Capacity of Two Ornamental Species (Iris sibirica and Zantedeschia aethiopica) to Take up, Translocate, and Accumulate Carbamazepine under Hydroponic Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tests to Assess CBZ Tolerance by I. sibirica and Z. aethiopica
2.2. Assessment of CBZ Uptake, Translocation, and Accumulation by I. sibirica and Z. aethiopica
2.3. Quantification of CBZ in Aqueous Samples and Plant Tissues
3. Results and Discussion
3.1. CBZ Tolerance by I. sibirica and Z. aethiopica
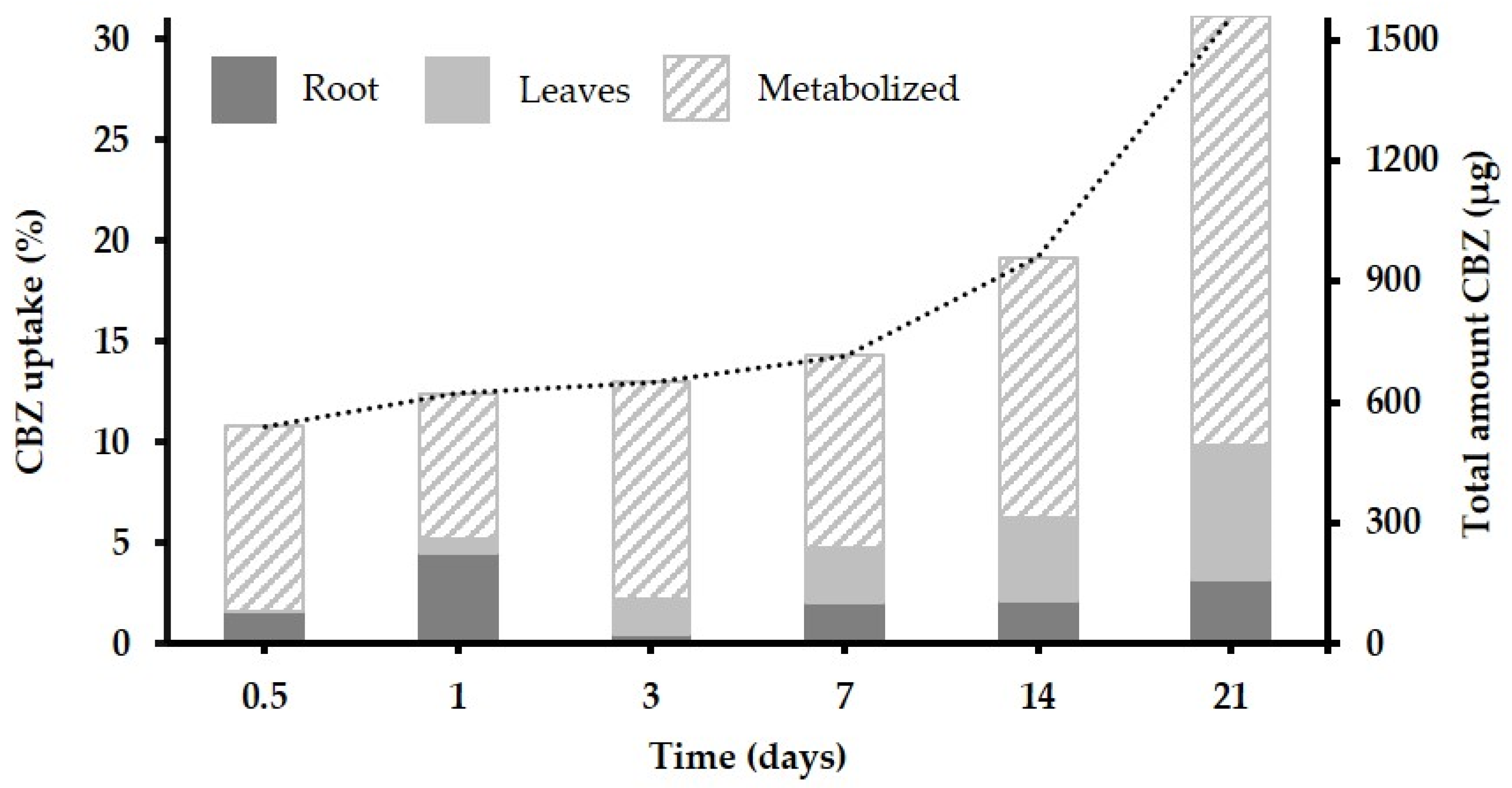
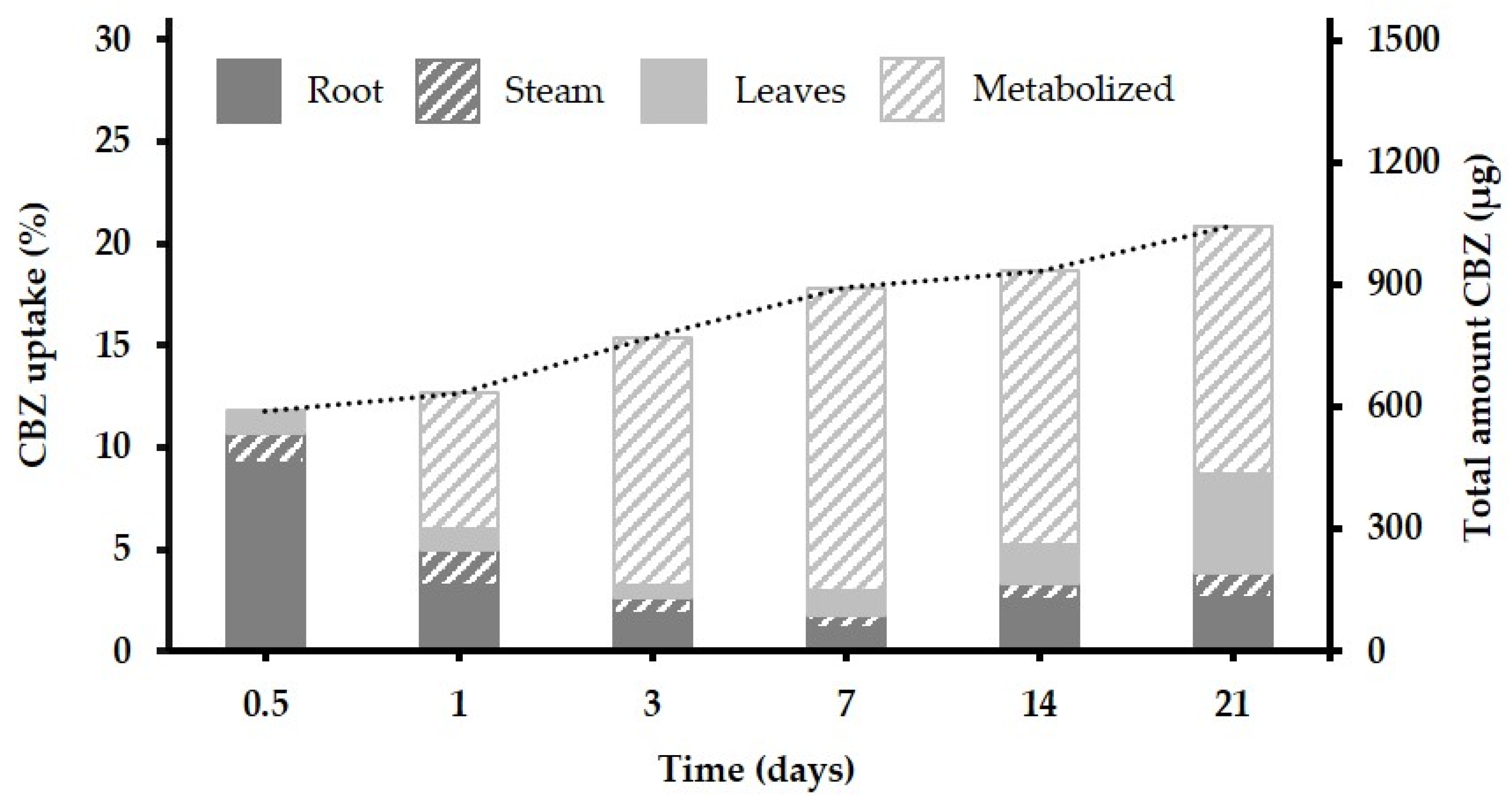
3.2. Uptake, Translocation and Accumulation of CBZ
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, D.Q.; Gersberg, R.M.; Hua, T.; Zhu, J.; Goyal, M.K.; Ng, W.J.; Tan, S.K. Fate of pharmaceutical compounds in hydroponic mesocosms planted with Scirpus validus. Environ. Pollut. 2013, 181, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Rienzie, R.; Ramanayaka, S.; Adassooriya, N.M. 12—Nanotechnology applications for the removal of environmental contaminants from pharmaceuticals and personal care products. In Pharmaceuticals and Personal Care Products: Waste Management and Treatment Technology; Prasad, M.N.V., Vithanage, M., Kapley, A., Eds.; Butterworth-Heinemann: Oxford, UK, 2019; pp. 279–296. [Google Scholar]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yin, T.; Kelly, B.C.; Gin, K.Y.-H. Bioaccumulation behaviour of pharmaceuticals and personal care products in a constructed wetland. Chemosphere 2019, 222, 275–285. [Google Scholar] [CrossRef]
- Zhang, Y.; Geißen, S.-U.; Gal, C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hu, Z.; Zhang, Y.; Zhuang, L.; Zhang, J.; Li, J.; Hu, H. Removal processes of carbamazepine in constructed wetlands treating secondary effluent: A review. Water 2018, 10, 1351. [Google Scholar] [CrossRef]
- Dordio, A.V.; Estêvão Candeias, A.J.; Pinto, A.P.; Teixeira da Costa, C.; Palace Carvalho, A.J. Preliminary media screening for application in the removal of clofibric acid, carbamazepine and ibuprofen by SSF-constructed wetlands. Ecol. Eng. 2009, 35, 290–302. [Google Scholar] [CrossRef]
