Effects of Waterlogging with Different Water Resources on Plant Growth and Tolerance Capacity of Four Herbaceous Flowers in a Bioretention Basin
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.1.1. Experiment 1: Waterlogging with Tap Water
2.1.2. Experiment 2: Waterlogging with Simulated RW
2.2. Measurements of Plant Growth and Physiological Parameters
2.3. Appearance
2.4. Statistical Analysis
3. Results
3.1. Effects of Waterlogging on Plant Growth and Physiological Parameters
3.2. Effects of Waterlogging by Simulated RW on Plant Growth and Physiological Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roy-Poirier, A.; Champagne, P.; Filion, Y. Review of Bioretention System Research and Design: Past, Present, and Future. J. Environ. Eng. 2010, 136, 878–889. [Google Scholar] [CrossRef]
- Pataki, D.E.; Carreiro, M.M.; Cherrier, J.; Grulke, N.E.; Jennings, V.; Pincetl, S.; Pouyat, R.V.; Whitlow, T.H.; Zipperer, W.C. Coupling biogeochemical cycles in urban environments: Ecosystem services, green solutions, and misconceptions. Front. Ecol. Environ. 2011, 9, 27–36. [Google Scholar] [CrossRef]
- Payne, E.G.I.; Pham, T.; Deletic, A.; Hatt, B.E.; Cook, P.L.M.; Fletcher, T.D. Which species? A decision-support tool to guide plant selection in stormwater biofilters. Adv. Water Resour. 2018, 113, 86–99. [Google Scholar] [CrossRef]
- Ao, D.; Luo, L.; Dzakpasu, M.; Chen, R.; Xue, T.; Wang, X.C. Replenishment of landscape water with reclaimed water: Optimization of supply scheme using transparency as an indicator. Ecol. Indic. 2018, 88, 503–511. [Google Scholar] [CrossRef]
- Pedrero, F.; Mounzer, O.; Alarcón, J.J.; Bayona, J.M.; Nicolás-Nicolás, E. The viability of irrigating mandarin trees with saline reclaimed water in a semi-arid Mediterranean region: A preliminary assessment. Irrig. Sci. 2013, 31, 759–768. [Google Scholar] [CrossRef]
- Pedersen, O.; Perata, P.; Voesenek, L.A. Flooding and low oxygen responses in plants. Funct. Plant Biol. 2017, 44, 3–5. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Chen, Y.Z.; Lu, H.Q.; Kong, X.Q.; Dai, J.L.; Li, Z.H.; Dong, H.Z. Growth, lint yield and changes in physiological attributes of cotton under temporal waterlogging. Field Crops Res. 2016, 194, 83–93. [Google Scholar] [CrossRef]
- Kumar, P.; Pal, M.; Joshi, R.; Sairam, R.K. Yield, growth and physiological responses of mung bean [Vigna radiata (L.) Wilczek] genotypes to waterlogging at vegetative stage. Physiol. Mol. Biol. Plants 2013, 19, 209–220. [Google Scholar] [CrossRef]
- Devitt, D.A.; Morris, R.L.; Fenstermaker, L.K. Foliar damage, spectral reflectance, and tissue ion concentrations of trees sprinkle irrigated with waters of similar salinity but different chemical composition. HortScience 2005, 40, 819–826. [Google Scholar] [CrossRef]
