Electrodialytic Hydrogen Production and Critical Raw Materials Recovery from Secondary Resources
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental
2.3. Methods
3. Results and Discussion
3.1. Characterization of Electrodialytic Experiments
3.2. Critical Raw Materials Extraction
3.3. Hydrogen Generation and Purity
3.4. Electrical Requirements and Savings
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eurostat Statistics Explained People in the EU-population Projections. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=People_in_the_EU_-_population_projections#Population_projections (accessed on 13 April 2020).
- European Comission. Communication from the Comission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions on the 2017 list of Critical Raw Materials for the EU; European Comission: Brussels, Belgium, 2017. [Google Scholar]
- European Commission. 2030 Climate and Energy Policy Framework; European Commission: Brussels, Belgium, 2014. [Google Scholar]
- Hart, P.S.; Feldman, L. Would it be better to not talk about climate change? The impact of climate change and air pollution frames on support for regulating power plant emissions. J. Environ. Psychol. 2018, 60, 1–8. [Google Scholar] [CrossRef]
- European Commission. Communication from the Comission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions-Closing the Loop-An EU Action Plan for the Circular Economy; European Commission: Brussels, Belgium, 2015. [Google Scholar]
- Ribeiro, A.B.; Mateus, E.P.; Ottosen, L.M.; Bech-Nielsen, G. Electrodialytic removal of Cu, Cr, and As from chromated copper arsenate-treated timber waste. Environ. Sci. Technol. 2000, 34, 784–788. [Google Scholar] [CrossRef]
