The Urgent Need for River Health Biomonitoring Tools for Large Tropical Rivers in Developing Countries: Preliminary Development of a River Health Monitoring Tool for Myanmar Rivers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Physico-Chemical Water Quality Variables
2.3. Macroinvertebrates Sampling and Identification
2.4. Rapid Assessment Index Methods—Field-Based Testing
2.5. Index and Statistical Analysis
3. Results
3.1. Physico-Chemical Water Quality Variables
3.2. Macroinvertebrates Richness and Abundance
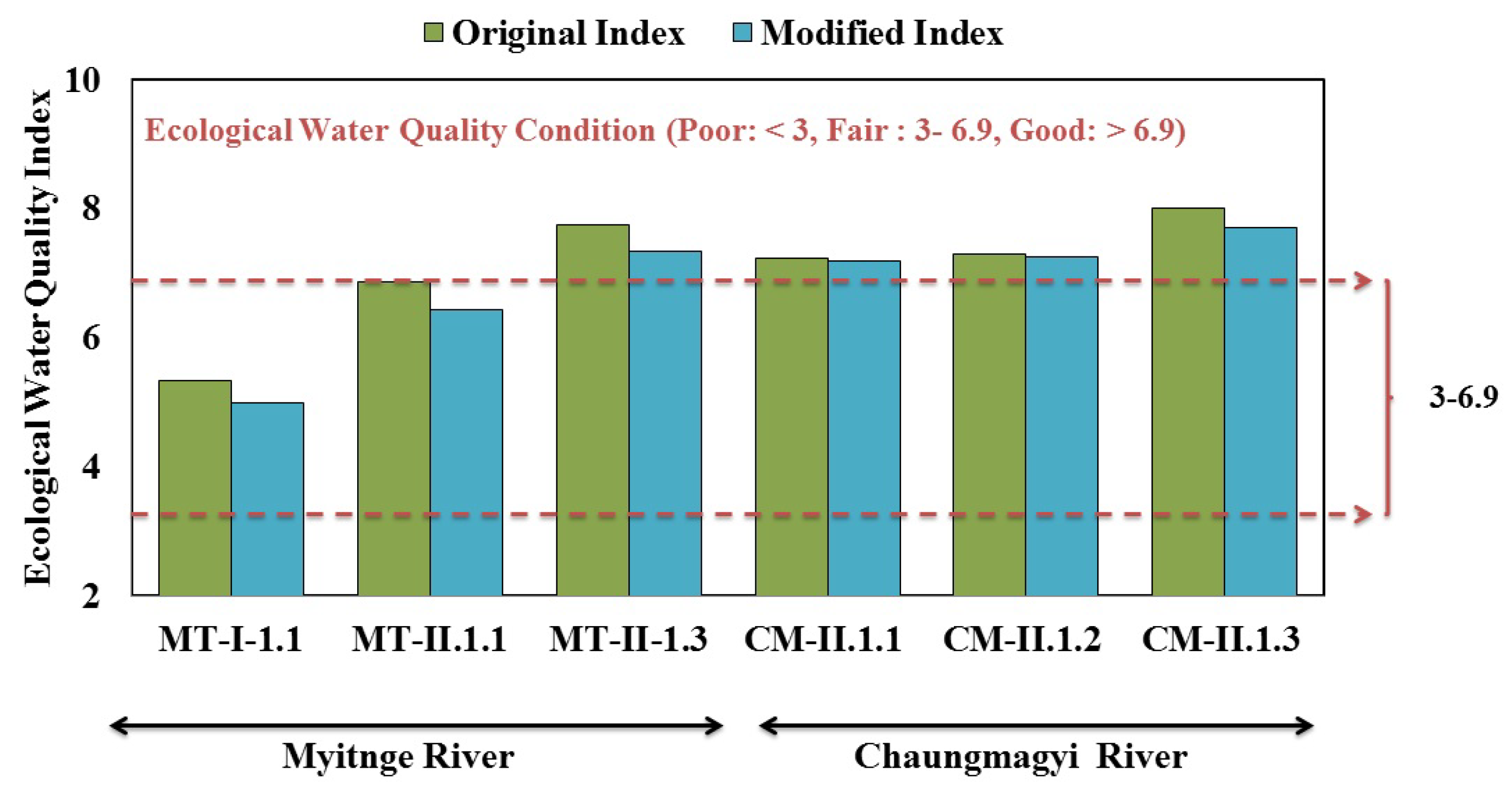
3.3. Ecological Water Quality Index and Statistical Analysis
3.4. Method Development and Modification–Myanmar Modified The Asia Foundation Index
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoang, H.T.T.; Duong, T.T.; Nguyen, K.T.; Le, Q.T.P.; Luu, M.T.N.; Trinh, D.A.; Le, A.H.; Ho, C.T.; Dang, K.D.; Némery, J.; et al. Impact of anthropogenic activities on water quality and plankton communities in the Day River (Red River Delta, Vietnam). Environ. Monit. Assess. 2018, 190, 67. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, C.; Renöfält, B.M. Linking flow regime and water quality in rivers: A challenge to adaptive catchment management. Ecol. Soc. 2008, 13, 2. [Google Scholar] [CrossRef]
- Dudgeon, D. Tropical Asian Streams: Zoobenthos, Ecology and Conservation; Hong Kong University Press: Hong Kong, China, 1999; Volume 1. [Google Scholar]
- Hughes, C.E. Are there many different routes to becoming a global biodiversity hotspot? Proc. Natl. Acad. Sci. USA 2017, 114, 4275–4277. [Google Scholar] [CrossRef]
- Mustow, S. Biological monitoring of rivers in Thailand: Use and adaptation of the BMWP score. Hydrobiologia 2002, 479, 191–229. [Google Scholar] [CrossRef]
- Boonsoong, B.; Sangpradub, N.; Barbour, M.T. Development of rapid bioassessment approaches using benthic macroinvertebrates for Thai streams. Environ. Monit. Assess. 2009, 155, 129–147. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.B.; Mugnai, R.; Castro, C.M.; Baptista, D.F. Determining subsampling effort for the development of a rapid bioassessment protocol using benthic macroinvertebrates in streams of Southeastern Brazil. Environ. Monit. Assess. 2011, 175, 75–85. [Google Scholar] [CrossRef]
- Buss, D.F.; Vitorino, A.S. Rapid bioassessment protocols using benthic macroinvertebrates in Brazil: Evaluation of taxonomic sufficiency. J. N. Am. Benthol. Soc. 2010, 29, 562–571. [Google Scholar] [CrossRef]
- Graham, P.M.; Dickens, C.W.; Taylor, R.J. miniSASS—A novel technique for community participation in river health monitoring and management. Afr. J. Aquat. Sci. 2004, 29, 25–35. [Google Scholar] [CrossRef]