- Mohapatra, D.P.; Brar, S.K.; Tyagi, R.D.; Picard, P.; Surampalli, R.Y. Carbamazepine in municipal wastewater and wastewater sludge: Ultrafast quantification by laser diode thermal desorption-atmospheric pressure chemical ionization coupled with tandem mass spectrometry. Talanta 2012, 99, 247–255. [Google Scholar] [CrossRef]
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef]
- Li, W.C. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ. Pollut. 2014, 187, 193–201. [Google Scholar] [CrossRef]
- Clara, M.; Strenn, B.; Ausserleitner, M.; Kreuzinger, N. Comparison of the behaviour of selected micropollutants in a membrane bioreactor and a conventional wastewater treatment plant. Water Sci. Technol. 2004, 50, 29–36. [Google Scholar] [CrossRef]
- Ferrari, B.; Paxéus, N.; Lo Giudice, R.; Pollio, A.; Garric, J. Ecotoxicological impact of pharmaceuticals found in treated wastewaters: Study of carbamazepine, clofibric acid, and diclofenac. Ecotoxicol. Environ. Saf. 2003, 55, 359–370. [Google Scholar] [CrossRef]
- Houeto, P.; Carton, A.; Guerbet, M.; Mauclaire, A.-C.; Gatignol, C.; Lechat, P.; Masset, D. Assessment of the health risks related to the presence of drug residues in water for human consumption: Application to carbamazepine. Regul. Toxicol. Pharmacol. 2012, 62, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Isidori, M.; Lavorgna, M.; Nardelli, A.; Pascarella, L.; Parrella, A. Toxic and genotoxic evaluation of six antibiotics on non-target organisms. Sci. Total Environ. 2005, 346, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Thacker, P.D. Pharmaceutical data elude researchers. Environ. Sci. Technol. 2005, 39, 193A–194A. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Triebskorn, R.; Casper, H.; Scheil, V.; Schwaiger, J. Ultrastructural effects of pharmaceuticals (carbamazepine, clofibric acid, metoprolol, diclofenac) in rainbow trout (Oncorhynchus mykiss) and common carp (Cyprinus carpio). Anal. Bioanal. Chem. 2007, 387, 1405–1416. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, G.; Ng, W.J.; Tan, S.K. A review on removing pharmaceutical contaminants from wastewater by constructed wetlands: Design, performance and mechanism. Sci. Total Environ. 2014, 468, 908–932. [Google Scholar] [CrossRef] [PubMed]
