Enhancement of the Activity of Electrochemical Oxidation of BPS by Nd-Doped PbO2 Electrodes: Performance and Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Electrodes Preparation
2.3. Characterizations of Electrodes
2.4. Electrochemical Oxidation of BPS
2.5. Analytical Methods
3. Results and Discussion
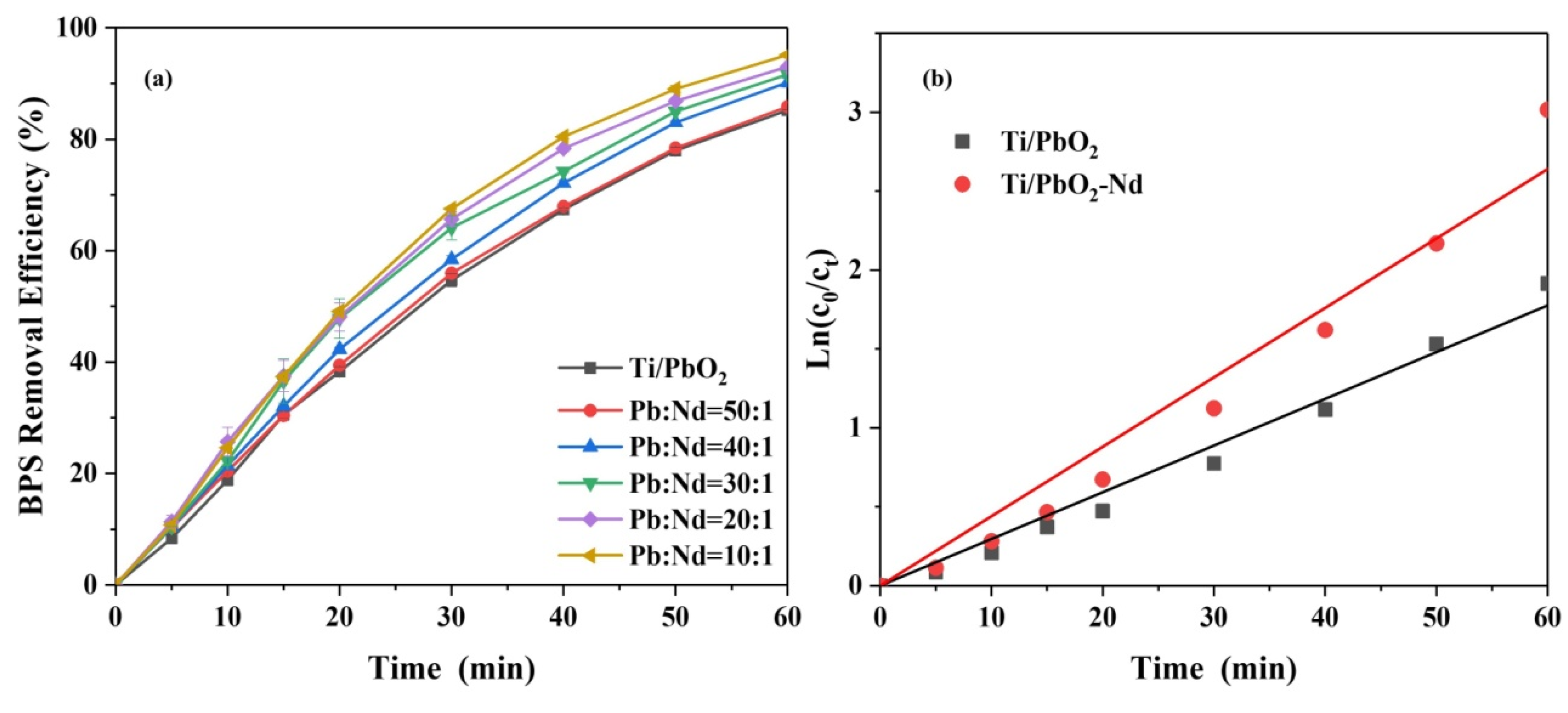
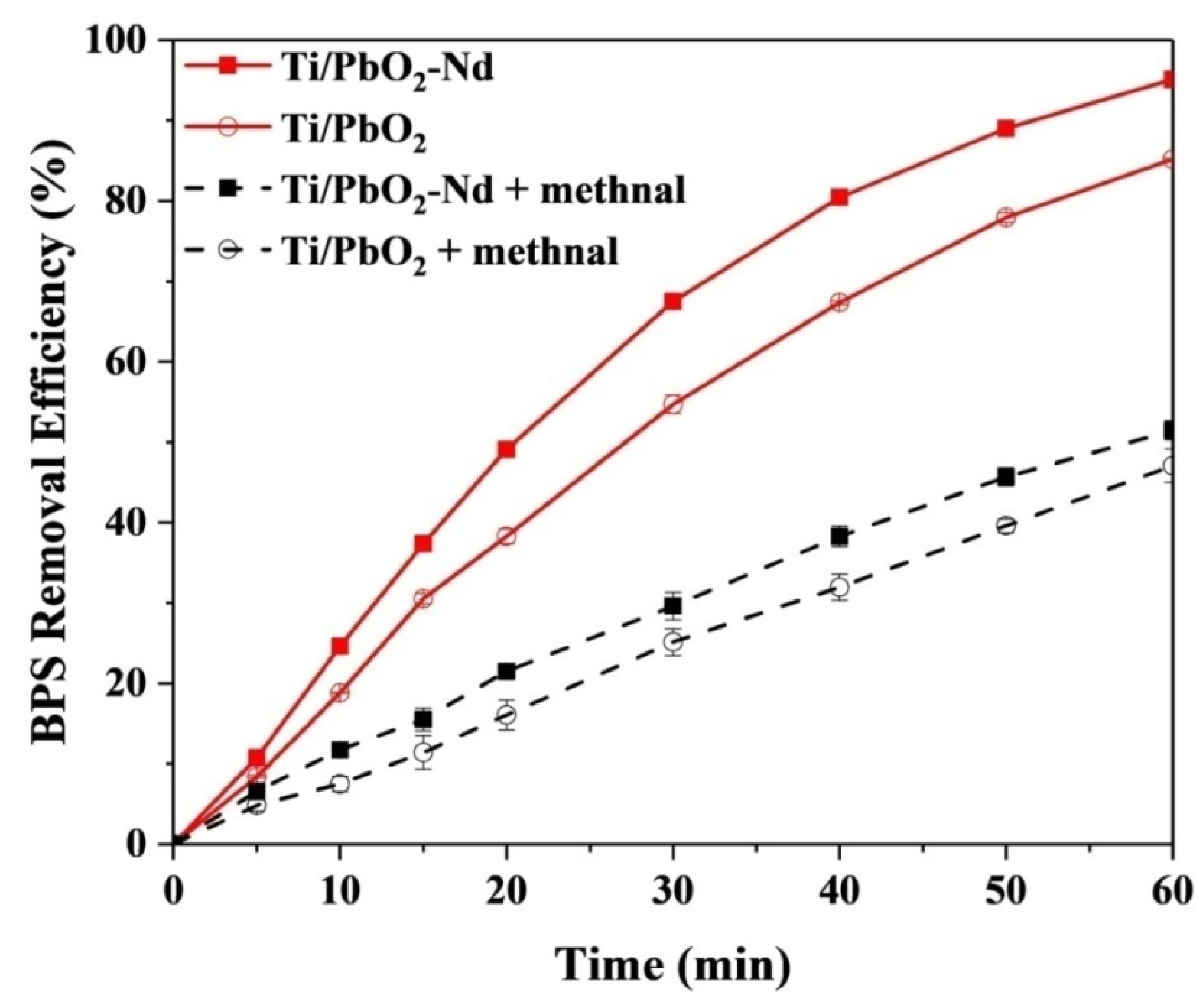
3.1. Electrode Optimization
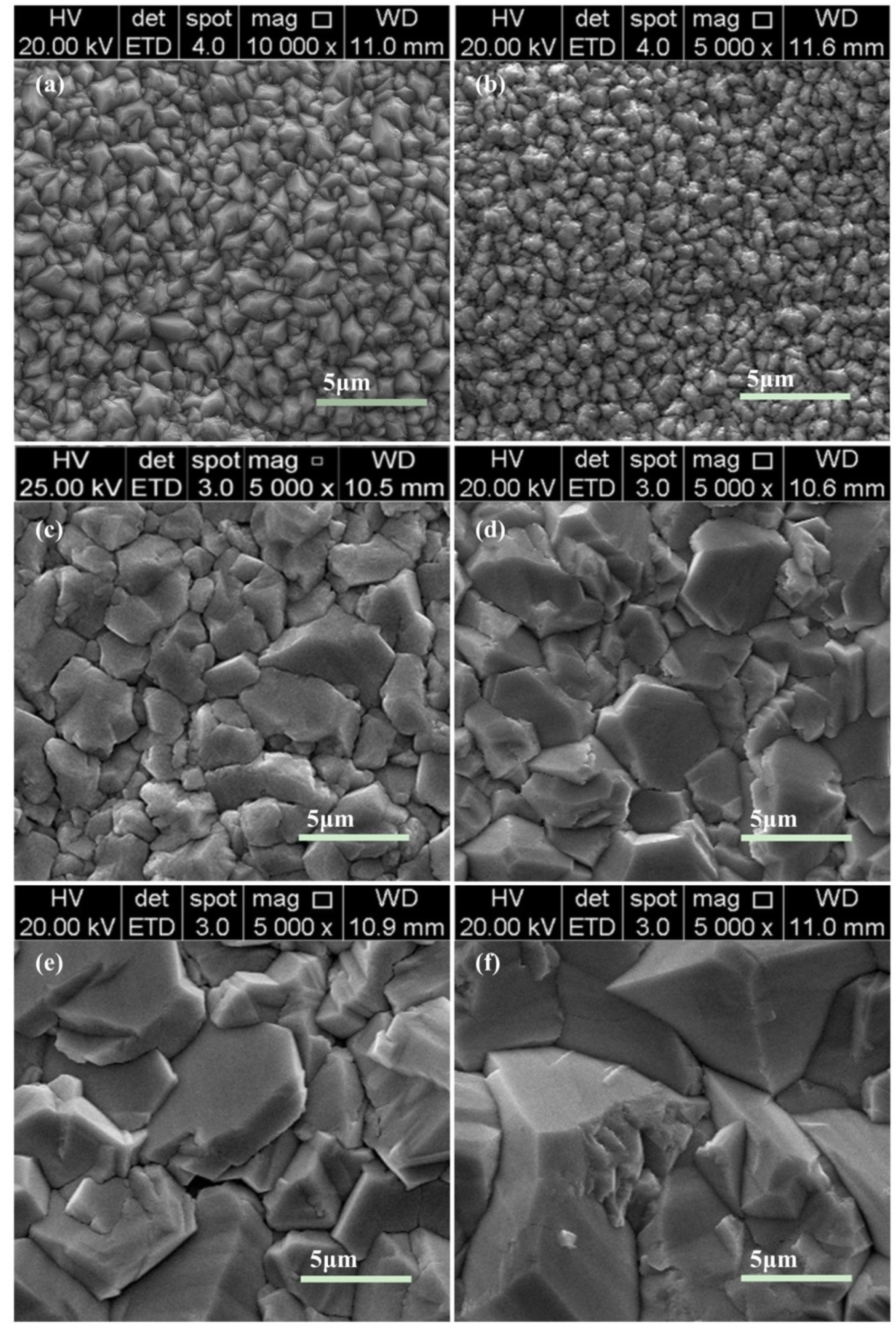
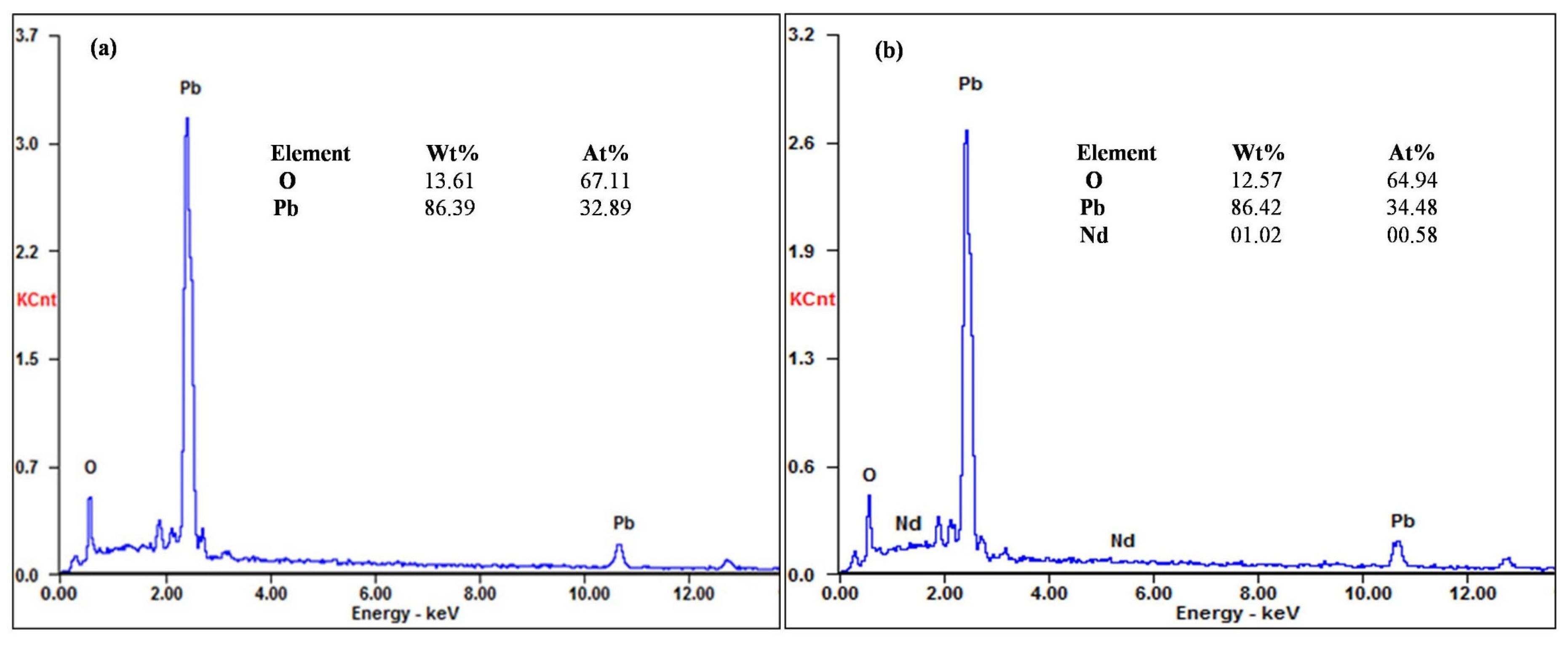
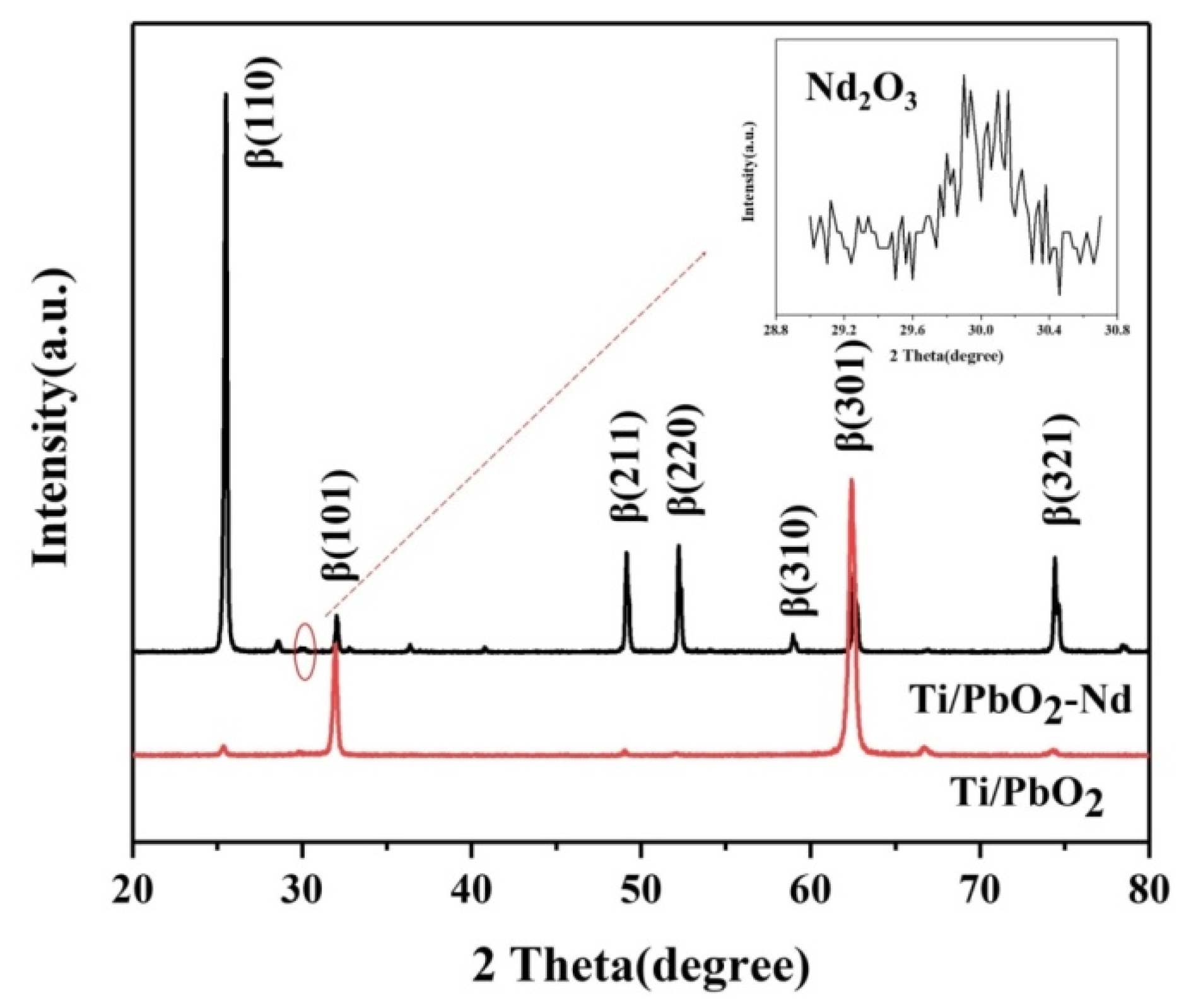
3.2. Morphology and Structure of Ti/PbO2-Nd Electrodes
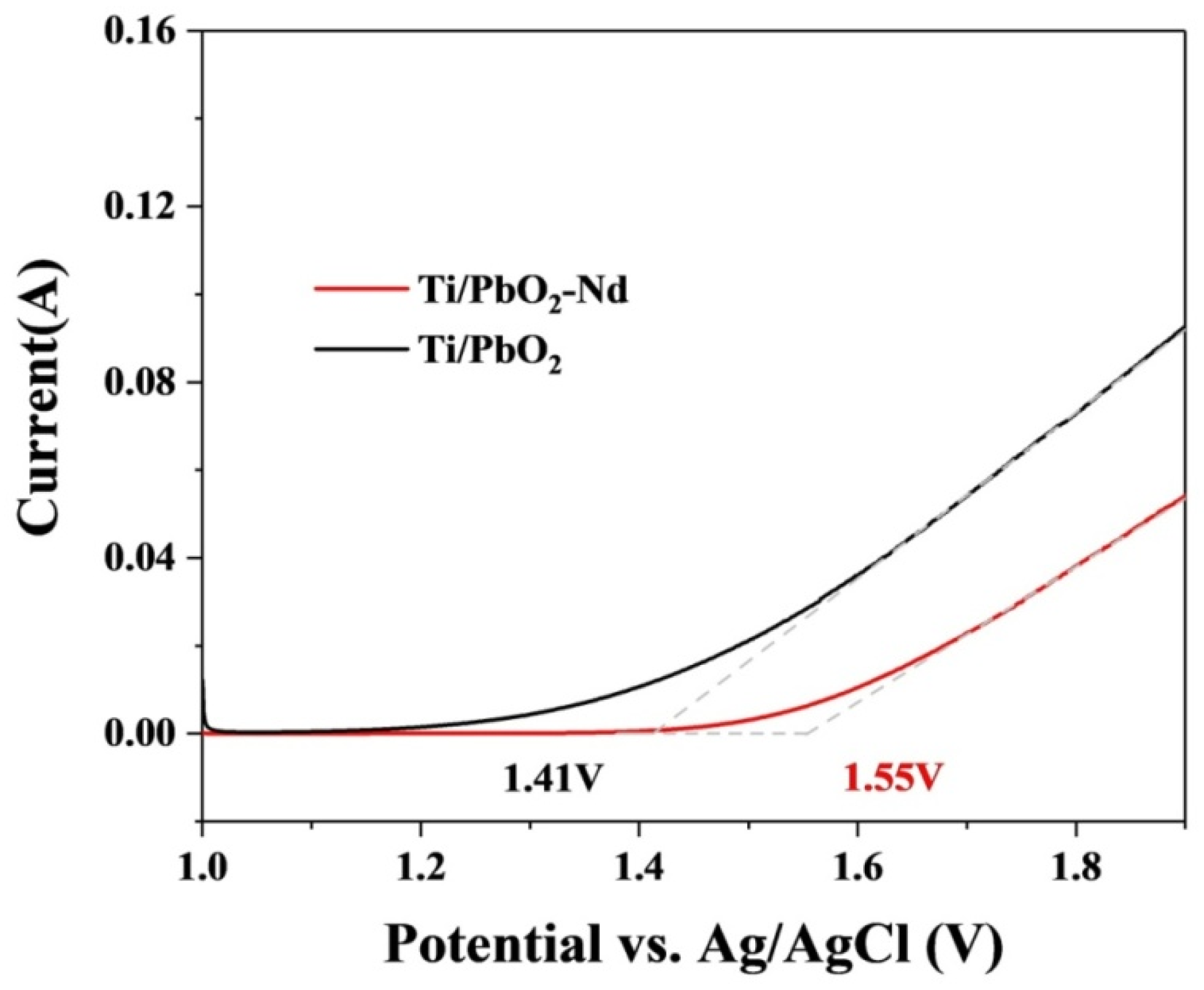
3.3. Electrochemical Property of Ti/PbO2-Nd Electrodes
3.4. Energy Consumption
3.5. Reusability Evaluation
3.6. Electrochemical Oxidation of BPS
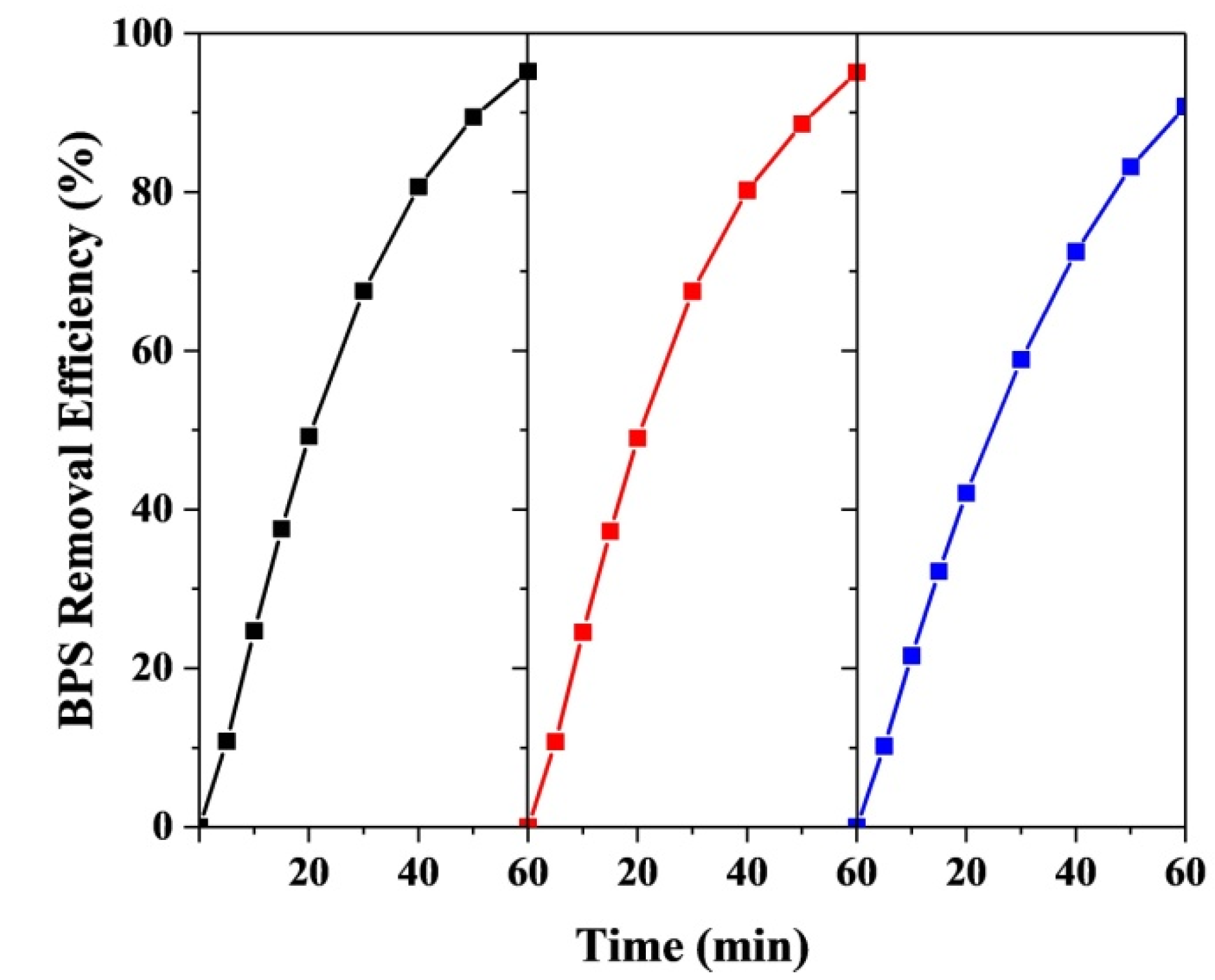
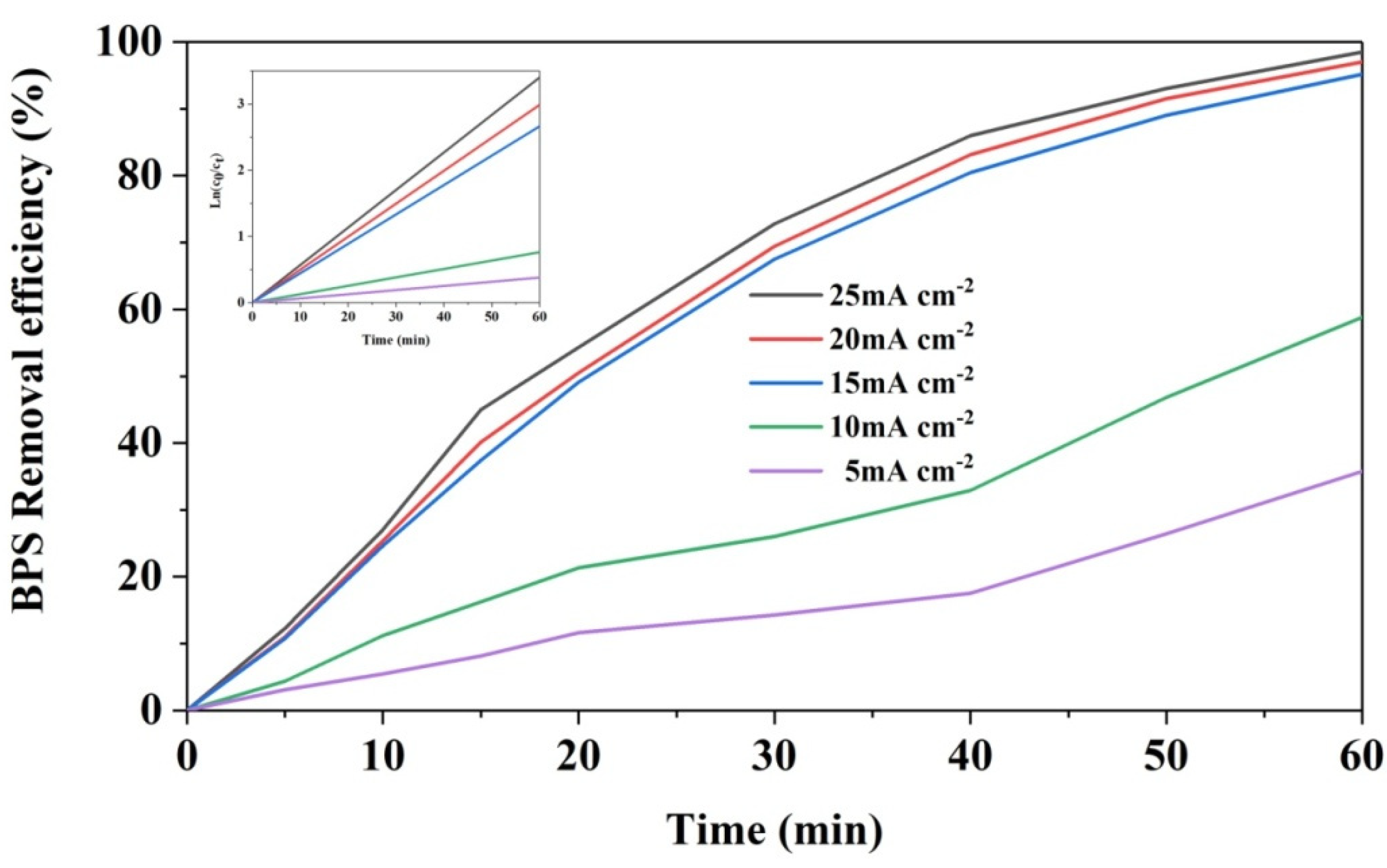
3.6.1. Effect of Current Density on BPS Removal
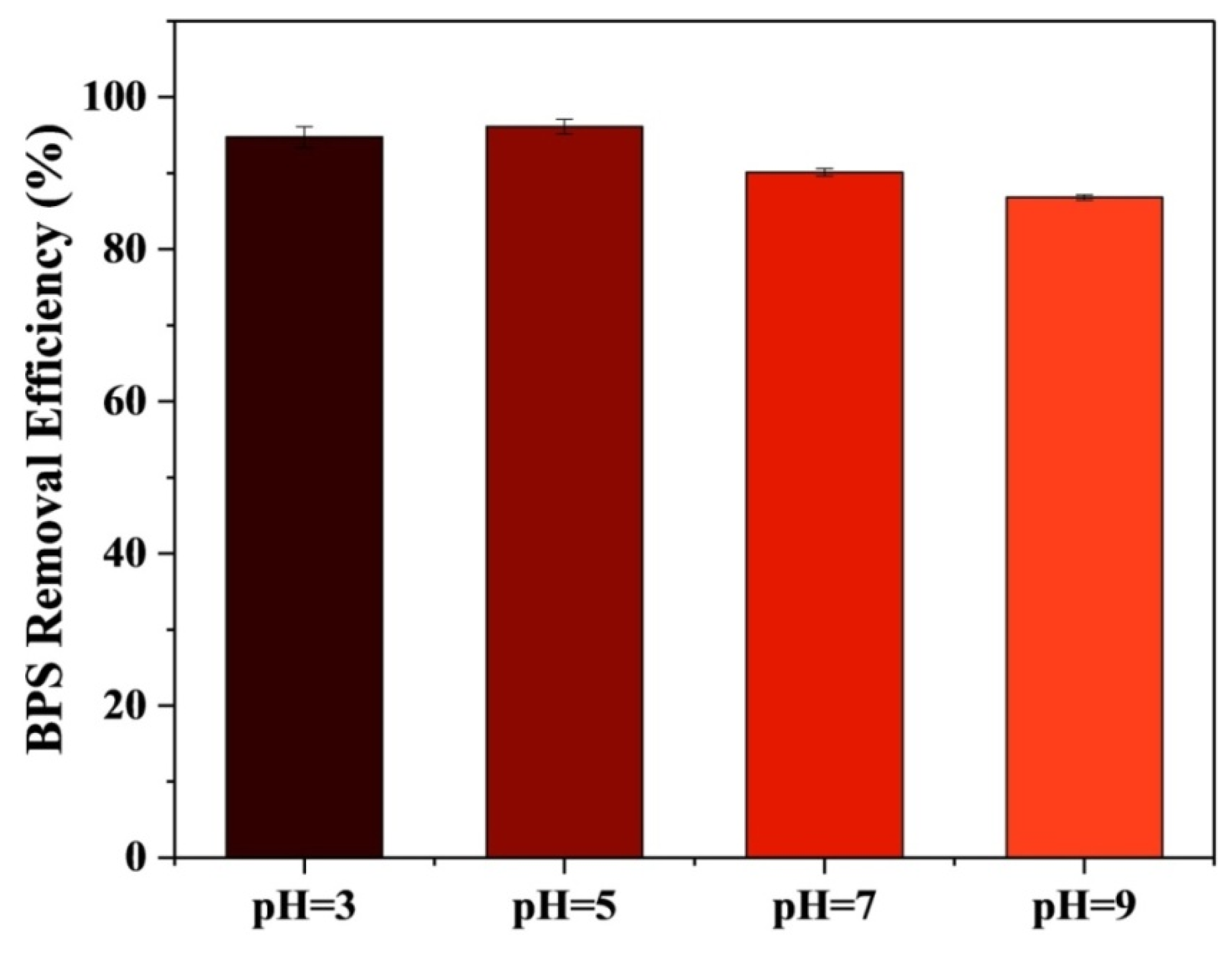
3.6.2. Effect of pH on BPS Removal
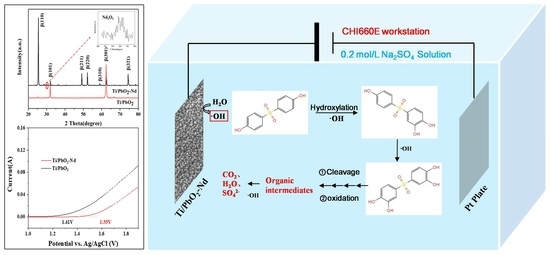
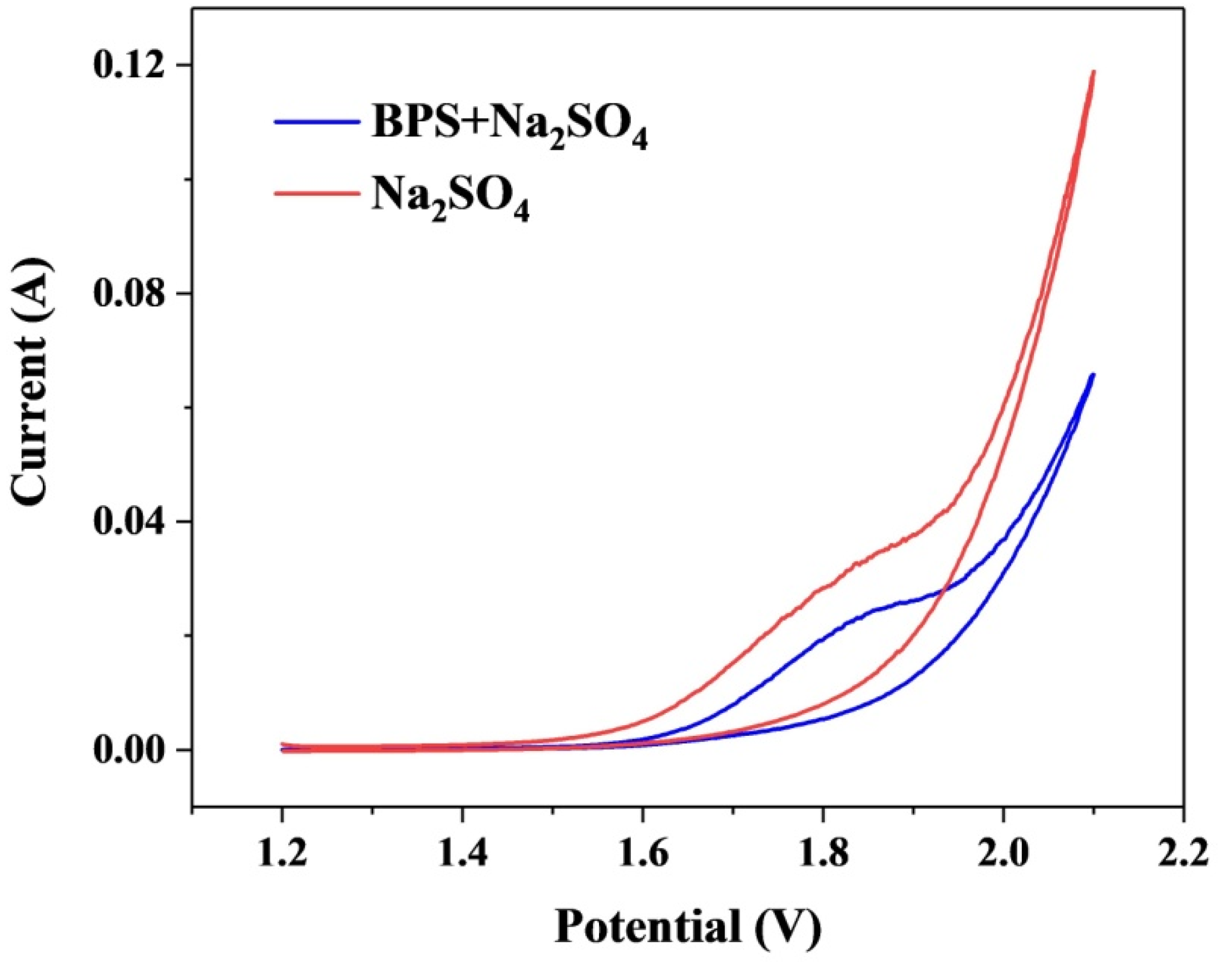
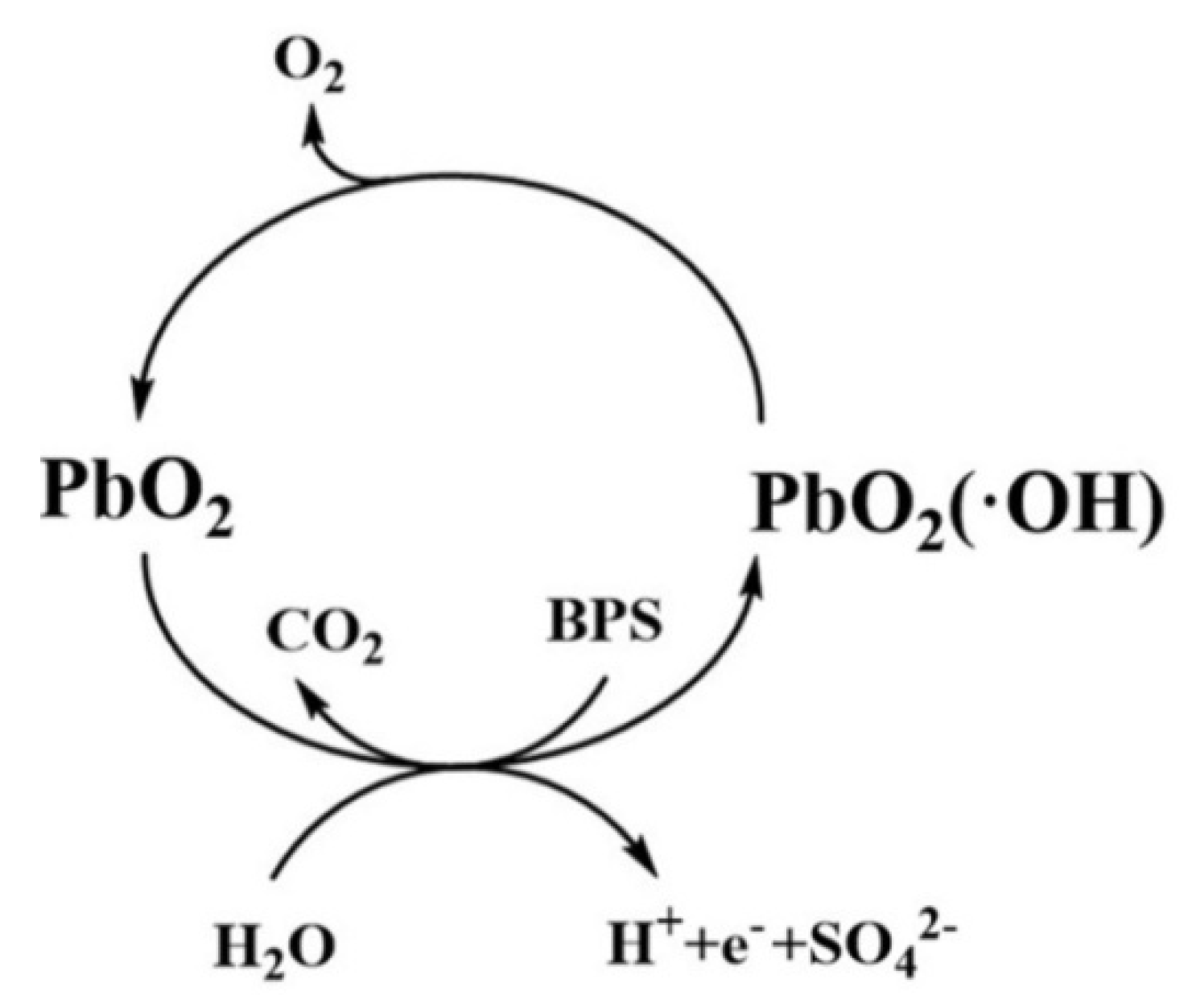
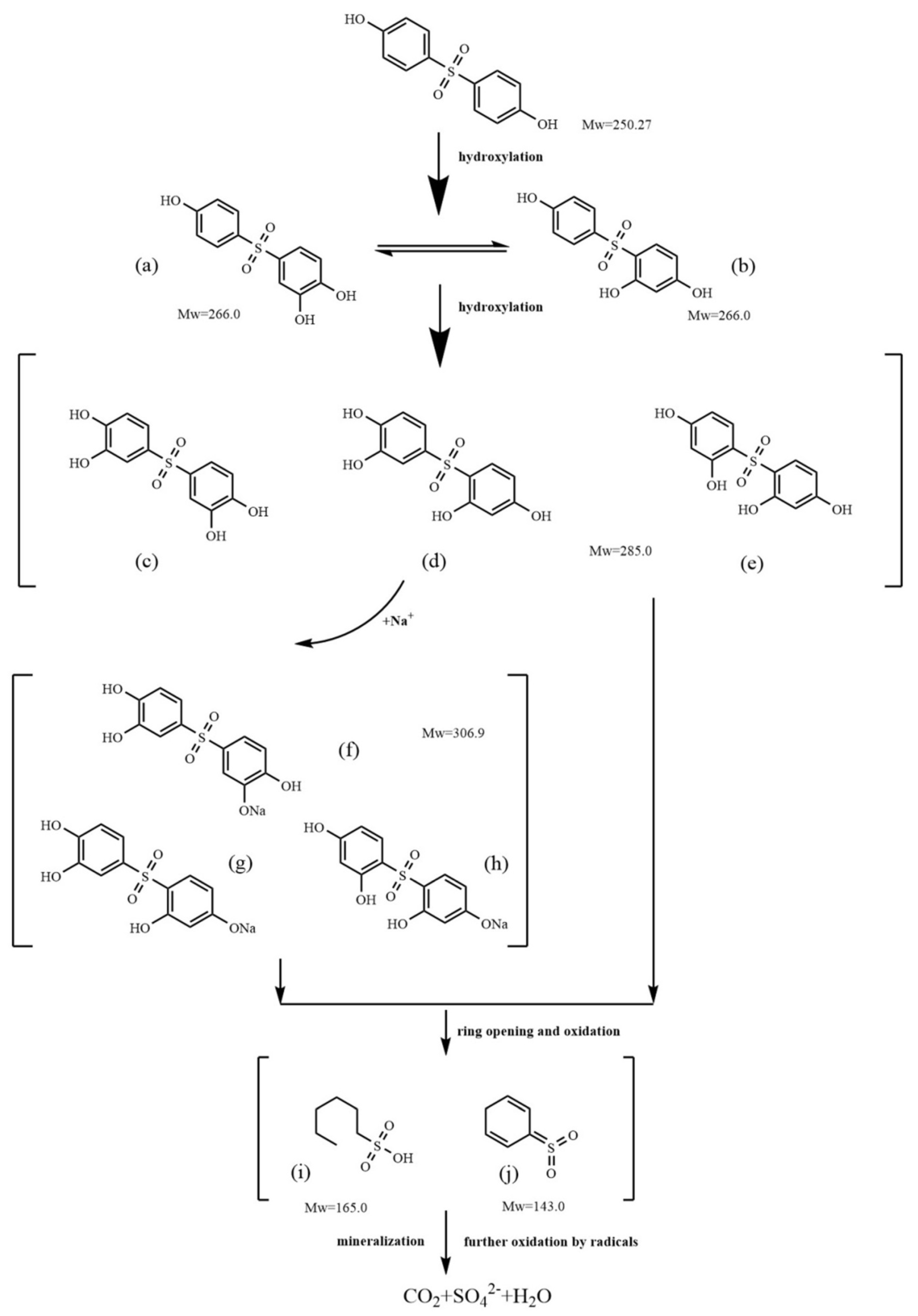
3.7. Mechanism of the Electrochemical Oxidation of BPS by Ti/PbO2-Nd
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, Q.Q.; Jia, J.B.; Wang, Y.; Zhang, K.G.; Zhang, H.; Liao, C.Y.; Jiang, G.B. Spatial distribution of parabens, triclocarban, triclosan, bisphenols, and tetrabromobisphenol A and its alternatives in municipal sewage sludges in China. Sci. Total Environ. 2019, 679, 61–69. [Google Scholar] [CrossRef]
