Effect of Temperature on the Size of Sedimentary Remains of Littoral Chydorids
Abstract
1. Introduction
2. Materials and Methods
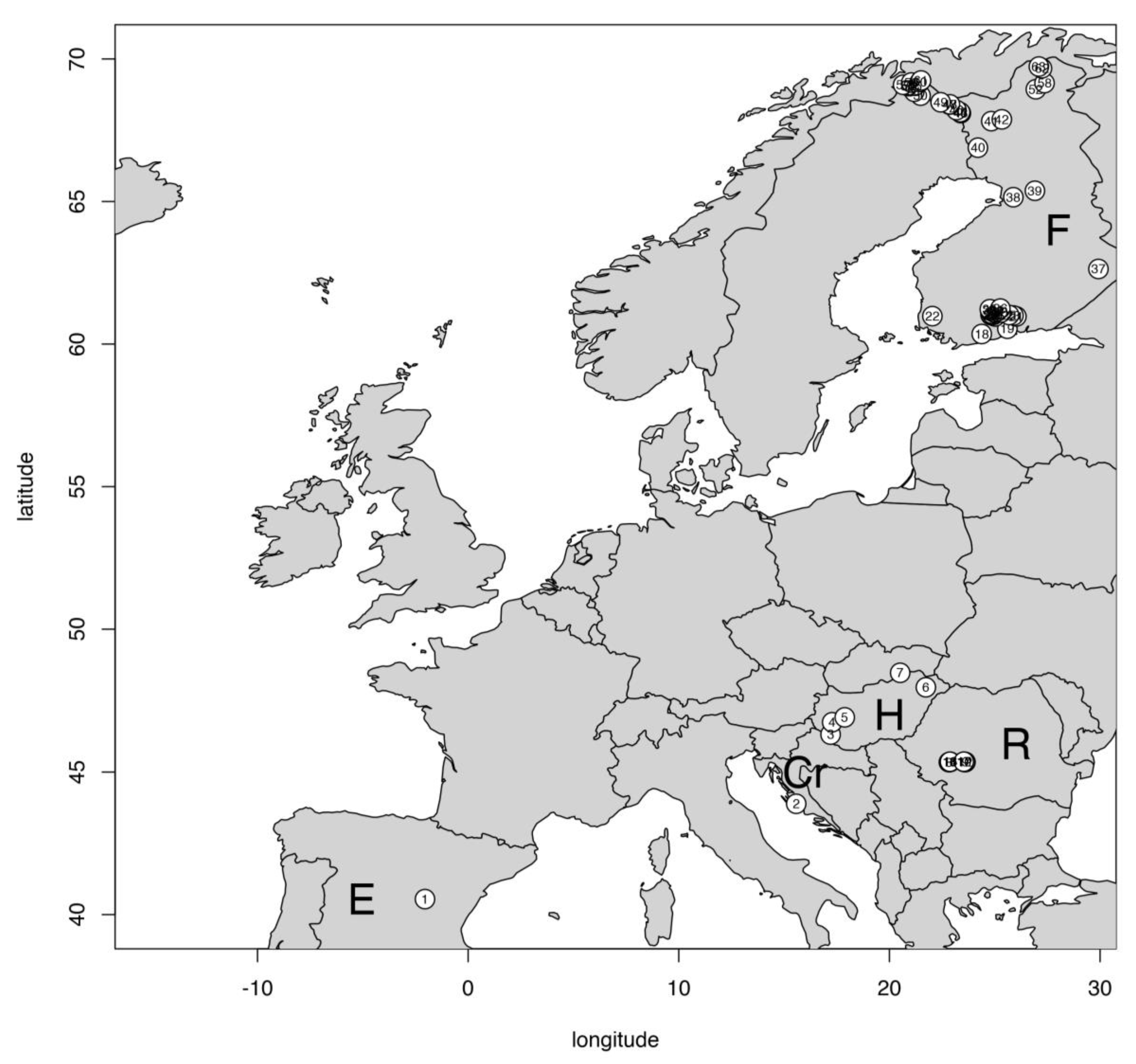
2.1. Study Sites
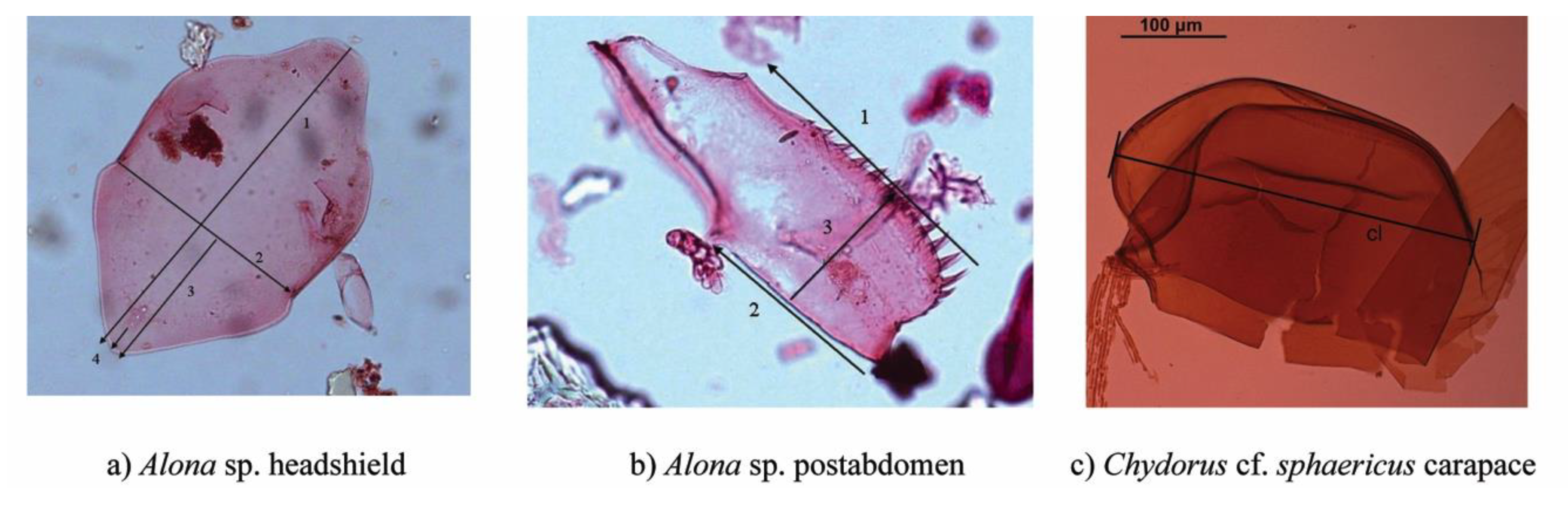
2.2. Subfossil Analyses
2.3. Data Analyses
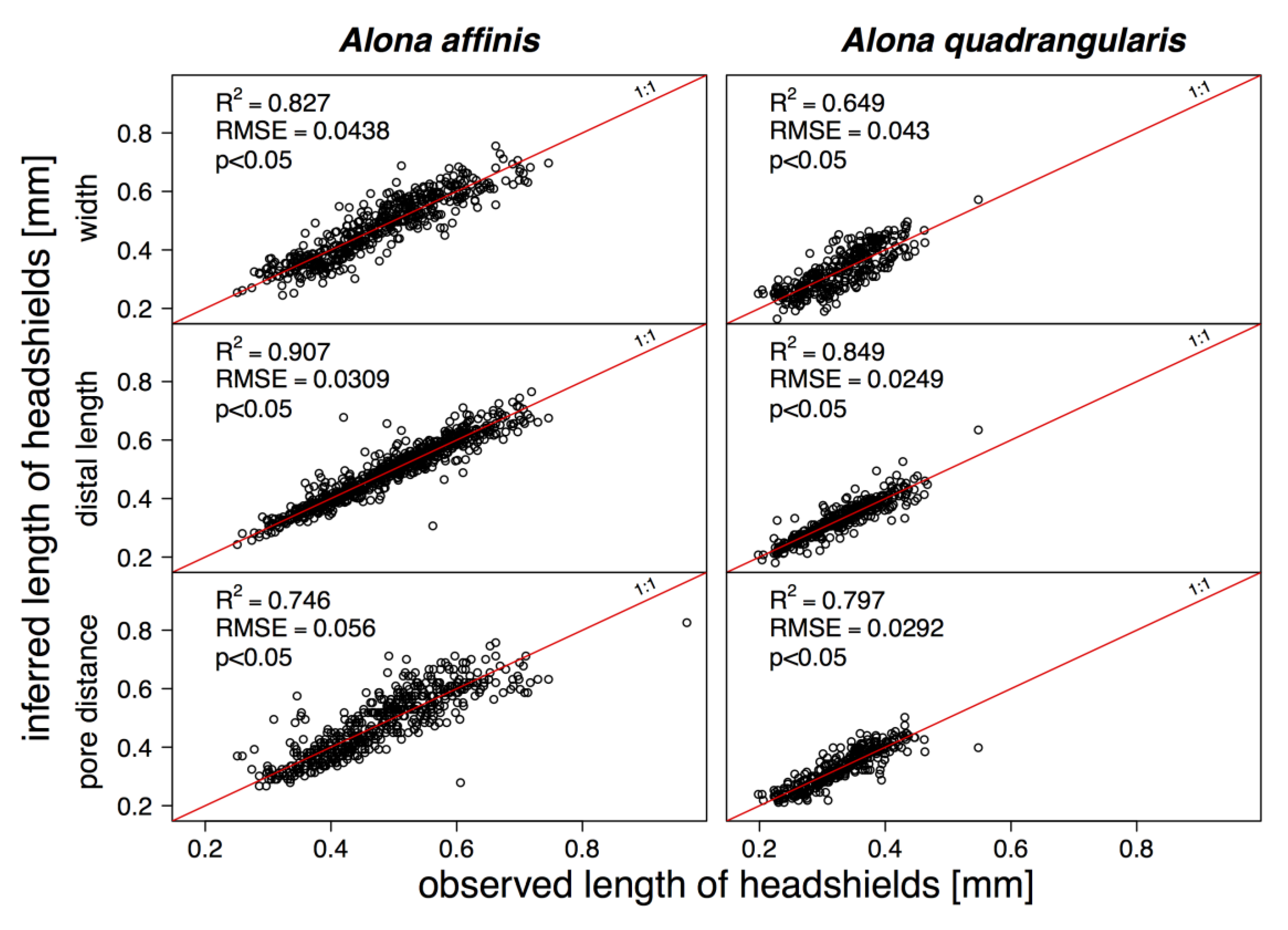
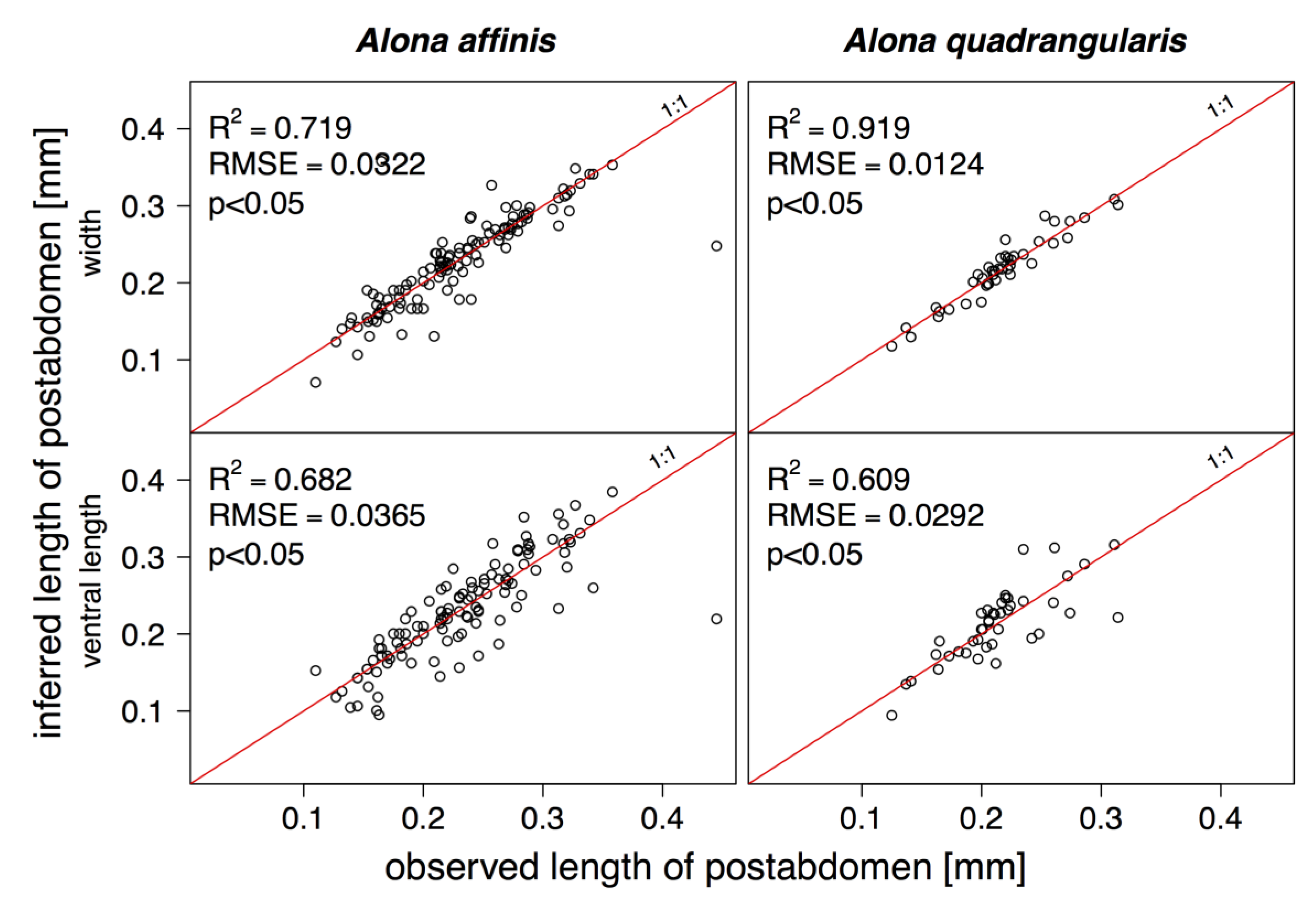
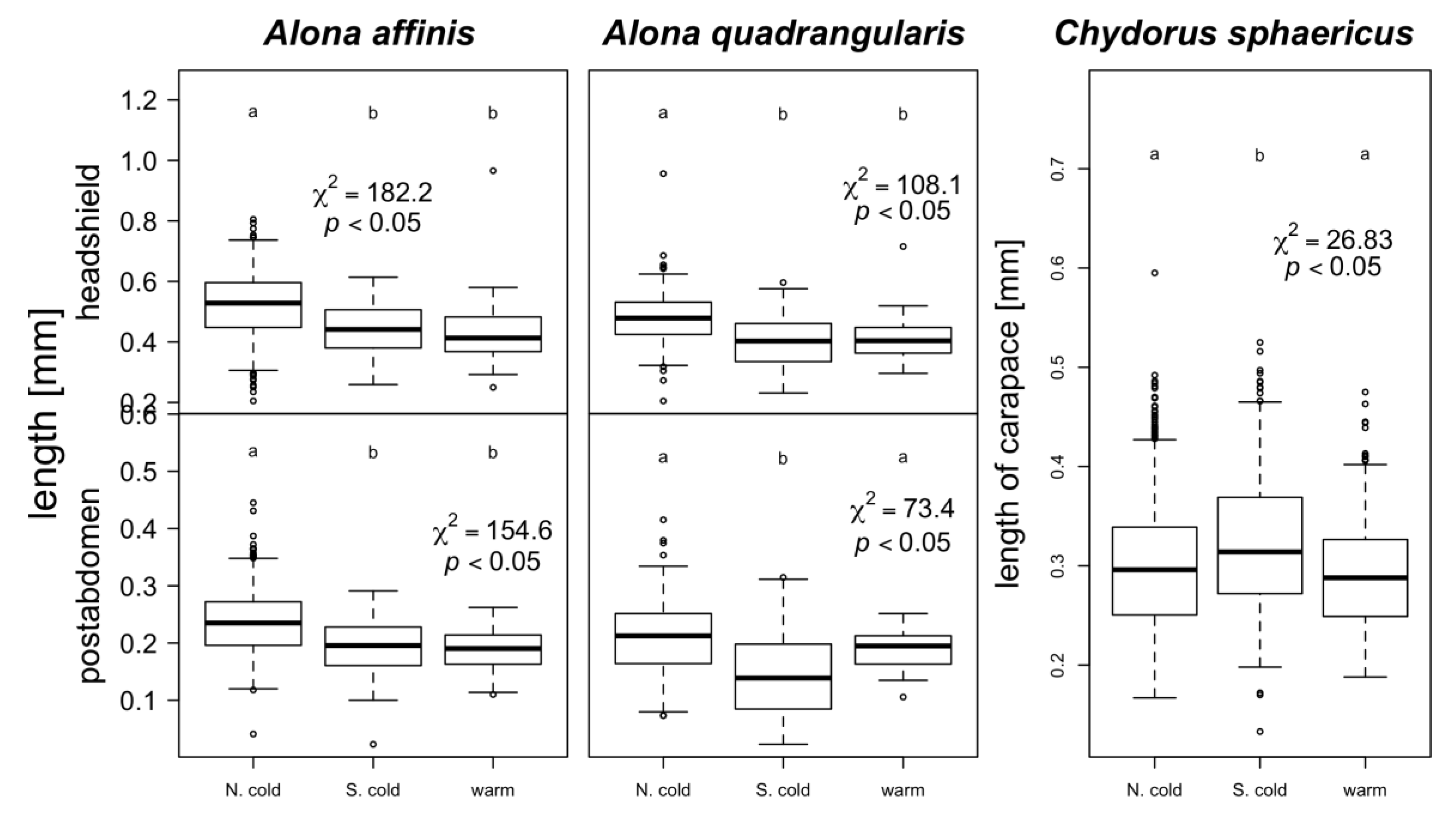
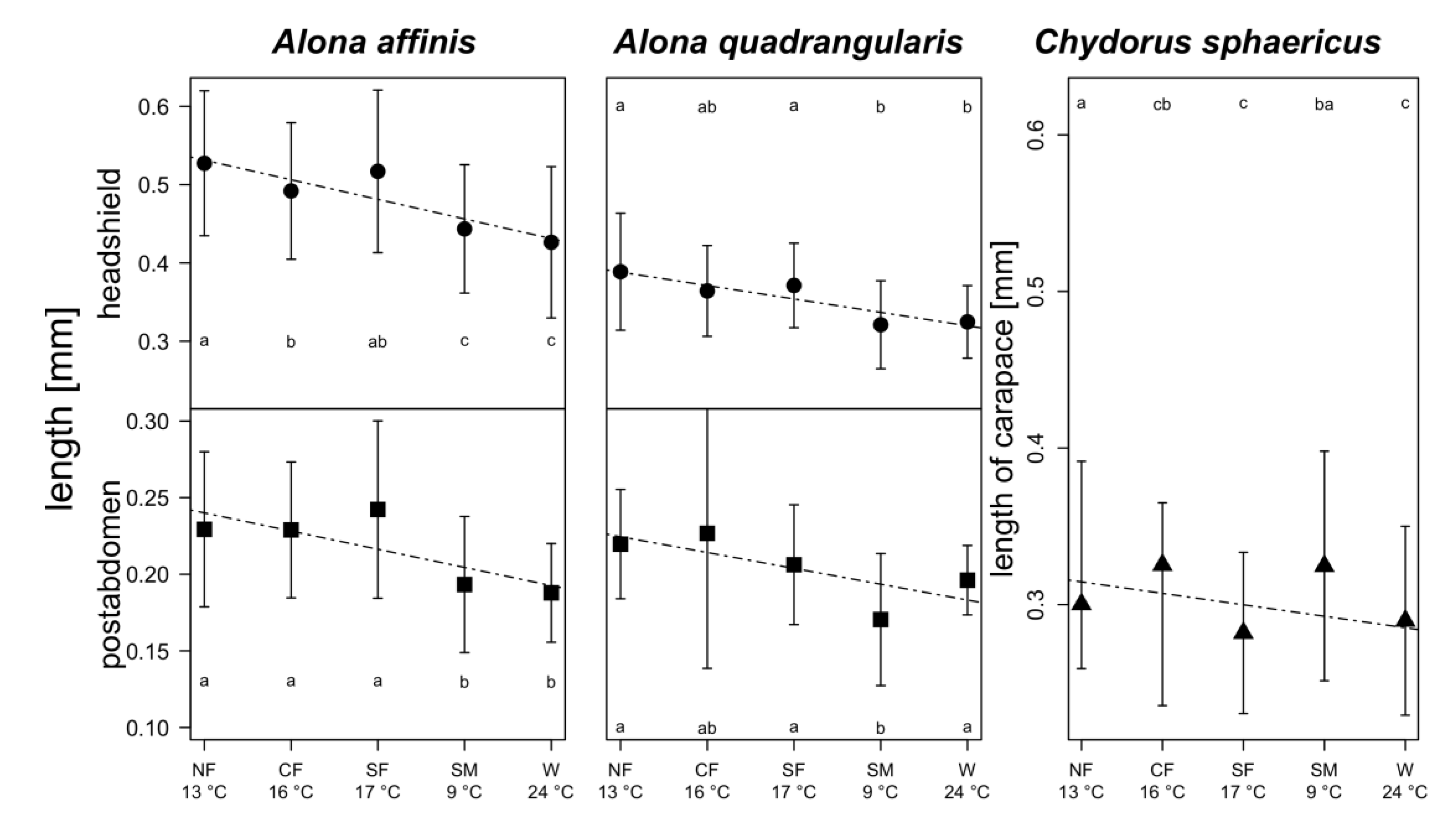
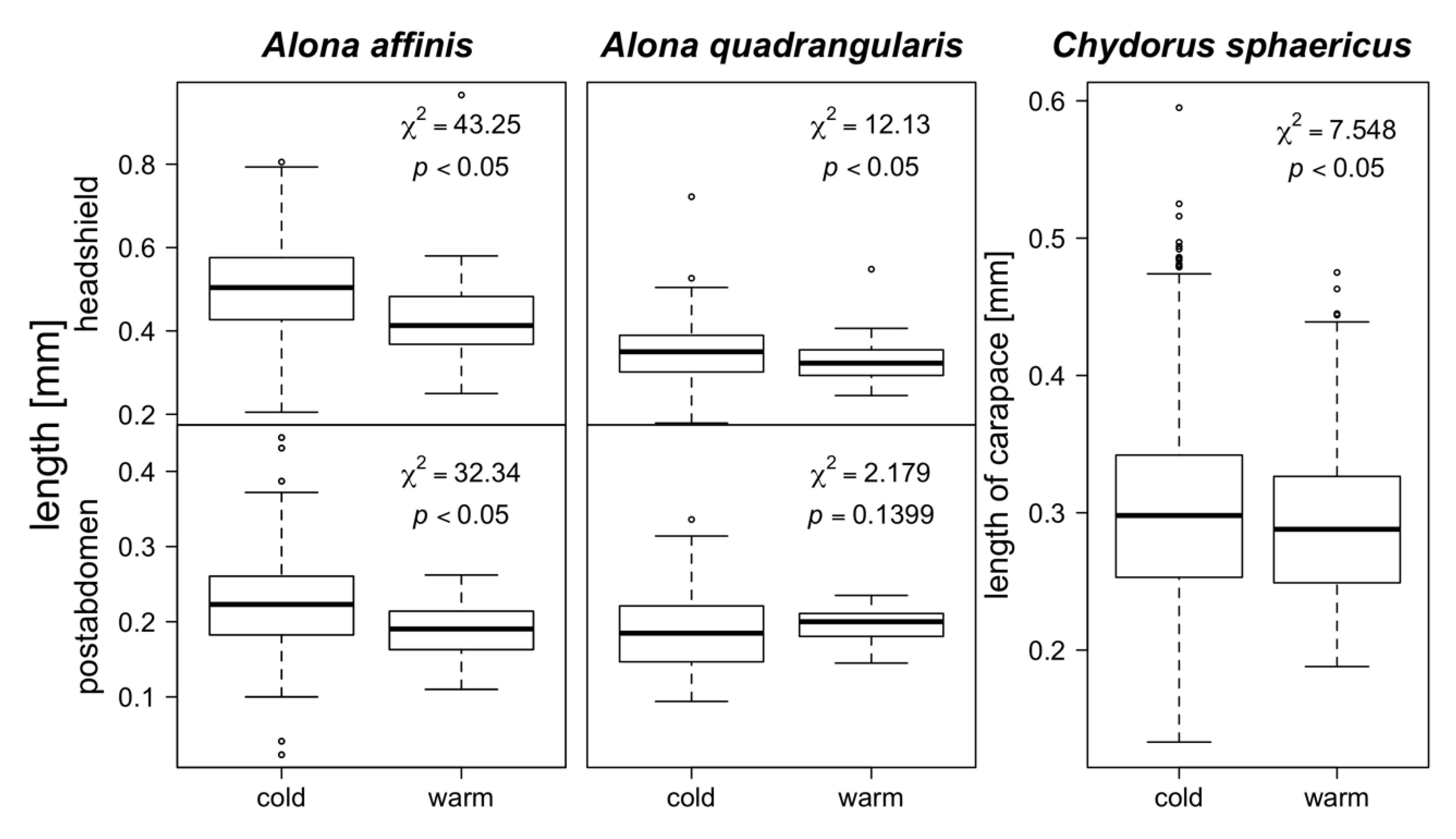
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Alona affinis | |||||
| headshield | a | b | t-value | R2 | p |
| width | 0.5465 | −0.00881 | 56.7427 | 0.8712 | <0.05 |
| distal length | 0.4232 | −0.00074 | 120.2307 | 0.9527 | <0.05 |
| pore distance | 0.0878 | −0.02046 | 46.6102 | 0.8165 | <0.05 |
| postabdomen | a | b | t-value | R2 | p |
| width | 0.4177 | 0.01548 | 32.5566 | 0.9013 | <0.05 |
| distal length | 0.5216 | 0.0205 | 20.2443 | 0.785 | <0.05 |
| Alona quadrangularis | |||||
| headshield | a | b | t-value | R2 | p |
| width | 0.5446 | 0.01065 | 26.8956 | 0.697 | <0.05 |
| distal length | 0.4074 | 0.00051 | 61.1244 | 0.9125 | <0.05 |
| pore distance | 0.151 | −0.0276 | 44.3056 | 0.8639 | <0.05 |
| postabdomen | a | b | t-value | R2 | p |
| width | 0.4192 | 0.01665 | 27.6451 | 0.9526 | <0.05 |
| ventral length | 0.5192 | 0.02206 | 10.3076 | 0.7296 | <0.05 |

| Akaike’s An Information Criterion (AIC) | |||
|---|---|---|---|
| Normal | Lognormal | Gamma | |
| Alona affinis | |||
| headshield | −2267 | −2230 | −2261 |
| postabdomen | −3150 | −3072 | −3137 |
| A. quadrangularis | |||
| headshield | −1609 | −1613 | −1620 |
| postabdomen | −1497 | −1489 | −1497 |
| Chydorus sphaericus | |||
| carapace | −4120 | −4211 | −4208 |
| Model | Parameter | Estimate | Std. Error | t Value | p-Value | AIC | |
|---|---|---|---|---|---|---|---|
| Alona affinis | |||||||
| headsield | all lake model | Intercept | 0.288 | 0.011 | 23.178 | <0.001 | −3491 |
| latitude | 0.002 | 0.000 | 13.87 | <0.001 | |||
| omit-model | Intercept | 0.291 | 0.021 | 12.075 | <0.001 | −2628 | |
| latitude | 0.002 | 0.000 | 7.473 | <0.001 | |||
| all lake model | Intercept | 0.475 | 0.008 | 47.63 | <0.001 | −3314 | |
| TJul | 0.001 | 0.001 | 1.692 | 0.091 | |||
| omit-model | Intercept | 0.637 | 0.011 | 43.813 | <0.001 | −2624 | |
| TJul | −0.005 | 0.001 | −7.19 | <0.001 | |||
| postabdomen | all lake model | Intercept | 0.120 | 0.009 | 12.202 | <0.001 | −3277 |
| latitude | 0.002 | 0.000 | 11.673 | <0.001 | |||
| omit-model | Intercept | 0.160 | 0.017 | 8.891 | <0.001 | −2280 | |
| latitude | 0.001 | 0.000 | 4.985 | <0.001 | |||
| all lake model | Intercept | 0.475 | 0.008 | 47.63 | <0.001 | −3314 | |
| TJul | 0.001 | 0.001 | 1.692 | 0.091 | |||