- Magro, C.C.; Guedes, P.R.; Kirkelund, G.M.; Jensen, P.E.; Ottosen, L.M.; Ribeiro, A.B. Incorporation of different fly ashes from mswi as substitute for cement in mortar: An overview of the suitability of electrodialytic pre-treatment. In Electrokinetics Across Disciplines and Continents; Ribeiro, A.B., Mateus, E.P., Couto, N., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 225–247. ISBN 9783319201795. [Google Scholar]
- Guedes, P.; Mateus, E.; Almeida, J.; Ferreira, A.; Couto, N.; Ribeiro, A. Electrodialytic treatment of sewage sludge: Current intensity influence on phosphorus recovery and organic contaminants removal. Chem. Eng. J. 2016, 306, 1058–1066. [Google Scholar] [CrossRef]
- Ribeiro, A.B.; Mateus, E.P.; Couto, N. (Eds.) Electrokinetics across disciplines and continents: New strategies for sustainable development; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; 427p, ISBN 9783319201795. [Google Scholar]
- Almeida, J.; Craveiro, R.; Faria, P.; Silva, A.S.; Mateus, E.P.; Barreiros, S.; Paiva, A.; Ribeiro, A.B. Electrodialytic removal of tungsten and arsenic from secondary mine resources—Deep eutectic solvents enhancement. Sci. Total Environ. 2020, 710, 136364. [Google Scholar] [CrossRef] [Green Version]
- Magro, C.; Paz-Garcia, J.M.; Ottosen, L.M.; Mateus, E.P.; Ribeiro, A.B. Sustainability of construction materials: Electrodialytic technology as a tool for mortars production. J. Hazard. Mater. 2019, 363, 421–427. [Google Scholar] [CrossRef]
- Ferreira, A.R.; Couto, N.; Guedes, P.; Pinto, J.; Mateus, E.P.; Ribeiro, A.B. Electrodialytic 2-compartment cells for emerging organic contaminants removal from effluent. J. Hazard. Mater. 2018, 358, 467–474. [Google Scholar] [CrossRef]
- Kacprzak, M.; Neczaj, E.; Fijałkowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Rorat, A.; Brattebo, H.; Almås, Å.; Singh, B.R. Sewage sludge disposal strategies for sustainable development. Environ. Res. 2017, 156, 39–46. [Google Scholar] [CrossRef]
