biochem. Physiol. 2012, 103, 74–80. [Google Scholar] [CrossRef]
- Kida, M.; Ziembowicz, S.; Koszelnik, P. Removal of organochlorine pesticides (OCPs) from aqueous solutions using hydrogen peroxide, ultrasonic waves, and a hybrid process. Sep. Purif. Technol. 2018, 192, 457–464. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.M.; Al-Kahtani, M.A.; Elmenshawy, O. Histopathological biomarkers in gills and liver of Oreochromis niloticus from polluted wetland environments, Saudi Arabia. Chemosphere 2012, 88, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, T.; Shillabeer, N.; Winter, M.; Pickford, D. Acute and chronic effects of carrier solvents in aquatic organisms: A critical review. Aquat. Toxicol. 2006, 76, 69–92. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zha, J.; Xu, Y.; Giesy, J.P.; Wang, Z. Toxicity of pentachlorophenol to native aquatic species in the Yangtze River. Environ. Sci. Pollut. Res. 2011, 19, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Marigoudar, S.R.; Nagarjuna, A.; Karthikeyan, P.; Mohan, D.; Sharma, K. Comparative toxicity of chlorpyrifos: Sublethal effects on enzyme activities and histopathology of Mugil cephalus and Chanos chanos. Chemosphere 2018, 211, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Kottelat, M.; Freyhof, J. Handbook of European freshwater fishes; Kottelat, Cornol, Switzerland and Freyhof: Berlin, Germany, 2007. [Google Scholar]
- Georgieva, E.; Atanasova, P.; Velcheva, I.; Stoyanova, S.; Yancheva, V. Histochemical effects of “Verita WG” on glycogen and lipid storage in common carp (Cyprinus carpio L.) liver. Ecol. Balk. 2013, 5, 91–97. [Google Scholar]
- Xu, P.; Zhang, X.; Wang, X.; Li, J.; Liu, G.; Kuang, Y.-Y.; Xu, J.; Zheng, X.; Ren, L.; Wang, G.; et al. Genome sequence and genetic diversity of the common carp, Cyprinus carpio. Nat. Genet. 2014, 46, 1212–1219. [Google Scholar] [CrossRef]
- Schultz, S.; Koussoroplis, A.-M.; Watzke, J.; Kainz, M.J.; Changizi-Magrhoor, Z. Fish oil-based finishing diets strongly increase long-chain polyunsaturated fatty acid concentrations in farm-raised common carp (Cyprinus carpio L.). Aquac. Res. 2014, 46, 2174–2184. [Google Scholar] [CrossRef]
- Sfakianakis, D.; Renieri, E.; Kentouri, M.; Tsatsakis, A. Effect of heavy metals on fish larvae deformities: A review. Environ. Res. 2015, 137, 246–255. [Google Scholar] [CrossRef]
- Khan, M.N.; Shahzad, K.; Chatta, A.; Sohail, M.; Piria, M.; Treer, T. A review of introduction of common carp Cyprinus carpio in Pakistan: Origin, purpose, impact and management. Croat. J. Fish. 2016, 74, 71–80. [Google Scholar] [CrossRef][Green Version]
- Macklin, R.; Brazier, B.; Harrison, S.; Chapman, D.; Vilizzi, L. A review of the status and range expansion of common carp (Cyprinus carpio L.) in Ireland. Aquat. Invasions 2016, 11, 75–82. [Google Scholar] [CrossRef]
- Vajargah, M.F.; Yalsuyi, A.M.; Hedayati, A.; Faggio, C. Histopathological lesions and toxicity in common carp (Cyprinus carpio L. 1758) induced by copper nanoparticles. Microsc. Res. Tech. 2018, 81, 724–729. [Google Scholar] [CrossRef]
