Sulfamethoxazole and Trimethoprim Degradation by Fenton and Fenton-Like Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Calibration Curve and Determination of SMX and TMP Concentration
2.3. Experimental Procedure
3. Results and Discussion
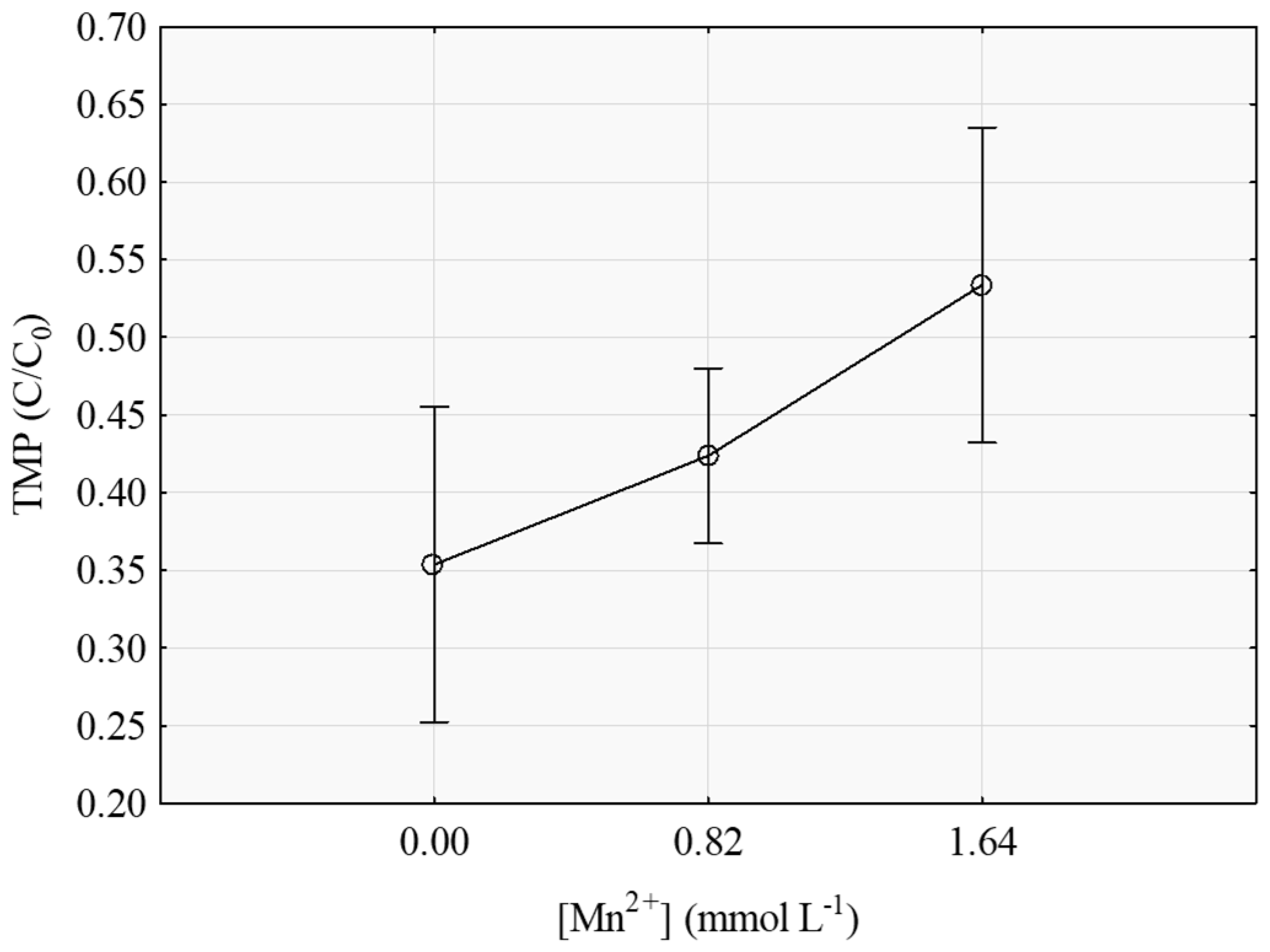
3.1. Degradation of TMP by the Fenton-Like Process
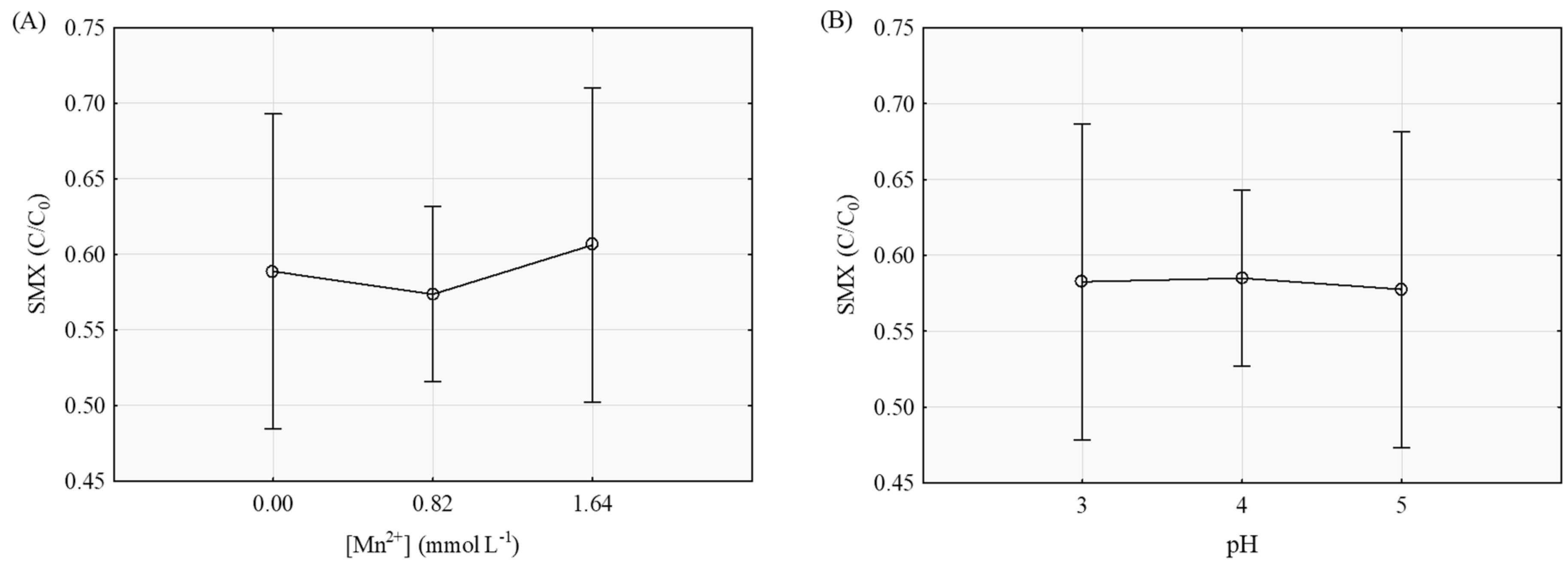
3.2. Degradation of SMX by the Fenton-Like Process
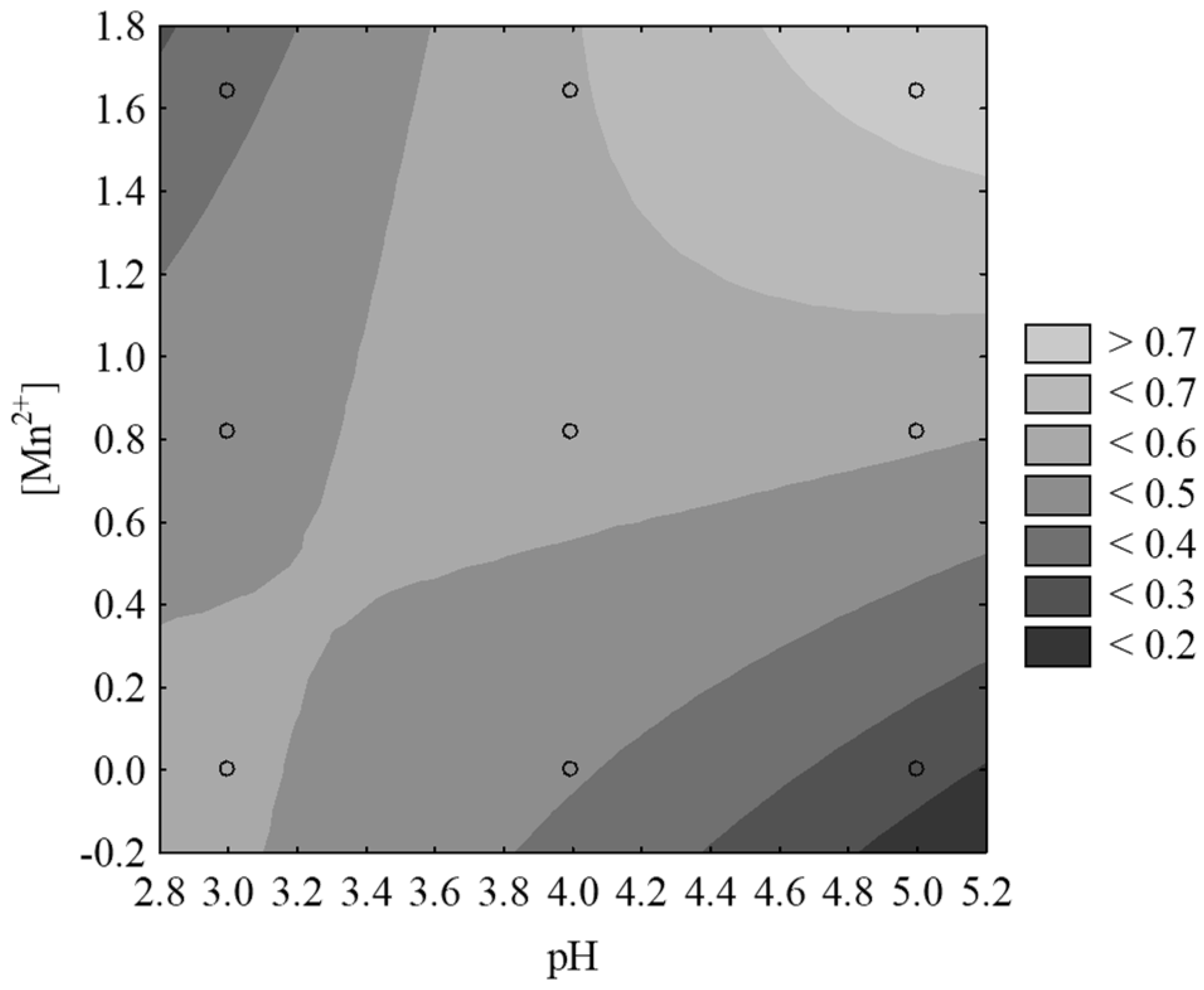
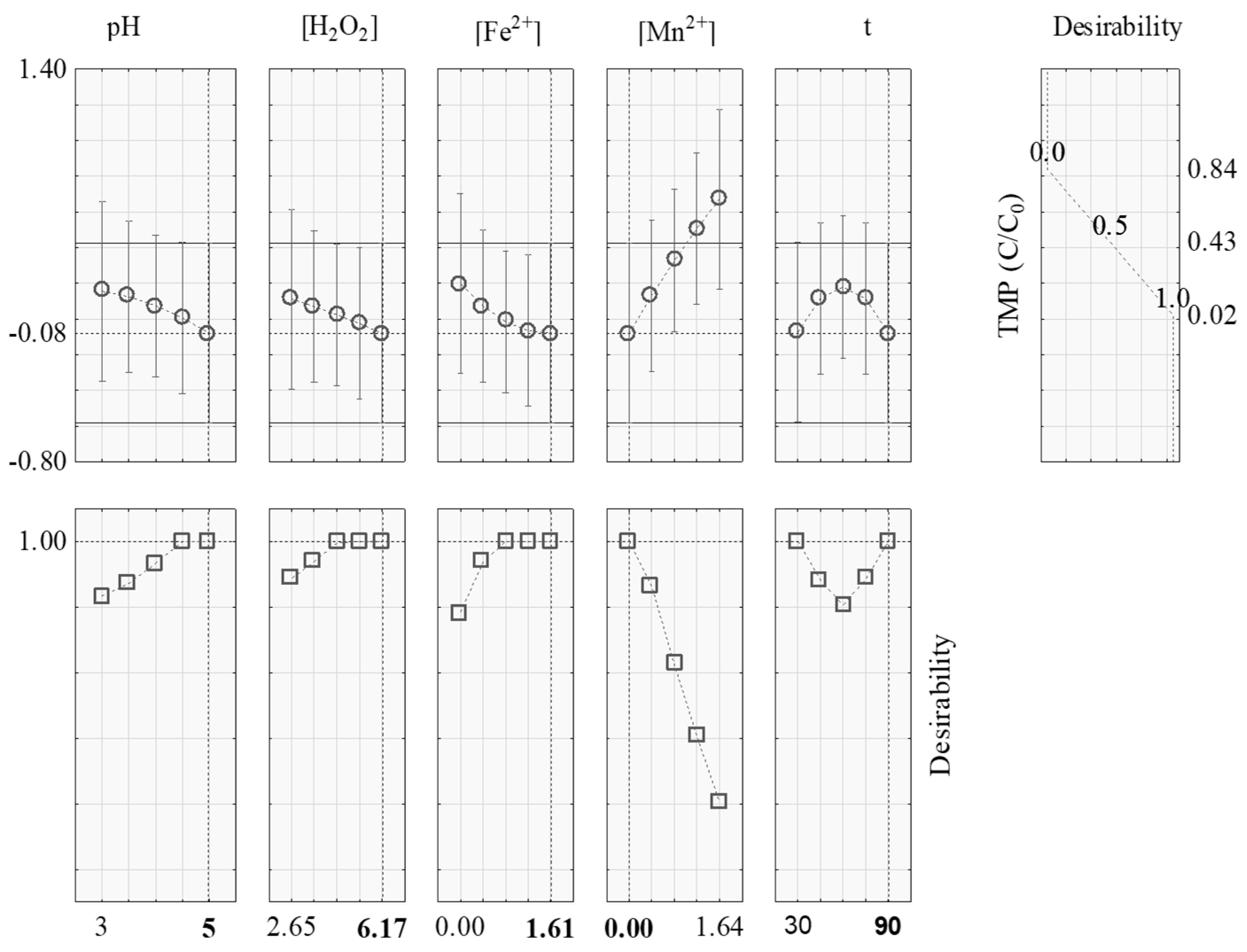
3.3. Optimization of the Fenton-Like Process
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yahyaoui, G.; Hendi, R.; Mahmoud, M. Prevalence of resistant Escherichia coli strain isolated from community acquired urinary infection in university hospital HASSAN II of Fez, Morocco. Saudi J. Pathol. Microbiol. 2017, 2, 1–6. [Google Scholar]
- Zhang, R.; Zhang, R.; Li, J.; Cheng, Z.; Luo, C.; Wang, Y.; Yu, K.; Zhang, G. Occurrence and distribution of antibiotics in multiple environmental media of the East River (Dongjiang) catchment, South China. Environ. Sci. Pollut. Res. 2017, 24, 9690–9701. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Gin, K.Y.-H.; Lin, A.Y.-C.; Reinhard, M. Impacts of emerging organic contaminants on freshwater resources: Review of recent occurrences, sources, fate and effects. Sci. Total. Environ. 2010, 408, 6062–6069. [Google Scholar] [CrossRef] [PubMed]
- Shraim, A.; Diab, A.; Alsuhaimi, A.; Niazy, E.; Metwally, M.; Amad, M.; Sioud, S.; Dawoud, A. Analysis of some pharmaceuticals in municipal wastewater of Almadinah Almunawarah. Arab. J. Chem. 2017, 10 (Suppl. 1), S719–S729. [Google Scholar] [CrossRef]
- Beretta, M.; Britto, V.; Tavares, T.M.; Silva, S.M.T.; Pletsch, A.L. Occurrence of pharmaceutical and personal care products (PPCPs) in marine sediments in the Todos os Santos Bay and the north coast of Salvador, Bahia, Brazil. J. Soils Sediments 2014, 14, 1278–1286. [Google Scholar] [CrossRef]
- Heberer, T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: A review of recent research data. Toxicol. Lett. 2002, 131, 5–17. [Google Scholar] [CrossRef]