- Tejeda, A.; Torres-Bojorges, Á.X.; Zurita, F. Carbamazepine removal in three pilot-scale hybrid wetlands planted with ornamental species. Ecol. Eng. 2017, 98, 410–417. [Google Scholar] [CrossRef]
- Vo, H.-N.-P.; Bui, X.-T.; Nguyen, T.-M.-H.; Koottatep, T.; Bandyopadhyay, A. Insights of the Removal Mechanisms of Pharmaceutical and Personal Care Products in Constructed Wetlands. Curr. Pollut. Rep. 2018, 4, 93–103. [Google Scholar] [CrossRef]
- Wu, X.; Dodgen, L.K.; Conkle, J.L.; Gan, J. Plant uptake of pharmaceutical and personal care products from recycled water and biosolids: A review. Sci. Total Environ. 2015, 536, 655–666. [Google Scholar] [CrossRef]
- Dordio, A.V.; Duarte, C.; Barreiros, M.; Carvalho, A.J.P.; Pinto, A.P.; da Costa, C.T. Toxicity and removal efficiency of pharmaceutical metabolite clofibric acid by Typha spp.—Potential use for phytoremediation? Bioresour. Technol. 2009, 100, 1156–1161. [Google Scholar] [CrossRef]
- Porra, R.J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 2002, 73, 149–156. [Google Scholar] [CrossRef]
- Turnbull, L.A.; Philipson, C.D.; Purves, D.W.; Atkinson, R.L.; Cunniff, J.; Goodenough, A.; Hautier, Y.; Houghton, J.; Marthews, T.R.; Osborne, C.P.; et al. Plant growth rates and seed size: A re-evaluation. Ecology 2012, 93, 1283–1289. [Google Scholar] [CrossRef]
- Dordio, A.; Carvalho, A.J.P.; Teixeira, D.M.; Dias, C.B.; Pinto, A.P. Removal of pharmaceuticals in microcosm constructed wetlands using Typha spp. and LECA. Bioresour. Technol. 2010, 101, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, S.; Kilibarda, V.; Stojanovic, T. Determination of carbamazepine in serum and saliva samples by high performance liquid chromatography with ultraviolet detection. Vojnosanit. Pregl. 2009, 66, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.; Kumpulainen, J. Determination of free and total phenolic acids in plant-derived foods by HPLC with diode-array detection. J. Agric. Food Chem. 2002, 50, 3660–3667. [Google Scholar] [CrossRef] [PubMed]