- Schmidt, S.; Berghau, W.; Hutten, A. From deposit to concentrate: The basics of tungsten mining Part 1: Project generation and project development. Int. Tungsten Ind. Assoc. 2012, 4, 1–20. [Google Scholar]
- Candeias, C.; Melo, R.; Ávila, P.F.; Ferreira da Silva, E.; Salgueiro, A.R.; Teixeira, J.P. Heavy metal pollution in mine-soil-plant system in S. Francisco de Assis-Panasqueira mine (Portugal). Appl. Geochem. 2014, 44, 12–26. [Google Scholar] [CrossRef] [Green Version]
- Ganiyu, S.O.; Martínez-Huitle, C.A.; Rodrigo, M.A. Renewable energies driven electrochemical wastewater/soil decontamination technologies: A critical review of fundamental concepts and applications. Appl. Catal. B Environ. 2020, 270, 118857. [Google Scholar] [CrossRef]
- Magro, C.; Almeida, J.; Paz-Garcia, J.M.; Mateus, E.P.; Ribeiro, A.B. Exploring hydrogen production for self-energy generation in electroremediation: A proof of concept. Appl. Energy 2019, 255, 113839. [Google Scholar] [CrossRef] [Green Version]
- Kappel, A.; Ottosen, L.M.; Kirkelund, G.M. Colour, compressive strength and workability of mortars with an iron rich sewage sludge ash. Constr. Build. Mater. 2017, 157, 1199–1205. [Google Scholar] [CrossRef] [Green Version]
- Almeida, J.; Ribeiro, A.B.; Silva, A.S.; Faria, P. Overview of mining residues incorporation in construction materials and barriers for full-scale application. J. Build. Eng. 2020, 29, 101215. [Google Scholar] [CrossRef]
- Diário da República. Decreto-Lei n.o 152/2017 de 7 de Dezembro; Diário da República: Lisbon, Portugal, 2017. [Google Scholar]
- Ugilt Sø, H. Adsorption of Arsenic and Phosphate onto the Surface of Calcite as Revealed by Batch Experiments and Surface Complexation Modelling; Technical University of Denmark: Lyngby, Denmark, 2011. [Google Scholar]