| omit-model | Intercept | 0.637 | 0.011 | 43.813 | <0.001 | −2624 | |
| TJul | −0.005 | 0.001 | −7.19 | <0.001 | |||
| Alona quadrangularis | |||||||
| headsield | all lake model | Intercept | 0.185 | 0.014 | 13.325 | <0.001 | −1729 |
| latitude | 0.003 | 0.000 | 11.593 | <0.001 | |||
| omit-model | Intercept | 0.196 | 0.024 | 8.342 | <0.001 | −940 | |
| latitude | 0.003 | 0.000 | 7.154 | <0.001 | |||
| all lake model | Intercept | 0.310 | 0.009 | 34.854 | <0.001 | −1623 | |
| TJul | 0.002 | 0.001 | 3.955 | <0.001 | |||
| omit-model | Intercept | 0.499 | 0.019 | 25.684 | <0.001 | −939 | |
| TJul | −0.008 | 0.001 | −7.087 | <0.001 | |||
| postabdomen | all lake model | Intercept | 0.080 | 0.010 | 7.585 | <0.001 | −1728 |
| latitude | 0.002 | 0.000 | 9.258 | <0.001 | |||
| omit-model | Intercept | 0.148 | 0.022 | 6.493 | <0.001 | −722 | |
| latitude | 0.001 | 0.000 | 2.366 | 0.019 | |||
| all lake model | Intercept | 0.135 | 0.006 | 20.307 | <0.001 | −1697 | |
| TJul | 0.003 | 0.000 | 7.076 | <0.001 | |||
| omit-model | Intercept | 0.257 | 0.017 | 13.76 | <0.001 | −722 | |
| TJul | −0.002 | 0.001 | −2.423 | 0.016 | |||
| Chydorus spharicus | |||||||
| carapace | all lake model | Intercept | 0.280 | 0.011 | 24.852 | <0.001 | −4132 |
| latitude | 0.000 | 0.000 | 1.909 | 0.056 | |||
| omit-model | Intercept | 0.190 | 0.014 | 13.313 | <0.001 | −3727 | |
| latitude | 0.002 | 0.000 | 7.617 | <0.001 | |||
| all lake model | Intercept | 0.378 | 0.007 | 55.581 | <0.001 | −4256 | |
| TJul | −0.005 | 0.000 | −11.543 | <0.001 | |||
| omit-model | Intercept | 0.386 | 0.008 | 46.03 | <0.001 | −3780 | |
| TJul | −0.006 | 0.001 | −10.69 | 0.000 | |||
References
- Litchman, E.; Ohman, M.D.; Kiørboe, T. Trait-Based Approaches to Zooplankton Communities. J. Plankton Res. 2013, 35, 473–484. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Kitchell, J.F. Consumer Control of Lake Productivity. BioScience 1988, 38, 764. [Google Scholar] [CrossRef]
- Kerfoot, W.C. Egg-Size Cycle of a Cladoceran. Ecology 1974, 55, 1259–1270. [Google Scholar] [CrossRef]
- Razak, S.A.; Saisho, T. Seasonal Variations in Egg Size, Brood Size and Body Length of Bosmina Longirostris (Crustacea: Cladocera) from Lake Ikeda, Japan. Malays. J. Sci. 2004, 23, 121–135. [Google Scholar]
- Havens, K.E.; Pinto-Coelho, R.M.; Beklioǧlu, M.; Christoffersen, K.S.; Jeppesen, E.; Lauridsen, T.L.; Mazumder, A.; Méthot, G.; Alloul, B.P.; Tavşanoğlu, U.N.; et al. Temperature Effects on Body Size of Freshwater Crustacean Zooplankton from Greenland to the Tropics. Hydrobiologia 2015, 743, 27–35. [Google Scholar] [CrossRef]
- Vijverberg, J.; Richter, A.F. Population Dynamics and Production of Daphnia Hyalina (Leydig) and Daphnia Cucullata Sars in Tjeukemeer. Hydrobiologia 1982, 9, 235–259. [Google Scholar] [CrossRef]