- Silva, C.G.A.; Collins, C.H. Aplicações de cromatografia líquida de alta eficiência para o estudo de poluentes orgânicos emergentes [Applications of high performance liquid chromatography for the study of emerging organic pollutants]. Quím. Nova 2011, 34, 665–676. [Google Scholar] [CrossRef]
- Kuster, M.; López de Alda, M.J.; Hernando, M.D.; Petrovic, M.; Martín-Alonso, J.; Barceló, D. Analysis and occurrence of pharmaceuticals, estrogens, progestogens and polar pesticides in sewage treatment plant effluents, river water and drinking water in the Llobregat river basin (Barcelona, Spain). J. Hydrol. 2008, 358, 112–123. [Google Scholar] [CrossRef]
- Li, Q.; Gao, J.; Zhang, Q.; Liang, L.; Tao, H. Distribution and Risk Assessment of Antibiotics in a Typical River in North China Plain. Bull. Environ. Contam. Toxicol. 2017, 98, 478–483. [Google Scholar] [CrossRef]
- Balabanič, D.; Rupnik, M.; Klemenčič, A.K. Negative impact of endocrine-disrupting compounds on human reproductive health. Reprod. Fertil. Dev. 2011, 23, 403–416. [Google Scholar] [CrossRef]
- Macku’ak, T.; Nagyová, K.; Faberová, M.; Grabic, R.; Koba, O.; Gál, M.; Birošová, L. Utilization of Fenton-like reaction for antibiotics and resistant bacteria elimination in different parts of WWTP. Environ. Toxicol. Pharmacol. 2015, 40, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Costa, J.I.; Rivera-Utrilla, J.; Leyva-Ramos, R.; Sánchez-Polo, M.; Velo-Gala, I.; Mota, A.J. Individual and simultaneous degradation of the antibiotics sulfamethoxazole and trimethoprim in aqueous solutions by Fenton, Fenton-like and photo-Fenton processes using solar and UV radiations. J. Photochem. Photobiol. A Chem. 2018, 360, 95–108. [Google Scholar] [CrossRef]
- Borowska, E.; Gomes, J.; Martins, R.; Quinta-Ferreira, R.; Horn, H.; Gmurek, M. Solar Photocatalytic Degradation of Sulfamethoxazole by TiO2 Modified with Noble Metals. Catalysts 2019, 9, 500. [Google Scholar] [CrossRef]
- Domingues, E.; Gomes, J.; Assunção, N.; Gmurek, M.; Quina, M.J.; Quinta-Ferreira, R.M.; Martins, R.C. Iron-based catalysts under solar and visible radiation for contaminants of emerging concern removal. Energy Rep. 2020, 6, 711–716. [Google Scholar] [CrossRef]