- Huang, C.; Zhao, S.; Wang, L.; Anjum, S.; Chen, M.; Zou, C. Alteration in chlorophyll fluorescence, lipid peroxidation and antioxidant enzymes activities in hybrid ramie (Boehmeria nivea L.) under drought stress. Aust. J. Crop Sci. 2013, 7, 594–599. [Google Scholar]
- Levizou, E.; Drilias, P.; Psaras, G.K.; Maneta, Y. Nondestructive assessment of leaf chemistry and physiology through spectral reflectance measurements may be misleading when changes in trichome density cooccur. New Phytol. 2005, 165, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Ballester, C.; Zarco-Tejada, P.J.; Nicola, E.; Alarco, J.J.; Fereres, E.; Intrigliolo, D.; Gonzalez-Dugo, V. Evaluating the performance of xanthophyll, chlorophyll and structure-sensitive spectral indices to detect water stress in five fruit tree species. Precis. Agric. 2018, 19, 178–193. [Google Scholar] [CrossRef]
- Hernández-Clemente, R.; Navarro-Cerrillo, R.; Morales, F.; Zarco-Tejada, P.J. Assessing structural effects on PRI for stress detection in conifer forests. Remote Sens. Environ. 2011, 115, 2360–2375. [Google Scholar] [CrossRef]
- Chen, F.D.; Yin, D.M.; Chen, S.M.; Guan, Z.Y.; Fang, W.M. Morpho-anatomical and physiological responses of two Dendranthema species to waterlogging. Environ. Exp. Bot. 2010, 68, 122–130. [Google Scholar]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damageand oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef]
- Biemelt, S.U.; Mock, H.P.; Grimm, B. Expression and activity of isoenzymes of superoxide dismutase in wheat roots in response to hypoxia and anoxia. Plant Cell Environ. 2000, 23, 135–144. [Google Scholar] [CrossRef]
- Chiou, R.J.; Chang, T.C.; Ouyang, C.F. Aspects of municipal wastewater reclamation and reuse for future water resource shortages in Taiwan. Water Sci. Technol. 2017, 55, 397–405. [Google Scholar] [CrossRef]
- Henson, D.Y.; Newman, S.E.; Hartley, D.E. Performance of selected herbaceous annual ornamentals grown at decreasing levels of irrigation. HortScience 2006, 41, 1481–1486. [Google Scholar] [CrossRef]
- Pandey, D.M.; Goswami, C.L.; Jain, S. Effect of growth regulators on photosynthetic metabolites in cotton under water stress. Biol. Plant 2002, 45, 445–448. [Google Scholar] [CrossRef]
- Balakhnina, T.; Gins, M.; Fomina, I. Oxidative stress development in the leaves of Amaranthus cruentus L. containing amaranthine under conditions of nighttime low temperatures, soil hypoxia and the combined effects of both stress factors. Int. Agrophys. 2019, 33, 511–516. [Google Scholar] [CrossRef]