- Moore, M.V.; Folt, C.L.; Stemberger, R.S. Consequences of Elevated Temperatures for Zooplankton Assemblages in Temperate Lakes. Arch. Hydrobiol. 1996, 135, 289–319. [Google Scholar]
- Chang, K.-H.; Hanazato, T. Seasonal and Reciprocal Succession and Cyclomorphosis of Two Bosmina Species (Cladocera, Crustacea) Co-Existing in a Lake: Their Relationship with Invertebrate Predators. J. Plankton Res. 2003, 25, 141–150. [Google Scholar] [CrossRef][Green Version]
- Deng, D.; Xie, P. Effect of Food and Temperature on the Growth and Development of Moina Irrasa (Cladocera: Moinidae). J. Freshw. Ecol. 2003, 18, 503–513. [Google Scholar] [CrossRef]
- Hart, R.C.; Bychek, E.A. Body Size in Freshwater Planktonic Crustaceans: An Overview of Extrinsic Determinants and Modifying Influences of Biotic Interactions. Hydrobiologia 2011, 668, 61–108. [Google Scholar] [CrossRef]
- Gillooly, J.F.; Dodson, S.I. Latitudinal Patterns in the Size Distribution and Seasonal Dynamics of New World, Freshwater Cladocerans. Limnol. Oceanogr. 2000, 45, 22–30. [Google Scholar] [CrossRef]
- Perrin, N. Why Are Offspring Born Larger When It Is Colder? Phenotypic for Offspring Plasticity Size in the Cladoceran Simocephalus Vetulus (Muller). Funct. Ecol. 1988, 2, 283–288. [Google Scholar] [CrossRef]
- Atkinson, D. Temperature and Organism Size-A Law for Ectotherms? Adv. Ecol. Res. 1994, 25, 1–58. [Google Scholar]
- Atkinson, D. Effects of Temperature on the Size of Aquatic Ectotherms: Exception to General Rule. J. Therm. Biol. 1995, 20, 61–71. [Google Scholar] [CrossRef]
- Smol, J.P. Pollution of Lakes and Rivers; Blackwell Publishing: Oxford, UK, 2002. [Google Scholar]
- Flower, R.J. Diatom Preservation: Experiments and Observations on Dissolution and Breakage in Modern and Fossil Material. Hydrobiologia 1993, 269–270, 473–484. [Google Scholar] [CrossRef]
- Battarbee, R.W.; Anderson, N.J.; Jeppesen, E.; Leavitt, P.R. Combining Palaeolimnological and Limnological Approaches in Assessing Lake Ecosystem Response to Nutrient Reduction. Freshw. Biol. 2005, 50, 1772–1780. [Google Scholar] [CrossRef]
- Kattel, G.R.; Battarbee, R.W.; Mackay, A.W.; Birks, H.J.B. Recent Ecological Change in a Remote Scottish Mountain Loch: An Evaluation of a Cladocera-Based Temperature Transfer-Function. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2008, 259, 51–76. [Google Scholar] [CrossRef]
- López-Blanco, C.; Miracle, M.R.; Vicente, E. Cladoceran Assemblages in a Karstic Lake as Indicators of Hydrological Alterations. Hydrobiologia 2011, 676, 249–261. [Google Scholar] [CrossRef]
- Korhola, A.; Rautio, M. 2. Cladocera and Other Branchiopod Crustaceans. In Tracking Environmental Change Using Lake Sediments. Volume 4: Zoological Indicators; Smol, J.P., Birks, H.J.B., Last, W.M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 5–41. [Google Scholar] [CrossRef]