- Armitage, P.; Moss, D.; Wright, J.; Furse, M. The performance of a new biological water quality score system based on macroinvertebrates over a wide range of unpolluted running-water sites. Water Res. 1983, 17, 333–347. [Google Scholar] [CrossRef]
- Dickens, C.W.; Graham, P.M. The South African Scoring System (SASS) Version 5 Rapid Bioassessment Method for Rivers. Afr. J. Aquat. Sci. 2002, 27, 1–10. [Google Scholar] [CrossRef]
- Buss, D.F.; Salles, F.F. Using Baetidae species as biological indicators of environmental degradation in a Brazilian river basin. Environ. Monit. Assess. 2007, 130, 365. [Google Scholar] [CrossRef] [PubMed]
- Buss, D.F.; Carlisle, D.M.; Chon, T.S.; Culp, J.; Harding, J.S.; Keizer-Vlek, H.E.; Robinson, W.A.; Strachan, S.; Thirion, C.; Hughes, R.M. Stream biomonitoring using macroinvertebrates around the globe: A comparison of large-scale programs. Environ. Monit. Assess. 2015, 187, 4132. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.A.; Chessman, B.C.; Fairweather, P.G.; Benson, L.J. Measuring the impact of sewage effluent on the macroinvertebrate community of an upland stream: The effect of different levels of taxonomic resolution and quantification. Aust. J. Ecol. 1995, 20, 142–149. [Google Scholar] [CrossRef]
- Ollis, D.J. Rapid Bioassessment of the Ecological Integrity of the Lourens, Palmiet and Hout Bay Rivers (South Western Cape, South Africa) Using Aquatic Macroinvertebrates. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2005. [Google Scholar]
- Stein, E.D.; Brown, J.S.; Mazor, R.D. Transferability of bioassessment indices among water body types and ecoregions: A California experiment in wetland assessment. Ecol. Indic. 2017, 81, 65–73. [Google Scholar] [CrossRef]
- Watson, M.; Dallas, H. Bioassessment in ephemeral rivers: Constraints and challenges in applying macroinvertebrate sampling protocols. Afr. J. Aquat. Sci. 2013, 38, 35–51. [Google Scholar] [CrossRef]
- Nixon, N.; Mustafa, M. Asia Foundation Supports Water Quality Monitoring Project in Mongolia. 2009. Available online: https://asiafoundation.org/2009/07/08/asia-foundation-supports-water-quality-monitoring-project-in-mongolia/ (accessed on 22 April 2019).
- HIC. Ayeyarwady SOBA 2017: Synthesis Report State of the Basin Assessment; The World Bank: Yangon, Myanmar, 2017. [Google Scholar]
- Dickens, C.; Cox, A.; Johnston, R.M.; Davison, S.; Henderson, D.; Meynell, P.; Shinde, V. Monitoring the Health of the Greater Mekong’s Rivers; CGIAR Research Program on Water, Land and Ecosystems: Patan, Nepal, 2018. [Google Scholar]
- Eriksen, T.E.; Nesheim, I.; Friberg, N.; Aung, T.T.; Myint, Z.W. Characterization of the Bago Sub-Basin Pilot Implementing the EU Water Framework Directive; NIVA-rapport; Ministry of Natural Rescources and Environmental Conservation: Naypyitaw, Myanmar, 2017.