- Poet, T.S.; Wu, H.; Kousba, A.A.; Timchalk, C. In Vitro Rat Hepatic and Intestinal Metabolism of the Organophosphate Pesticides Chlorpyrifos and Diazinon. Toxicol. Sci. 2003, 72, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Verma, R.S.; Srivastava, N. Chlorpyrifos-induced DNA damage in rat liver and brain. Environ. Mol. Mutagen. 2008, 49, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Banaee, M.; Sureda, A.; Mirvaghefi, A.R.; Ahmadi, K. Biochemical and histological changes in the liver tissue of rainbow trout (Oncorhynchus mykiss) exposed to sub-lethal concentrations of diazinon. Fish Physiol. Biochem. 2012, 39, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Guptha, J.S.; Renuka, M.; Suneetha, Y.; Srinivasulu Reddy, M. Evaluation of antioxidant defence system during xenobiotic induced oxidative stress in freshwater fish Oreochromis mossambicus. Int. J. Fish Aquat. Stud. 2016, 4, 379–385. [Google Scholar]
- Ghorpade, N.; Mehta, V.; Khare, M.; Sinkar, P.; Krishnan, S.; Rao, C.V. Toxicity Study of Diethyl Phthalate on Freshwater Fish Cirrhina mrigala. Ecotoxicol. Environ. Saf. 2002, 53, 255–258. [Google Scholar] [CrossRef]
- Gültekin, F.; Delibas, N.; Yasar, S.; Kilinc, I. In vivo changes in antioxidant systems and protective role of melatonin and a combination of vitamin C and vitamin E on oxidative damage in erythrocytes induced by chlorpyrifos-ethyl in rats. Arch. Toxicol. 2001, 75, 88–96. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, H.; Wang, X.; Wu, J.; Xue, Y. Effects of chronic exposure of 2,4-dichlorophenol on the antioxidant system in liver of freshwater fish Carassius auratus. Chemosphere 2004, 55, 167–174. [Google Scholar] [CrossRef]
- Peixoto, F.; Alves-Fernandes, D.; Santos, D.; Fontaínhas-Fernandes, A. Toxicological effects of oxyfluorfen on oxidative stress enzymes in tilapia Oreochromis niloticus. Pestic. Biochem. Physiol. 2006, 85, 91–96. [Google Scholar] [CrossRef]
- Mansour, S.; Mossa, A.-T.H. Lipid peroxidation and oxidative stress in rat erythrocytes induced by chlorpyrifos and the protective effect of zinc. Pestic. Biochem. Physiol. 2009, 93, 34–39. [Google Scholar] [CrossRef]
- Devi, Y.; Mishra, A. Histopathological alterations in gill and liver anatomy of fresh water, air breathing fish Channa punctatus after pesticide Hilban® (Chlorpyrifos) treatment. Adv. BioRes. 2013, 4, 57–62. [Google Scholar]
- Kunjamma, A.K.P.; Philip, B.; Bhanu, S.V.; Jose, J. Histopathological effects on Oreochromis mossambicus (tilapia) exposed to chlorpyrifos. J. Environ. Res. Dev. 2008, 2, 553–559. [Google Scholar]
- Van Der Oost, R.; Beyer, J.; Vermeulen, N.P. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar] [CrossRef]
- Stentiford, G.D.; Longshaw, M.; Lyons, B.; Jones, G.; Green, M.; Feist, S.W. Histopathological biomarkers in estuarine fish species for the assessment of biological effects of contaminants. Mar. Environ. Res. 2003, 55, 137–159. [Google Scholar] [CrossRef]