- Goreau, T.J.; Larson, R.W.; Campe, J. Geotherapy: Innovative methods of soil fertility restoration, carbon sequestration, and reversing CO2 increase. Choice Rev. Online 2015, 53, 1302. [Google Scholar]
- Petzet, S.; Peplinski, B.; Bodkhe, S.Y.; Cornel, P. Recovery of phosphorus and aluminium from sewage sludge ash by a new wet chemical elution process (SESAL-Phos-recovery process). Water Sci. Technol. 2011, 64, 693–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackenzie, A.F.; Amer, S.A. Reactions of iron, aluminum and calcium phosphates in six Ontario soils. Plant Soil 1964, 21, 17–25. [Google Scholar] [CrossRef]
- Kouzbour, S.; Gourich, B.; Gros, F.; Vial, C.; Allam, F.; Stiriba, Y. Comparative analysis of industrial processes for cadmium removal from phosphoric acid: A review. Hydrometallurgy 2019, 188, 222–247. [Google Scholar] [CrossRef]
- Dermatas, D.; Braida, W.; Christodoulatos, C.; Strigul, N.; Panikov, N.; Los, M.; Larson, S. Solubility, sorption, and soil respiration effects of tungsten and tungsten alloys. Environ. Forensics 2004, 5, 5–13. [Google Scholar] [CrossRef]
- Wang, S.; Gao, H.; Sun, G.; Li, Y.; Wang, Y.; Liu, H.; Chen, C.; Yang, L. Structure characterization, optical and photoluminescence properties of scheelite-type CaWO4 nanophosphors: Effects of calcination temperature and carbon skeleton. Opt. Mater. 2020, 99, 109562. [Google Scholar] [CrossRef]
- Miyazaki, H.; Nose, A.; Suzuki, H.; Ota, T. Phosphorus solid solution effects of electric and dielectric properties on sintered WO3 ceramics. J. Ceram. Soc. Jpn. 2011, 8, 650–653. [Google Scholar] [CrossRef] [Green Version]
- Anik, M. pH-dependent anodic reaction behavior of tungsten in acidic phosphate solutions. Electrochim. Acta 2009, 54, 3943–3951. [Google Scholar] [CrossRef]
