Elemental Contaminants in Surface Sediments from Jiulong River Estuary, China: Pollution Level and Ecotoxicological Risk Assessment
Abstract
1. Introduction
2. Materials and Methods
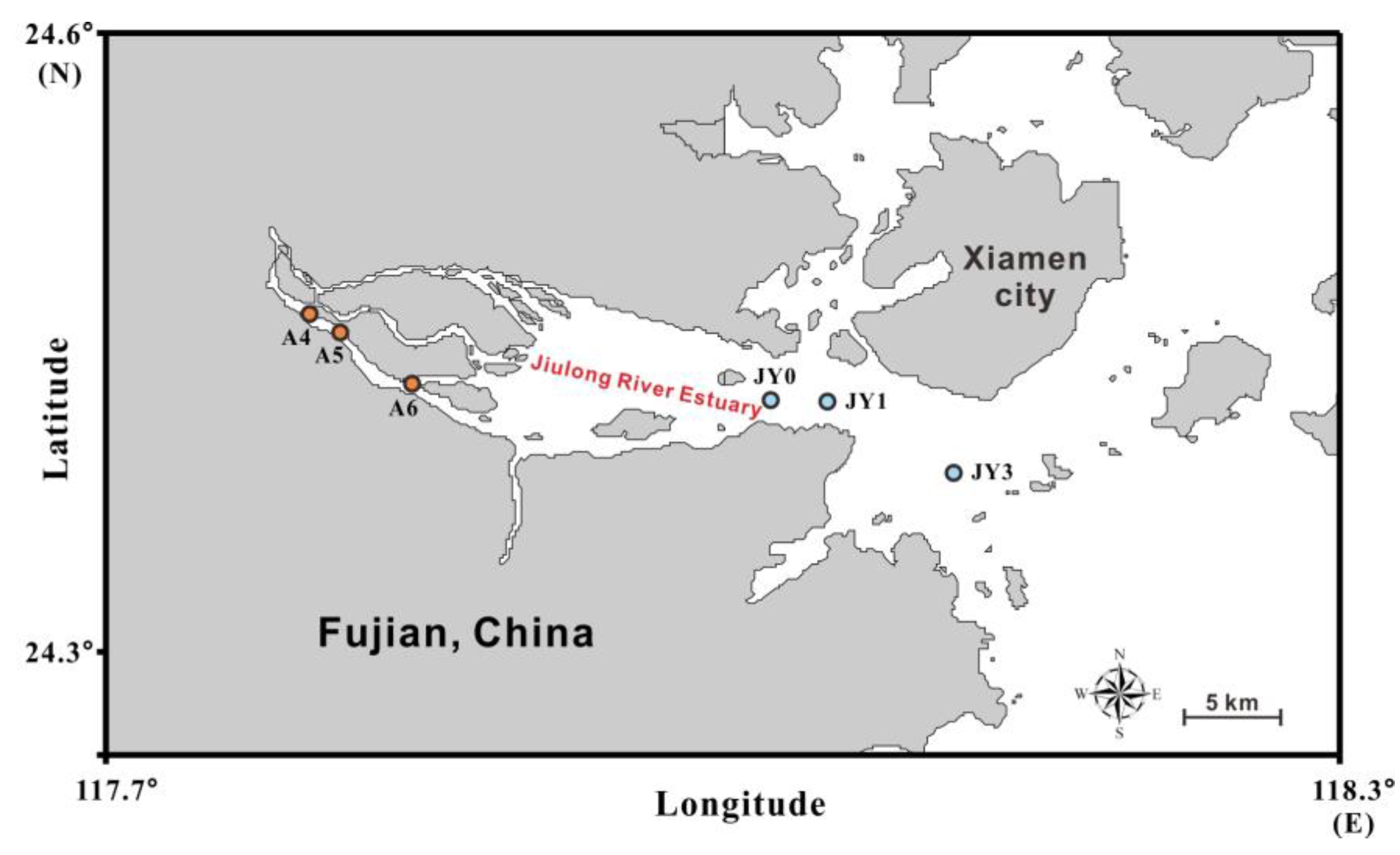
2.1. Description of the Study Area
2.2. Sample Collection and Physicochemical Characterization
2.3. Reagent and Materials
2.4. Extraction and Analysis of Metals
2.5. Quality Assurance and Quality Control (QA/QC)
2.6. Characteristic Parameters of Rare Earth Elements (REEs)
2.7. Pollution and Ecological Risk Assessment
2.8. Statistical Analysis
3. Results and Discussions
3.1. Physicochemical Characteristics of Sediments
3.2. Status and Characteristics of REEs
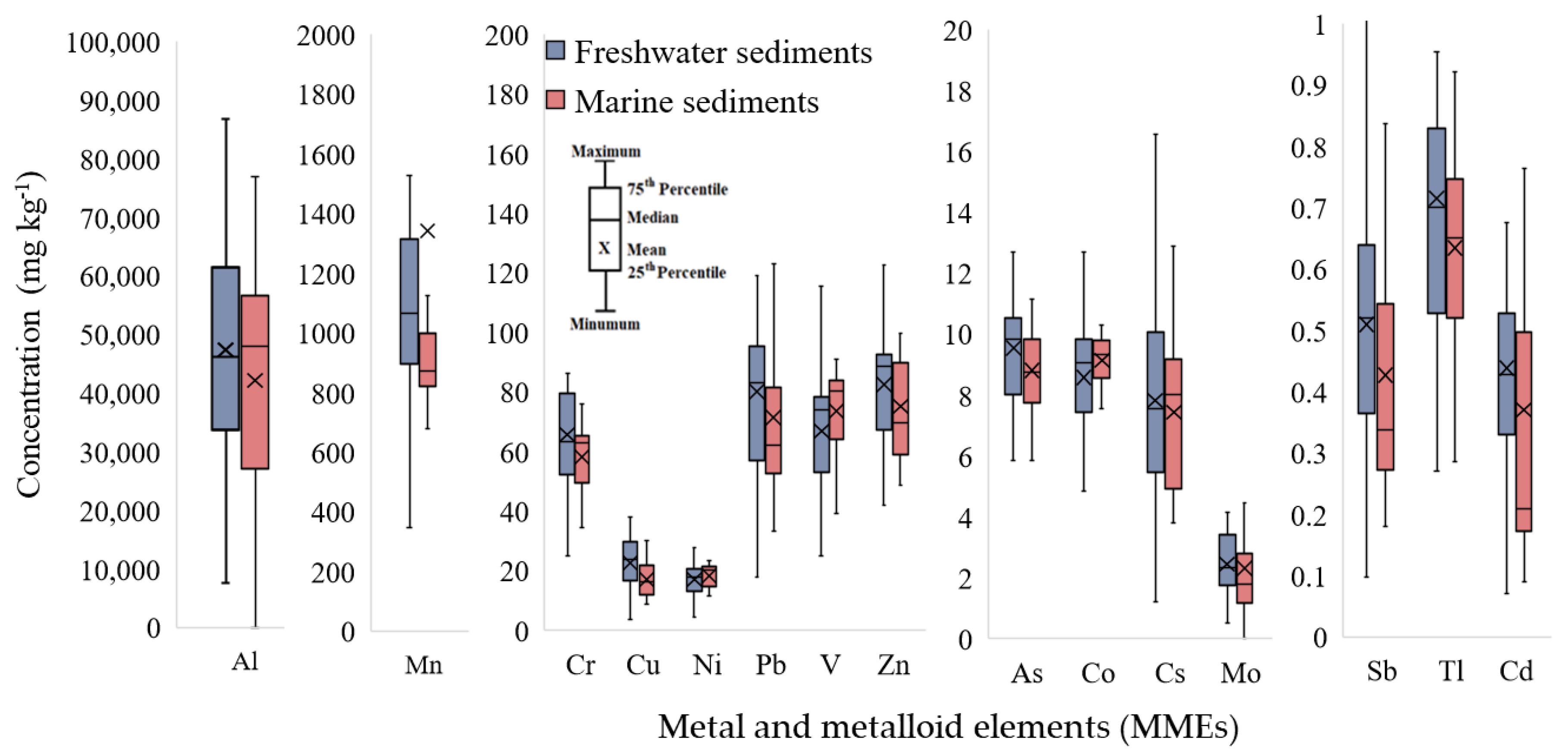
3.3. Status and Characteristics of MMEs
3.4. Temporal Analysis of REEs and MMEs
3.5. Spatial Structure Analysis of REEs and MMEs
3.6. Geoaccumulation, Enrichment and Ecological Risk Assessment of REEs and MMEs
3.6.1. Geoaccumulation and Enrichment
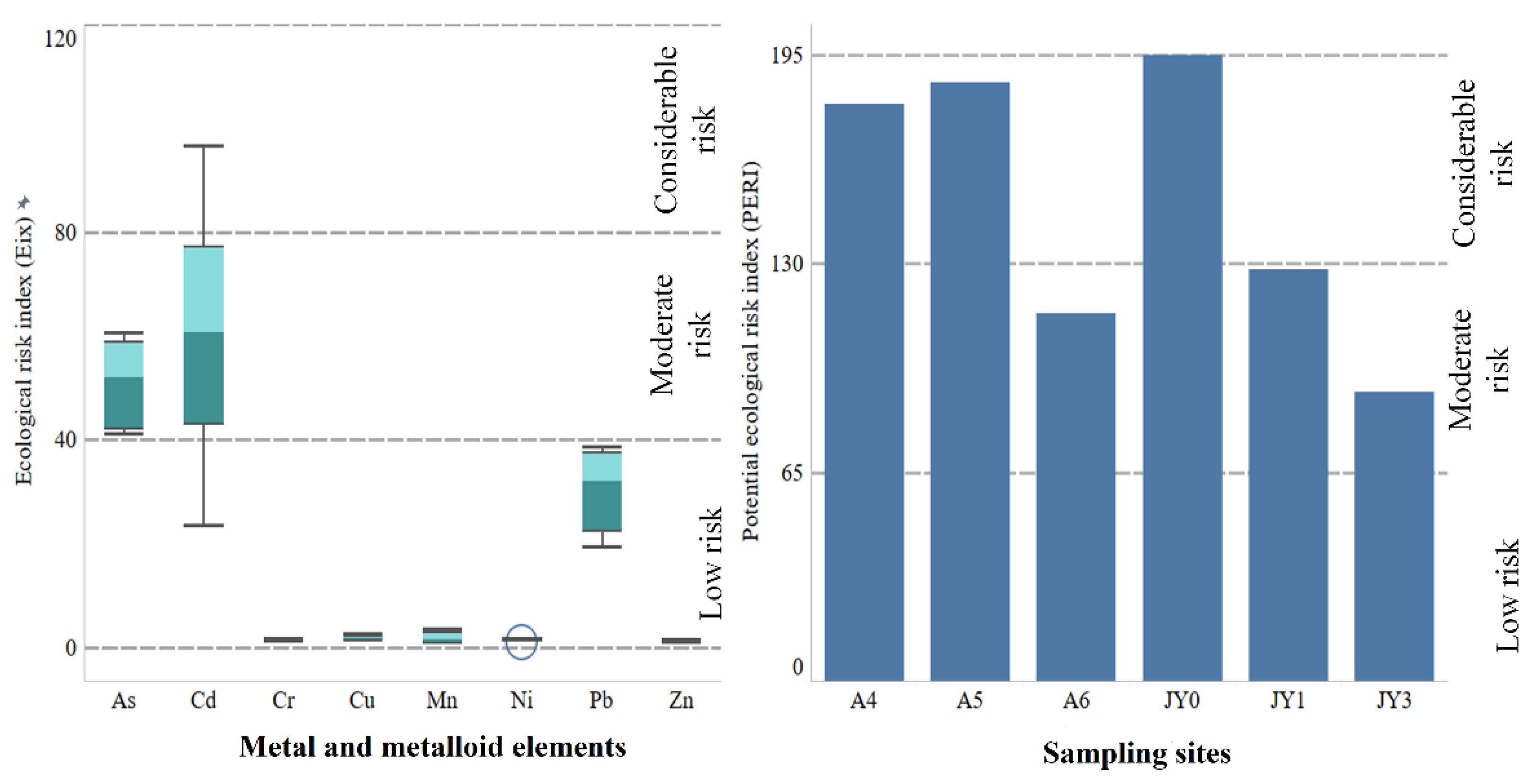
3.6.2. Ecotoxicological Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chitrarasu, P.; Ali, A.J.; Babuthangadurai, T.; Manickam, N. Studies on the heavy metal analysis of sediment at Ennore Estuary in Southeast coast of India. Curr. Bot. 2013, 7, 1–7. [Google Scholar]
- Wang, J.; Liu, R.; Zhang, P.; Yu, W.; Shen, Z.; Feng, C. Spatial variation, environmental assessment and source identification of heavy metals in sediments of the Yangtze River Estuary. Mar. Pollut. Bull. 2014, 87, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Naifar, I.; Pereira, F.; Zmemla, R.; Bouaziz, M.; Elleuch, B.; Garcia, D. Spatial distribution and contamination assessment of heavy metals in marine sediments of the southern coast of Sfax, Gabes Gulf, Tunisia. Mar. Pollut. Bull. 2018, 131, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Song, J.; Duan, L.; Yuan, H.; Li, X.; Li, N.; Qu, B.; Wang, Q.; Xing, J. Source identification and risk assessment based on fractionation of heavy metals in surface sediments of Jiaozhou Bay, China. Mar. Pollut. Bull. 2018, 128, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Kulaksiz, S.; Bau, M. Rare earth elements in the Rhine River, Germany: First case of anthropogenic lanthanum as a dissolved microcontaminant in the hydrosphere. Environ. Int. 2011, 37, 973–979. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, X. Rare earth elements in surface sediments of a marine coast under heavy anthropogenic influence: The Bohai Bay, China. Estuar. Coast. Shelf Sci. 2015, 164, 86–93. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, X.; Arthur Chen, C.T. Rare earth elements in intertidal sediments of Bohai Bay, China: Concentration, fractionation and the influence of sediment texture. Ecotoxicol. Environ. Saf. 2014, 105, 72–79. [Google Scholar] [CrossRef]
