Alien Crayfish Species in the Deep Subalpine Lake Maggiore (NW-Italy), with a Focus on the Biometry and Habitat Preferences of the Spiny-Cheek Crayfish
Abstract
1. Introduction
2. Materials and Methods
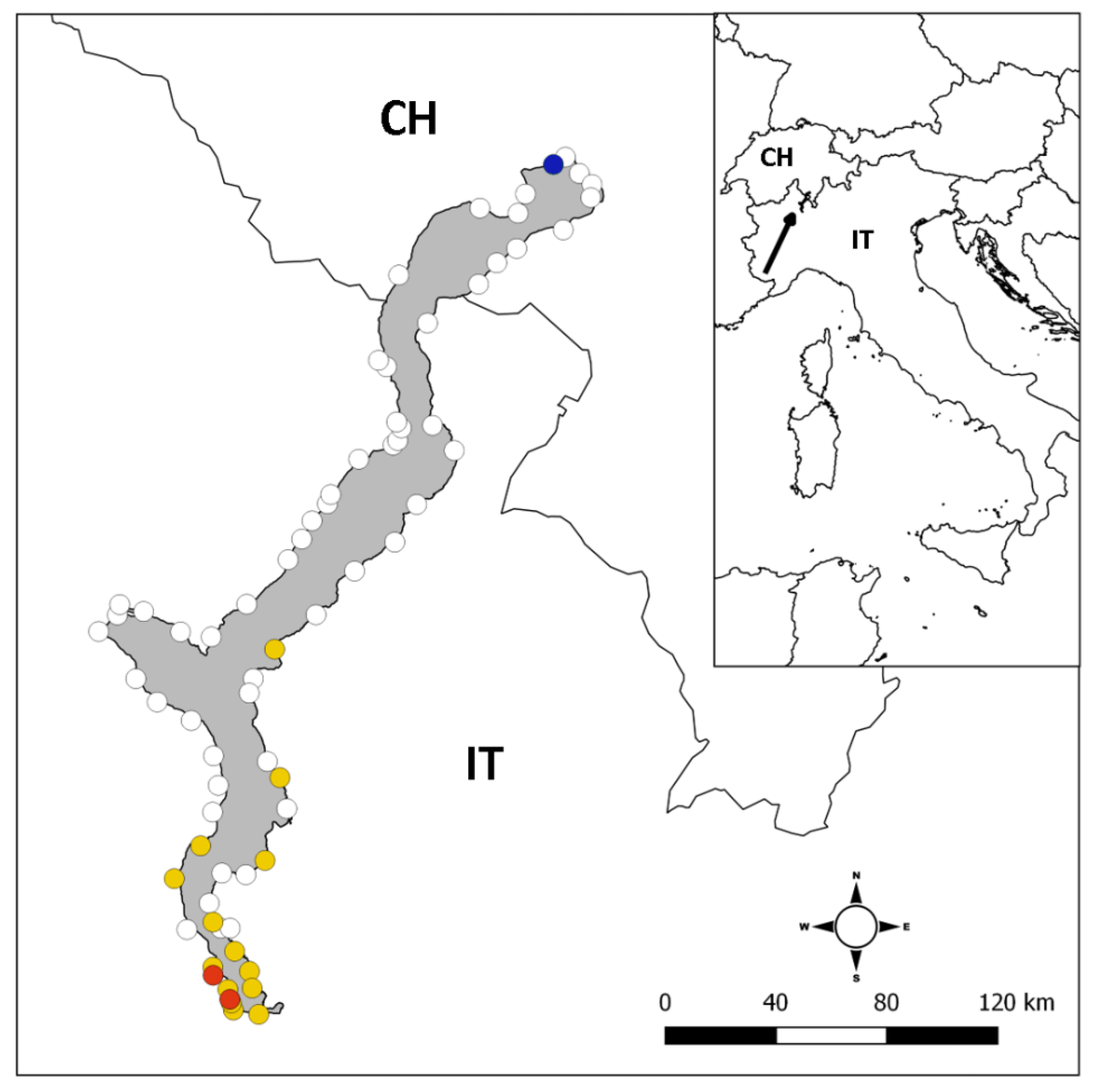
2.1. Study Site
2.2. Sampling Procedures
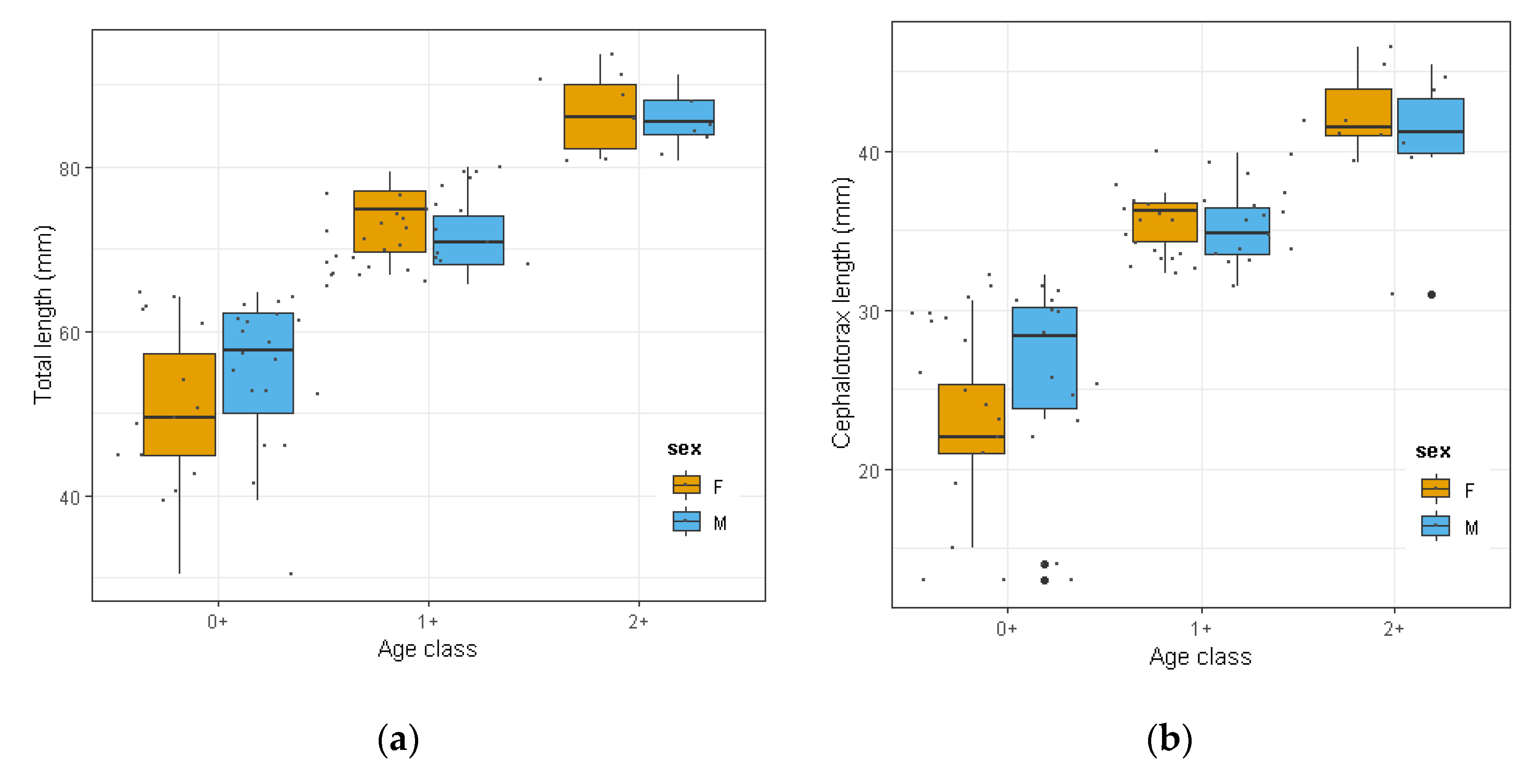
2.3. Biometric Analysis
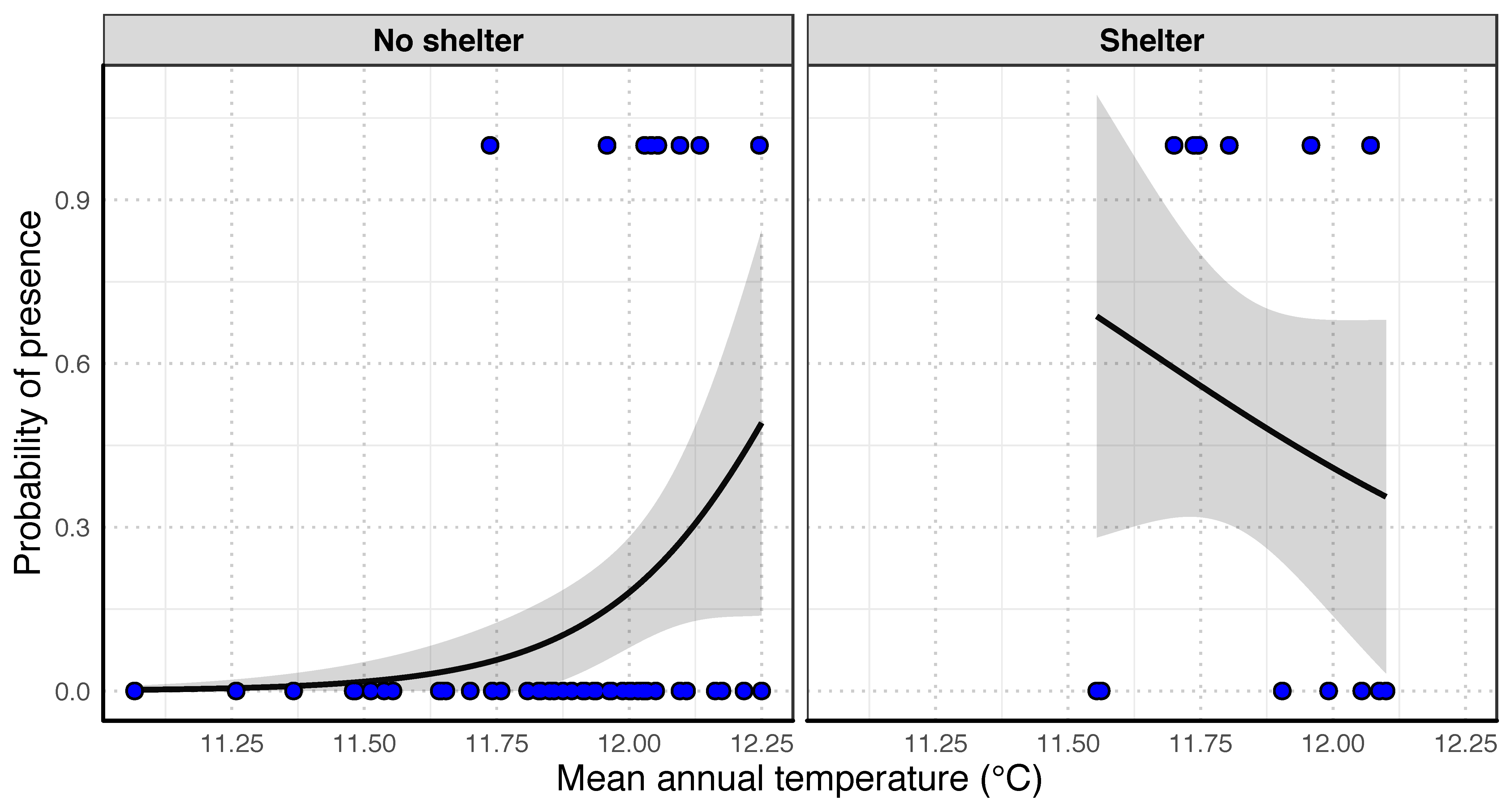
2.4. Statistical Analyses and Models
3. Results
3.1. Occurrence of Alien Crayfish
3.2. Biometric Analyses and Population Size Structure of O. Limosus
3.3. Influence of Environmental Factors on O. limosus Distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Butchart, S.H.; Walpole, M.; Collen, B.; Van Strien, A.; Scharlemann, J.P.; Almond, R.E.; Baillie, J.E.M.; Bomhard, B.; Brown, C.; Bruno, J.; et al. Global biodiversity: Indicators of recent declines. Science 2010, 328, 1164–1168. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G. Ecosystem consequences of biological invasions. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 59–80. [Google Scholar] [CrossRef]
- Bellard, C.; Cassey, P.; Blackburn, T.M. Alien species as a driver of recent extinctions. Biol. Lett. 2016, 12, 20150623. [Google Scholar] [CrossRef]
- IPBES 2019. Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. Available online: www.ipbes.net (accessed on 9 September 2019).
- Westman, K. Alien crayfish in Europe: Negative and positive impacts and interactions with native crayfish. In Invasive Aquatic Species of Europe. Distribution, Impacts and Management; Leppäkoski, E., Gollasch, S., Olenin, S., Eds.; Springer: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Gherardi, F. Crayfish in Europe as Alien Species; CRC Press: Boca Raton, FL, USA; Routledge: London, UK, 1999; p. 310. [Google Scholar]
- Cantonati, M.; Poikane, S.; Pringle, C.M.; Stevens, L.E.; Turak, E.; Heino, J.; Richardson, J.S.; Bolpagni, R.; Borrini, A.; Cid, N.; et al. Characteristics, main impacts, and stewardship of natural and artificial freshwater environments: Consequences for biodiversity conservation. Water 2020, 12, 260. [Google Scholar] [CrossRef]
- Solimini, A.; Cardoso, A.C.; Heiskanen, A.S. Indicators and methods for the Ecological Status Assessment under the Water Framework Directive. In Linkages between Chemical and Biological Quality of Surface Waters; EUR 22314 EN; European Commission: Rome, Italy, 2006; p. 248. [Google Scholar]
- Nõges, P.; van de Bund, W.; Cardoso, A.C.; Heiskanen, A.S. Impact of climatic variability on parameters used in typology and ecological quality assessment of surface waters—Implications on the Water Framework Directive. Hydrobiologia 2007, 584, 373–379. [Google Scholar] [CrossRef]
- Gherardi, F.; Aquiloni, L.; Diéguez-Uribeondo, J.; Tricarico, E. Managing invasive crayfish: Is there a hope? Aquat. Sci. 2011, 73, 185–200. [Google Scholar] [CrossRef]
- Chucholl, C.; Daudey, T. First record of Orconectes juvenilis (Hagen, 1870) in eastern France: Update to the species identity of a recently introduced orconectid crayfish (Crustacea: Astacida). Aquat. Invasions 2008, 3, 105–107. [Google Scholar] [CrossRef]
- EU 2017 Invasive Alien Species of Union Concern. Available online: https://ec.europa.eu/environment/nature/pdf/IAS_brochure_species.pdf (accessed on 27 March 2020).