- Choÿ, S.; Thompsons, P. River Bioassessment and monitoring using benthic macroinvertebrate community structure: The Monitoring River Heath initiative. In Downstream Effects of Landuse; Department of Natural Resources: Brisbane, Australia, 1996; pp. 53–55. [Google Scholar]
- Department of Meteorology and Hydrology; Myanmar. Available online: https://www.moezala.gov.mm/ (accessed on 12 September 2019).
- Narumon, S.; Boonsoong, B. Identification of Freshwater Invertebrates of the Mekong River and Tributaries; Faculty of Science, Appllied Taxonomic Research Center Khon Kean University: Khon Kean, Thailand, 2004. [Google Scholar]
- Clarke, K.; Gorley, R. PRIMER v6: User Manual/Tutorial, Primer E: Plymouth; Plymouth Marine Laboratory: Plymouth, UK, 2006. [Google Scholar]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Chessman, B.C. New sensitivity grades for Australian river macroinvertebrates. Mar. Freshw. Res. 2003, 54, 95–103. [Google Scholar] [CrossRef]
- Soldán, T.; Braasch, D. Two new species of the genus Prosopistoma (Ephemeroptera, Prosopistomatidae) from Vietnam. Acta Entomol. Bohemoslov. 1984, 81, 370–376. [Google Scholar]
- Suter, P.J. The Taxonomy and Ecology of the Ephemeroptera (Mayflies) of South Australia. Ph.D. Thesis, University of Adelaide, Department of Zoology, Adelaide, Australia, 1980. [Google Scholar]
- Carter, J.L.; Resh, V.H.; Hannaford, M.J. Macroinvertebrates as biotic indicators of environmental quality. In Methods in Stream Ecology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 293–318. [Google Scholar]
- Fairweather, P.G. State of environment indicators of ‘river health’: Exploring the metaphor. Freshw. Biol. 1999, 41, 211–220. [Google Scholar] [CrossRef]
- Norris, R.H.; Thoms, M.C. What is river health? Freshw. Biol. 1999, 41, 197–209. [Google Scholar] [CrossRef]