- Jeppesen, E.; Leavitt, P.; De Meester, L.; Jensen, J.P. Functional Ecology and Palaeolimnology: Using Cladoceran Remains to Reconstruct Anthropogenic Impact. Trends Ecol. Evol. 2001, 16, 191–198. [Google Scholar] [CrossRef]
- Jeppesen, E.; Nõges, P.; Davidson, T.A.; Haberman, J.; Nõges, T.; Blank, K.; Lauridsen, T.L.; Søndergaard, M.; Sayer, C.; Laugaste, R.; et al. Zooplankton as Indicators in Lakes: A Scientific-Based Plea for Including Zooplankton in the Ecological Quality Assessment of Lakes According to the European Water Framework Directive (WFD). Hydrobiologia 2011, 676, 279–297. [Google Scholar] [CrossRef]
- Korosi, J.B.; Paterson, A.M.; Desellas, A.M.; Smol, J.P. Linking Mean Body Size of Pelagic Cladocera to Environmental Variables in Precambrian Shield Lakes: A Paleolimnological Approach. J. Limnol. 2008, 67, 22–34. [Google Scholar] [CrossRef]
- Bjerring, R.; Becares, E.; Declerck, S.; Gross, E.M.; Hansson, L.-A.; Kairesalo, T.; Nykänen, M.; Halkiewicz, A.; Kornijów, R.; Conde-Porcuna, J.M.; et al. Subfossil Cladocera in Relation to Contemporary Environmental Variables in 54 Pan-European Lakes. Freshw. Biol. 2009, 54, 2401–2417. [Google Scholar] [CrossRef]
- Korponai, J.; Magyari, E.K.; Buczkó, K.; Iepure, S.; Namiotko, T.; Czakó, D.; Kövér, C.; Braun, M. Cladocera Response to Late Glacial to Early Holocene Climate Change in a South Carpathian Mountain Lake. Hydrobiologia 2011, 676, 223–235. [Google Scholar] [CrossRef]
- Nevalainen, L.; Luoto, T.P.; Kultti, S.; Sarmaja-Korjonen, K. Spatio-Temporal Distribution of Sedimentary Cladocera (Crustacea: Branchiopoda) in Relation to Climate. J. Biogeogr. 2013, 40, 1548–1559. [Google Scholar] [CrossRef]
- Nevalainen, L.; Rantala, M.V.; Luoto, T.P. Sedimentary Cladoceran Assemblages and Their Functional Attributes Record Late Holocene Climate Variability in Southern Finland. J. Paleolimnol. 2015, 1–14. [Google Scholar] [CrossRef]
- Burks, R.L.; Lodge, D.M.; Jeppesen, E.; Lauridsen, T.L. Diel Horizontal Migration of Zooplankton: Costs and Benefits of Inhabiting the Littoral. Freshw. Biol. 2002, 47, 343–365. [Google Scholar] [CrossRef]
- Sagrario, G.; de los Ángeles, M.; Balseiro, E.; Ituarte, R.; Spivak, E. Macrophytes as Refuge or Risky Area for Zooplankton: A Balance Set by Littoral Predacious Macroinvertebrates. Freshw. Biol. 2009, 54, 1042–1053. [Google Scholar] [CrossRef]
- Jeppesen, E.; Jensen, J.P.; Søndergaard, M.; Lauridsen, T.; Pedersen, L.J.; Jensen, L. Top-down Control in Freshwater Lakes: The Role of Nutrient State, Submerged Macrophytes and Water Depth. Hydrobiologia 1997, 342, 151–164. [Google Scholar] [CrossRef]