- Feng, H.; Jiang, H.; Gao, W.; Weinstein, M.P.; Zhang, Q.; Zhang, W.; Yu, L.; Yuan, D.; Tao, J. Metal contamination in sediments of the western Bohai Bay and adjacent estuaries, China. J. Environ. Manag. 2011, 92, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Q.; Li, G.; Liu, Z. Distribution, source and pollution level of heavy metals in river sediments from South China. Catena 2018, 170, 386–396. [Google Scholar] [CrossRef]
- Gao, L.; Wang, Z.; Shan, J.; Chen, J.; Tang, C.; Yi, M.; Zhao, X. Distribution characteristics and sources of trace metals in sediment cores from a trans-boundary watercourse: An example from the Shima River, Pearl River Delta. Ecotoxicol. Environ. Saf. 2016, 134, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzi, B.; Mokhtarzadeh, Z.; Moore, F.; Rastegari Mehr, M.; Lahijanzadeh, A.; Rostami, S.; Kaabi, H. Heavy metals and polycyclic aromatic hydrocarbons in surface sediments of Karoon River, Khuzestan Province, Iran. Environ. Sci. Pollut. Res. 2015, 22, 19077–19092. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sun, Q.; Yi, L.; Yin, X.; Wang, A.; Li, Y.; Chen, J. The source of natural and anthropogenic heavy metals in the sediments of the Minjiang River Estuary (SE China): Implications for historical pollution. Sci. Total Environ. 2014, 493, 729–736. [Google Scholar] [CrossRef]
- Wang, L.; Long, X.; Chong, Y.; Yu, G. Potential risk assessment of heavy metals in sediments during the denitrification process enhanced by calcium nitrate addition: Effect of AVS residual. Ecol. Eng. 2016, 87, 333–339. [Google Scholar] [CrossRef]
- He, Z.; Li, F.; Dominech, S.; Wen, X.; Yang, S. Heavy metals of surface sediments in the Changjiang (Yangtze River) Estuary: Distribution, speciation and environmental risks. J. Geochem. Explor. 2019, 198, 18–28. [Google Scholar] [CrossRef]
- Gillan, D.C.; Pede, A.; Sabbe, K.; Gao, Y.; Leermakers, M.; Baeyens, W.; Louriño Cabana, B.; Billon, G. Effect of bacterial mineralization of phytoplankton-derived phytodetritus on the release of arsenic, cobalt and manganese from muddy sediments in the Southern North Sea. A microcosm study. Sci. Total Environ. 2012, 419, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.F.; Song, Y.H.; Yuan, P.; Cui, X.Y.; Qiu, G.L. The remediation of heavy metals contaminated sediment. J. Hazard. Mater. 2009, 161, 633–640. [Google Scholar] [CrossRef]