- Chessman, B.C.; Royal, M.J. Bioassessment without reference sites: Use of environmental filters to predict natural assemblages of river macroinvertebrates. J. N. Am. Benthol. Soc. 2004, 23, 599–615. [Google Scholar] [CrossRef]
- Prommi, T.; Thamsenanupap, P. Diversity and Structure of Trichoptera Communities and Water Quality Variables in Streams, Northern Thailand. World Acad. Sci. Eng. Technol. Int. J. Environ. Chem. Ecol. Geol. Geophys. Eng. 2015, 9, 1065–1072. [Google Scholar]
- Nesemann, H.; Shah, R.D.T.; Shah, D.N. Key to the larval stages of common Odonata of Hindu Kush Himalaya, with short notes on habitats and ecology. J. Threatened Taxa 2011, 2045–2060. [Google Scholar] [CrossRef]
- Abdo, A.S.S.; MD Rawi, C.S.; Ahmad, A.H.; Rosmahanie Madrus, M. Biodiversity of stream insects in the M alaysian P eninsula: Spatial patterns and environmental constraints. Ecol. Entomol. 2013, 38, 238–249. [Google Scholar] [CrossRef]
- Ward, J.V. Aquatic Insect Ecology. 1. Ecology and Habitat; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1992. [Google Scholar]
- Thatoe Nwe Win, T.; Bogaard, T.; van de Giesen, N. A low-cost water quality monitoring system for the Ayeyarwady River in Myanmar using a participatory approach. Water 2019, 11, 1984. [Google Scholar] [CrossRef]
- Dim, A.B.H.; Rutten, W.Z.; Hoes, O. Estimation of Hydropoweer Potential in Myanmar. Glob. J. Eng. Technol. Rev. 2017, 2, 78–85. [Google Scholar]
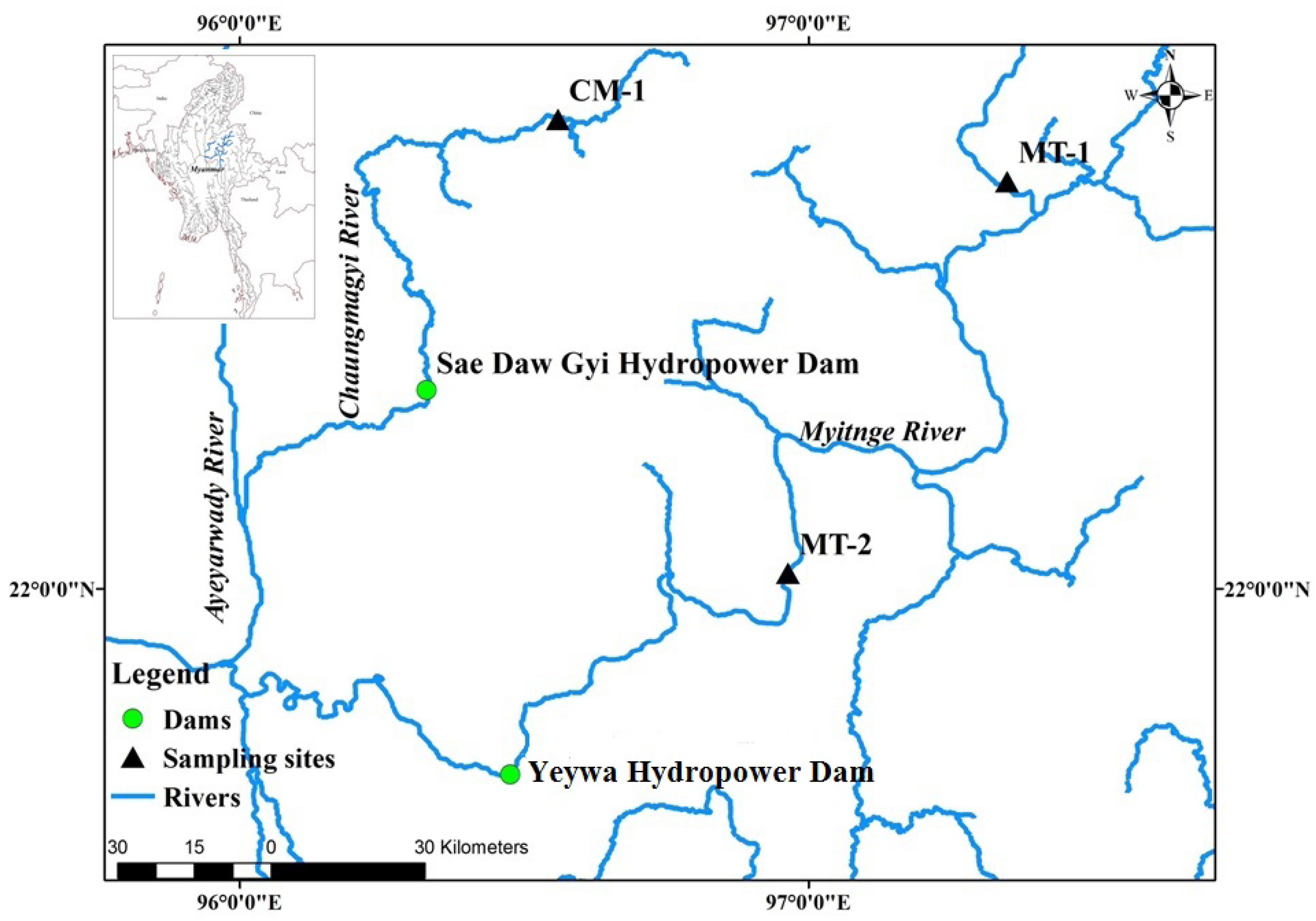
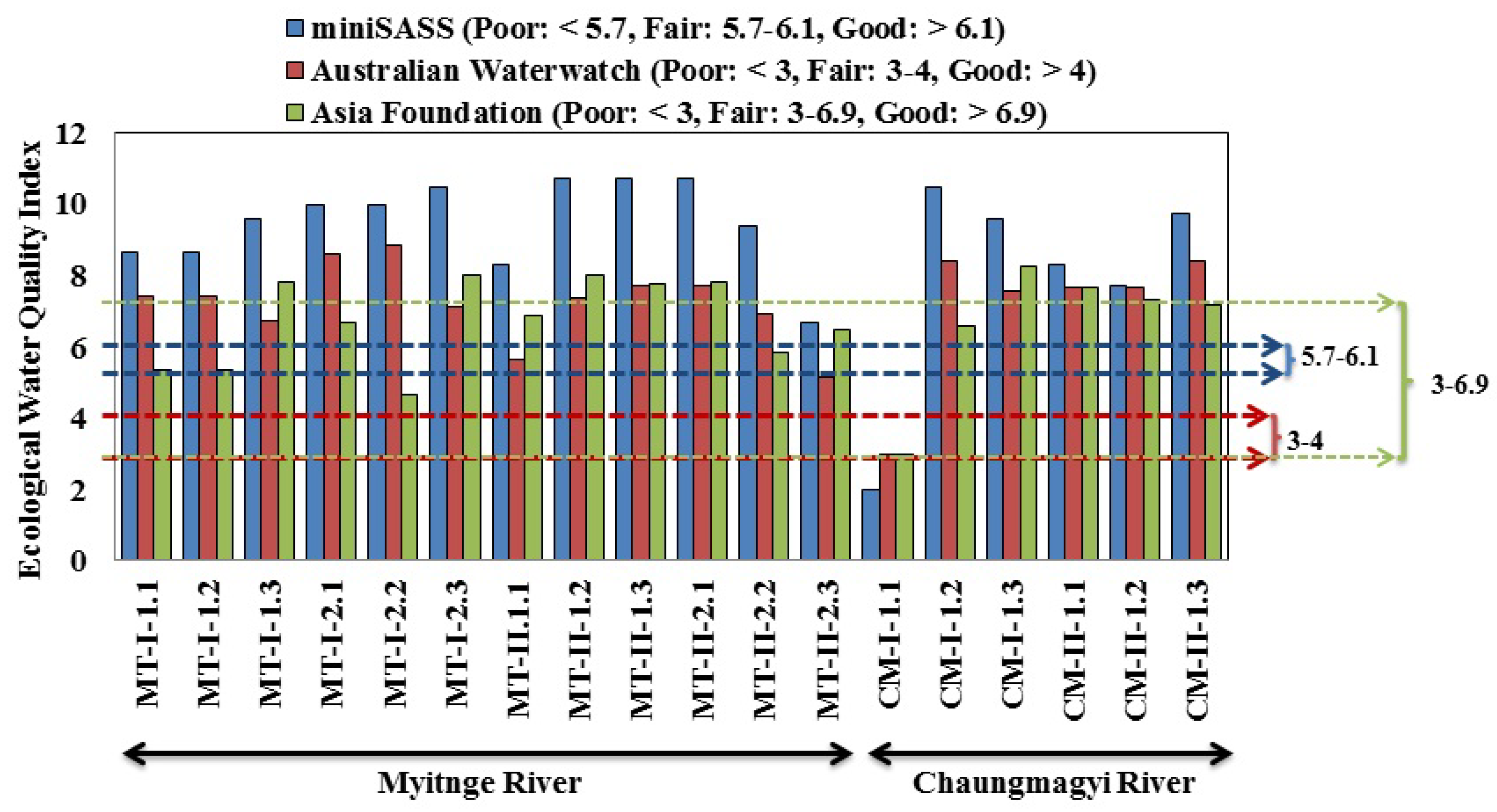
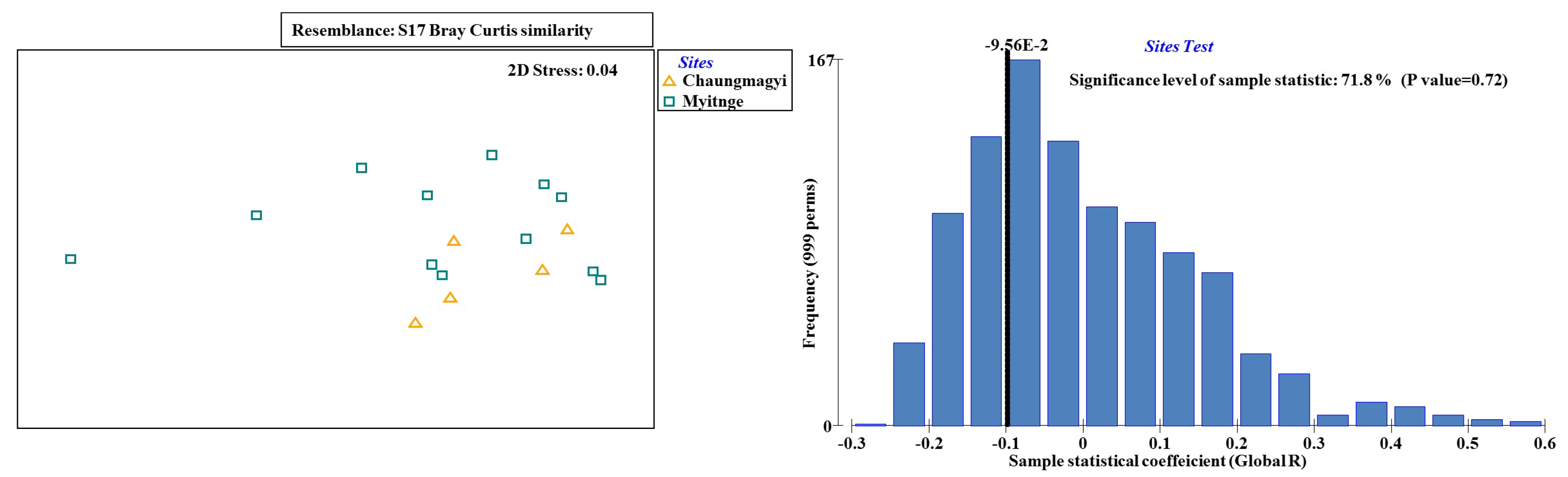
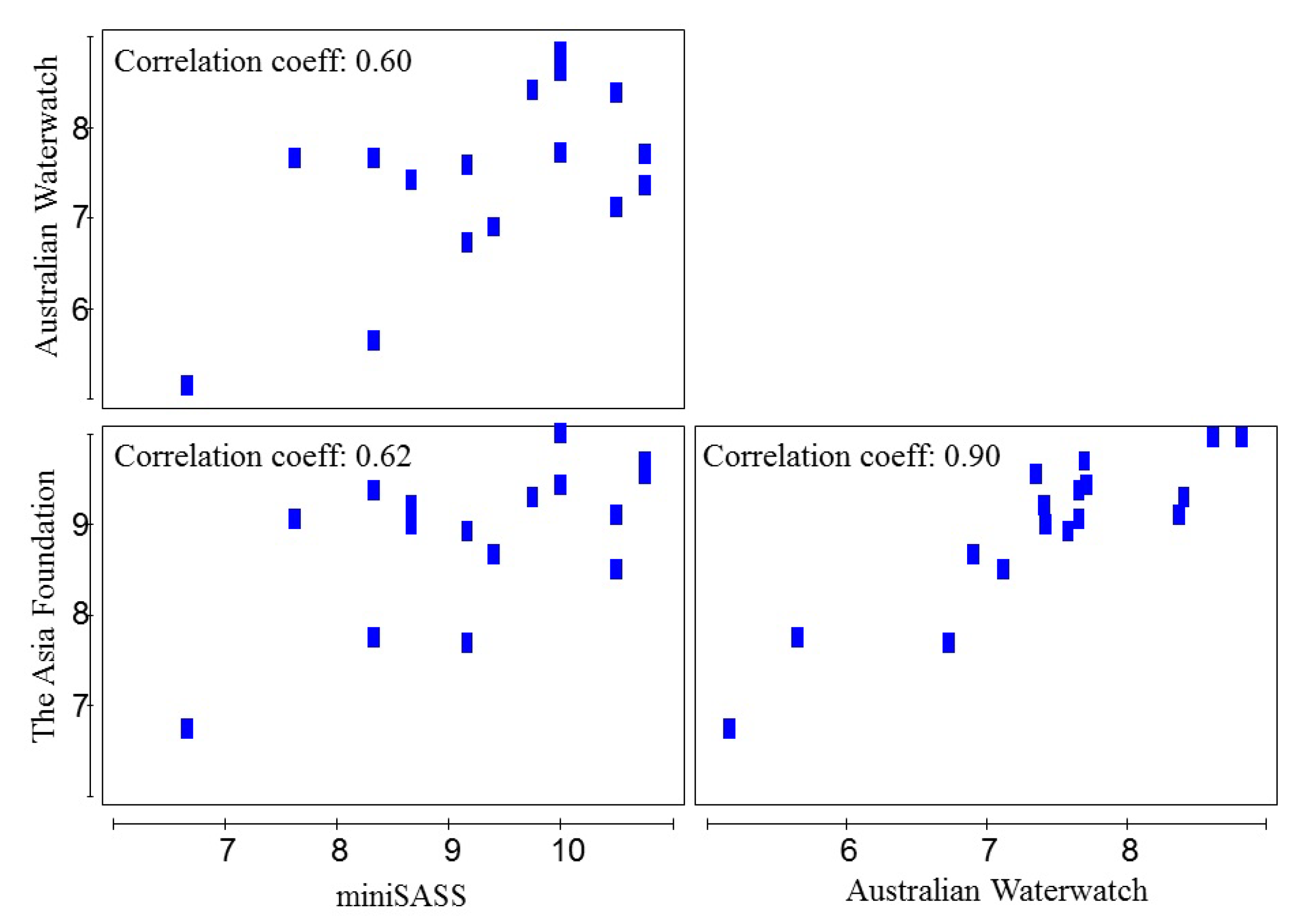
| Site Observation | Occasion | Locations | Samples | Total Taxa | Total Abundance | pH | Turbidity (NTU) | EC (μS cm−1) | Temp (°C) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All | EPTO | All | EPTO | ||||||||