- De Los Ángeles, M.; Sagrario, G.; Balseiro, E. The Role of Macroinvertebrates and Fish in Regulating the Provision by Macrophytes of Refugia for Zooplankton in a Warm Temperate Shallow Lake. Freshw. Biol. 2010, 55, 2153–2166. [Google Scholar] [CrossRef]
- Jeppesen, E.; Søndergaard, M.; Søndergaard, M.; Christoffersen, K. The Structuring Role of Submerged Macrophytes in Lakes; Jeppesen, E., Søndergaard, M., Søndergaard, M., Christoffersen, K., Eds.; Ecological Studies; Springer: New York, NY, USA, 1998; Volume 131. [Google Scholar] [CrossRef]
- Scheffer, M. Ecology of Shallow Lakes; Springer: Dordrecht, The Netherlands, 2004. [Google Scholar] [CrossRef]
- Płaska, W.; Mieczan, T. Effects of Water Bugs on Crustacean Zooplankton in a Shallow Littoral Zone. Knowl. Manag. Aquat. Ecosyst. 2018. [Google Scholar] [CrossRef]
- Adamczuk, M. The Effect of Habitat Complexity on the Contribution of Some Littoral-Benthic Cladocera to the Pelagic Food Web. Mar. Freshw.Res. 2013, 64, 1049–1057. [Google Scholar] [CrossRef]
- Adamczuk, M. Niche Separation by Littoral-Benthic Chydoridae (Cladocera, Crustacea) in a Deep Lake—Potential Drivers of Their Distribution and Role in Littoral-Pelagic Coupling. J. Limnol. 2014, 73, 490–501. [Google Scholar] [CrossRef]
- Choedchim, W.; Damme, K.; Van Maiphae, S. Spatial and Temporal Variation of Cladocera in a Tropical Shallow Lake. Ann. Limnol. 2017, 53, 233–252. [Google Scholar] [CrossRef]
- Basińska, A.M.; Antczak, M.; Świdnicki, K.; Jassey, V.E.J.; Kuczyńska-Kippen, N. Habitat Type as Strongest Predictor of the Body Size Distribution of Chydorus Sphaericus (O. F. Müller) in Small Water Bodies. Int. Rev. Hydrobiol. 2014, 99, 382–392. [Google Scholar] [CrossRef]
- Rivera-De la Parra, L.; Sarma, S.S.S.; Nandini, S. Effects of Predation by Hydra (Cnidaria) on Cladocerans (Crustacea: Cladocera). J. Limnol. 2016, 75 (Suppl. 1), 39–47. [Google Scholar] [CrossRef]
- Rabus, M.; Söllradl, T.; Clausen-Schaumann, H.; Laforsch, C. Uncovering Ultrastructural Defences in Daphnia Magna—An Interdisciplinary Approach to Assess the Predator-Induced Fortification of the Carapace. PLoS ONE 2013, 8, e67856. [Google Scholar] [CrossRef]
- Laforsch, C.; Ngwa, W.; Grill, W.; Tollrian, R. An Acoustic Microscopy Technique Reveals Hidden Morphological Defenses in Daphnia. Proc. Natl. Acad. Sci. USA 2004, 101, 15911–15914. [Google Scholar] [CrossRef]
- Rizo, E.Z.; Xu, S.; Tang, Q.; Papa, R.D.S.; Dumont, H.J.; Qian, S.S.; Han, B.P. A Global Analysis of Cladoceran Body Size and Its Variation Linking to Habitat, Distribution and Taxonomy. Zool. J. Linn. Soc. 2019, 187, 1119–1130. [Google Scholar] [CrossRef]
- Pirinen, P.; Simola, H.; Aalto, J.; Kaukoranta, J.-P.; Karlsson, P.; Ruuhela, R. Tilastoja Suomen Ilmastosta 1981–2010; Ilmatieteen laitos: Helsinki, Finland, 2012; Volume 1.