- Deltombe, E.; De Zoubov, N.; Pourbaix, M. Tungsten. In Atlas of Electrochemical Equilibria in-Aqueous Solutions; Pourbaix, M., Ed.; NACE: Houston, TX, USA, 1974; pp. 280–285. [Google Scholar]
- Guedes, P.; Couto, N.; Almeida, J.; Rodrigues, A.M.; Mateus, E.P.; Ribeiro, A.B. Electrodialytic treatment of sewage sludge: Influence on microbiological community. Int. J. Environ. Sci. Technol. 2018, 15, 1103–1112. [Google Scholar] [CrossRef]
- Rivera, I.; Schröder, U.; Patil, S.A. Microbial electrolysis for biohydrogen production. In Microbial Electrochemical Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 871–898. [Google Scholar]
- Li, P.C.H.; Harrison, D.J. Transport, manipulation, and reaction of biological cells on-chip using electrokinetic effects. Anal. Chem. 1997, 69, 1564–1566. [Google Scholar] [CrossRef]
- Gill, R.T.; Harbottle, M.J.; Smith, J.W.N.; Thornton, S.F. Electrokinetic-enhanced bioremediation of organic contaminants: A review of processes and environmental applications. Chemosphere 2014, 107, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Szumski, M.; Kłodzińska, E.; Buszewski, B. Separation of microorganisms using electromigration techniques. J. Chromatogr. A 2005, 1084, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Ebbers, B.; Ottosen, L.M.; Jensen, P.E. Electrodialytic treatment of municipal wastewater and sludge for the removal of heavy metals and recovery of phosphorus. Electrochim. Acta 2015, 181, 90–99. [Google Scholar] [CrossRef]
- Salameh, Z. Energy storage. In Renewable Energy System Design; Elsevier: Amsterdam, The Netherlands, 2014; pp. 201–298. ISBN 9780123749918. [Google Scholar]
- Staffell, I.; Green, R.; Kendall, K. Cost targets for domestic fuel cell CHP. J. Power Sources 2008, 181, 339–349. [Google Scholar] [CrossRef]
- Seo, J.G.; Kwon, J.T.; Kim, J.; Kim, W.S.; Jung, J.T. Impurity effect on proton exchange membrane fuel cell. In Proceedings of the 2007 International Forum on Strategic Technology; IFOST: Ulsan, Korea, 2007; pp. 484–487. [Google Scholar]