- NBO Marine Environmental Quality Bulletin of China-2012 (In Chinese); State Oceanic Administration: Beijing, China, 2013.
- Chakraborty, P.; Ramteke, D.; Chakraborty, S.; Nagender Nath, B. Changes in metal contamination levels in estuarine sediments around India—An assessment. Mar. Pollut. Bull. 2014, 78, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Li, F.; Liu, C.; Gao, J.; Tong, H.; Chen, M. Fractionation characteristics of rare earth elements (REEs) linked with secondary Fe, Mn, and Al minerals in soils. Acta Geochim. 2016, 35, 329–339. [Google Scholar] [CrossRef]
- Essington, M.E.; Mattigod, S.V. Element Partitioning in Size- and Density-Fractionated Sewage Sludge and Sludge-Amended Soil. Soil Sci. Soc. Am. J. 1990, 54, 385–394. [Google Scholar] [CrossRef]
- Pagano, G.; Aliberti, F.; Guida, M.; Oral, R.; Siciliano, A.; Trifuoggi, M.; Tommasi, F. Rare earth elements in human and animal health: State of art and research priorities. Environ. Res. 2015, 142, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.K.; Probst, A.; Orange, D.; Gilbert, F.; Elger, A.; Kallerhoff, J.; Laurent, F.; Bassil, S.; Duong, T.T.; Gerino, M. Bioturbation effects on bioaccumulation of cadmium in the wetland plant Typha latifolia: A nature-based experiment. Sci. Total Environ. 2018, 618, 1284–1297. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.S.; Xue, Y.; Wang, Y.L.; Cang, L.; Xu, B.; Ding, J. Source identification and apportionment of heavy metals in urban soil profiles. Chemosphere 2015, 127, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Rengel, Z. Heavy Metals as Essential Nutrients. In Heavy Metal Stress in Plants; Prasad, M.N.V., Hagemeyer, J., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 1999; pp. 231–251. ISBN 978-3-662-07745-0. [Google Scholar]
- Sun, Z.; Mou, X.; Tong, C.; Wang, C.; Xie, Z.; Song, H.; Sun, W.; Lv, Y. Spatial variations and bioaccumulation of heavy metals in intertidal zone of the Yellow River estuary, China. Catena 2015, 126, 43–52. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, J.; Xiao, R.; Zhao, Q.; Jia, J.; Cui, B.; Liu, X. Heavy metal fractions and ecological risk assessment in sediments from urban, rural and reclamation-affected rivers of the Pearl River Estuary, China. Chemosphere 2017, 184, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzi, B.; Hassanaghaei, M.; Moore, F.; Rastegari Mehr, M.; Soltanian, S.; Lahijanzadeh, A.R.; Sorooshian, A. Heavy metal contamination and health risk assessment in three commercial fish species in the Persian Gulf. Mar. Pollut. Bull. 2018, 129, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Onder, S.; Dursun, S. Air borne heavy metal pollution of Cedrus libani (A. Rich.) in the city centre of Konya (Turkey). Atmos. Environ. 2006, 40, 1122–1133. [Google Scholar] [CrossRef]
- Zhao, S.; Feng, C.; Wang, D.; Liu, Y.; Shen, Z. Salinity increases the mobility of Cd, Cu, Mn, and Pb in the sediments of Yangtze Estuary: Relative role of sediments’ properties and metal speciation. Chemosphere 2013, 91, 977–984. [Google Scholar] [CrossRef]
- Acosta, J.A.; Jansen, B.; Kalbitz, K.; Faz, A.; Martínez-Martínez, S. Salinity increases mobility of heavy metals in soils. Chemosphere 2011, 85, 1318–1324. [Google Scholar] [CrossRef]
- Lv, M.; Sun, Q.; Hu, A.; Hou, L.; Li, J.; Cai, X.; Yu, C.P. Pharmaceuticals and personal care products in a mesoscale subtropical watershed and their application as sewage markers. J. Hazard. Mater. 2014, 280, 696–705. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Xu, X.R.; Zhou, G.J.; Liu, S.S.; Yue, W.Z.; Sun, K.F.; Ying, G.G. Antibiotics in the coastal environment of the Hailing Bay region, South China Sea: Spatial distribution, source analysis and ecological risks. Mar. Pollut. Bull. 2015, 95, 365–373. [Google Scholar] [CrossRef]
- Lin, C.; Yu, R.; Hu, G.; Yang, Q.; Wang, X. Contamination and isotopic composition of Pb and Sr in offshore surface sediments from Jiulong River, Southeast China. Environ. Pollut. 2016, 218, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Hou, L.; Yu, C.P. Biogeography of Planktonic and Benthic Archaeal Communities in a Subtropical Eutrophic Estuary of China. Microb. Ecol. 2015, 70, 322–335. [Google Scholar] [CrossRef]
- Westerhoff, P.; Lee, S.; Yang, Y.; Gordon, G.W.; Hristovski, K.; Halden, R.U.; Herckes, P. Characterization, Recovery Opportunities, and Valuation of Metals in Municipal Sludges from U.S. Wastewater Treatment Plants Nationwide. Environ. Sci. Technol. 2015, 49, 9479–9488. [Google Scholar] [CrossRef] [PubMed]
- USEPA. 3051A Microwave Assisted Acid Digestion of Sediments, Sludge, Soils, and Oils; US Environtal Protection Agency: Cincinnati, OH, USA, 2007; pp. 1–30.