- Shao, P.H.; Ren, Z.J.; Tian, J.Y.; Gao, S.S.; Luo, X.B.; Shi, W.X.; Yan, B.Y.; Li, J.; Cui, F.Y. Silica hydrogel-mediated dissolution-recrystallization strategy for synthesis of ultrathin A-Fe2O3 nanosheets with highly exposed (1 1 0) facets: A superior photocatalyst for degradation of bisphenol S. Chem. Eng. J. 2017, 323, 64–73. [Google Scholar] [CrossRef]
- Liao, C.Y.; Liu, F.; Moon, H.B.; Yamashita, N.; Yun, S.H.; Kannan, K. Bisphenol analogues in sediments from industrialized areas in the United States, Japan, and Korea: Spatial and temporal distributions. Environ. Sci. Technol. 2012, 46, 11558–11565. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.B.; Zhu, L.Y. Occurrence and partitioning of bisphenol analogues in water and sediment from Liaohe River Basin and Taihu Lake, China. Water Res. 2016, 103, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.; Sreedhanya, S.; Manoj, P.; Aravindakumar, C.T.; Aravind, U.K. Exploring the interaction of bisphenol-S with serum albumins: A better or worse alternative for bisphenol A? J. Phys. Chem. B 2014, 118, 3832–3843. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.Y.; Liu, F.; Alomirah, H.; Loi, V.D.; Mohd, M.A.; Moon, H.B.; Nakata, H.; Kannan, K. Bisphenol S in urine from the United States and seven Asian countries: Occurrence and human exposures. Environ. Sci. Technol. 2012, 46, 6860–6866. [Google Scholar] [CrossRef]