- Boggero, A.; Basset, A.; Austoni, M.; Barbone, E.; Bartolozzi, L.; Bertani, I.; Campanaro, A.; Cattaneo, A.; Cianferoni, F.; Corriero, G.; et al. Weak effects of habitat type on susceptibility to invasive freshwater species: An Italian case study. Aquat. Conserv. 2014, 24, 841–852. [Google Scholar] [CrossRef]
- Colangelo, P.; Fontaneto, D.; Marchetto, A.; Ludovisi, A.; Basset, A.; Bartolozzi, L.; Bertani, I.; Campanaro, A.; Cattaneo, A.; Cianferoni, F.; et al. Alien species in Italian freshwater ecosystems: A macroecological assessment of invasion drivers. Aquat. Invasions 2017, 12, 299–309. [Google Scholar] [CrossRef]
- Guilizzoni, P.; Levine, S.N.; Manca, M.; Marchetto, A.; Lami, A.; Ambrosetti, W.; Brauer, A.; Gerli, S.; Carrara, E.A.; Rolla, A.; et al. Ecological effects of multiple stressors on a deep lake (Lago Maggiore, Italy) integrating neo and palaeolimnological approaches. J. Limnol. 2012, 71, 1–22. [Google Scholar] [CrossRef]
- Callieri, C.; Piscia, R. Photosynthetic efficiency and seasonality of autotrophic picoplankton in Lago Maggiore after its recovery. Freshw. Biol. 2002, 47, 941–956. [Google Scholar] [CrossRef]
- Mosello, R.; Lami, A. Climate Change and Related Effects on Water Quality: Examples from Lake Maggiore (Italy). Glob. Bioeth. 2011, 24, 95–98. [Google Scholar] [CrossRef][Green Version]
- Volta, P.; Jepsen, N. The recent invasion of Rutilus rutilus (L.)(Pisces: Cyprinidae) in a large South-Alpine lake: Lago Maggiore. J. Limnol. 2008, 67, 163–170. [Google Scholar] [CrossRef]
- Fenoglio, S.; Bo, T.; Cucco, M.; Mercalli, L.; Malacarne, G. Effects of global climate change on freshwater biota: A review with special emphasis on the Italian situation. Ital. J. Zool. 2010, 77, 374–383. [Google Scholar] [CrossRef]
- Kamburska, L.; Lauceri, R.; Beltrami, M.; Boggero, A.; Cardeccia, A.; Guarneri, I.; Manca, M.; Riccardi, N. Establishment of Corbicula fluminea (OF Müller, 1774) in Lake Maggiore: A spatial approach to trace the invasion dynamics. Bioinvasions Rec. 2013, 2, 105–117. [Google Scholar] [CrossRef]
- Delmastro, G.B. Il gambero della Louisiana Procambarus clarkii (Girard, 1852) in Piemonte: Nuove osservazioni su distribuzione, biologia, impatto e utilizzo (Crustacea: Decapoda: Cambaridae). Rivista Piemontese Di Storia Naturale 2017, 38, 61–129. [Google Scholar]
- Bazzoni, P. Censimento E Studio Delle Popolazioni Di Gambero d’Acqua Dolce Nell’Area Del Verbano-Cusio-Ossola; Azienda Agricola Ossolana Acque: Verbano, Italy, 2006; p. 25. [Google Scholar]
- Saidi, H.; Ciampittiello, M.; Dresti, C.; Ghiglieri, G. Assessment of trends in extreme precipitation events: A case study in Piedmont (North-West Italy). Water Resour. Manage. 2015, 29, 63–80. [Google Scholar] [CrossRef]
- Bain, M.B.; Stevenson, N.J. Aquatic habitat assessment. In Asian Fisheries Society; Bethesda: Rockville, MD, USA, 1999. [Google Scholar]
- Bonk, M.; Bobrek, R.; Dołęga, J.; Strużyński, W. Evaluation of visual encounter surveys of the noble crayfish, Astacus astacus, and the spiny-cheek crayfish, Orconectes limosus. Fish. Aquat. Life 2019, 27, 112–117. [Google Scholar] [CrossRef]
- Tricarico, E.; Inghilesi, A.F.; Ferretti, G.; Johovic, I.; Ruberti, V.; Scapini, F. Monitoraggio e quarto intervento di controllo del gambero rosso della Louisiana Procambarus clarkii; Quinta relazione tecnica A2C2 per il progetto LIFE+ 2011 SOS TUSCAN WETLANDS; Italy, 2017; Available online: http://www.life-sostuscanwetlands.eu/index.php/it/pubblicazioni/ (accessed on 1 March 2020).