| Myitnge River | November–December 2016 | MT-I-1 | MT-I-1.1 | 5 | 5 | 50 | 50 | 7.5 | 14.0 | 289 | 23 |
| MT-I-1.2 | 4 | 4 | 25 | 25 | |||||||
| MT-I-1.3 | 12 | 7 | 32 | 22 | |||||||
| MT-I-2 | MT-I-2.1 | 4 | 4 | 29 | 29 | 6.5 | 14.0 | 354 | 29 | ||
| MT-I-2.2 | 4 | 4 | 25 | 25 | |||||||
| MT-I-2.3 | 5 | 5 | 16 | 13 | |||||||
| February–March 2017 | MT-II-1 | MT-II.1.1 | 17 | 13 | 119 | 55 | 7.3 | 4.3 | 327 | 22 | |
| MT-II-1.2 | 9 | 9 | 380 | 380 | |||||||
| MT-II-1.3 | 13 | 13 | 267 | 267 | |||||||
| MT-II-2 | MT-II-2.1 | 14 | 13 | 304 | 300 | 6.8 | 6.0 | 427 | 23 | ||
| MT-II-2.2 | 8 | 7 | 33 | 30 | |||||||
| MT-II-2.3 | 4 | 2 | 12 | 6 | |||||||
| Chaungmagyi River | November–December 2016 | CM-I-1 | CM-I-1.1 | 1 | 0 | 1 | 0 | 5.5 | 307.8 | 72 | 24 |
| CM-I-1.2 | 10 | 9 | 124 | 123 | |||||||
| CM-I-1.3 | 12 | 10 | 103 | 96 | |||||||
| February–March 2017 | CM-II-1 | CM-II-1.1 | 19 | 17 | 148 | 146 | 7.6 | 108.0 | 65 | 23 | |
| CM-II-1.2 | 19 | 15 | 245 | 236 | |||||||
| CM-II-1.3 | 9 | 8 | 38 | 37 | |||||||
| The Asia Foundation | SIGNAL 2 | Rivers | |||||
|---|---|---|---|---|---|---|---|
| Order | Family | Species | Original | Modify | Grades | Myitnge | Chaungmagyi |
| Ephemeroptera * | Baetidae * * | Platybaetis sp 1 | 5 | 5 | 5 | 67 | 189 |
| Baetis sp 1 | 5 | 5 | 5 | 814 | 183 | ||
| Procloeon sp 1 | 5 | 5 | 5 | 6 | 0 | ||
| Prosopistomatidae ** | Prosopistoma sp 1 | N.A | 4 | 4 | 4 | 0 | |
| Ephemeridae ** | Ephemera sp 1 | 6 | 6 | N.A | 3 | 0 | |
| Heptageniidae ** | Asionurus sp 1 | 10 | 10 | N.A | 18 | 27 | |
| Asionurus sp 2 | 10 | 10 | N.A | 0 | 9 | ||
| Caenidae ** | Caenis sp 1 | 4 | 4 | 4 | 2 | 0 | |
| Leptophlebiidae ** | Chloroterpes sp 1 | 10 | 10 | 8 | 20 | 3 | |