- Eladak, S.; Grisin, T.; Moison, D.; Guerquin, M.J.; N’Tumba-Byn, T.; Pozzi-Gaudin, S.; Benachi, A.; Livera, G.; Rouiller-Fabre, V.; Habert, R. A new chapter in the bisphenol a story: Bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil. Steril. 2015, 103, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.Q.; Zhang, H.M. Effects of bisphenol s on the structures and activities of trypsin and pepsin. J. Agric. Food Chem. 2014, 62, 11303–11311. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, H.G.; Zhang, Y.L.; Cheng, X.; Zhou, P.; Wang, J.Q.; Li, W. Fe@C carbonized resin for peroxymonosulfate activation and bisphenol S degradation. Environ. Pollut. 2019, 252, 1042–1050. [Google Scholar] [CrossRef]
- Zhang, T. Research Progress on Peroxymonosulfate Activation Technology. Guangdong Chem. Ind. 2019, 46, 122–123. [Google Scholar]
- López-Ramón, M.V.; Ocampo-Pérez, R.; Bautista-Toledo, M.I.; Rivera-Utrilla, J.; Moreno-Castilla, C.; Sánchez-Polo, M. Removal of bisphenols A and S by adsorption on activated carbon clothes enhanced by the presence of bacteria. Sci. Total Environ. 2019, 669, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, H.Y.; Liu, W.J.; Tu, Y.; Zhang, Y.H.; Han, W.Q.; Wang, L.J. Electrochemical degradation of nitrobenzene by anodic oxidation on the constructed TiO2-NTs/SnO2-Sb/PbO2 electrode. Chemosphere. 2014, 113, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.N.; Huang, W.M.; Wang, X.; Gao, Y.; Lin, H.B. Feasibility and advantage of biofilm-electrode reactor for phenol degradation. J. Environ. Sci. 2009, 21, 1181–1185. [Google Scholar] [CrossRef]