- Boggero, A.; Dugaro, M.; Migliori, L.; Garzoli, L. Prima segnalazione del gambero invasivo Pacifastacus leniusculus (Dana 1852) nel Lago Maggiore (Cantone Ticino, Svizzera). Bollettino Della Società Ticinese Di Scienze Naturali 2018, 106, 103–106. [Google Scholar]
- Souty-Grosset, C.; Holdich, D.M.; Noël, P.Y.; Reynolds, J.D.; Haffner, P. Atlas of Crayfish in Europe; Muséum national d’Histoire naturelle: Paris, France, 2006; p. 187. [Google Scholar]
- Pieplow, U. Fischereiwissenschaftliche Monographie Von Cambarus Affinis; Say. Neumann: Berlin, Germany, 1939; p. 92. [Google Scholar]
- R Core Team R: A language and environment for statistical computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. Available online: https://www.R-project.org/ (accessed on 13 May 2020).
- Wickham, H.; Chang, W.; Wickham, M.H. Package ’ggplot2’. In Create Elegant Data Visualisations Using the Grammar of Graphics; 2016; Volume 2, pp. 1–189. Available online: https://ggplot2.tidyverse.org/ (accessed on 13 May 2020).
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N. A protocol for conducting and presenting results of regression-type analyses. Methods Ecolo. Evol. 2016, 7, 636–645. [Google Scholar] [CrossRef]
- . Zuur, A.F.; Ieno, E.N.; Elphick, S.C. A protocol for data exploration to avoid common statistical problem. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Savaliev, A.A.; Smith, G.M. Mixed Effect Models and Extensions in Ecology with R.; Springer: Berlin, Germany, 2009; p. 574. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2002; p. 488. [Google Scholar]
- Johnson, J.B.; Omland, K.S. Model selection in ecology and evolution. Trends Ecol. Evol. 2004, 19, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Hurvich, C.M.; Tsai, C.L. Regression and time series model selection in small samples. Biometrika 1989, 76, 297–307. [Google Scholar] [CrossRef]
- Howkins, D.M. The problem of overfitting. J. Chem. Inf. Comput. Sci. 2004, 44, 1–12. [Google Scholar] [CrossRef]
- Hefti, D.; Stucki, P. Crayfish management for Swiss waters. Bulletin Français De La Pêche Et De La Pisciculture 2006, 380–381, 937–950. [Google Scholar] [CrossRef]
- Maddalena, T.; Zanini, M.; Torriani, D.; Marchesi, P.; Jann, B.; Paltrinieri, L. Inventario dei gamberi d’acqua dolce del Cantone Ticino (Svizzera). Bollettino Della Società Ticinese Di Scienze Naturali 2009, 97, 19–25. [Google Scholar]
- Vedia, I.; Galicia, D.; Baquero, E.; Oscoz, J.; Miranda, R. Environmental factors influencing the distribution and abundance of the introduced signal crayfish in the north of Iberian Peninsula. Mar. Freshw. Res. 2017, 68, 900–908. [Google Scholar] [CrossRef]
- Aquiloni, L.; Tricarico, E.; Gherardi, F. Crayfish in Italy: Distribution, threats and management. Int. Aquat. Res. 2010, 2, 1–14. [Google Scholar]
- Maceda-Veiga, A.; De Sostoa, A.; Sanchez-Espada, S. Factors affecting the establishment of the invasive crayfish Procambarus clarkii (Crustacea, Decapoda) in the Mediterranean rivers of the northeastern Iberian Peninsula. Hydrobiologia 2013, 703, 33–45. [Google Scholar] [CrossRef]
- Piscia, R.; Volta, P.; Boggero, A.; Manca, M. The invasion of Lake Orta (Italy) by the red swamp crayfish Procambarus clarkii (Girard, 1852): A new threat to an unstable environment. Aquat. Invasions 2011, 6, S45–S48. [Google Scholar] [CrossRef]
- Gherardi, F.; Baldaccini, G.N.; Barbaresi, S.; Ercolini, P.; De Luise, G.; Mazzoni, D.; Mori, M. The situation in Italy. In Crayfish in Europe as Alien Species. How to Make the Best of a Bad Situation? Gherardi, F., Holdich, D.M., Balkema, A.A., Eds.; Crustacean Issues: Rotterdam, The Netherlands, 1999; pp. 107–128. [Google Scholar]
- Groppali, R Sulla presenza del gambero americano Orconectes limosus (Rafinesque) in acque della Pianura Pavese (Crustacea Decapoda Cambaridae). Riv. Piem. St. Nat. 1993, 14, 93–96.