- Korponai, J.; Varga, K.A.; Lengré, T.; Papp, I.; Tóth, A.; Braun, M. Paleolimnological Reconstruction of the Trophic State in Lake Balaton (Hungary) Using Cladocera Remains. Hydrobiologia 2011, 676, 237–248. [Google Scholar] [CrossRef]
- Livingstone, D.M.; Lotter, A.F. The Relationship between Air and Water Temperatures in Lakes of the Swiss Plateau: A Case Study with Palæolimnological Implications. J. Paleolimnol. 1998, 19, 181–198. [Google Scholar] [CrossRef]
- Livingstone, D.M.; Padisák, J. Large-Scale Coherence in the Response of Lake Surface-Water Temperatures to Synoptic-Scale Climate Forcing during Summer. Limnol. Oceanogr. 2007, 52, 896–902. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very High Resolution Interpolated Climate Surfaces for Global Land Areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. Dismo: Species Distribution Modeling; 2016. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Szeroczyńska, K.; Sarmaja-Korjonen, K. Atlas of Subfossil Cladocera from Central and Northern Europe; Friends of Lower Vistula Society: Swiecie, Poland, 2007. [Google Scholar]
- Pohlert, T. Package ‘PMCMRplus’: Calculate Pairwise Cultiple Comparisons of Mean Rank Sums Extended; R-Project: Vienna, Austria, 2018. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2015. [Google Scholar]
- Gamble, A.E.; Lloyd, R.; Aiken, J.; Johannsson, O.E.; Mills, E.L. Using Zooplankton Biomass Size Spectra to Assess Ecological Change in a Well-Studied Freshwater Lake Ecosystem: Oneida Lake, New York. Can. J. Fish. Aquat. Sci. 2006, 63, 2687–2699. [Google Scholar] [CrossRef]
- Brooks, J.L.; Dodson, S.I. Predation, Body Size, and Composition of Plankton. Science 1965, 150, 28–35. [Google Scholar] [CrossRef]
- Cousyn, C.; De Meester, L.; Colbourne, J.K.; Brendonck, L.; Verschuren, D.; Volckaert, F. Rapid, Local Adaptation of Zooplankton Behavior to Changes in Predation Pressure in the Absence of Neutral Genetic Changes. Proc. Natl. Acad. Sci. USA 2001, 98, 6256–6260. [Google Scholar] [CrossRef]
- Sweetman, J.N.; Finney, B.P. Differential Responses of Zooplankton Populations (Bosmina Longirostris) to Fish Predation and Nutrient-Loading in an Introduced and a Natural Sockeye Salmon Nursery Lake on Kodiak Island, Alaska, USA. J. Paleolimnol. 2003, 30, 183–193. [Google Scholar] [CrossRef]
- Lotter, A.F.; Birks, H.J.B.; Hofmann, W.; Marchetto, A. Modern Diatom, Cladocera, Chironomid, and Chrysophyte Cyst Assemblages as Quantitative Indicators for the Reconstruction of Past Environmental Conditions in the Alps. I. Climate. J. Paleolimnol. 1997, 18, 395–420. [Google Scholar] [CrossRef]
- Manca, M.; Comoli, P. Reconstructing Long-Term Changes in Daphnia’s Body Size from Subfossil Remains in Sediments of a Small Lake in the Himalayas. J. Paleolimnol. 2004, 32, 95–107. [Google Scholar] [CrossRef]
- Atkinson, D.; Morley, S.A.; Hughes, R.N. From Cells to Colonies: At What Levels of Body Organization Does the “temperature-Size Rule” Apply? Evol. Dev. 2006, 8, 202–214. [Google Scholar] [CrossRef]
- Whiteside, M.C.; Williams, J.B.; White, C.P. Seasonal Abundance and Pattern of Chydorid, Cladocera in Mud and Vegetative Habitats. Ecology 1978, 59, 1177–1188. [Google Scholar] [CrossRef]
- Tremel, B.; Frey, S.E.L.; Yan, N.D.; Somers, K.M.; Pawson, T.W. Habitat Specificity of Littoral Chydoridae (Crustacea, Branchiopoda, Anomopoda) in Plastic Lake, Ontario, Canada. Hydrobiologia 2000, 432, 195–205. [Google Scholar] [CrossRef]
- Smirnov, N. The Chydorinae and Sayciinae (Chydoridae) of the World; Guides to the Identification of the Microinvertebrates of the Continental Waters of the World; SPB Academic: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Alonso, M. Crustacea, Branchiopoda, Fauna Iberica. Vol. 7; Museo Nacional de Ciencias Naturales, Consejo Superior de Investigaciones Cientificas: Madrid, Spain, 1996. [Google Scholar] [CrossRef][Green Version]
- Fryer, G. Evolution and Adaptive Radiation in the Chydoridae (Crustacea: Cladocera): A Study in Comparative Functional Morphology and Ecology. Philos. Trans. R. Soc. B Biol. Sci. 1968, 254, 224–387. [Google Scholar]
- Van Damme, K.; Brancelj, A.; Dumont, H.J. Adaptations to the Hyporheic in Aloninae (Crustacea: Cladocera): Allocation of Alona Protzi Hartwig, 1900 and Related Species to Phreatalona Gen. Nov. Hydrobiologia 2009, 618, 1–34. [Google Scholar] [CrossRef]
- Nevalainen, L.; Helama, S.; Luoto, T.P. Hydroclimatic Variations over the Last Millennium in Eastern Finland Disentangled by Fossil Cladocera. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 378, 13–21. [Google Scholar] [CrossRef]
- Kingsolver, J.G.; Huey, R.B. Size, Temperature, and Fitness: Three Rules. Evol. Ecol. Res. 2008, 10, 251–268. [Google Scholar]
- Pop, M. Mechanisms of the Filtering Area Adaptation in Daphnia. Hydrobiologia 1991, 225, 169–176. [Google Scholar] [CrossRef]
- Macháček, J.; Seda, J. Over-Wintering Daphnia: Uncoupling the Effects of Temperature and Food on Offspring Size and Filtering Screen Morphology in D. Galeata. J. Plankton Res. 2013, 35, 1069–1079. [Google Scholar] [CrossRef]
- Gliwicz, Z.M. Food Thresholds and Body Size in Cladocerans. Nature 1990, 343, 638–640. [Google Scholar] [CrossRef]
- Lampert, W. Phenotypic Plasticity of the Filter Screen in Daphnia: Adapatation to a Low-Food Environment. Limnol. Oceanogr. 1994, 39, 997–1006. [Google Scholar] [CrossRef]
- Repka, S.; Veen, A.; Vijverberg, J. Morphological Adaptations in Filtering Screens of Daphnia Galeata to Food Quantity and Food Quality. J. Plankton Res. 1999, 21, 971–989. [Google Scholar] [CrossRef]
- Boersma, M.; Boriss, H.; Mitchell, S.E. Maternal Effects after Sexual Reproduction in Daphnia Magna. J. Plankton Res. 2000, 22, 279–285. [Google Scholar] [CrossRef][Green Version]