- Chen, J.M.; Xia, Y.J.; Dai, Q.Z. Electrochemical degradation of chloramphenicol with a novel Al doped PbO2 electrode: Performance, kinetics and degradation mechanism. Electrochim. Acta 2015, 165, 277–287. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Q.; Yan, W.; Chu, W.; Zhang, L.F. Preparation and characterization of PbO2 electrodes doped with TiO2 and its degradation effect on azo dye wastewater. Int. J. Electrochem. Sci. 2013, 8, 5382–5395. [Google Scholar]
- Li, X.L.; Li, X.M.; Yang, W.J.; Chen, X.H.; Li, W.L.; Luo, B.B.; Wang, K.L. Preparation of 3D PbO2 nanospheres@SnO2 nanowires/Ti Electrode and Its Application in Methyl Orange Degradation. Electrochim. Acta 2014, 146, 15–22. [Google Scholar] [CrossRef]
- Kotyk, J.F.K.; Chen, C.; Sheehan, S.W. Corrosion Potential Modulation on Lead Anodes Using Water Oxidation Catalyst Coatings. Coatings. 2018, 8, 246. [Google Scholar] [CrossRef] [Green Version]
- Li, S.P.; Wang, H.B.; Qiao, P.; Zhou, X.Y. Study on the treatment of printing and dyeing wastewater with the new type of Ni—Doped PbO2/Ti electrode. Ind. Water Treat. 2008, 28, 48–51. [Google Scholar]
- Xu, H.; Li, J.J.; Yan, W.; Chu, W. Preparation and characterization of titanium based PbO2 electrodes doped with some common elements. Rare Met. Mater. Eng. 2013, 42, 885–890. [Google Scholar]
- Dai, Q.Z.; Zhou, J.Z.; Weng, M.L.; Luo, X.B.; Feng, D.L.; Chen, J.M. Electrochemical oxidation metronidazole with Co modified PbO2 electrode: Degradation and mechanism. Sep. Purif. Technol. 2016, 166, 109–116. [Google Scholar] [CrossRef]