| Chloroterpes sp 2 | 10 | 10 | 8 | 2 | 0 | ||
| Potamanthidae ** | Potamanthindus sp 1 | 5 | 5 | 6 | 0 | 1 | |
| Polymitarcyidae ** | Ephoron | N.A | 6 | 6 | 0 | 31 | |
| Isonychiidae ** | Isonychia | N.A | 8 | 8 | 0 | 1 | |
| Ephemerellidae ** | Torleya | 10 | 10 | 9 | 0 | 1 | |
| Cincticostella gosei sp 1 | 10 | 10 | 9 | 0 | 3 | ||
| Plecoptera * | Perlidae ** | Neoperla sp 1 | 10 | 10 | 10 | 11 | 14 |
| Neoperla sp 2 | 10 | 10 | 10 | 3 | 1 | ||
| Neoperla sp 3 | 10 | 10 | 10 | 0 | 1 | ||
| Etrocorema sp 1 | 10 | 10 | 10 | 1 | 1 | ||
| Etrocorema sp 2 | 10 | 10 | 10 | 9 | 1 | ||
| Togoperla sp 1 | 10 | 10 | 10 | 0 | 12 | ||
| Togoperla sp 2 | 10 | 10 | 10 | 0 | 1 | ||
| Togoperla sp 3 | 10 | 10 | 10 | 0 | 23 | ||
| Trichoptera * | Hydropsychidae ** | Potamyia sp1 | 5 | 5 | 6 | 105 | 27 |
| Oestropsyche sp 1 | 5 | 5 | 6 | 1 | 0 | ||
| Macrostemum sp 1 | 5 | 5 | 6 | 40 | 35 | ||
| Amphipsyche sp 1 | 5 | 5 | 6 | 0 | 1 | ||
| Parapsyche sp 1 | 5 | 5 | 6 | 0 | 1 | ||
| Trichomacronema sp 1 | 5 | 5 | 6 | 0 | 46 | ||
| Helicopsychidae ** | Helicopsyche sp 1 | 10 | 10 | 8 | 1 | 0 | |
| Odontoceridae ** | Marilia sp 1 | 10 | 10 | 7 | 18 | 3 | |
| Dipseudopsidae ** | Pseudoneureclipsis sp 1 | 10 | 10 | 9 | 4 | 6 | |
| Molannidae ** | Molanna sp 1 | 10 | 10 | N.A | 7 | 0 | |
| Leptoceridae ** | Triplectides sp 1 | 10 | 10 | 6 | 1 | 0 | |
| Stenopsychidae ** | Stenopsyche sp 1 | 10 | 10 | N.A | 0 | 5 | |
| Stenopsyche sp 2 | 10 | 10 | N.A | 0 | 2 | ||
| Odonata * | Gomphidae ** | Gomphidae sp 1 | 6 | 6 | 5 | 61 | 7 |
| Megapodagrionidae ** | Megapodagrionidae sp 1 | 6 | 6 | 5 | 3 | 2 | |
| Euphaeidae ** | Euphaeidae sp 1 | 6 | 6 | 5 | 0 | 1 | |
| Megaloptera | Corydalidae ** | Corydalidae sp 1 | 10 | 10 | 7 | 6 | 8 |
| Diptera * | Chironomidae | Orthocladinae sp 1 | 3 | 3 | 3 | 36 | 0 |