- Nagaraju, B.; Rathnamma, V.V. Histopathological changes in the gill and liver of freshwater fish Labeo rohita (Hamilton) exposed to Novaluron. Innoriginal. Int. J. Sci. 2014, 1, 16–18. [Google Scholar]
- Ksheerasagar, R.L.; Hiremath, M.B.; Kaliwa, B.B. Impairment of hepatic biochemical contents and enzymes activities during carbosulfan intoxication in albino mice. Int. J. Multidiscip. Res. Dev. 2011, 1, 6–15. [Google Scholar]
- Slaninova, A.; Smutna, M.; Modra, H.; Svobodova, Z. A review: Oxidative stress in fish induced by pesticides. Neuro Endocrinol. Lett. 2009, 30, 2–12. [Google Scholar]
- Bantu, N.; Vakita, V.R.; Karra, S. Effect of Chlorantraniliprole on biochemical and certain biomarkers in various tissues of freshwater fish Labeo rohita (Hamilton). Environ. Ecol. Res. 2013, 1, 205–215. [Google Scholar] [CrossRef]
- Kurutaş, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef]
- Yancheva, V.; Velcheva, I.; Georgieva, E.; Mollov, I.; Stoyanova, S. Chlorpyrifos induced changes on the physiology of common carp (cyprinus carpio Linnaeus, 1785): A laboratory exposure study. Appl. Ecol. Environ. Res. 2019, 17, 5139–5157. [Google Scholar] [CrossRef]
- Kamrin, M.A. Pesticide Profiles: Toxicity, Environmental Impact, and Fate; Lewis Publishers: Boca Raton, FL, USA, 1997. [Google Scholar]
- De Moura, F.R.; Brentegani, K.R.; Gemelli, A.; Sinhorin, A.P.; Sinhorin, V.D.G. Oxidative stress in the hybrid fish jundiara (Leiarius marmoratus × Pseudoplatystoma reticulatum) exposed to Roundup Original®. Chemosphere 2017, 185, 445–451. [Google Scholar] [CrossRef]
- Modesto, K.A.; Martinez, C.B. Roundup® causes oxidative stress in liver and inhibits acetylcholinesterase in muscle and brain of the fish Prochilodus lineatus. Chemosphere 2010, 78, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.G.; Martinez, C.B. Atrazine promotes biochemical changes and DNA damage in a Neotropical fish species. Chemosphere 2012, 89, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.A.A.; Klosterhoff, M.D.C.; Romano, L.A.; Pires, D.M. Histological evaluation of vital organs of the livebearer Jenynsia multidentata (Jenyns, 1842) exposed to glyphosate: A comparative analysis of Roundup® formulations. Chemosphere 2018, 217, 914–924. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Rosseland, B.O.; Massabuau, J.-C.; Grimalt, J.; Hofer, R.; Lackner, R.; Raddum, G.; Rognerud, S.; Vives, I. Fish ecotoxicology: European mountain lake ecosystems regionalisation, diagnostic and socio-economic evaluation (EMERGE). In Fish Sampling Manual for Live Fish; Norwegian Institute for Water Research (NIVA): Oslo, Norway, 2003; pp. 1–7. [Google Scholar]
- Gautier, J.-C. Drug Safety Evaluation: Methods and Protocols, Methods in Molecular Biology; Springer Science Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Bernet, D.; Schmidt, H.; Meier, W.; Burkhardt-Holm, P.; Wahli, T. Histopathology in fish: Proposal for a protocol to assess aquatic pollution. J. Fish Dis. 1999, 22, 25–34. [Google Scholar] [CrossRef]