- Xiao, Y.; Jie, Z.L.; Wang, M.; Lin, G.H.; Wang, W.Q. Leaf and stem anatomical responses to periodical waterlogging in simulated tidal floods in mangrove Avicennia marina seedlings. Aquatic Bot. 2009, 91, 231–237. [Google Scholar] [CrossRef]
- Seelig, H.D.; Wolter, A.; Schröder, F.G. Leaf thickness and turgor pressure in bean during plant desiccation. Sci. Hort. 2015, 184, 55–62. [Google Scholar] [CrossRef]
- Sauter, M. Root responses to flooding. Curr. Opin. Plant Biol. 2013, 16, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, I.; Nazir, N. Effect of waterlogging and drought stress in plants. Int. J. Water Resour. Environ. Sci. 2013, 2, 34–40. [Google Scholar]
- Wei, W.; Li, D.; Wang, L.; Ding, X.; Zhang, Y.; Gao, Y.; Zhang, X. Morpho-anatomical and physiological responses to waterlogging of sesame (Sesamum indicum L.). Plant Sci. 2013, 208, 102–111. [Google Scholar] [CrossRef]
- Liu, M.X.; Jiang, Y.W. Genotypic variation in growth and metabolic responses of perennial ryegrass exposed to short-term waterlogging and submergence stress. Plant Physiol. Biochem. 2015, 95, 57–64. [Google Scholar] [CrossRef]
- Nezamia, A.; Khazaeia, H.R.; Rezazadehb, Z.; Hosseinic, A. Effects of drought stress and defoliation on sunflower (Helianthus annuus) in controlled conditions. Desert 2008, 12, 99–104. [Google Scholar]
- Whitehead, D.; Boelman, N.; Turnbull, M.; Hunt, J.; Richardson, S.; Peltzer, D. Photosynthesis and reflectance indices for rainforest species in ecosystems undergoing progression and retrogression along a soil fertility chronosequence in New Zealand. Oecologia 2005, 144, 233–244. [Google Scholar] [CrossRef]
- González-Fernández, A.B.; Rodríguez-Pérez, J.R.; Marcelo, V.; Valenciano, J.B. Using field spectrometry and a plant probe accessory to determine leaf water content in commercial vineyards. Agric. Water Manag. 2015, 156, 43–50. [Google Scholar] [CrossRef]
- Liu, L.; Yang, X.; Zhou, H.; Liu, S.; Zhou, L.; Li, X.; Yang, J.; Wu, J. Relationship of root zone soil moisture with solar-induced chlorophyll fluorescence and vegetation indices in winter wheat: A comparative study based on continuous ground-measurements. Ecol. Indic. 2018, 90, 9–17. [Google Scholar] [CrossRef]
- Smillie, R.M.; Hetherington, S.E. Stress tolerance and stress-induced injury in crop plants measured by chlorophyll fluorescence in vivo: Chilling, freezing, ice cover, heat, and high light. Plant Physiol. 1983, 72, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Buschmann, C.; Knapp, M. How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio RFd of leaves with the PAM fluorometer. Photosynthetica 2005, 43, 379–393. [Google Scholar] [CrossRef]