- Andreozzi, R.; Marotta, R.; Sanchirico, R. Manganese-catalysed ozonation of glyoxalic acid in aqueous solutions. J. Chem. Technol. Biotechnol. 2000, 75, 59–65. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, C.-H. Oxidative transformation of triclosan and chlorophene by manganese oxides. Environ. Sci. Technol. 2003, 37, 2421–2430. [Google Scholar] [CrossRef]
- Fernandez, J.; Maruthamuthu, P.; Renken, A.; Kiwi, J. Bleaching and photobleaching of Orange II within seconds by the oxone/Co2+ reagent in Fenton-like processes. Appl. Catal. B Environ. 2004, 49, 207–215. [Google Scholar] [CrossRef]
- Zheng, Z.W.; Lei, L.C.; Xu, S.J.; Cen, P.L. Heterogeneous UV/Fenton catalytic degradation of wastewater containing phenol with Fe-Cu-Mn-Y catalyst. J. Zhejiang Univ. Sci. 2004, 5, 206–211. [Google Scholar] [CrossRef]
- Friedrich, L.C.; Mendes, M.A.; Silva, V.O.; Zanta, C.L.P.S.; Machulek, A., Jr.; Quina, F.H. Mechanistic implications of zinc(II) ions on the degradation of phenol by the fenton reaction. J. Braz. Chem. Soc. 2012, 23, 1372–1377. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Gandhimathi, R. Comparative Removal of Rhodamine B from Aqueous Solution by Electro-Fenton and Electro-Fenton-Like Processes. CLEAN Soil Air Water 2014, 42, 779–784. [Google Scholar] [CrossRef]
- Haber, F.; Weiss, J. Über die Katalyse des Hydroperoxydes. Naturwissenschaften 1932, 20, 948–950. [Google Scholar] [CrossRef]
- Walling, C.; Goosen, A. Mechanism of the ferric ion catalyzed decomposition of hydrogen peroxide. Effect of organic substrates. J. Am. Chem. Soc. 1973, 95, 2987–2991. [Google Scholar] [CrossRef]
- Fenton, H.J.H. LXXIII.—Oxidation of tartaric acid in presence of iron. J. Chem. Soc. Trans. 1894, 65, 899–910. [Google Scholar] [CrossRef]
- Hermosilla, D.; Cortijo, M.; Huang, C.P. Optimizing the treatment of landfill leachate by conventional Fenton and photo-Fenton processes. Sci. Total Environ. 2009, 407, 3473–3481. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, S.; Ye, X.; Zhao, R.; Chen, D. Oxidation and coagulation removal of humic acid using Fenton process. Colloids Surf. A Physicochem. Eng. Asp. 2011, 379, 151–156. [Google Scholar] [CrossRef]