- Englund, G.; Krupa, J.J. Habitat use by crayfish in stream pools: Influence of predators, depth and body size. Freshw. Biol. 2000, 43, 75–83. [Google Scholar] [CrossRef]
- Vlach, P.; Valdmanová, L. Morphometry of the stone crayfish (Austropotamobius torrentium) in the Czech Republic: Allometry and sexual dimorphism. Knowl. Manag. Aquat. Ec. 2015, 416, 16–50. [Google Scholar] [CrossRef]
- Ďuriš, Z.; Drozd, P.; Horká, I.; Kozák, P.; Policar, T. Biometry and demography of the invasive crayfish Orconectes limosus in the Czech Republic. Bulletin Français De La Pêche Et De La Pisciculture 2006, 380–381, 1215–1228. [Google Scholar] [CrossRef]
- Pilotto, F.; Free, G.; Crosa, G.; Sena, F.; Ghiani, M.; Cardoso, A.C. The invasive crayfish Orconectes limosus in Lake Varese: Estimating abundance and population size structure in the context of habitat and methodological constraints. J. Crustacean Biol. 2008, 28, 633–640. [Google Scholar] [CrossRef][Green Version]
- Buřič, M.; Kouba, A.; Kozák, P. Intra-sex dimorphism in crayfish females. Zoology 2010, 113, 301–307. [Google Scholar] [CrossRef]
- Usio, N.; Nakajima, H.; Kamiyama, R.; Wakana, I.; Hiruta, S.; Takamura, N. Predicting the distribution of invasive crayfish (Pacifastacus leniusculus) in a Kusiro Moor marsh (Japan) using classification and regression trees. Ecol. Res. 2006, 21, 271–277. [Google Scholar] [CrossRef]
- Liu, X.; Guo, Z.; Ke, Z.; Wang, S.; Li, Y. Increasing potential risk of a global aquatic invader in Europe in contrast to other continents under future climate change. PLoS ONE 2011, 6, e18429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Capinha, C.; Usio, N.; Weterings, R.; Liu, X.; Li, Y.; Landeira, J.; Zhou, Q.; Yokota, M. Impacts of climate change on the global potential distribution of two notorious invasive crayfishes. Freshw. Biol. 2020, 65, 353–365. [Google Scholar] [CrossRef]
- Zhang, Z.; Mammola, S.; McLay, C.L.; Capinha, C.; Yokota, M. To invade or not to invade? Exploring the niche-based processes underlying the failure of a biological invasion using the invasive Chinese mitten crab. Sci. Total Environ. 2020, 728, 138815. [Google Scholar] [CrossRef] [PubMed]
- Jowett, I.G.; Parkyn, S.M.; Richardson, J. Habitat characteristics of crayfish (Paranephrops planifrons) in New Zealand streams using generalised additive models (GAMs). Hydrobiologia 2008, 596, 353–365. [Google Scholar] [CrossRef]
- Holdich, D.; Black, J. The spiny-cheek crayfish, Orconectes limosus (Rafinesque, 1817)Crustacea: Decapoda: Cambaridae., digs into the UK. Aquat. Invasions 2007, 2, 1–15. [Google Scholar] [CrossRef]