- Saraiva, A.; Costa, J.; Serrão, J.; Cruz, C.; Eiras, J.C. A histology-based fish health assessment of farmed seabass (Dicentrarchus labrax L.). Aquaculture 2015, 448, 375–381. [Google Scholar] [CrossRef]
- Zimmerli, S.; Bernet, D.; Burkhardt-Holm, P.; Schmidt-Posthaus, H.; Vonlanthen, P.; Wahli, T.; Segner, H. Assessment of fish health status in four Swiss rivers showing a decline of brown trout catches. Aquat. Sci. 2007, 69, 11–25. [Google Scholar] [CrossRef]
- Yancheva, V. Toxicity of two organophosphorous pesticides on bighead carp (Aristichthys nobilis Richardson, 1845) liver. Appl. Ecol. Environ. Res. 2016, 14, 397–410. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Vassault, A. Methods of Enzymatic Analysis; Academic Press: New York, NY, USA, 1983; pp. 118–126. [Google Scholar]
- Reitman, S.; Frankel, S. A Colorimetric Method for the Determination of Serum Glutamic Oxalacetic and Glutamic Pyruvic Transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Burtis, C.A.; Ashwood, E.R. Textbook of clinical chemistry. Eur. J. Clin. Chem. Clin. Biochem. 1992, 30, 162–170. [Google Scholar]
- Wendel, A. Enzymatic Basis of Detoxication, 1st ed.; Academic Press: New York, NY, USA, 1980; p. 333. [Google Scholar]
- Hundet, A.; Prabhat, B. Histopathological alterations in hepatopancreas of a carp fish, C. carpio due to endosulfan toxicity. Cibtech J. Zool. 2014, 3, 7–11. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2020; Food and Agriculture Organization of the United Nations (FAO): Roma, Italy, 2020. [Google Scholar]
- Cuevas, N.; Zorita, I.; Franco, J.; Costa, P.M.; Larreta, J.; Zuazo, N.C. Multi-organ histopathology in gobies for estuarine environmental risk assessment: A case study in the Ibaizabal estuary (SE Bay of Biscay). Estuar. Coast. Shelf Sci. 2016, 179, 145–154. [Google Scholar] [CrossRef]
- Deb, N.; Das, S. Chlorpyrifos toxicity in fish: A Review. Curr. World Environ. 2013, 8, 77–84. [Google Scholar] [CrossRef]
- Bukhari, A.S.; Mohamed, H.S.; Broos, K.; Stalin, A.; Singhal, R.K.; Venubabu, P. Histological variations in liver of freshwater fish Oreochromis mossambicus exposed to 60Co gamma irradiation. J. Environ. Radioact. 2012, 113, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Sevgiler, Y.; Oruç, E.Ö.; Üner, N. Evaluation of etoxazole toxicity in the liver of Oreochromis niloticus. Pestic. Biochem. Physiol. 2004, 78, 1–8. [Google Scholar] [CrossRef]
- Saravanan, M.; Kumar, K.P.; Ramesh, M. Haematological and biochemical responses of freshwater teleost fish Cyprinus carpio (Actinopterygii: Cypriniformes) during acute and chronic sublethal exposure to lindane. Pestic. Biochem. Physiol. 2011, 100, 206–211. [Google Scholar] [CrossRef]
- Velisek, J.; Stara, A.; Kolárová, J.; Svobodová, Z. Biochemical, physiological and morfological responses in common carp (Cyprinus carpio L.) after long-term exposure to terbutryn in real environmental concentration. Pestic. Biochem. Physiol. 2011, 100, 305–313. [Google Scholar] [CrossRef]