- Dai, Q.Z.; Xia, Y.J.; Chen, J.M. Mechanism of enhanced electrochemical degradation of highly concentrated aspirin wastewater using a rare earth La-Y co-doped PbO2 electrode. Electrochim. Acta 2016, 188, 871–881. [Google Scholar] [CrossRef]
- Xu, M.; Wang, Z.C.; Wang, F.W.; Hong, P.; Wang, C.Y.; Ouyang, X.M.; Zhu, C.G.; Wei, Y.J.; Hun, Y.H.; Fang, W.Y. Fabrication of cerium doped Ti/nanoTiO2/PbO2 electrode with improved electrocatalytic activity and its application in organic degradation. Electrochim. Acta 2016, 201, 240–250. [Google Scholar] [CrossRef]
- Feng, Y.J.; Ding, H.; Zhang, W.J. Research on electrocatalytic properties of rare earth doped Ti/SnO2-Sb electrodes by CV and Tafel curves. Mater. Sci. Technol. 2009, 17, 278–280+284. [Google Scholar]
- Sun, Z.R.; Zhang, H.; Wei, X.F.; Du, R.; Hu, X. Fabrication and electrochemical properties of a SnO2-Sb anode doped with Ni-Nd for phenol oxidation. J. Electrochem. Soc. 2015, 162, H590–H596. [Google Scholar] [CrossRef]
- Yao, Y.W.; Zhao, C.M.; Zhao, M.M.; Wang, X. Electrocatalytic degradation of methylene blue on PbO2-ZrO2 nanocomposite electrodes prepared by pulse electrodeposition. J. Hazard. Mater. 2013, 263, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.W.; Zhao, M.M.; Zhao, C.M.; Wang, X. Preparation and Characterization of PbO2 Electrodes Prepared by Pulse Electrodeposition with Different Pulse Frequency. J. Electrochem. Soc. 2013, 160, D553–D557. [Google Scholar] [CrossRef]