| Ceratopogonidae | Ceratopogonidae sp 1 | 3 | 3 | 4 | 1 | 0 | |
| Tipulidae (L) | Tipulidae (L) | 3 | 3 | 5 | 1 | 0 | |
| Tipulidae-Limnoninae | Tipulidae Limnoninae sp 1 | 3 | 3 | 5 | 0 | 4 | |
| Tipulidae | Antocha | 3 | 3 | 5 | 0 | 1 | |
| Athericidae | Athericidae sp 1 | N.A | 8 | 8 | 0 | 1 | |
| Coleoptera * | Noteridae ** | Noteridae sp 1 | 7 | 7 | 4 | 1 | 0 |
| Gyrinidae ** | Gyrinidae sp 1 | 7 | 7 | 4 | 1 | 0 | |
| Elmidae (L) ** | Elmidae (L) sp 1 | 8 | 8 | 7 | 20 | 1 | |
| Elmidae (L) sp 3 | 8 | 8 | 7 | 0 | 1 | ||
| Elimidae (A) | 8 | 8 | 7 | 1 | 0 | ||
| Psephenidae ** | Psephenidae sp 1 | 7 | 7 | 6 | 1 | 0 | |
| Lepidoptera * | Crambidae | Eoephyla | 5 | 5 | 3 | 0 | 1 |
| Hemiptera * | Naucoridae ** | Naucoridae sp 1 | 10 | 10 | 2 | 2 | 3 |
| Aphelocheiridae ** | Aphelocheirus sp 1 | 10 | 10 | N.A | 12 | 0 | |
| Micronectidae | Micronectidae sp 1 | 1 | 1 | 2 | 0 | 1 | |
| Mesogastropoda * | Thiaridae ** | Thiaridae sp 1 | 1 | 1 | 4 | 6 | 0 |
| Viviparidae ** | Viviparidae sp 1 | 1 | 1 | 4 | 2 | 0 | |
| Total abundance | 1291 | 659 | |||||
| Number of sites | 2 | 1 | |||||
| Nymber of occasion | 2 | 2 | |||||
| Group CM | ||||||
| Average similarity: 95.92 | ||||||
| Species | Average Abundance | Average Similarity | Similarity/standard deviation | Contribution % | Cumulative.% | |
| The Asia Foundation | 9.15 | 34.55 | 30.73 | 36.02 | 36.02 | |
| miniSASS | 9.08 | 31.9 | 14.96 | 33.26 | 69.28 | |
| Australian Waterwatch | 7.94 | 29.47 | 35.17 | 30.72 | 100 | |
| Group MT | ||||||
| Average similarity: 92.43 | ||||||
| Species | Average.Abundance | Average.Similarity | Similarity/standard deviation | Contribution % | Cumulative.% | |
| miniSASS | 9.41 | 34.17 | 13.38 | 36.97 | 36.97 | |
| The Asia Foundation | 8.85 | 32.36 | 17.64 | 35.01 | 71.98 | |