- Dordio, A.V.; Belo, M.; Martins Teixeira, D.; Palace Carvalho, A.J.; Dias, C.M.B.; Picó, Y.; Pinto, A.P. Evaluation of carbamazepine uptake and metabolization by Typha spp., a plant with potential use in phytotreatment. Bioresour. Technol. 2011, 102, 7827–7834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Q.; Hua, T.; Gersberg, R.M.; Zhu, J.; Ng, W.J.; Tan, S.K. Carbamazepine and naproxen: Fate in wetland mesocosms planted with Scirpus validus. Chemosphere 2013, 91, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Haehnel, W. Photosynthetic electron transport in higher plants. Annu. Rev. Plant Physiol. 1984, 35, 659–693. [Google Scholar] [CrossRef]
- Yamori, W.; Shikanai, T. Physiological functions of cyclic electron transport around photosystem I in sustaining photosynthesis and plant growth. Annu. Rev. Plant Biol. 2016, 67, 81–106. [Google Scholar] [CrossRef]
- Rodríguez-Celma, J.; Ceballos-Laita, L.; Grusak, M.A.; Abadía, J.; López-Millán, A.-F. Plant fluid proteomics: Delving into the xylem sap, phloem sap and apoplastic fluid proteomes. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2016, 1864, 991–1002. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Falcón, M.; Hernández, M.; Alvarez, C.E.; Borges, A.A. Variation in nutrition along time and relative chlorophyll content of Leucospermum cordifolium cv. ‘High Gold’, and their relationship with chlorotic sypmptoms. Sci. Hortic. 2006, 107, 373–379. [Google Scholar] [CrossRef]
- Carvalho, P.N.; Basto, M.C.P.; Almeida, C.M.R.; Brix, H. A review of plant–pharmaceutical interactions: From uptake and effects in crop plants to phytoremediation in constructed wetlands. Environ. Sci. Pollut. Res. 2014, 21, 11729–11763. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Treatments | |||
|---|---|---|---|---|
| Control | 5 mg/L | 10 mg/L | 15 mg/L | |
| Relative Chlorophyll Content (mg/g) | ||||
| Chl a + b | 0.62 ± 0.018 a | 0.60 ± 0.018 a,b | 0.60 ± 0.018 a,b | 0.58 ± 0.018 b |