- Duan, X.Y.; Ma, F.; Yuan, Z.X.; Chang, L.M.; Jin, X.T. Comparative studies on the electro-catalytic oxidation performance of surfactant-carbon nanotube-modified PbO2 electrodes. J. Electroanal. Chem. 2012, 677, 90–100. [Google Scholar] [CrossRef]
- Feng, J.R.; Johnson, D.C. Electrocatalysis of anodic oxygen-transfer reactions: Titanium substrates for pure and doped lead dioxide films. J.Electrochem. Soc. 1991, 138, 3328–3337. [Google Scholar] [CrossRef]
- He, Z.Q.; Zhou, J.J.; Huang, X.W.; Zhang, S.H.; Song, S. Enhancement of the Activity and Stability of PbO2 Electrodes by Modifying with Polydimethylsiloxane. J. Electrochem. Soc. 2018, 165, H717–H724. [Google Scholar] [CrossRef]
- Qiao, Q.C.; Singh, S.; Lo, S.L.; Li, Y.; Jin, J.R.; Wang, L.Z. Electrochemical oxidation of acid orange 7 dye with Ce, Nd, and Co-modified PbO2 electrodes: Preparation, characterization, optimization, and mineralization. J. Taiwan Inst. Chem. Eng. 2018, 84, 110–122. [Google Scholar] [CrossRef]
- Darabizad, G.; Rahmanifar, M.S.; Mousavi, M.F.; Pendashteh, A. Electrodeposition of morphology- and size-tuned PbO2 nanostructures in the presence of PVP and their electrochemical studies. Mater. Chem. Phys. 2015, 156, 121–128. [Google Scholar] [CrossRef]
- Zhao, B.; Yu, H.B.; Lu, Y.; Qu, J.; Zhu, S.Y.; Huo, M.X. Polyethylene glycol assisted synthesis of a praseodymium-doped PbO2 electrode and its enhanced electrocatalytic oxidation performance. J. Taiwan Inst. Chem. Eng. 2019, 100, 144–150. [Google Scholar] [CrossRef]