- Oruç, E. Özcan; Üner, N. Combined effects of 2,4-D and azinphosmethyl on antioxidant enzymes and lipid peroxidation in liver of Oreochromis niloticus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2000, 127, 291–296. [Google Scholar] [CrossRef]
- Maduenho, L.P.; Martinez, C.B. Acute effects of diflubenzuron on the freshwater fish Prochilodus lineatus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2008, 148, 265–272. [Google Scholar] [CrossRef]
- Tilak, K.S.; Koteswara Rao, D.; Veeraiah, K. Effects of chlorpyrifos on histopathology of the fish Catla catla. J. Ecotox. Environ. Monitor. 2005, 15, 127–140. [Google Scholar]
- Mohamed, F.A.S. Histopathological studies on Tilapia zillii and Solea vulgaris from Lake Qarun, Egypt. World J. Fish Mar. Sci. 2009, 1, 29–39. [Google Scholar]
- Kostić, J.; Kolarević, S.; Kračun-Kolarević, M.; Aborgiba, M.; Gačić, Z.; Paunović, M.; Višnjić-Jeftić, Ž.; Rašković, B.; Poleksić, V.; Lenhardt, M.; et al. The impact of multiple stressors on the biomarkers response in gills and liver of freshwater breams during different seasons. Sci. Total Environ. 2017, 601, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Stoyanova, S.; Yancheva, V.; Iliev, I.; Vasileva, T.; Bivolarski, V.; Velcheva, I.; Georgieva, E. Glyphosate induces morphological and enzymatic changes in common carp (Cyprinus carpio L.) liver. Bulg. J. Agric. Sci. 2015, 21, 409–412. [Google Scholar]
- Stoyanova, S. Biochemical, histological and histochemical changes in Aristichthys nobilis Rich. Liver exposed to thiamethoxam. Period. Biol. 2016, 118, 29–36. [Google Scholar] [CrossRef]
- Payne, J.; Mathieu, A.; Melvin, W.; Fancey, L. Acetylcholinesterase, an old biomarker with a new future? Field trials in association with two urban rivers and a paper mill in Newfoundland. Mar. Pollut. Bull. 1996, 32, 225–231. [Google Scholar] [CrossRef]
- Assis, C.R.D.; Bezerra, R.S.; Carvalho, L.B., Jr. Fish cholinesterases as biomarkers of organophosphorus and carbamate pesticides, pesticides in the modern world. In Pests Control and Pesticides Exposure and Toxicity Assessment; Stoytcheva, M., Ed.; InTech: London, UK, 2011; ISBN 978-953-307-457-3. [Google Scholar]
- Ahmad, I.; Hamid, T.; Fatima, M.; Chand, H.S.; Jain, S.K.; Athar, M.; Raisuddin, S. Induction of hepatic antioxidants in freshwater catfish (Channa punctatus Bloch) is a biomarker of paper mill effluent exposure. Biochim. et Biophys. Acta (BBA) Gen. Subj. 2000, 1523, 37–48. [Google Scholar] [CrossRef]
- Yousafzai, A.M.; Shakoori, A.R. Hepatic response of a fresh water fish against aquatic pollution. Pak. J. Zool. 2011, 43, 209–221. [Google Scholar]
- Akhgari, M.; Abdollahi, M.; Kebryaeezadeh, A.; Hosseini, R.; Sabzevari, O. Biochemical evidence for free radical induced lipid peroxidation as a mechanism for subchronic toxicity of malathion in blood and liver of rats. Hum. Exp. Toxicol. 2003, 22, 205–211. [Google Scholar] [CrossRef]