- Nkinahamira, F.; Suanon, F.; Chi, Q.; Li, Y.; Feng, M.; Huang, X.; Yu, C.P.; Sun, Q. Occurrence, geochemical fractionation, and environmental risk assessment of major and trace elements in sewage sludge. J. Environ. Manag. 2019, 249, 109427. [Google Scholar] [CrossRef] [PubMed]
- Longjiang, M.; Duowen, M.; Ke, H.; Jinghong, Y. Distribution of the rare earth elements in the surface sediments from the lower Wuding River of China. J. Radioanal. Nucl. Chem. 2010, 285, 359–364. [Google Scholar] [CrossRef]
- Hu, G.; Lin, C.; Yang, Q.; Yan, Y.; Huang, H.; Yu, R.; Cui, J.; Yan, Y. Distribution and source appointment of rare earth elements in offshore sediments of Western Xiamen Bay, Southeast China. J. Geochem. Explor. 2019, 201, 31–39. [Google Scholar] [CrossRef]
- Hamdoun, H.; Van-Veen, E.; Basset, B.; Lemoine, M.; Coggan, J.; Leleyter, L.; Baraud, F. Characterization of harbor sediments from the English Channel: Assessment of heavy metal enrichment, biological effect and mobility. Mar. Pollut. Bull. 2015, 90, 273–280. [Google Scholar] [CrossRef]
- Taylor, S.R.; Mclennan, S.M. The geochemical evolution of the continental crust. Am. Geophys. Union 1995, 33, 241–265. [Google Scholar] [CrossRef]
- Suanon, F.; Chi, Q.; Yang, X.; Wang, H.; Rashid, A.; Asefi, B.; Mama, D.; Yu, C.-P.; Sun, Q. Diagnosis and ecotoxicological risk assessment of 49 elements in sludge from wastewater treatment plants of Chongqing and Xiamen cities, China. Environ. Sci. Pollut. Res. 2018, 25, 29006–29016. [Google Scholar] [CrossRef]
- Luo, W.; Lu, Y.; Giesy, J.P.; Wang, T.; Shi, Y.; Wang, G.; Xing, Y. Effects of land use on concentrations of metals in surface soils and ecological risk around Guanting Reservoir, China. Environ. Geochem. Health 2007, 29, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Hakanson, L. An ecological risk index for aquatic pollution control.a sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Long, E.R.; Macdonald, D.D.; Smith, S.L.; Calder, F.D. Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ. Manag. 1995, 19, 81–97. [Google Scholar] [CrossRef]
- Sabater-Liesa, L.; Ginebreda, A.; Barceló, D. Shifts of environmental and phytoplankton variables in a regulated river: A spatial-driven analysis. Sci. Total Environ. 2018, 642, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Tableau Software Inc. Tableau Desktop: Public Edition 10.4.2 (10400.17.1206.1554) 32-Bit 2017; Tableau Software Inc.: Seattle, WA, USA, 2017. [Google Scholar]