| Australian Waterwatch | 7.22 | 25.9 | 12.69 | 28.02 | 100 | |
| Groups CM & MT | ||||||
| Average dissimilarity = 6.13 | ||||||
| Group Chaungmagyi | Group Myitnge | |||||
| Species | Average Abundance | Average Abundance | Average Dissimilarity | Dissimilarity/standard deviation | Contribution% | Cumulative % |
| miniSASS | 9.08 | 9.41 | 2.5 | 1.34 | 40.83 | 40.83 |
| Australian Waterwatch | 7.94 | 7.22 | 2 | 1.11 | 32.72 | 73.55 |
| The Asia Foundation | 9.15 | 8.85 | 1.62 | 1.12 | 26.45 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, N.T.; Suter, P.; Conallin, J.; Rutten, M.; Bogaard, T. The Urgent Need for River Health Biomonitoring Tools for Large Tropical Rivers in Developing Countries: Preliminary Development of a River Health Monitoring Tool for Myanmar Rivers. Water 2020, 12, 1408. https://doi.org/10.3390/w12051408
Ko NT, Suter P, Conallin J, Rutten M, Bogaard T. The Urgent Need for River Health Biomonitoring Tools for Large Tropical Rivers in Developing Countries: Preliminary Development of a River Health Monitoring Tool for Myanmar Rivers. Water. 2020; 12(5):1408. https://doi.org/10.3390/w12051408
Chicago/Turabian StyleKo, Nyein Thandar, Phil Suter, John Conallin, Martine Rutten, and Thom Bogaard. 2020. "The Urgent Need for River Health Biomonitoring Tools for Large Tropical Rivers in Developing Countries: Preliminary Development of a River Health Monitoring Tool for Myanmar Rivers" Water 12, no. 5: 1408. https://doi.org/10.3390/w12051408
APA StyleKo, N. T., Suter, P., Conallin, J., Rutten, M., & Bogaard, T. (2020). The Urgent Need for River Health Biomonitoring Tools for Large Tropical Rivers in Developing Countries: Preliminary Development of a River Health Monitoring Tool for Myanmar Rivers. Water, 12(5), 1408. https://doi.org/10.3390/w12051408