- Abdollahi, M.; Ranjbar, A.; Shadnia, S.; Nikfar, S.; Rezaie, A. Pesticides and oxidative stress: A review. Med. Sci. Monit. 2004, 10, 141–147. [Google Scholar]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Di Giulio, R.T.; Meyer, J.N. Reactive oxygen species and oxidative stress. In the Toxicology of Fishes; Di Giulio, R.T., Hinton, D.E., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2008; pp. 273–324. [Google Scholar]
- Uchendu, C. The organophosphate, chlorpyrifos, oxidative stress and the role of some antioxidants: A review. Afr. J. Agric. Res. 2012, 7, 2720–2728. [Google Scholar] [CrossRef]
- Narra, M.R.; Rajender, K.; Reddy, R.R.; Murty, U.S.; Begum, G. Insecticides induced stress response and recuperation in fish: Biomarkers in blood and tissues related to oxidative damage. Chemosphere 2017, 168, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Clasen, B.; Loro, V.L.; Murussi, C.R.; Tiecher, T.L.; Moraes, B.; Zanella, R. Bioaccumulation and oxidative stress caused by pesticides in Cyprinus carpio reared in a rice-fish system. Sci. Total Environ. 2018, 626, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.E.; Müller, T.E.; Murussi, C.; Amaral, A.M.D.; Gomes, J.L.; Marins, A.T.; Leitemperger, J.; Rodrigues, C.C.; Fiuza, T.L.; Costa, M.D.; et al. Oxidative effects of the acute exposure to a pesticide mixture of cypermethrin and chlorpyrifos on carp and zebrafish—A comparative study. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 2018, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Yonar, M.E. Chlorpyrifos-induced biochemical changes in Cyprinus carpio: Ameliorative effect of curcumin. Ecotoxicol. Environ. Saf. 2018, 151, 49–54. [Google Scholar] [CrossRef] [PubMed]
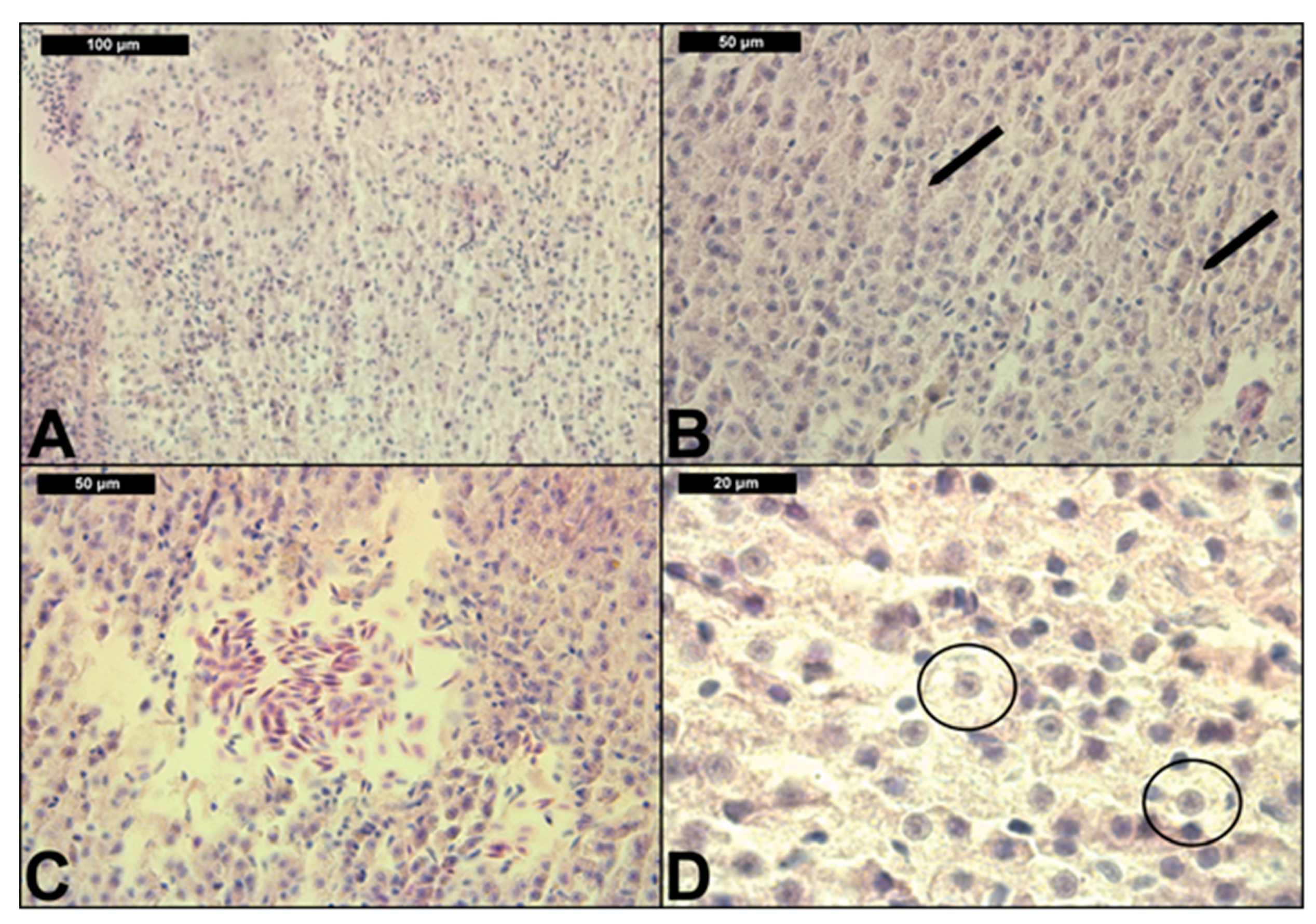


| Test Tank | pH | T (°C) | Conductivity (μS/cm) | Dissolved Oxygen (mg/L) | Dissolved Oxygen Saturation (%) |
|---|---|---|---|---|---|
| Control | 8.3 ± 0.5 | 16.5 ± 1.5 | 370 ±5.5 | 8.5 ± 1.5 | 86.99 ± 12.82 |
| 0.009 μg/L CPF | 8.0 ± 0.5 | 17.3 ± 2.0 | 365 ± 3.5 | 8.3 ± 0.3 | 86.38 ± 3.02 |
| 0.015 μg/L CPF | 7.5 ± 1.5 | 18.5 ± 0.5 | 410 ± 0.5 | 7.0 ± 0.5 | 74.67 ± 5.38 |
| 0.030 μg/L CPF | 8.5 ± 0.5 | 19.0 ± 0.5 | 353.3 ± 0.3 | 9.1 ± 1.0 | 98.06 ± 10.55 |
| Reaction Pattern | Functional Unit of the Tissue | Alteration | Importance Factor | Score Value | Index for Each Group (0.009; 0.015; 0.03 μg/L) | |||
|---|---|---|---|---|---|---|---|---|
| Control | 0.009 μg/L | 0.015 μg/L | 0.03 μg/L | |||||
| Circulatory Disturbances * | Liver | Hyperaemia | WLC1 = 1 | 0 | 0 | 2 | 2 | ILC = 0 ILC = 2 ILC = 2 |
| Intercellular oedema | 0 | 0 | 0 | 0 | ||||
| Regressive Lesions * | Liver | Granular degeneration | WLR1 = 1 | 1 | 2 | 2 | 2 | ILR = 4 ILR = 11 ILR = 19 |
| Deposits (lipids) | WLR3 = 1 | 0 | 0 | 0 | 0 | |||
| Nuclear alterations | WLR4 = 2 | 0 | 0 | 1 | 2 | |||
| Necrosis | WLR5 = 3 | 0 | 0 | 1 | 2 | |||
| Vacuolar degeneration | WLR6 = 2 | 0 | 1 | 2 | 3 | |||
| Interstitial tissue | Architectural and structural alterations | WLR7 = 1 | 0 | 0 | 0 | 1 | ||