- De Almeida, J.; Tezara, W.; Herrera, A. Physiological responses to drought and experimental water deficitand waterlogging of four clones of cacao (Theobroma cacao L.) selectedfor cultivation in Venezuela. Agric. Water Manag. 2016, 171, 80–88. [Google Scholar] [CrossRef]
- Malik, A.; Colmer, T.D.; Lambers, H.; Setter, L.T.; Schortemeyer, M. Short-term waterlogging has long-term effects on the growth and physiology of wheat. New Phytol. 2002, 153, 225–236. [Google Scholar] [CrossRef]
- Pang, J.Y.; Zhou, M.X.; Mendham, N.; Shabala, S. Growth and physiological responses of six barley genotypes to waterlogging and subsequent recovery. Aust. J. Agric. Res. 2004, 55, 895–906. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, L.; Zhou, Q.; Huang, X. Stomatal and non-stomatal factors regulated the photosynthesis of soybean seedlings in the present of exogenous bisphenol A. Ecotoxicol. Environ. Saf. 2017, 145, 150–160. [Google Scholar] [CrossRef]
- Irfan, M.; Hayat, S.; Hayat, Q.; Afroz, S.; Ahmad, A. Physiological and biochemical changes in plants under waterlogging. Protoplasma 2010, 241, 3–17. [Google Scholar] [CrossRef]
- Arbona, V.; Hossain, Z.; López-Climent, M.F.; Pérez-Clemente, R.M.; Gómez-Cadenas, A. Antioxidant enzymatic activity is linked to waterlogging stress tolerance in citrus. Physiol. Plant 2008, 132, 452–466. [Google Scholar] [CrossRef]
- Yordanova, R.Y.; Popova, L.P. Waterlogging-induced changes in photosynthesis and oxidative status in maize plants. Acta Physiol. Plant 2007, 29, 535–541. [Google Scholar] [CrossRef]
- Yeager, T.H.; von Merveldt, J.K.; Larsen, C.A. Ornamental plant response to percentage of reclaimed water irrigation. HortScience 2010, 45, 1610–1615. [Google Scholar] [CrossRef]
- Gómez-Bellot, M.J.; Ortuño, M.F.; Nortes, P.A.; Vicente-Sánchez, J.; Bañón, S.; Sánchez-Blanco, M.J. Mycorrhizal euonymus plants and reclaimed water: Biomass, water status and nutritional responses. Sci. Hortic. 2015, 186, 61–69. [Google Scholar]
- Yuan, J.; Dunnett, N. Plant selection for rain gardens: Response to simulated cyclical flooding of 15 perennial species. Urban For. Urban Green. 2018, 35, 57–65. [Google Scholar] [CrossRef]
- Petousi, I.; Fountoulakis, M.; Saru, M.; Nikolaidis, N.; Fletcher, L.; Stentiford, E.; Manios, T. Effects of reclaimed wastewater irrigation on olive (Olea europaea L. cv ‘Koroneiki’) trees. Agric. Water Manag. 2015, 160, 33–40. [Google Scholar] [CrossRef]
- Shuster, W.; Darner, R.; Schifman, L.; Herrmann, D. Factors Contributing to the Hydrologic Effectiveness of a Rain Garden Network (Cincinnati OH USA). Infrastructures 2017, 2, 11. [Google Scholar] [CrossRef]

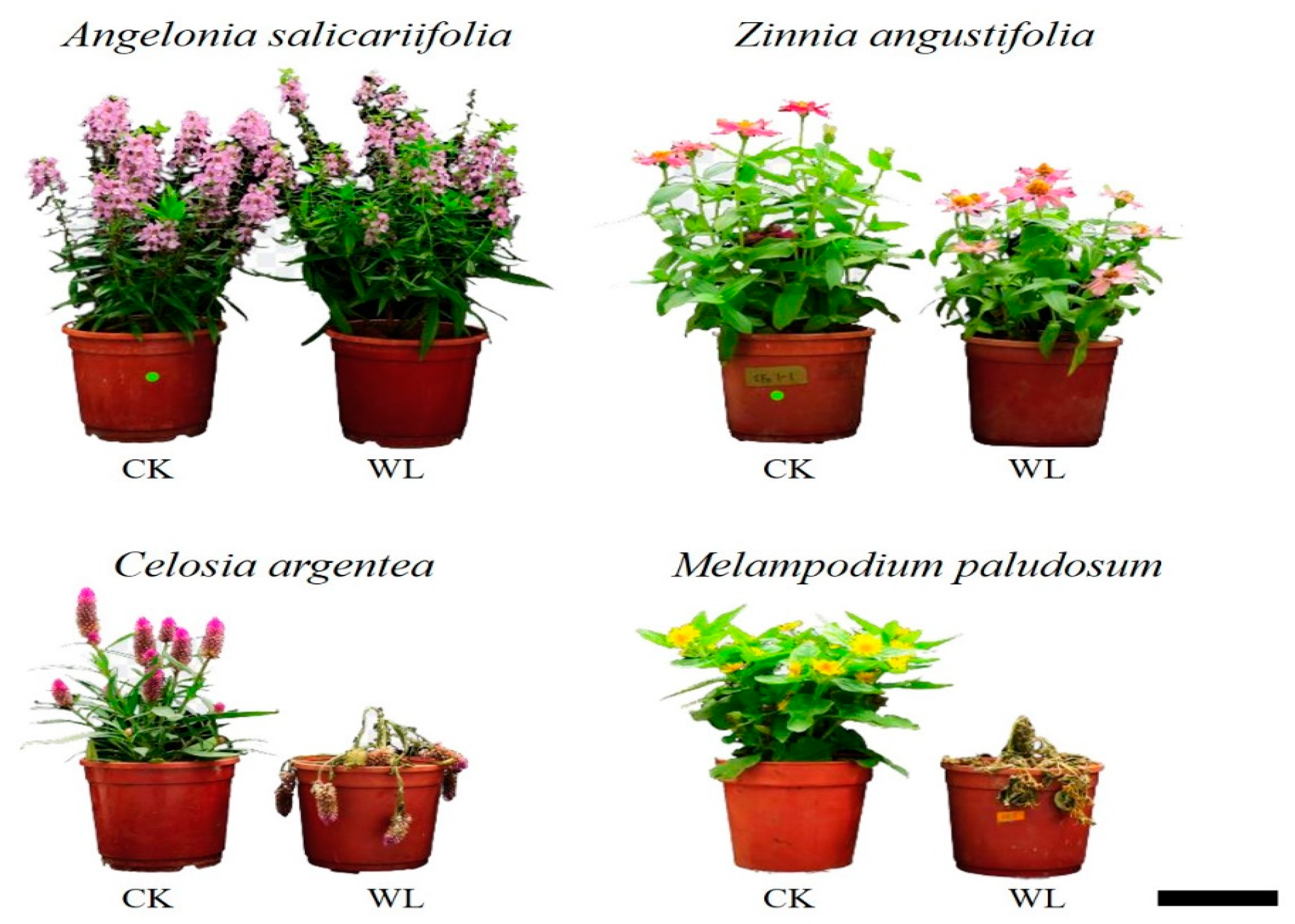




| Treatment | Height (cm) | Dry Weight (g) | Water Content (%) | Leaf Thickness (mm) | SPAD Value | NDVI | MDA (nmol·g−1) | Plant Quality | |