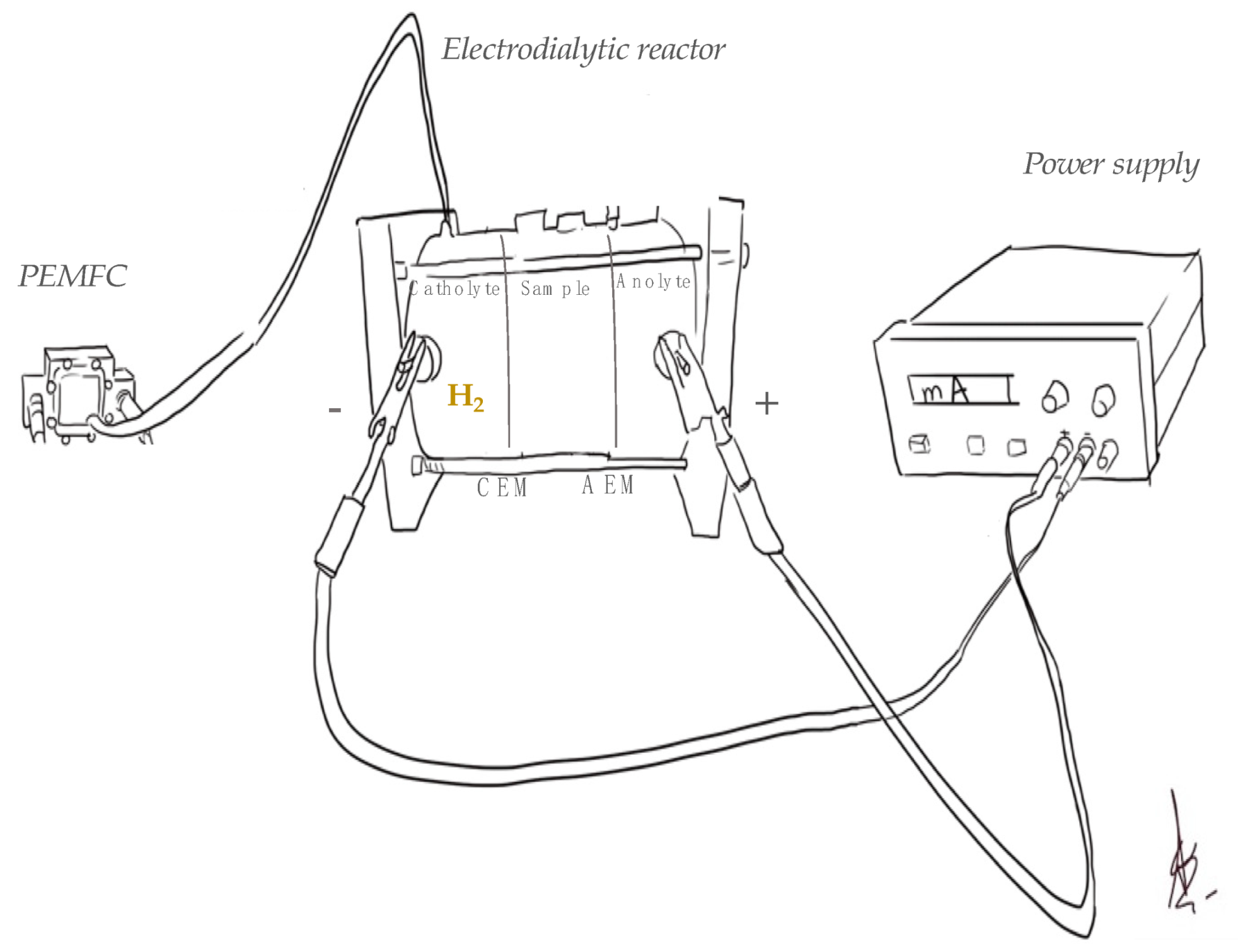
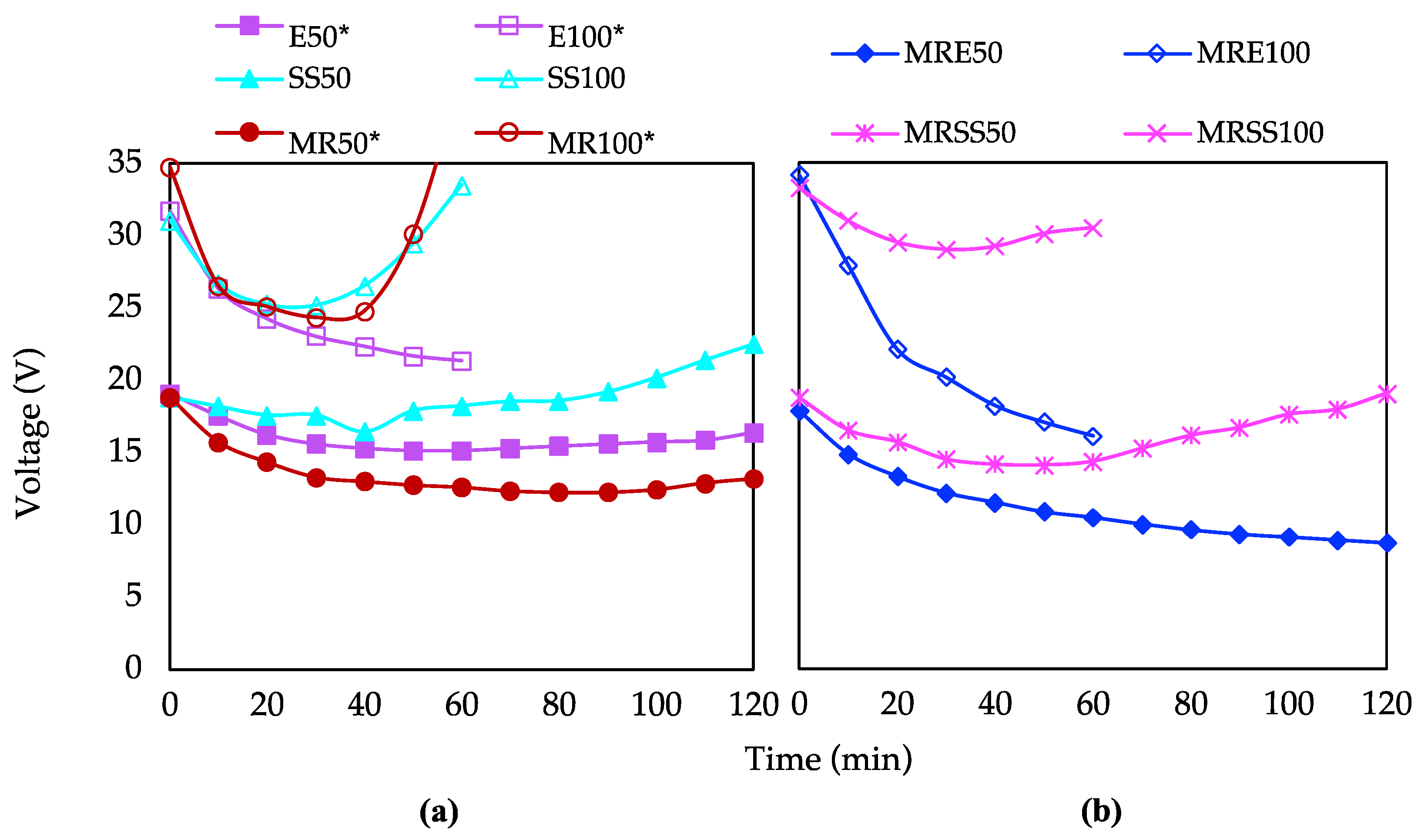
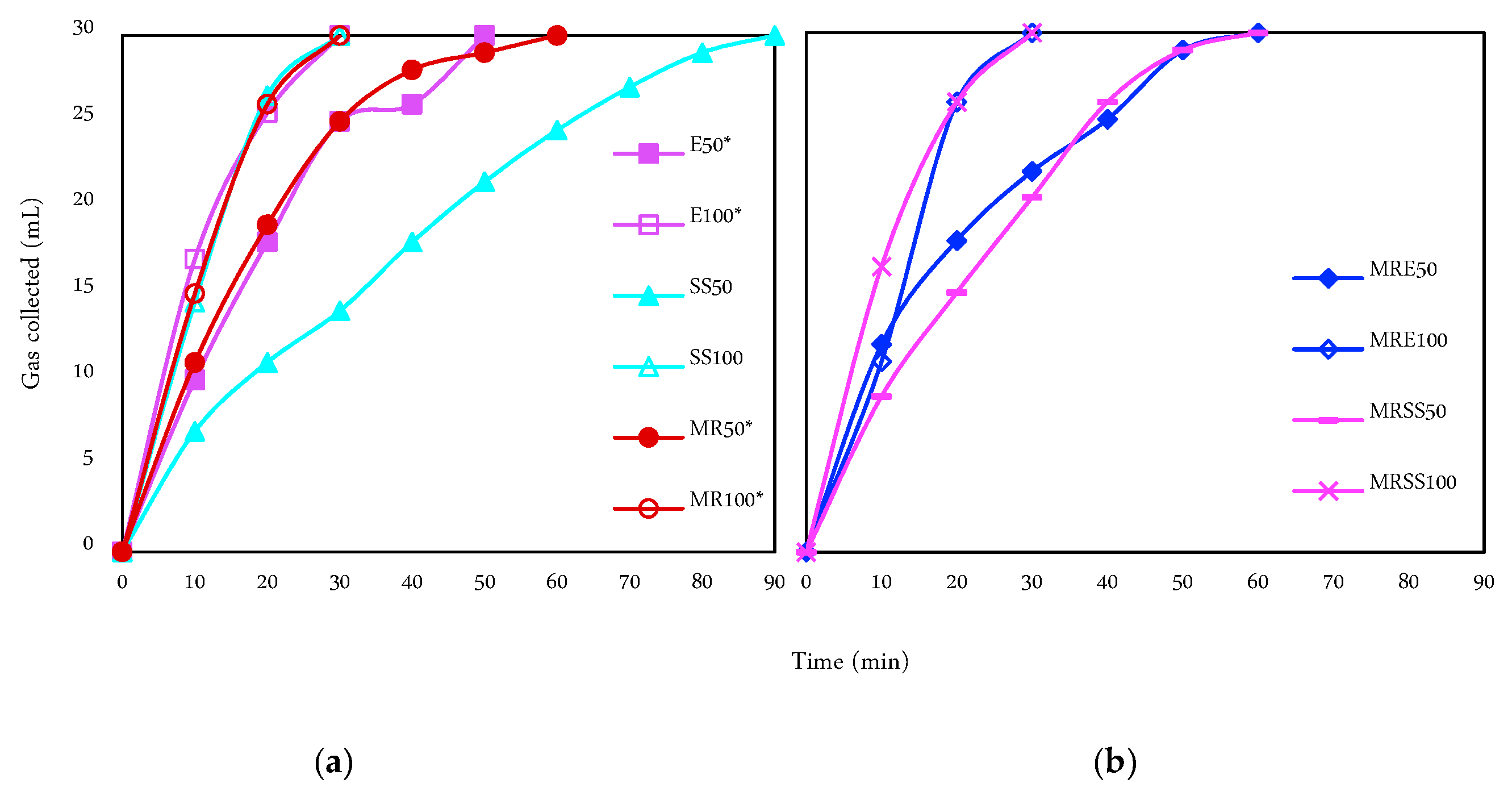
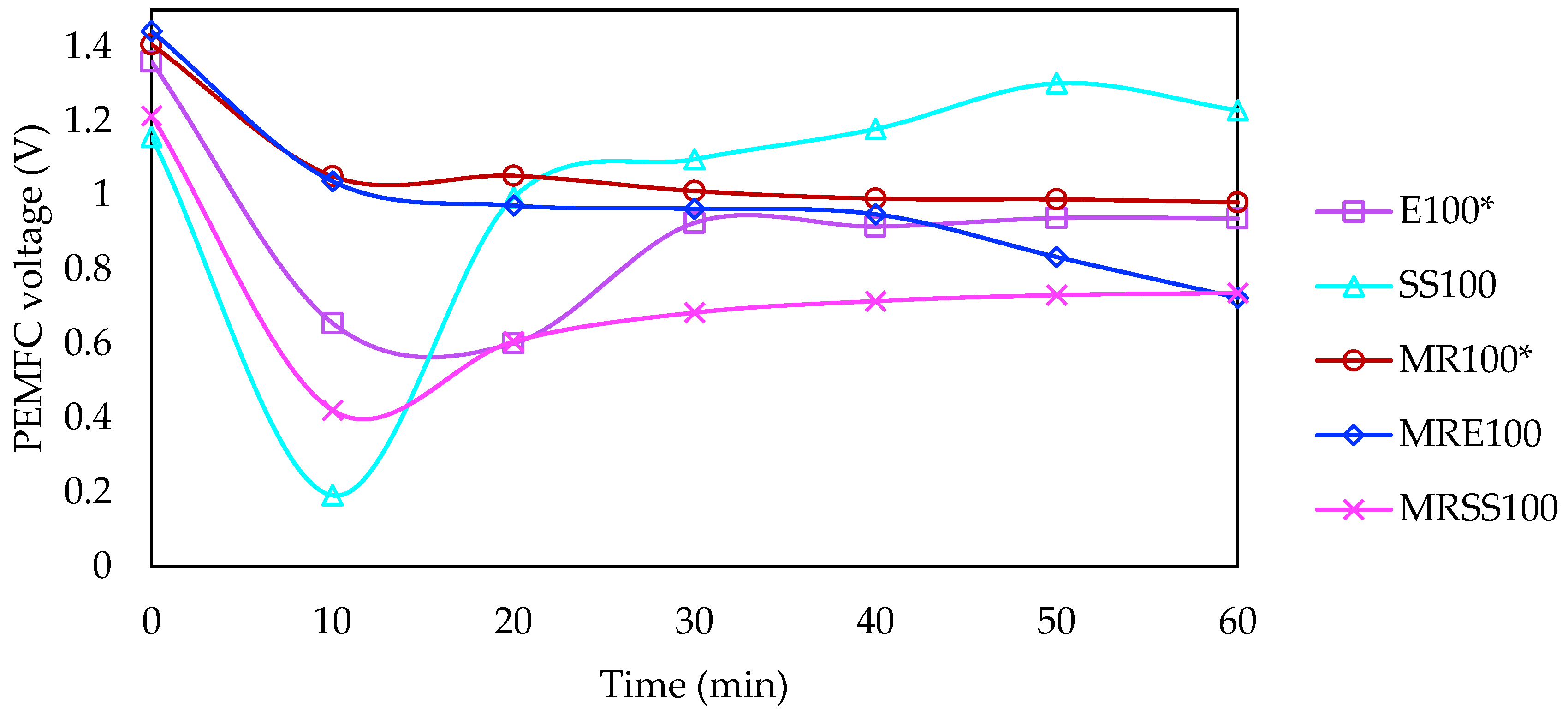
| Code | Operation Time (min) | Current Intensity (mA) | Matrix |
|---|---|---|---|
| E50 * | 120 | 50 | Effluent |
| E100 * | 60 | 100 | |
| SS50 | 120 | 50 | Sewage Sludge |
| SS100 | 60 | 100 | |
| MR50 * | 120 | 50 | Mining Residues and Briny Water |
| MR100 * | 60 | 100 | |
| MRE50 | 120 | 50 | Mining Residues and Effluent |
| MRE100 | 60 | 100 | |
| MRSS50 | 120 | 50 | Mining Residues and Sewage Sludge |
| MRSS100 | 60 | 100 |
| Element | Matrix | ||
|---|---|---|---|
| Effluent (mg/L) * | Sewage Sludge (mg/L) | Mining Residues (mg/kg) * | |
| As | n.d. | 0.06 ± 0.01 | 218.57 ± 132.31 |
| Ca | 51.74 ± 18.34 | 158.98 ± 48.21 | 91.11 ± 27.08 |
| Cu | 0.04 ± 0.00 | 1.90 ± 0.48 | 76.82 ± 39.30 |
| K | 47.80 ± 29.10 | 25.51 ± 1.30 | n.d. |
| Mg | 118.00 ± 137.17 | 14.87 ± 3.35 | n.d. |
| Na | 524.55 ± 532.55 | 23.63 ± 0.07 | n.d. |
| S | 84.89 ± 65.27 a | 78.35 ± 17.45b | 789.59 ± 214.13 A,B |
| Sn | n.d. | 0.17 ± 0.04 c | 1.95 ± 0.53 C |
| Zn | 0.07 ± 0.06 e | 7.20 ± 0.99 E | n.d. |
| Cl- | 908.1 ± 1013.7 | 81 # | 5.6 ± 2.3 |
| Experiment | Compartment | pH | Conductivity (mS/cm) | ||
|---|---|---|---|---|---|
| Initial | Final | Initial | Final | ||
| E50 * | Anode | 6.46 ± 0.55 | 2.20 ± 0.02 | 0.90 ± 0.06 | 2.70 ± 0.00 j |
| Cathode | 12.21± 0.01 a | 2.45 ± 0.21 | |||
| Sample | 7.67 ± 0.16 | 4.54 ± 2.55 | 2.41 ± 2.12 | 1.80 ± 0.86 | |
| E100 * | Anode | 6.46 ± 0.55 | 2.21 ± 0.04 | 0.90 ± 0.06 | 2.85 ± 1.06 k |
| Cathode | 12.11 ± 0.01 b | 1.94 ± 0.23 | |||
| Sample | 7.67 ± 0.16 | 2.85 ± 0.20 o | 2.41 ± 2.12 | 1.49 ± 0.15 | |