- Jiang, C.; Gao, Z.; Qu, H.; Li, J.; Wang, X.; Li, P.; Liu, H. A new insight into Fenton and Fenton-like processes for water treatment: Part II. Influence of organic compounds on Fe(III)/Fe(II) interconversion and the course of reactions. J. Hazard. Mater. 2013, 250–251, 76–81. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, X.; Fang, Y.; Wang, Q.; Lau, K. Degradation of 2, 4-D acid using Mn2+ as catalyst under UV irradiation. Wuhan Univ. J. Nat. Sci. 2009, 14, 262–266. [Google Scholar] [CrossRef]
- Davies, G.; Kirschenbaum, L.J.; Kustin, K. Kinetics and stoichiometry of the reaction between manganese(III) and hydrogen peroxide in acid perchlorate solution. Inorg. Chem. 1968, 7, 146–154. [Google Scholar] [CrossRef]
- Tekindal, M.; Bayrak, H.; Özkaya, B.; Yavuz, Y. Box-Behnken experimental design in factorial experiments: The importance of bread for nutrition and health. Turkish J. Field Crops 2012, 17, 115–123. [Google Scholar]
- Maruyama, S.A.; Palombini, S.V.; Claus, T.; Carbonera, F.; Montanher, P.F.; Souza, N.E.d.; Visentainer, J.V.; Gomes, S.T.M.; Matsushita, M. Application of Box-Behnken design to the study of fatty acids and antioxidant activity from enriched white bread. J. Braz. Chem. Soc. 2013, 24, 1520–1529. [Google Scholar] [CrossRef]
- Honary, S.; Zolfaghari, A.; Ghasemitabar, M. Preparation of gold nanoparticles for biomedical applications using chemometric technique. Trop. J. Pharm. Res. 2013, 12, 295–298. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; Silva, E.G.P.; Portugal, L.A.; Reis, P.S.; Souza, A.S.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef]
- Amorim, K.P.; Romualdo, L.L.; Andrade, L.S. Performance and kinetic-mechanistic aspects in the electrochemical degradation of sulfadiazine on boron-doped diamond electrode. J. Braz. Chem. Soc. 2014, 25, 1484–1492. [Google Scholar] [CrossRef]
- NIST/SEMATECH. e-Handbook of Statistical Methods. Available online: http://www.itl.nist.gov/div898/handbook/ (accessed on 15 March 2017).
- Box, G.E.P.; Behnken, D.W. Some new three level designs for the study of quantitative variables. Technometrics 1960, 2, 455–475. [Google Scholar] [CrossRef]
- Sarmento, A.P.; Borges, A.C.; Matos, A.T.; Romualdo, L.L. Phenol degradation by Fenton-like process. Environ. Sci. Pollut. Res. 2016, 23, 18429–18438. [Google Scholar] [CrossRef]
- Sarmento, A.P.; Borges, A.C.; Matos, A.T.; Romualdo, L.L. Humic acid degradation by fenton-like process using Fe2+ and Mn4+. Water Pract. Technol. 2018, 13, 388–399. [Google Scholar] [CrossRef]