- Ramesh, R.; Ramanathan, A.L.; James, R.A.; Subramanian, V.; Jacobsen, S.B.; Holland, H.D. Rare earth elements and heavy metal distribution in estuarine sediments of east coast of India. Hydrobiologia 1999, 397, 89–99. [Google Scholar] [CrossRef]
- Maulana, A.; Yonezu, K.; Watanabe, K. Geochemistry of rare earth elements (REE) in the weathered crusts from the granitic rocks in Sulawesi Island, Indonesia. J. Earth Sci. 2014, 25, 460–472. [Google Scholar] [CrossRef]
- Chen, Z.J.; Chen, C.X.; Liu, Y.Q.; Wu, Y.D.; Yang, S.K.; Lu, C.Y. Study of soil environmental background values in Fujian Province. Chin. J. Environ. Sci. 1992, 13, 70–75. [Google Scholar]
- Bayon, G.; Toucanne, S.; Skonieczny, C.; André, L.; Bermell, S.; Cheron, S.; Dennielou, B.; Etoubleau, J.; Freslon, N.; Gauchery, T.; et al. Rare earth elements and neodymium isotopes in world river sediments revisited. Geochim. Cosmochim. Acta 2015, 170, 17–38. [Google Scholar] [CrossRef]
- Yu, R.; Lin, C.; Yan, Y.; Hu, G.; Huang, H.; Wang, X. Distribution and provenance implication of rare earth elements and Sr-Nd isotopes in surface sediments of Jiulong River, Southeast China. J. Soils Sediments 2019, 19, 1499–1510. [Google Scholar] [CrossRef]
- CESP. Background Values of Element in Soil of China (in Chinese); China Environtal Science Press: Beijing, China, 1990; pp. 418–451. [Google Scholar]
- Zhang, Q.; Han, G.; Liu, M.; Wang, L. Geochemical characteristics of rare earth elements in soils from Puding Karst Critical Zone Observatory, Southwest China. Sustainability 2019, 11, 4963. [Google Scholar] [CrossRef]
- Aide, M.T.; Aide, C. Rare Earth Elements: Their Importance in Understanding Soil Genesis. ISRN Soil Sci. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Garzanti, E.; Wang, J.G.; Vezzoli, G.; Limonta, M. Tracing provenance and sediment fluxes in the Irrawaddy River basin (Myanmar). Chem. Geol. 2016, 440, 73–90. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, X.; Zhao, Z. The Geochemistry of Rare Earth Elements (in Chinese), 1st ed.; Science Press: Beijing, China, 1989; pp. 322–323. [Google Scholar]
- Jahan, S.; Strezov, V. Comparison of pollution indices for the assessment of heavy metals in the sediments of seaports of NSW, Australia. Mar. Pollut. Bull. 2018, 128, 295–306. [Google Scholar] [CrossRef] [PubMed]
- CSBTS The People’s Republic of China National Standards GB 18668-2002-Marine Sediment Quality. China State Bur. Qual. Tech. Superv. 2002, p. 10. Available online: https://www.codeofchina.com/standard/GB18668-2002.html (accessed on 18 December 2019).