| SS50 | Anode | 7.54 ± 0.38 | 2.27 ± 0.19 | 0.54 ± 0.06 | 1.24 ± 0.14 l |
| Cathode | 11.77 ± 0.13 A,c | 0.97 ± 0.02 | |||
| Sample | 6.68 ± 0.02 | 5.96 ± 0.18 | 0.81 ± 0.02 | 0.37 ± 0.01 | |
| SS100 | Anode | 7.54 ± 0.38 | 2.15 ± 0.11 | 0.54 ± 0.06 | 0.94 ± 0.17 K |
| Cathode | 11.51 ± 0.08 B,d | 1.06 ± 0.21 | |||
| Sample | 6.68 ± 0.02 | 5.86 ± 0.14 | 0.81 ± 0.02 | 0.30 ± 0.01 | |
| MR50 * | Anode | 6.46 ± 0.55 | 2.02 ± 0.11 | 0.90 ± 0.06 | 3.25 ± 0.21 L |
| Cathode | 12.30 ± 0.01 C,e | 2.40 ± 0.14 | |||
| Sample | 4.57 ± 1.74 | 5.91 ± 0.45 | 1.82 ± 0.54 | 1.41 ± 0.49 | |
| MR100* | Anode | 6.46 ± 0.55 | 1.99 ± 0.04f | 0.90 ± 0.06 | 2.55 ± 0.07 |
| Cathode | 12.21 ± 0.25 D,g | 2.08 ± 0.46 | |||
| Sample | 4.57 ± 1.74 | 4.42 ± 1.33 | 1.82 ± 0.54 | 0.24 ± 0.16 | |
| MRE50 | Anode | 6.46 ± 0.55 | 2.00 ± 0.02 | 0.90 ± 0.06 | 2.70 ± 0.14 n |
| Cathode | 12.31 ± 0.02 C,h | 2.12 ± 0.54 | |||
| Sample | 7.24 ± 0.12 | 6.50 ± 0.22 | 2.55 ± 1.92 | 1.17 ± 0.60 | |
| MRE100 | Anode | 6.46 ± 0.55 | 2.08 ± 0.01 | 0.90 ± 0.06 | 4.00 ± 0.99 |
| Cathode | 12.25 ± 0.03 D,i | 2.20 ± 0.14 | |||
| Sample | 7.24 ± 0.12 | 7.51 ± 0.12 O | 2.55 ± 1.92 | 1.68 ± 0.32 | |
| MRSS50 | Anode | 7.54 ± 0.38 | 2.36 ± 0.11 | 0.54 ± 0.06 | 1.00 ± 0.50 J,N |
| Cathode | 11.65 ± 0.16 A,E,H | 1.12 ± 0.28 | |||
| Sample | 7.18 ± 0.04 | 6.38 ± 0.23 | 0.65 ± 0.01 | 0.61 ± 0.37 | |
| MRSS100 | Anode | 7.54 ± 0.38 | 2.47 ± 0.18 F | 0.54 ± 0.06 | 1.05 ± 0.30 K |
| Cathode | 11.57 ± 0.11 B,G,I | 0.91 ± 0.04 M | |||
| Sample | 7.18 ± 0.04 | 6.31 ± 0.01 | 0.65 ± 0.01 | 0.43 ± 0.01 | |
| Experiment | Phosphorus | Tungsten | |||
|---|---|---|---|---|---|
| Initial | Final | Initial | Final | ||
| E50 * | mg/L | 3.21 ± 0.04 a | 2.84 ± 0.51 b | nd # | nd # |
| E100 * | 3.30 ± 0.14 c | nd # | |||
| SS50 | mg/L | 65.83 ± 19.60 | 156.97 ± 30.73 B,d | nd # | nd # |