- Wu, T.; Zhao, G.H.; Lei, Y.Z.; Li, P.Q. Distinctive tin dioxide anode fabricated by pulse electrodeposition: High oxygen evolution potential and efficient electrochemical degradation of fluorobenzene. J. Phys. Chem. C. 2011, 115, 3888–3898. [Google Scholar] [CrossRef]
- Comninellis, C. Electrocatalysis in the electrochemical conversion/combustion of organic pollutants for waste water treatment. Electrochim. Acta 1994, 39, 1857–1862. [Google Scholar] [CrossRef]
- Shih, Y.J.; Huang, Y.H.; Huang, C.P. Oxidation of ammonia in dilute aqueous solutions over graphite-supported α- and β-lead dioxide electrodes (PbO2@G). Electrochim. Acta 2017, 257, 444–454. [Google Scholar] [CrossRef]
- Yang, X.P.; Zou, R.Y.; Huo, F.; Cai, D.C.; Xiao, D. Preparation and characterization of Ti/SnO2-Sb2O3-Nb2O5/PbO2 thin film as electrode material for the degradation of phenol. J. Hazard. Mater. 2009, 164, 367–373. [Google Scholar] [CrossRef]
- He, Z.; Hayat, M.D.; Huang, S.F.; Wang, X.G.; Cao, P. PbO2 electrodes prepared by pulse reverse electrodeposition and their application in benzoic acid degradation. J. Electroanal. Chem. 2018, 812, 74–81. [Google Scholar] [CrossRef]
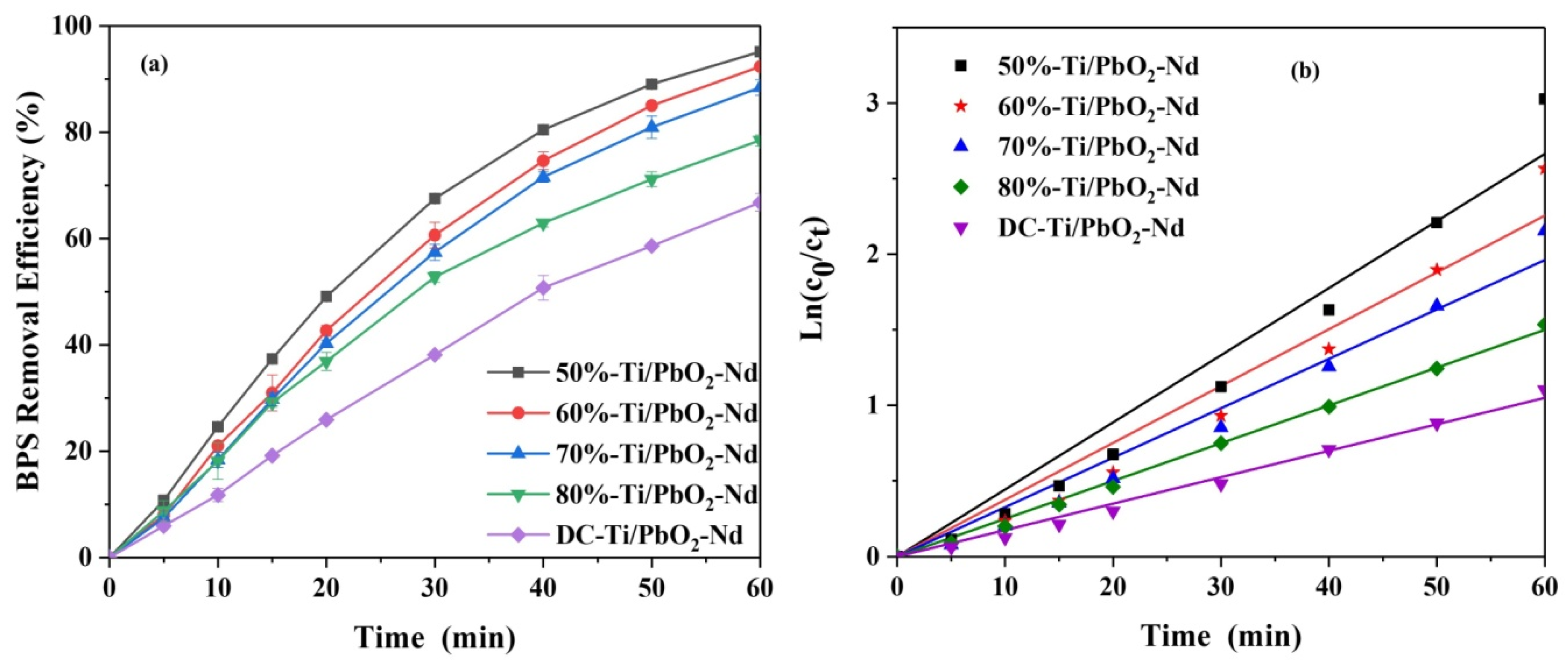

| Duty Cycle | Kinetics Coefficientsk (10−3min−1) | Correlation Coefficient R2 |
|---|---|---|
| 50% | 49.3 | 0.976 |
| 60% | 42.2 | 0.974 |
| 70% | 36.1 | 0.986 |
| 80% | 25.8 | 0.999 |
| 100% (DC) | 18.7 | 0.995 |
| Retention Time (min) | Symbol | Mass (m/z) | Molecular Formula | Chemical Structure |
|---|---|---|---|---|