| Chl a | 0.34 ± 0.009 a | 0.33 ± 0.009 a,b | 0.33 ± 0.009 a,b | 0.32 ± 0.009 b |
| Chl b | 0.28 ± 0.009 a | 0.27 ± 0.009 a,b | 0.27 ± 0.009 a,b | 0.26 ± 0.009 b |
| RGR (d−1) | 0.010 ± 0.001 | 0.010 ± 0.001 | 0.003 ± 0.018 | 0.003 ± 0.018 |
| ET (mm/d) | 5.07 ± 0.90 a | 3.81 ± 0.53 a,b | 3.57 ± 0.55 b | 2.48 ± 0.26 c |
| pH | 5.51 ± 0.25 a | 5.90 ± 0.21 a,b | 6.11 ± 0.07 b | 6.09 ± 0.07 b |
| Parameters | Treatments | |||
|---|---|---|---|---|
| Control | 5 mg/L | 10 mg/L | 15 mg/L | |
| Relative Chlorophyll Content (mg/g) | ||||
| Chl a + b | 0.36 ± 0.011 a | 0.33 ± 0.011 b,c | 0.35 ± 0.010 a,b | 0.32 ± 0.010 c |
| Chl a | 0.14 ± 0.004 a | 0.14 ± 0.004 b,c | 0.14 ± 0.003 a,b | 0.13 ± 0.003 c |
| Chl b | 0.21 ± 0.006 a | 0.20 ± 0.006 b,c | 0.21 ± 0.005 a,b | 0.19 ± 0.005 c |
| RGR (d−1) | 0.021 ± 0.012 | 0.020 ± 0.001 | 0.020 ± 0.001 | 0.017 ± 0.014 |
| ET (mm/d) | 3.04 ± 0.19 a | 3.12 ± 0.25 a | 2.81 ± 0.44 a,b | 2.42 ± 0.22 b |
| pH | 5.98 ± 0.07 | 5.96 ± 0.08 | 6.01 ± 0.08 | 5.96 ± 0.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejeda, A.; Zurita, F. Capacity of Two Ornamental Species (Iris sibirica and Zantedeschia aethiopica) to Take up, Translocate, and Accumulate Carbamazepine under Hydroponic Conditions. Water 2020, 12, 1272. https://doi.org/10.3390/w12051272
Tejeda A, Zurita F. Capacity of Two Ornamental Species (Iris sibirica and Zantedeschia aethiopica) to Take up, Translocate, and Accumulate Carbamazepine under Hydroponic Conditions. Water. 2020; 12(5):1272. https://doi.org/10.3390/w12051272
Chicago/Turabian StyleTejeda, Allan, and Florentina Zurita. 2020. "Capacity of Two Ornamental Species (Iris sibirica and Zantedeschia aethiopica) to Take up, Translocate, and Accumulate Carbamazepine under Hydroponic Conditions" Water 12, no. 5: 1272. https://doi.org/10.3390/w12051272
APA StyleTejeda, A., & Zurita, F. (2020). Capacity of Two Ornamental Species (Iris sibirica and Zantedeschia aethiopica) to Take up, Translocate, and Accumulate Carbamazepine under Hydroponic Conditions. Water, 12(5), 1272. https://doi.org/10.3390/w12051272