- Momot, W.T. Orconectes in North America and elsewhere. In Freshwater Crayfish: Biology, Management and Exploitation; Holdich, D.M., Lowery, R.S., Eds.; Croom Helm Ltd.: Kent, UK, 1998; pp. 262–283. [Google Scholar]
- Fulton, C.J.; Starrs, D.; Ruibal, M.P.; Ebner, B.C. Counting crayfish: Active searching and baited cameras trump conventional hoop netting in detecting Euastacus armatus. Endanger Species Res. 2012, 19, 39–45. [Google Scholar] [CrossRef][Green Version]
- Alderman, D.J.; Polglase, J.L. Aphanomyces astaci: Isolation and culture. J. Fish Dis. 1986, 9, 367–379. [Google Scholar] [CrossRef]


| Age | Body Length (mm) |
|---|---|
| 0+ | Up to 40–65 |
| 1+ | 65–80 |
| 2+ | 80–95 |
| 3+ | 95–110 |
| Sex | Total Body Lenght | Total Weight | Cephalo-Thorax Length | ||||||
|---|---|---|---|---|---|---|---|---|---|
| L min | L max | L mean | W min | W max | W mean | CL min | CL max | CL mean | |
| M | 3.94 | 10.57 | 6.66 ± 1.19 | 1.75 | 27.20 | 10.07 ± 5.37 | 1.3 | 4.54 | 3.15 ± 0.73 |
| F | 3.04 | 9.88 | 6.86 ± 1.71 | 0.80 | 30.43 | 11.30 ± 7.47 | 1.1 | 4.89 | 3.24 ± 0.98 |
| Total M+F | 3.04 | 10.57 | 6.71 ± 1.35 | 0.80 | 30.43 | 10.41 ± 6.03 | 1.1 | 4.89 | 3.18 ± 0.80 |
| Model Structure | Df | AICc | ∆AICc | wi(AIC) |
|---|---|---|---|---|
| y ~ Tmean * Shelters + Substrate + Algae + Macrophytes | 8 | 74.00 | 5.27 | 0.02 |
| y ~ Tmean * Shelters + Algae + Macrophytes | 6 | 70.27 | 1.54 | 0.18 |
| y ~ Tmean * Shelters + Macrophytes | 5 | 68.81 | 0.08 | 0.38 |
| y ~ Tmean * Shelters | 4 | 68.73 | 0.00 | 0.40 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garzoli, L.; Mammola, S.; Ciampittiello, M.; Boggero, A. Alien Crayfish Species in the Deep Subalpine Lake Maggiore (NW-Italy), with a Focus on the Biometry and Habitat Preferences of the Spiny-Cheek Crayfish. Water 2020, 12, 1391. https://doi.org/10.3390/w12051391
Garzoli L, Mammola S, Ciampittiello M, Boggero A. Alien Crayfish Species in the Deep Subalpine Lake Maggiore (NW-Italy), with a Focus on the Biometry and Habitat Preferences of the Spiny-Cheek Crayfish. Water. 2020; 12(5):1391. https://doi.org/10.3390/w12051391
Chicago/Turabian StyleGarzoli, Laura, Stefano Mammola, Marzia Ciampittiello, and Angela Boggero. 2020. "Alien Crayfish Species in the Deep Subalpine Lake Maggiore (NW-Italy), with a Focus on the Biometry and Habitat Preferences of the Spiny-Cheek Crayfish" Water 12, no. 5: 1391. https://doi.org/10.3390/w12051391
APA StyleGarzoli, L., Mammola, S., Ciampittiello, M., & Boggero, A. (2020). Alien Crayfish Species in the Deep Subalpine Lake Maggiore (NW-Italy), with a Focus on the Biometry and Habitat Preferences of the Spiny-Cheek Crayfish. Water, 12(5), 1391. https://doi.org/10.3390/w12051391