- Khalid, A.; Khan, A.S.; Nazli, Z.-I.-H.; Mahmood, T.; Siddique, M.T.; Mahmood, S.; Arshad, M. Post-treatment of aerobically pretreated poultry litter leachate using fenton and photo-fenton processes. Int. J. Agric. Biol. 2011, 13, 439–443. [Google Scholar]
- Mortazavi, S.; Sabzali, A.; Rezaee, A. Sequence-fenton reaction for decreasing phenol formation during benzene chemical conversion in aqueous solutions. Iran. J. Environ. Health Sci. Eng. 2005, 2, 62–71. [Google Scholar]
- Kang, S.-F.; Liao, C.-H.; Chen, M.-C. Pre-oxidation and coagulation of textile wastewater by the Fenton process. Chemosphere 2002, 46, 923–928. [Google Scholar] [CrossRef]
- Oliveira, L.C.A.; Gonçalves, M.; Oliveira, D.Q.L.; Guarieiro, A.L.N.; Pereira, M.C. Síntese e propriedades catalíticas em reações de oxidação de goethitas contendo nióbio [Synthesis and catalytic properties on oxidation reaction of goethite containing niobium]. Quím. Nova 2007, 30, 925–929. [Google Scholar] [CrossRef][Green Version]
- Liu, W.; Andrews, S.A.; Stefan, M.I.; Bolton, J.R. Optimal methods for quenching H2O2 residuals prior to UFC testing. Water Res. 2003, 37, 3697–3703. [Google Scholar] [CrossRef]
- Hasan, D.U.B.; Abdul Aziz, A.; Daud, W. Application of response surface methodology in process parameters optimization for phenol mineralization using Fenton’s peroxidation. Afr. J. Biotechnol. 2011, 10, 10218–10231. [Google Scholar]
- Marinho, B.A. Estudo da Potencialidade da Fotocatálise Heterogênea e dos Processos Fenton Para Degradação de Micropoluentes em Águas Residuárias (Esgoto Tratado); Mestrado em Química Analítica, Universidade Federal do Paraná: Curitiba, Brazil, 2012. [Google Scholar]
- Wu, Y.; Zhou, S.; Qin, F.; Peng, H.; Lai, Y.; Lin, Y. Removal of humic substances from landfill leachate by Fenton oxidation and coagulation. Process Saf. Environ. Protect. 2010, 88, 276–284. [Google Scholar] [CrossRef]
- Balci, B.; Oturan, M.A.; Oturan, N.; Sirés, I. Decontamination of Aqueous Glyphosate, (Aminomethyl)phosphonic Acid, and Glufosinate Solutions by Electro-Fenton-like Process with Mn2+ as the Catalyst. J. Agric. Food Chem. 2009, 57, 4888–4894. [Google Scholar] [CrossRef] [PubMed]