- Bergmann, C. Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Grösse; Verlagsort: Göttingen, Germany, 1848. [Google Scholar]
- Forster, J.; Hirst, A.G.; Atkinson, D. Warming-Induced Reductions in Body Size Are Greater in Aquatic than Terrestrial Species. Proc. Natl. Acad. Sci. USA 2012, 109, 19310–19314. [Google Scholar] [CrossRef]
- Horne, C.R.; Hirst, A.G.; Atkinson, D. Temperature-Size Responses Match Latitudinal-Size Clines in Arthropods, Revealing Critical Differences between Aquatic and Terrestrial Species. Ecol. Lett. 2015. [Google Scholar] [CrossRef] [PubMed]
- Angilletta, M.J.; Steury, T.D.; Sears, M.W. Temperature, Growth Rate, and Body Size in Ectotherms: Fitting Pieces of a Puzzle. Integr. Comp. Biol. 2004, 44, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Karpowicz, M.; Ejsmont-Karabin, J.; Kozłowska, J.; Feniova, I.; Dzialowski, A.R. Zooplankton Community Responses to Oxygen Stress. Water 2020, 12, 706. [Google Scholar] [CrossRef]
- Verberk, W.C.E.P.; Bilton, D.T.; Calosi, P.; Spicer, J.I. Oxygen Supply in Aquatic Ectotherms: Partial Pressure and Solubility Together Explain Biodiversity and Size Patterns. Ecology 2011, 92, 1565–1572. [Google Scholar] [CrossRef]
- Verberk, W.C.E.P.; Atkinson, D. Why Polar Gigantism and Palaeozoic Gigantism Are Not Equivalent: Effects of Oxygen and Temperature on the Body Size of Ectotherms. Funct. Ecol. 2013, 27, 1275–1285. [Google Scholar] [CrossRef]
- Fang, X.; Stefan, G. Temperature Variability in Lake Sediments Depth. Water Resour. 1998, 34, 717–729. [Google Scholar] [CrossRef]
- Salmaso, N. Effects of Climatic Fluctuations and Vertical Mixing on the Interannual Trophic Variability of Lake Garda, Italy. Limnol. Oceanogr. 2005, 50, 553–565. [Google Scholar] [CrossRef]
- Luoto, T.P. Hydrological Change in Lakes Inferred from Midge Assemblages through Use of an Intralake Calibration Set. Ecol. Monogr. 2010, 80, 303–329. [Google Scholar] [CrossRef]
- Luoto, T.P. Intra-Lake Patterns of Aquatic Insect and Mite Remains. J. Paleolimnol. 2012, 47, 141–157. [Google Scholar] [CrossRef]
- Nykänen, M.; Kairesalo, T.; Mäkelä, S.; Huitu, E.; Ala-Opas, P.; Mannio, J. A Typology and Ecological Classification System for Finnish Lakes: Applicability of the ECOFRAME Scheme. Boreal Environ. Res. 2005, 10, 159–179. [Google Scholar]
- Decei, P. Lacuri de Munte. Drumetie Si Pescuit; Ditura Sport Turism: Bucharest, Romania, 1981. [Google Scholar]
- Frost, W.E. The Natural History of the Minnow, Phoxinus Phoxinus. J. Anim. Ecol. 1943, 12, 139–162. [Google Scholar] [CrossRef]
- Straskraba, M.; Chiar, J.; Frank, S.; Hruska, V. Contribution to the Problem of Food Competition among the Sculpin, Minnow and Brown-Trout. J. Anim. Ecol. 1966, 35, 303–311. [Google Scholar] [CrossRef]
- Museth, J.; Borgstrøm, R.; Brittain, J.E. Diet Overlap between Introduced European Minnow (Phoxinus Phoxinus) and Young Brown Trout (Salmo Trutta) in the Lake, Øvre Heimdalsvatn: A Result of Abundant Resources or Forced Niche Overlap? Hydrobiologia 2010, 642, 93–100. [Google Scholar] [CrossRef]
- Rautio, M.; Vincent, W.F. Benthic and Pelagic Food Resources for Zooplankton in Shallow High-Latitude Lakes and Ponds. Freshw. Biol. 2006, 51, 1038–1052. [Google Scholar] [CrossRef]
- Milardi, M.; Siitonen, S.; Lappalainen, J.; Liljendahl, A.; Weckström, J. The Impact of Trout Introductions on Macro- and Micro-Invertebrate Communities of Fishless Boreal Lakes. J. Paleolimnol. 2016, 55, 273–287. [Google Scholar] [CrossRef]
- Helminen, H.; Karjalainen, J.; Kurkilahti, M.; Rask, M.; Sarvala, J. Eutrophication and Fish Biodiversity in Finnish Lakes. Verh. Int. Ver. Theor. Angew. Limnol. 2000, 27, 194–199. [Google Scholar] [CrossRef]
- Moss, B.; Stephen, D.; Alvarez, C.; Becares, E.; Van De Bund, W.; Collings, S.E.; Van Donk, E.; De Eyto, E.; Feldmann, T.; Fernández-Aláez, C.; et al. The Determination of Ecological Status in Shallow Lakes—A Tested System (ECOFRAME) for Implementation of the European Water Framework Directive. Aquat. Conserv. Mar. Freshw. Ecosyst. 2003, 13, 507–549. [Google Scholar] [CrossRef]
- Belyaeva, M.; Taylor, D.J. Cryptic Species within the Chydorus Sphaericus Species Complex (Crustacea: Cladocera) Revealed by Molecular Markers and Sexual Stage Morphology. Mol. Phylogenet. Evol. 2009, 50, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Frey, D.G. On the Plurality of Chydorus Sphaericus (0. F. Müller) (Cladocera, Chydoridae), and Designation of a Neotype from Sjaelsø, Denmark. Hydrobiologia 1980, 69, 83–123. [Google Scholar] [CrossRef]
- Van Damme, K.; Eggermont, H. The Afromontane Cladocera (Crustacea: Branchiopoda) of the Rwenzori (Uganda–D. R. Congo): Taxonomy, Ecology and Biogeography. Hydrobiologia 2011, 676, 57–100. [Google Scholar] [CrossRef]
 warm lakes,
warm lakes,  Southern mountain lakes,
Southern mountain lakes,  Finnish lakes, solid line: all-lake model, dashed line: omit-SM model; Table A3).
Finnish lakes, solid line: all-lake model, dashed line: omit-SM model; Table A3).
 warm lakes,
warm lakes,  Southern mountain lakes,
Southern mountain lakes,  Finnish lakes, solid line: all-lake model, dashed line: omit-SM model; Table A3).
Finnish lakes, solid line: all-lake model, dashed line: omit-SM model; Table A3).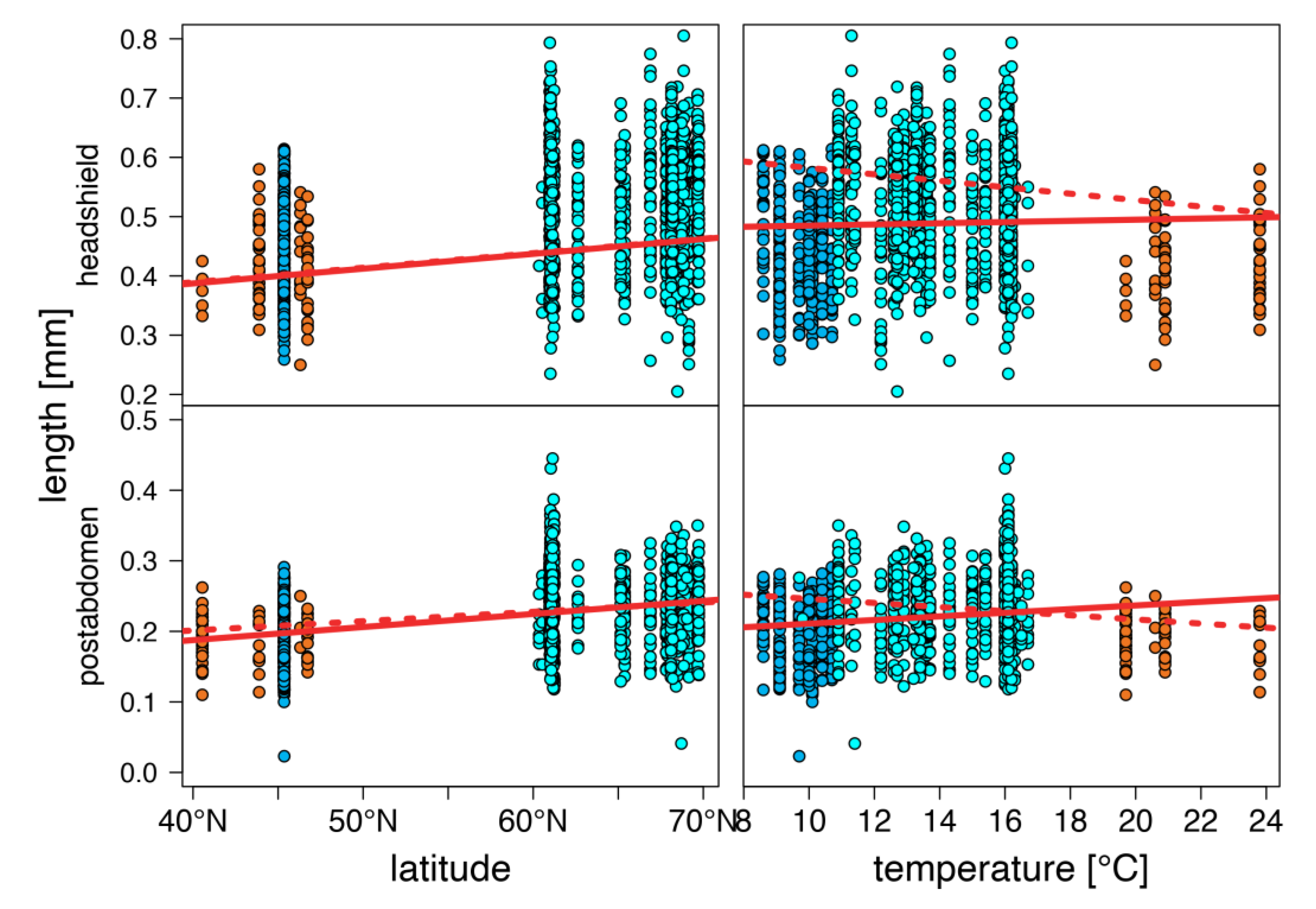
 warm lakes,
warm lakes,  Southern mountain lakes,
Southern mountain lakes,  Finnish lakes, solid line: all-lake model, dashed line: omit-SM model; Table A3).
Finnish lakes, solid line: all-lake model, dashed line: omit-SM model; Table A3).
 warm lakes,
warm lakes,  Southern mountain lakes,
Southern mountain lakes,  Finnish lakes, solid line: all-lake model, dashed line: omit-SM model; Table A3).