- Brooks, G.R.; Larson, R.A.; Schwing, P.T.; Romero, I.; Moore, C.; Reichart, G.J.; Jilbert, T.; Chanton, J.P.; Hastings, D.W.; Overholt, W.A.; et al. Sedimentation pulse in the NE Gulf of Mexico following the 2010 DWH blowout. PLoS ONE 2015, 10, e0132341. [Google Scholar] [CrossRef]
- Ergin, M. Possible sources and mechanisms of manganese enrichment in the deep-sea sediments of the Marmara Trough depressions (NE-Mediterranean, Turkey). Oceanol. Acta 1994, 17, 535–546. [Google Scholar]
- Hastings, D.W.; Schwing, P.T.; Brooks, G.R.; Larson, R.A.; Morford, J.L.; Roeder, T.; Quinn, K.A.; Bartlett, T.; Romero, I.C.; Hollander, D.J. Changes in sediment redox conditions following the BP DWH blowout event. Deep Sea Res. Part II Top. Stud. Oceanogr. 2016, 129, 167–178. [Google Scholar] [CrossRef]
- Macdonald, D.D.; Carr, R.S.; Calder, F.D.; Long, E.R.; Ingersoll, C.G. Development and evaluation of sediment quality guidelines for Florida coastal waters. Ecotoxicology 1996, 5, 253–278. [Google Scholar] [CrossRef]
- Huang, X.; Hu, J.; Qin, F.; Quan, W.; Cao, R.; Fan, M.; Wu, X. Heavy metal pollution and ecological assessment around the Jinsha coal-fired power plant (China). Int. J. Environ. Res. Public Health 2017, 14, 1589. [Google Scholar] [CrossRef]
- Zhu, C.; Tian, H.; Cheng, K.; Liu, K.; Wang, K.; Hua, S.; Gao, J.; Zhou, J. Potentials of whole process control of heavy metals emissions from coal-fired power plants in China. J. Clean. Prod. 2016, 114, 343–351. [Google Scholar] [CrossRef]





| This Study | Fujian Soil | World River Sediment | Jiulong River Average | Chinese River Sediment Average | East Coast of India | Granitic Rock Indonesia | Chondrite | Crustal Average | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | Range | |||||||||
| La | 47.5 | 0–151.4 | 41.1 | 37.8 | 55.13 | 39.7 | 21.4 | 110.5 | 0.32 | 30 |
| Ce | 105.6 | 0.01–296.2 | 89.8 | 78 | 106.1 | 68.4 | 41.89 | 165.5 | 0.81 | 60 |
| Pr | 10.5 | 1.5–31.3 | 9.4 | 8.8 | 11.07 | 12.5 | 5.03 | 22.9 | 0.12 | 8.2 |
| Nd | 44.7 | 10.4–120.6 | 33.1 | 32.7 | 45.6 | 26.4 | 15.89 | 84.7 | 0.59 | 28 |
| Sm | 8.5 | 1.95–23.2 | 6.12 | 6.2 | 8.3 | 5.22 | 3.21 | 16.6 | 0.19 | 6 |
| Eu | 1.4 | 0.3–3.6 | 1.21 | 1.2 | 1.4 | 1.03 | 0.58 | 2.8 | 0.072 | 1.2 |
| Gd | 8.0 | 1.9–22.0 | 5.21 | 5.2 | 7.6 | 7.2 | 3.53 | 12.3 | 0.26 | 5.4 |
| Tb | 1.3 | 0.3–2.0 | 0.72 | 0.8 | 1.3 | 0.63 | 0.45 | 2.3 | 0.047 | 0.9 |
| Dy | 6.0 | 1.5–16.0 | 4.6 | 4.9 | 2.91 | 6.5 | 2.91 | 14.6 | 0.33 | 3 |
| Ho | 1.1 | 0.3–2.9 | 0.91 | 1.02 | 1.4 | 1.3 | 0.66 | 2.83 | 0.072 | 1.2 |
| Er | 3.6 | 0.9–9.0 | 2.4 | 2.97 | 1.49 | 4.4 | 1.49 | 8.2 | 0.21 | 2.8 |
| Tm | 0.4 | 0.1–1.1 | 0.39 | - | 0.7 | 0.56 | 0.23 | 1.09 | 0.033 | 0.48 |
| Yb | 3.2 | 0.8–7.8 | 2.6 | 3.01 | 1.3 | 2.44 | 1.3 | 6.6 | 0.208 | 3 |
| Lu | 0.5 | 0.16–1.5 | 0.37 | 0.46 | 0.7 | 0.36 | - | 0.9 | 0.032 | 0.5 |
| ∑REEs | 230.8 | 58.2–688.2 | 197.9 | 183.1 | - | 176.6 | - | 451.8 | 3.3 | 150.7 |
| ∑LREE | 209.7 | 51.9–626.3 | 180.7 | 164.7 | 227.6 | 153.2 | 17.48 | 403.0 | 2.1 | 133.4 |
| ∑HREE | 24.7 | 6.2–61.9 | 17.2 | 18.4 | 26.7 | 23.4 | 1.37 | 48.8 | 1.2 | 17.3 |
| ∑LREE/∑HREE | 9.9 | 2.2–12.0 | 10.5 | 9.0 | 8.5 | 6.6 | - | 1.8 | 7.7 | |
| (La/Yb)N | 12.1 | 0–16.7 | 10.68 | 8.49 | 9.31 | 10.99 | - | - | 1.04 | 6.76 |
| (La/Sm)N | 3.9 | 0–4.7 | 4.23 | 3.84 | 4.18 | 4.79 | - | - | 1.06 | 3.15 |
| (Gd/Yb)N | 2.2 | 1.6–2.9 | 1.62 | 1.40 | 1.54 | 2.33 | - | - | 1.01 | 1.46 |