- Ben, W.; Qiang, Z.; Pan, X.; Chen, M. Removal of veterinary antibiotics from sequencing batch reactor (SBR) pretreated swine wastewater by Fenton’s reagent. Water Res. 2009, 43, 4392–4402. [Google Scholar] [CrossRef]
| Variables | Symbol | Code | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Initial pH | X1 | 3 | 4 | 5 |
| [H2O2] (mmol L−1) | X2 | 2.65 | 4.41 | 6.17 |
| [Fe2+] (mmol L−1) | X3 | 0.00 | 0.81 | 1.61 |
| [Mn2+] (mmol L−1) | X4 | 0.00 | 0.82 | 1.64 |
| t—reaction time (minutes) | X5 | 30 | 60 | 90 |
| Run | pH | [H2O2] | [Fe2+] | [Mn2+] | t | TMP | SMX | ||
|---|---|---|---|---|---|---|---|---|---|
| Efficiency | C/C0 | Efficiency | C/C0 | ||||||
| (mmol L−1) | (min) | (%) | (%) | ||||||
| 1 | 3 | 2.65 | 0.81 | 0.82 | 60 | 74.02 | 0.26 | 47.28 | 0.53 |
| 2 | 5 | 2.65 | 0.81 | 0.82 | 60 | 36.38 | 0.64 | 33.36 | 0.67 |
| 3 | 3 | 6.17 | 0.81 | 0.82 | 60 | 66.76 | 0.33 | 38.28 | 0.62 |
| 4 | 5 | 6.17 | 0.81 | 0.82 | 60 | 52.79 | 0.47 | 23.64 | 0.76 |
| 5 | 4 | 4.41 | 0.00 | 0.00 | 60 | 39.38 | 0.61 | 31.70 | 0.68 |
| 6 | 4 | 4.41 | 1.61 | 0.0 | 60 | 55.49 | 0.45 | 34.82 | 0.65 |
| 7 | 4 | 4.41 | 0.00 | 1.64 | 60 | 30.17 | 0.70 | 19.55 | 0.80 |
| 8 | 4 | 4.41 | 1.61 | 1.64 | 60 | 62.40 | 0.38 | 37.78 | 0.62 |
| 9 | 4 | 2.65 | 0.81 | 0.82 | 30 | 85.57 | 0.14 | 89.57 | 0.10 |
| 10 | 4 | 6.17 | 0.81 | 0.82 | 30 | 84.00 | 0.16 | 89.85 | 0.10 |
| 11 | 4 | 2.65 | 0.81 | 0.82 | 90 | 62.13 | 0.38 | 29.20 | 0.71 |
| 12 | 4 | 6.17 | 0.81 | 0.82 | 90 | 59.04 | 0.41 | 24.65 | 0.75 |
| 13 | 3 | 4.41 | 0.00 | 0.82 | 60 | 23.16 | 0.77 | 17.50 | 0.83 |
| 14 | 5 | 4.41 | 0.00 | 0.82 | 60 | 23.30 | 0.77 | 41.37 | 0.59 |
| 15 | 3 | 4.41 | 1.61 | 0.82 | 60 | 56.28 | 0.44 | 40.91 | 0.59 |
| 16 | 5 | 4.41 | 1.61 | 0.82 | 60 | 42.62 | 0.57 | 17.40 | 0.83 |
| 17 | 4 | 4.41 | 0.81 | 0.00 | 30 | 94.38 | 0.06 | 74.37 | 0.26 |
| 18 | 4 | 4.41 | 0.81 | 1.64 | 30 | 89.49 | 0.11 | 88.39 | 0.12 |
| 19 | 4 | 4.41 | 0.81 | 0.00 | 90 | 81.80 | 0.18 | 31.12 | 0.69 |
| 20 | 4 | 4.41 | 0.81 | 1.64 | 90 | 22.49 | 0.78 | 38.08 | 0.62 |
| 21 | 4 | 2.65 | 0.00 | 0.82 | 60 | 39.79 | 0.60 | 10.24 | 0.90 |
| 22 | 4 | 6.17 | 0.00 | 0.82 | 60 | 25.51 | 0.74 | 13.24 | 0.87 |
| 23 | 4 | 2.65 | 1.61 | 0.82 | 60 | 44.20 | 0.56 | 25.95 | 0.74 |