Finnish lakes, solid line: all-lake model, dashed line: omit-SM model; Table A3).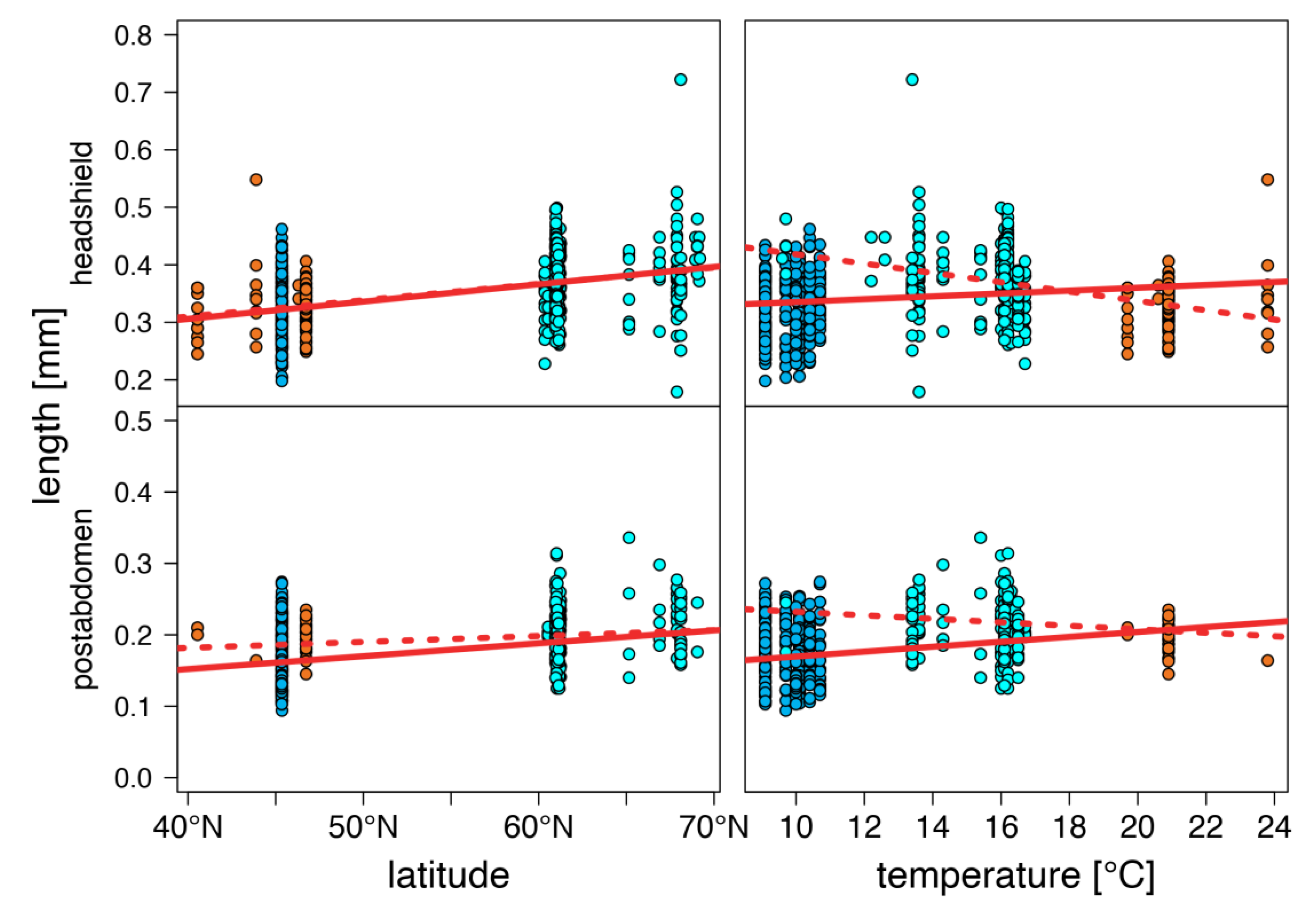
 warm lakes,
warm lakes,  Southern mountain lakes,
Southern mountain lakes,  Finnish lakes, solid line: all-lake model, dashed line: omit-SM model; Table A3).
Finnish lakes, solid line: all-lake model, dashed line: omit-SM model; Table A3).
 warm lakes,
warm lakes,  Southern mountain lakes,
Southern mountain lakes,  Finnish lakes, solid line: all-lake model, dashed line: omit-SM model; Table A3).
Finnish lakes, solid line: all-lake model, dashed line: omit-SM model; Table A3).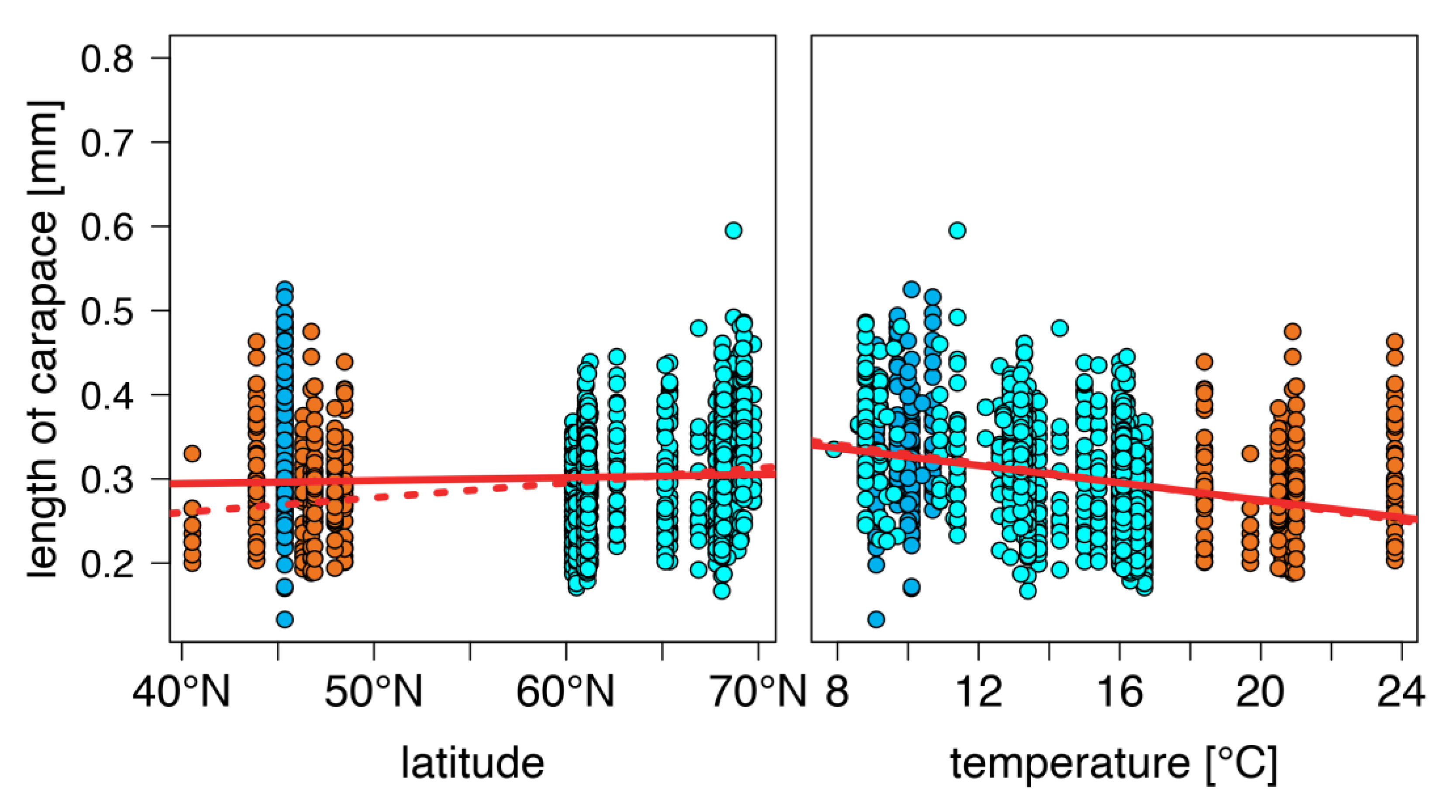
| Lakes | Latitude | Altitude (m a.s.l.) | No. of Lakes * | Depth (m) | Region | Temp. Regime |
|---|---|---|---|---|---|---|
| Finland | 60–70° N | 39–1009 | 46 | 0.5–25 | Nordic | cold |
| Hungary | 46° N | 104–384 | 5 | 3.5 | Southern | warm |
| Romania (South-Carpathians) | 45.34–45.36° N | 1909–2129 | 10 | 2.5–30 | Southern Mountain | cold |
| Croatia (Lake Vrana) | 43° N | 0 | 1 | 2.8 | Southern | warm |
| Spain (El Tobar) | 40.5° N | 1250 | 1 | 20 | Southern | warm |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korponai, J.L.; Kövér, C.; López-Blanco, C.; Gyulai, I.; Forró, L.; Katalinic, A.; Ketola, M.; Nevalainen, L.; Luoto, T.P.; Sarmaja-Korjonen, K.; et al. Effect of Temperature on the Size of Sedimentary Remains of Littoral Chydorids. Water 2020, 12, 1309. https://doi.org/10.3390/w12051309
Korponai JL, Kövér C, López-Blanco C, Gyulai I, Forró L, Katalinic A, Ketola M, Nevalainen L, Luoto TP, Sarmaja-Korjonen K, et al. Effect of Temperature on the Size of Sedimentary Remains of Littoral Chydorids. Water. 2020; 12(5):1309. https://doi.org/10.3390/w12051309
Chicago/Turabian StyleKorponai, János L., Csilla Kövér, Charo López-Blanco, István Gyulai, László Forró, Ana Katalinic, Mirva Ketola, Liisa Nevalainen, Tomi P. Luoto, Kaarina Sarmaja-Korjonen, and et al. 2020. "Effect of Temperature on the Size of Sedimentary Remains of Littoral Chydorids" Water 12, no. 5: 1309. https://doi.org/10.3390/w12051309
APA StyleKorponai, J. L., Kövér, C., López-Blanco, C., Gyulai, I., Forró, L., Katalinic, A., Ketola, M., Nevalainen, L., Luoto, T. P., Sarmaja-Korjonen, K., Magyari, E. K., Weckström, J., Urák, I., Vadkerti, E., & Buczkó, K. (2020). Effect of Temperature on the Size of Sedimentary Remains of Littoral Chydorids. Water, 12(5), 1309. https://doi.org/10.3390/w12051309