| SS100 | 146.83 ± 0.25 C,e | nd # | |||
| MR50 * | mg/kg | 36.57 ± 18.74 | 20.73 ± 23.81 D | 5.30 ± 1.56 | 5.64 ± 0.43 f |
| MR100 * | 30.29 ± 4.88 E | 4.88 ± 1.58 g | |||
| MRE50 | mg/kg | 39.80 ± 20.61 | 4.22 ± 0.64 D | 5.30 ± 1.56 | 3.89 ± 0.13 h |
| MRE100 | 17.81 ± 22.15 E | 3.76 ± 0.27 | |||
| MRSS50 | mg/kg | 108.66 ± 38.34 A | 29.59 ± 1.11 D | 5.30 ± 1.56 | 1.71 ± 0.41 F,H |
| MRSS100 | 33.51 ± 0.66 E | 1.63 ± 0.02 G | |||
| Experiment | Hydrogen | ||
|---|---|---|---|
| Flow mL/min (Slope) | R-Square (Slope) | Purity w/w (%) | |
| E50 * | 0.68 | 0.97 | - |
| E100 * | 1.13 | 0.96 | 90.4 ± 0.3 a |
| SS50 | 0.33 | 0.97 | - |
| SS100 | 1.02 | 0.94 | 32.6 ± 0.5 b |
| MR50 * | 0.62 | 0.94 | - |
| MR100 * | 1.12 | 0.97 | 72.4 ± 0.7 A,B |
| MRE50 | 0.47 | 0.91 | - |
| MRE100 | 1.05 | 0.96 | 71.3 ± 0.7 A,B,c |
| MRSS50 | 0.50 | 0.94 | - |
| MRSS100 | 1.0 | 0.93 | 33.6 ± 4.9 A,B,C |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, J.; Magro, C.; Mateus, E.P.; Ribeiro, A.B. Electrodialytic Hydrogen Production and Critical Raw Materials Recovery from Secondary Resources. Water 2020, 12, 1262. https://doi.org/10.3390/w12051262
Almeida J, Magro C, Mateus EP, Ribeiro AB. Electrodialytic Hydrogen Production and Critical Raw Materials Recovery from Secondary Resources. Water. 2020; 12(5):1262. https://doi.org/10.3390/w12051262
Chicago/Turabian StyleAlmeida, Joana, Cátia Magro, Eduardo P. Mateus, and Alexandra B. Ribeiro. 2020. "Electrodialytic Hydrogen Production and Critical Raw Materials Recovery from Secondary Resources" Water 12, no. 5: 1262. https://doi.org/10.3390/w12051262
APA StyleAlmeida, J., Magro, C., Mateus, E. P., & Ribeiro, A. B. (2020). Electrodialytic Hydrogen Production and Critical Raw Materials Recovery from Secondary Resources. Water, 12(5), 1262. https://doi.org/10.3390/w12051262