| δCe | 1.0 | 0.01–1.2 | 1.07 | 1.00 | 1.01 | 0.72 | - | - | 0.97 | 0.90 |
| δEu | 0.5 | 0.4–0.6 | 0.65 | 0.65 | 0.54 | 0.52 | - | - | 0.99 | 0.64 |
| This Study | Fujian Soil | Chinese River Sediment Average | World River Sediment Average | * MQS GB18668-2002 | Crustal Average | TEL/PEL | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | Mean | Range | MSQ-1 | MSQ-2 | MSQ-3 | ||||||
| V | 76.1 | 70.3 | 25.07–115.3 | 79.5 | n.a | n.a | n.a | n.a | n.a. | n.a | n.a |
| Cr | 63.1 | 61.9 | 25.05–138.1 | 44 | 61 | 40 | 80 | 150 | 200 | 270 | 52.3/160 |
| Mn | 936.3 | 1788.9 | 345.6–15,780 | 301 | 583 | 975.3 | n.a | n.a | n.a. | n.a | n.a |
| Co | 9.3 | 8.9 | 3.7–12.7 | 8.8 | n.a | n.a | n.a | n.a | n.a. | n.a | n.a |
| Ni | 19.5 | 17.6 | 4.53–27.8 | 18.2 | 26.9 | 20 | n.a. | n.a. | n.a. | 40 | 15.9/42.8 |
| Cu | 19.1 | 19.8 | 3.8–38.03 | 22.8 | 22.6 | 30 | 35 | 100 | 200 | 200 | 18.7/108 |
| Zn | 80.0 | 78.8 | 41.9–147.4 | 86.1 | 74.2 | 90 | 150 | 350 | 600 | 600 | 124/271 |
| As | 9.5 | 9.2 | 3.6–15.6 | 6.3 | 11.2 | 7.2 | 20 | 65 | 93 | 93 | 7.2/41.6 |
| Mo | 2.1 | 2.4 | 0–7.19 | n.a | n.a | n.a | n.a | n.a | n.a. | n.a | n.a |
| Cd | 0.4 | 0.4 | 0.07–1.68 | 0.07 | 0.1 | 0.35 | 0.5 | 1.5 | 5 | 5 | 0.68/4.21 |
| Sb | 0.4 | 0.5 | 0.1–1.08 | n.a | n.a | n.a | n.a | n.a | n.a. | n.a | n.a |
| Cs | 7.8 | 7.6 | 1.2–16.6 | n.a | n.a. | n.a | n.a | n.a | n.a. | n.a | n.a |
| Pb | 71.0 | 75.8 | 17.9–191.1 | 41.3 | 26 | 230.7 | 60 | 130 | 250 | 250 | 30.2/112 |
| Tl | 0.7 | 0.7 | 0.27–1.95 | 0.82 | n.a | n.a | n.a | n.a | n.a. | n.a | n.a |
| Al | 47,160.0 | 44,754 | 2.75–86,700 | n.a | n.a | n.a | n.a | n.a | n.a. | n.a | n.a |
| Cr | Ni | Cu | Zn | As | Cd | Pb | |
|---|---|---|---|---|---|---|---|
| A4 | 0.90 | 0.91 | 0.73 | 1.06 | 1.29 | 0.42 | 1.94 |
| A5 | 0.88 | 0.91 | 0.78 | 1.13 | 1.32 | 0.43 | 2.06 |
| A6 | 0.65 | 0.63 | 0.49 | 0.88 | 0.90 | 0.26 | 1.20 |
| JY0 | 0.74 | 0.86 | 0.66 | 1.16 | 1.18 | 0.54 | 2.01 |
| JY1 | 0.73 | 0.93 | 0.45 | 0.86 | 1.10 | 0.24 | 1.49 |
| JY3 | 0.68 | 0.83 | 0.38 | 0.74 | 0.92 | 0.13 | 1.02 |
| AEI ≤ 1, safe for biota | AEI ≥ 1, adverse for biota | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peter, P.O.; Rashid, A.; Hou, L.; Nkinahamira, F.; Kiki, C.; Sun, Q.; Yu, C.-P.; Hu, A. Elemental Contaminants in Surface Sediments from Jiulong River Estuary, China: Pollution Level and Ecotoxicological Risk Assessment. Water 2020, 12, 1640. https://doi.org/10.3390/w12061640
Peter PO, Rashid A, Hou L, Nkinahamira F, Kiki C, Sun Q, Yu C-P, Hu A. Elemental Contaminants in Surface Sediments from Jiulong River Estuary, China: Pollution Level and Ecotoxicological Risk Assessment. Water. 2020; 12(6):1640. https://doi.org/10.3390/w12061640
Chicago/Turabian StylePeter, Philomina Onyedikachi, Azhar Rashid, Liyuan Hou, François Nkinahamira, Claude Kiki, Qian Sun, Chang-Ping Yu, and Anyi Hu. 2020. "Elemental Contaminants in Surface Sediments from Jiulong River Estuary, China: Pollution Level and Ecotoxicological Risk Assessment" Water 12, no. 6: 1640. https://doi.org/10.3390/w12061640
APA StylePeter, P. O., Rashid, A., Hou, L., Nkinahamira, F., Kiki, C., Sun, Q., Yu, C.-P., & Hu, A. (2020). Elemental Contaminants in Surface Sediments from Jiulong River Estuary, China: Pollution Level and Ecotoxicological Risk Assessment. Water, 12(6), 1640. https://doi.org/10.3390/w12061640