| Deposits | WLR8 = 1 | 0 | 0 | 0 | 0 | |||
| Nuclear alterations | WLR9 = 2 | 0 | 0 | 0 | 0 | |||
| Necrosis | WLR10 = 3 | 0 | 0 | 0 | 0 | |||
| Progressive Lesions * | Liver | Hypertrophy | WLP1 = 1 | 0 | 1 | 1 | 2 | ILP = 1 ILP = 1 ILP = 3 |
| Interstitial tissue | Hypertrophy | WLP2 = 1 | 0 | 0 | 0 | 1 | ||
| Inflammation * | Liver | Activation of the reticuloendothelial system in the parenchyma (RES) | WLI1 = 1 | 0 | 0 | 0 | 1 | ILI = 0 ILI = 0 ILI = 3 |
| Infiltration | WLI2 = 2 | 0 | 0 | 0 | 1 | |||
| Index organ | IC = 1 | I0.009 = 5 | I0.015 = 14 | I0.03 = 27 | ||||
| Histopathological Alterations | Prevalence, % | |||||
|---|---|---|---|---|---|---|
| Control | 0.009 μg/L | 0.015 μg/L | 0.030 μg/L | |||
| Circulatory Disturbances | Liver | Hyperaemia | 0 | 0 | 32 | 38 |
| Intercellular oedema | 0 | 0 | 0 | 0 | ||
| Average, % | 0 | 0 | 16 | 19 | ||
| Regressive Lesions | Liver | Granular degeneration | 11 | 36 | 41 | 47 |
| Deposits (lipids) | 0 | 0 | 0 | 0 | ||
| Nuclear alterations | 0 | 0 | 16 | 42 | ||
| Necrosis | 0 | 0 | 21 | 36 | ||
| Vacuolar degeneration | 0 | 17 | 36 | 47 | ||
| Interstitial tissue | Architectural and structural alterations | 0 | 0 | 0 | 14 | |
| Deposits | 0 | 0 | 0 | 0 | ||
| Nuclear alterations | 0 | 0 | 0 | 0 | ||
| Necrosis | 0 | 0 | 0 | 0 | ||
| Average, % | 1.2 | 5.9 | 12.7 | 20.7 | ||
| Progressive Lesions | Liver | Hypertrophy | 0 | 13 | 21 | 32 |
| Interstitial tissue | Hypertrophy | 0 | 0 | 0 | 12 | |
| Average, % | 0 | 6.5 | 10.5 | 22 | ||
| Inflammation | Liver | Activation of the reticuloendothelial system in the parenchyma (RES) | 0 | 0 | 0 | 25 |
| Infiltration | 0 | 0 | 0 | 37 | ||
| Average, % | 0 | 0 | 0 | 31 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoyanova, S.; Georgieva, E.; Velcheva, I.; Iliev, I.; Vasileva, T.; Bivolarski, V.; Tomov, S.; Nyeste, K.; Antal, L.; Yancheva, V. Multi-Biomarker Assessment in Common Carp (Cyprinus carpio, Linnaeus 1758) Liver after Acute Chlorpyrifos Exposure. Water 2020, 12, 1837. https://doi.org/10.3390/w12061837
Stoyanova S, Georgieva E, Velcheva I, Iliev I, Vasileva T, Bivolarski V, Tomov S, Nyeste K, Antal L, Yancheva V. Multi-Biomarker Assessment in Common Carp (Cyprinus carpio, Linnaeus 1758) Liver after Acute Chlorpyrifos Exposure. Water. 2020; 12(6):1837. https://doi.org/10.3390/w12061837
Chicago/Turabian StyleStoyanova, Stela, Elenka Georgieva, Iliana Velcheva, Ilia Iliev, Tonka Vasileva, Veselin Bivolarski, Stoil Tomov, Krisztián Nyeste, László Antal, and Vesela Yancheva. 2020. "Multi-Biomarker Assessment in Common Carp (Cyprinus carpio, Linnaeus 1758) Liver after Acute Chlorpyrifos Exposure" Water 12, no. 6: 1837. https://doi.org/10.3390/w12061837
APA StyleStoyanova, S., Georgieva, E., Velcheva, I., Iliev, I., Vasileva, T., Bivolarski, V., Tomov, S., Nyeste, K., Antal, L., & Yancheva, V. (2020). Multi-Biomarker Assessment in Common Carp (Cyprinus carpio, Linnaeus 1758) Liver after Acute Chlorpyrifos Exposure. Water, 12(6), 1837. https://doi.org/10.3390/w12061837