|---|---|---|---|---|---|---|---|---|---|
| Shoot | Root | ||||||||
| Angelonia salicariifolia | |||||||||
| Control | 26.34 | 3.86 | 1.40 | 86.0 | 0.26 | 45.22 | 0.5340 | 13.36 | 5.0 |
| Waterlog | 27.10 NS | 4.05 NS | 1.69 NS | 86.1 NS | 0.24 NS | 49.42 NS | 0.5370 NS | 18.27 NS | 4.8 NS |
| Zinnia angustifolia | |||||||||
| Control | 22.04 | 4.03 | 0.63 | 87.3 | 0.32 | 43.04 | 0.5513 | 47.09 | 5.0 |
| Waterlog | 18.34 ** | 4.16 NS | 0.78 NS | 83.3 *** | 0.34 NS | 45.76 NS | 0.5210 ** | 100.08 ** | 4.6 NS |
| Celosia argentea | |||||||||
| Control | 17.64 | 3.78 | 0.99 | 87.1 | 0.63 | 68.24 | 0.5035 | 63.71 | 5.0 |
| Waterlog | 16.18 * | 3.64 NS | 0.88 NS | 73.7 *** | 0.36 *** | 60.64 *** | 0.4955 NS | 458.77 ** | 1.8 *** |
| Melampodium paludosum | |||||||||
| Control | 15.74 | 3.54 | 2.09 | 87.7 | 0.25 | 35.22 | 0.5291 | 32.31 | 5.0 |
| Waterlog | 10.94 *** | 2.71 ** | 0.47 ** | 76.4 *** | 0.20 *** | 36.60 NS | 0.5033 * | 166.17 * | 1.4 *** |
| Treatment | Height (cm) | Dry Weight (g) | Leaf Thickness (mm) | SPAD | NDVI | MDA (nmol·g−1) | |
|---|---|---|---|---|---|---|---|
| Shoot | Root | ||||||
| Angelonia salicariifolia | |||||||
| Control | 31.90 | 5.46 | 3.28 | 32.68 | 43.44 | 0.5512 | 65.53 |
| RW | 32.42 NS | 5.36 NS | 2.76 NS | 32.54 NS | 44.24 NS | 0.5573 NS | 53.40 * |
| Zinnia angustifolia | |||||||
| Control | 34.86 | 8.95 | 2.92 | 32.46 | 35.70 | 0.5006 | 53.99 |
| RW | 34.87 NS | 9.50 NS | 2.80 NS | 31.80 NS | 36.38 NS | 0.5017 NS | 61.92 NS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.-C.; Lin, K.-H.; Wu, C.-W.; Chang, Y.-J.; Chang, Y.-S. Effects of Waterlogging with Different Water Resources on Plant Growth and Tolerance Capacity of Four Herbaceous Flowers in a Bioretention Basin. Water 2020, 12, 1619. https://doi.org/10.3390/w12061619
Yang W-C, Lin K-H, Wu C-W, Chang Y-J, Chang Y-S. Effects of Waterlogging with Different Water Resources on Plant Growth and Tolerance Capacity of Four Herbaceous Flowers in a Bioretention Basin. Water. 2020; 12(6):1619. https://doi.org/10.3390/w12061619
Chicago/Turabian StyleYang, Wen-Chi, Kuan-Hung Lin, Chun-Wei Wu, Yu-Jie Chang, and Yu-Sen Chang. 2020. "Effects of Waterlogging with Different Water Resources on Plant Growth and Tolerance Capacity of Four Herbaceous Flowers in a Bioretention Basin" Water 12, no. 6: 1619. https://doi.org/10.3390/w12061619
APA StyleYang, W.-C., Lin, K.-H., Wu, C.-W., Chang, Y.-J., & Chang, Y.-S. (2020). Effects of Waterlogging with Different Water Resources on Plant Growth and Tolerance Capacity of Four Herbaceous Flowers in a Bioretention Basin. Water, 12(6), 1619. https://doi.org/10.3390/w12061619