| 7.533 | a | 267.1 | C12H11O5S+ | 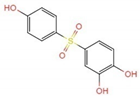 +H+ +H+ |
| b | 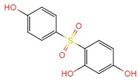 +H+ +H+ | |||
| 2.071–3.053 | c | 285.0 | C12H13O6S3+ | 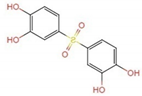 +3H+ +3H+ |
| d | 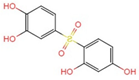 +3H+ +3H+ | |||
| e |  +3H+ +3H+ | |||
| f | 306.9 | C12H11O6SNa2+ | 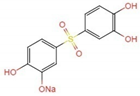 +2H+ +2H+ | |
| g | 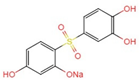 +2H+ +2H+ | |||
| h | 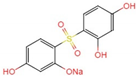 +2H+ +2H+ | |||
| i | 165 | C6H14O3S- |  −H+ −H+ | |
| j | 143 | C6H7O2S+ |  +H+ +H+ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Ni, Z.; Yao, J. Enhancement of the Activity of Electrochemical Oxidation of BPS by Nd-Doped PbO2 Electrodes: Performance and Mechanism. Water 2020, 12, 1317. https://doi.org/10.3390/w12051317
Zhang Y, Ni Z, Yao J. Enhancement of the Activity of Electrochemical Oxidation of BPS by Nd-Doped PbO2 Electrodes: Performance and Mechanism. Water. 2020; 12(5):1317. https://doi.org/10.3390/w12051317
Chicago/Turabian StyleZhang, Yan, Zhili Ni, and Jie Yao. 2020. "Enhancement of the Activity of Electrochemical Oxidation of BPS by Nd-Doped PbO2 Electrodes: Performance and Mechanism" Water 12, no. 5: 1317. https://doi.org/10.3390/w12051317
APA StyleZhang, Y., Ni, Z., & Yao, J. (2020). Enhancement of the Activity of Electrochemical Oxidation of BPS by Nd-Doped PbO2 Electrodes: Performance and Mechanism. Water, 12(5), 1317. https://doi.org/10.3390/w12051317