| 24 | 4 | 6.17 | 1.61 | 0.82 | 60 | 37.06 | 0.63 | 20.31 | 0.80 |
| 25 | 3 | 4.41 | 0.81 | 0.00 | 60 | 44.86 | 0.55 | 15.27 | 0.85 |
| 26 | 5 | 4.41 | 0.81 | 0.00 | 60 | 97.97 | 0.02 | 98.46 | 0.02 |
| 27 | 3 | 4.41 | 0.81 | 1.64 | 60 | 45.94 | 0.54 | 42.80 | 0.57 |
| 28 | 5 | 4.41 | 0.81 | 1.64 | 60 | 34.92 | 0.65 | 21.28 | 0.79 |
| 29 | 4 | 4.41 | 0.00 | 0.82 | 30 | 91.35 | 0.09 | 87.93 | 0.12 |
| 30 | 4 | 4.41 | 1.61 | 0.82 | 30 | 92.20 | 0.08 | 90.16 | 0.10 |
| 31 | 4 | 4.41 | 0.00 | 0.82 | 90 | 31.04 | 0.69 | 23.65 | 0.76 |
| 32 | 4 | 4.41 | 1.61 | 0.82 | 90 | 70.37 | 0.30 | 41.94 | 0.58 |
| 33 | 3 | 4.41 | 0.81 | 0.82 | 30 | 92.39 | 0.08 | 90.69 | 0.09 |
| 34 | 5 | 4.41 | 0.81 | 0.82 | 30 | 94.94 | 0.05 | 89.22 | 0.11 |
| 35 | 3 | 4.41 | 0.81 | 0.82 | 90 | 62.81 | 0.37 | 41.90 | 0.58 |
| 36 | 5 | 4.41 | 0.81 | 0.82 | 90 | 46.52 | 0.53 | 15.23 | 0.85 |
| 37 | 4 | 2.65 | 0.81 | 0.00 | 60 | 47.75 | 0.52 | 10.12 | 0.90 |
| 38 | 4 | 6.17 | 0.81 | 0.00 | 60 | 55.89 | 0.44 | 33.80 | 0.66 |
| 39 | 4 | 2.65 | 0.81 | 1.64 | 60 | 45.95 | 0.54 | 41.05 | 0.59 |
| 40 | 4 | 6.17 | 0.81 | 1.64 | 60 | 42.56 | 0.57 | 25.72 | 0.74 |
| 41 | 4 | 4.41 | 0.81 | 0.82 | 60 | 48.25 | 0.52 | 22.45 | 0.78 |
| 42 | 4 | 4.41 | 0.81 | 0.82 | 60 | 48.58 | 0.51 | 16.46 | 0.84 |
| 43 | 4 | 4.41 | 0.81 | 0.82 | 60 | 16.49 | 0.84 | 24.00 | 0.76 |
| 44 | 4 | 4.41 | 0.81 | 0.82 | 60 | 52.36 | 0.48 | 25.76 | 0.74 |
| 45 | 4 | 4.41 | 0.81 | 0.82 | 60 | 51.45 | 0.49 | 25.04 | 0.75 |
| 46 | 4 | 4.41 | 0.81 | 0.82 | 60 | 64.64 | 0.35 | 30.30 | 0.70 |
| Variation Source | Degrees of Freedom | Square Sum | Average Square | F Calculated |
|---|---|---|---|---|
| Regression | 20 | 1.8364 | 0.0918 | 5.1307 * |
| Residue | 25 | 0.4474 | 0.0179 | |
| Lack of fit | 20 | 0.3143 | 0.0157 | 0.5905 n.s. |
| Pure error | 5 | 0.1331 | 0.0266 | |
| Total | 45 | 2.2838 |
| Parameter | Coefficient | Standard Error | t (25) | p-Value |
|---|---|---|---|---|
| Intercept | −0.910762 | 1.397793 | −0.65157 | 0.520623 |
| pH | 0.178586 | 0.432823 | 0.41261 | 0.683411 |
| pH vs. pH | −0.036667 | 0.045284 | −0.80971 | 0.425750 |
| [H2O2] | 0.153310 | 0.220854 | 0.69417 | 0.493976 |
| [H2O2] vs. [H2O2] | −0.002421 | 0.014619 | −0.16562 | 0.869787 |
| [Fe] | −0.126336 | 0.450193 | −0.28063 | 0.781306 |
| [Fe] vs. [Fe] | 0.114806 | 0.069883 | 1.64282 | 0.112939 |
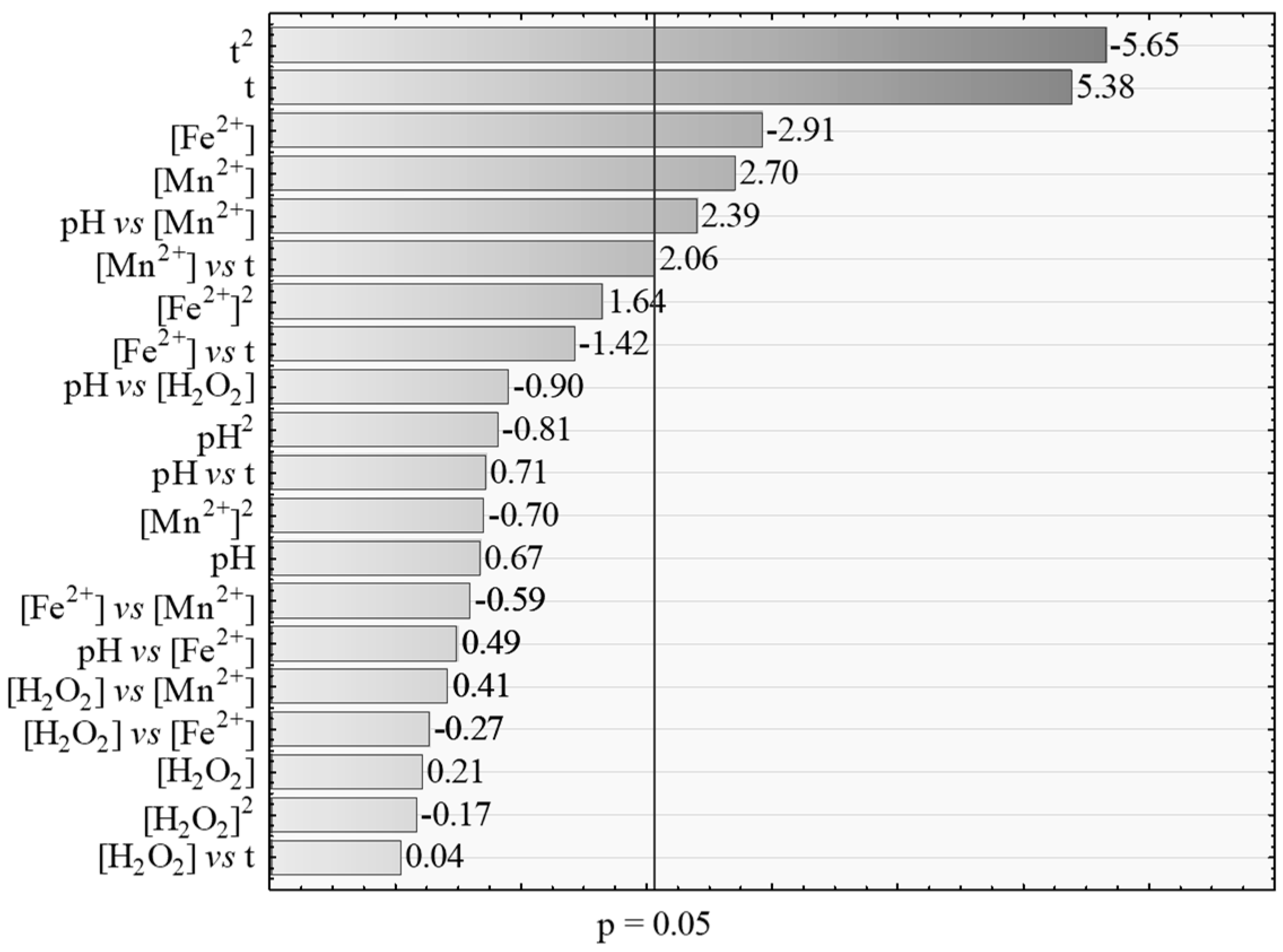
| [Mn] * | −0.964534 | 0.442079 | −2.18181 | 0.038734 |
| [Mn] vs. [Mn] | −0.047095 | 0.067347 | −0.69929 | 0.490826 |
| t * | 0.032151 | 0.012587 | 2.55429 | 0.017114 |
| t vs. t * | −0.000284 | 0.000050 | −5.64953 | 0.000007 |
| pH vs. [H2O2] | −0.034091 | 0.038005 | −0.89701 | 0.378271 |
| pH vs. [Fe] | 0.040294 | 0.083091 | 0.48495 | 0.631938 |
| pH vs. [Mn] * | 0.195122 | 0.081572 | 2.39203 | 0.024595 |
| pH vs. t | 0.001583 | 0.002230 | 0.71013 | 0.484193 |
| [H2O2] vs. [Fe] | −0.012551 | 0.047211 | −0.26586 | 0.792527 |
| [H2O2] vs. [Mn] | 0.019055 | 0.046348 | 0.41113 | 0.684480 |
| [H2O2] vs. t | 0.000047 | 0.001267 | 0.03738 | 0.970483 |
| [Fe] vs. [Mn] | −0.059795 | 0.101330 | −0.59011 | 0.560416 |
| [Fe] vs. t | −0.003940 | 0.002770 | −1.42263 | 0.167204 |
| [Mn] vs. t | 0.005589 | 0.002719 | 2.05565 | 0.050402 |
| Variation Source | Degrees of Freedom | Square Sum | Average Square | F calculated |
|---|---|---|---|---|
| Regression | 20 | 2.7544 | 0.1377 | 10.2963 * |
| Residue | 25 | 0.3344 | 0.0134 | |
| Lack of fit | 20 | 0.3235 | 0.0162 | 7.4314 * |
| Pure error | 5 | 0.0109 | 0.0022 | |
| Total | 45 | 3.0888 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarmento, A.P.; Borges, A.C.; Matos, A.T.d.; Romualdo, L.L. Sulfamethoxazole and Trimethoprim Degradation by Fenton and Fenton-Like Processes. Water 2020, 12, 1655. https://doi.org/10.3390/w12061655
Sarmento AP, Borges AC, Matos ATd, Romualdo LL. Sulfamethoxazole and Trimethoprim Degradation by Fenton and Fenton-Like Processes. Water. 2020; 12(6):1655. https://doi.org/10.3390/w12061655
Chicago/Turabian StyleSarmento, Antover Panazzolo, Alisson Carraro Borges, Antonio Teixeira de Matos, and Lincoln Lucílio Romualdo. 2020. "Sulfamethoxazole and Trimethoprim Degradation by Fenton and Fenton-Like Processes" Water 12, no. 6: 1655. https://doi.org/10.3390/w12061655
APA StyleSarmento, A. P., Borges, A. C., Matos, A. T. d., & Romualdo, L. L. (2020). Sulfamethoxazole and Trimethoprim Degradation by Fenton and Fenton-Like Processes. Water, 12(6), 1655. https://doi.org/10.3390/w12061655





