Effects of Transverse Groynes on Meso-Habitat Suitability for Native Fish Species on a Regulated By-Passed Large River: A Case Study along the Rhine River
Abstract
:1. Introduction
2. Materials and Methods
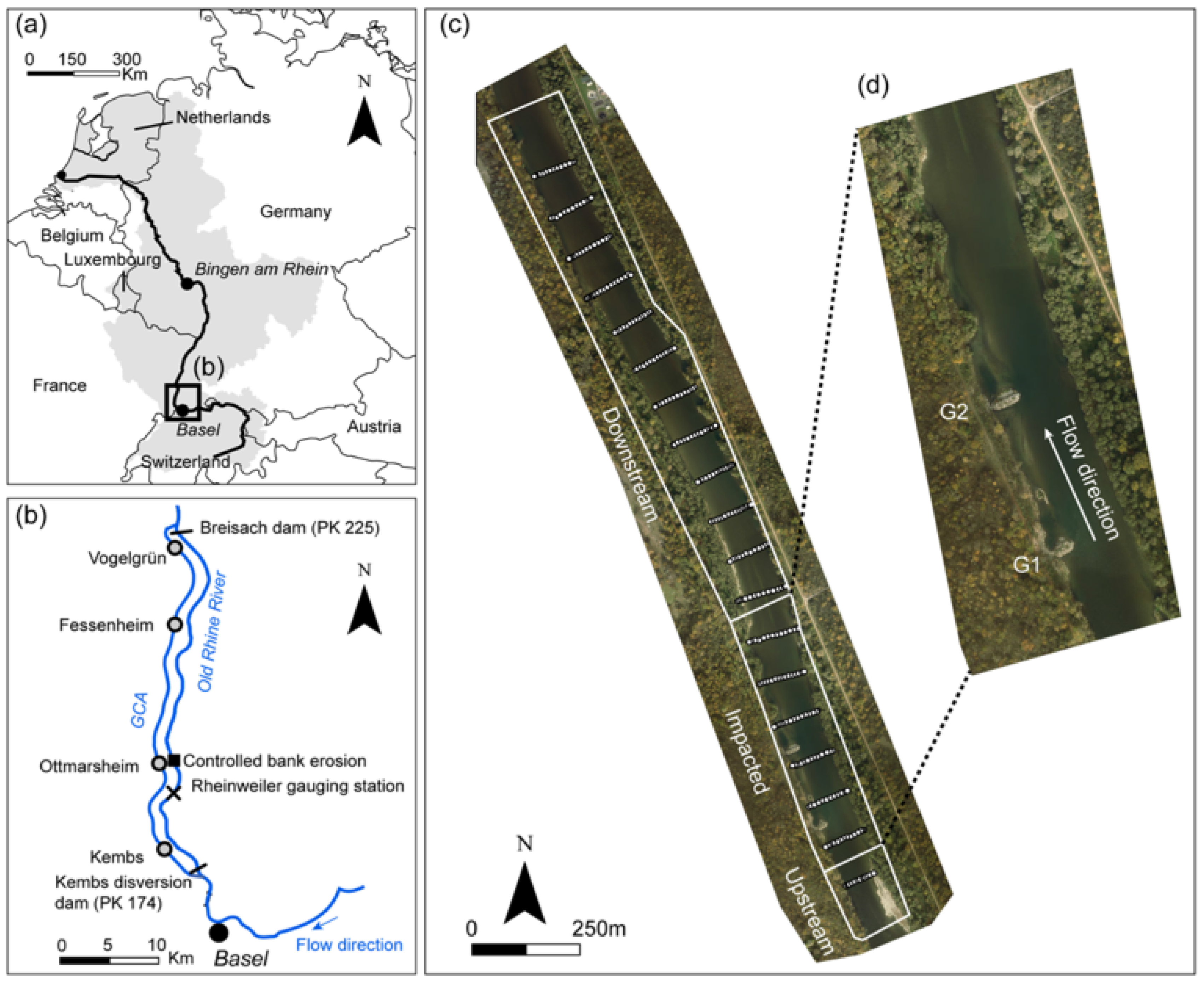
2.1. Study Area
2.2. Data Collection and Processing
2.2.1. Field Monitoring
2.2.2. Grain Size Quantification
2.2.3. Hydraulic Models
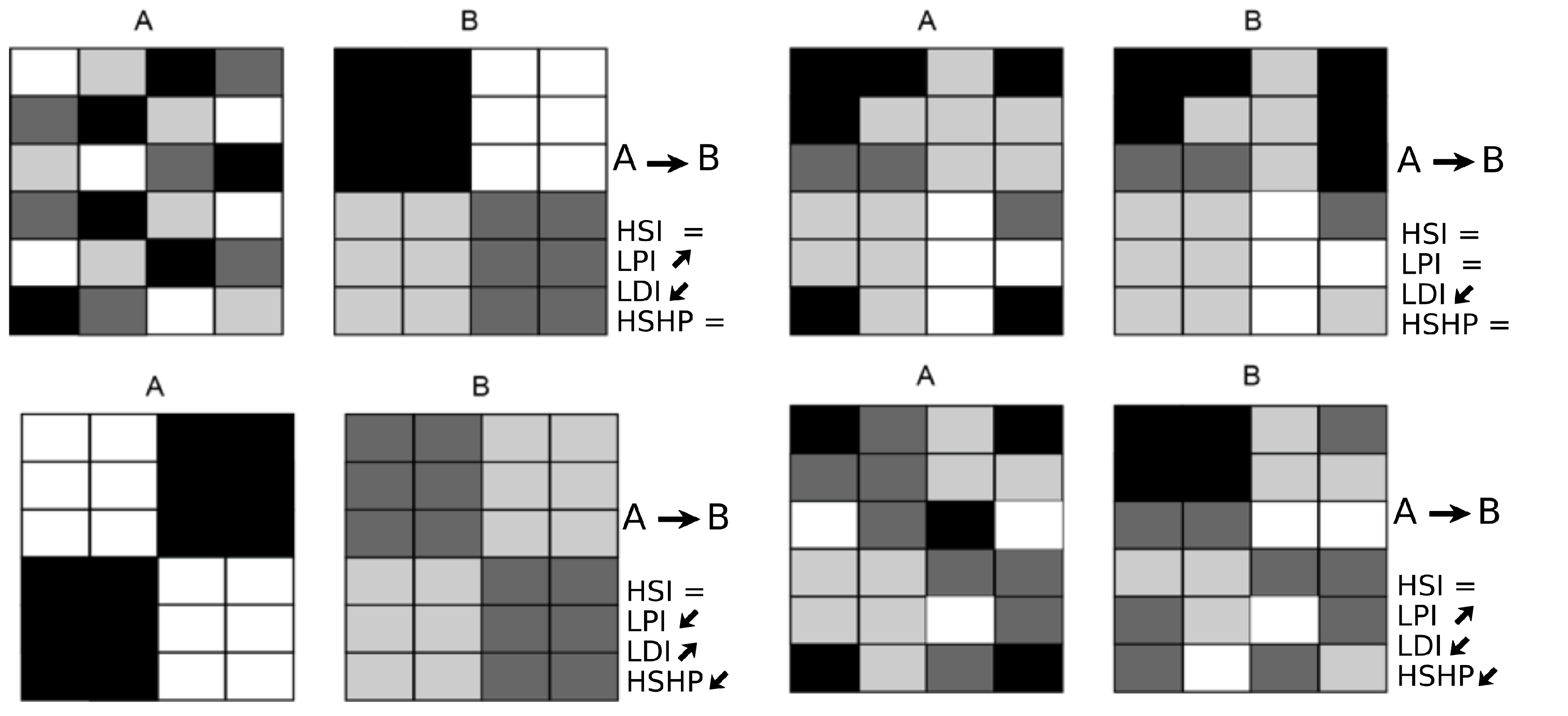
2.2.4. Estimation of Aquatic Habitat Heterogeneity
2.2.5. Fish Habitat Models and Metrics
- i
- The highly suitable habitat proportion (HSHP):
- ii
- The largest patch index (LPI):
- iii
- The landscape division index (LDI):where is equal to the surface area of patch ij and A is the cross-sectional area of the section being studied. These three metrics were calculated for the highly suitable habitat, which corresponds to a CSI equal to or greater than 0.5. All of the metrics were estimated for the upstream, impacted, and downstream sections and for each S (Figure 1c and Figure 3).
3. Results
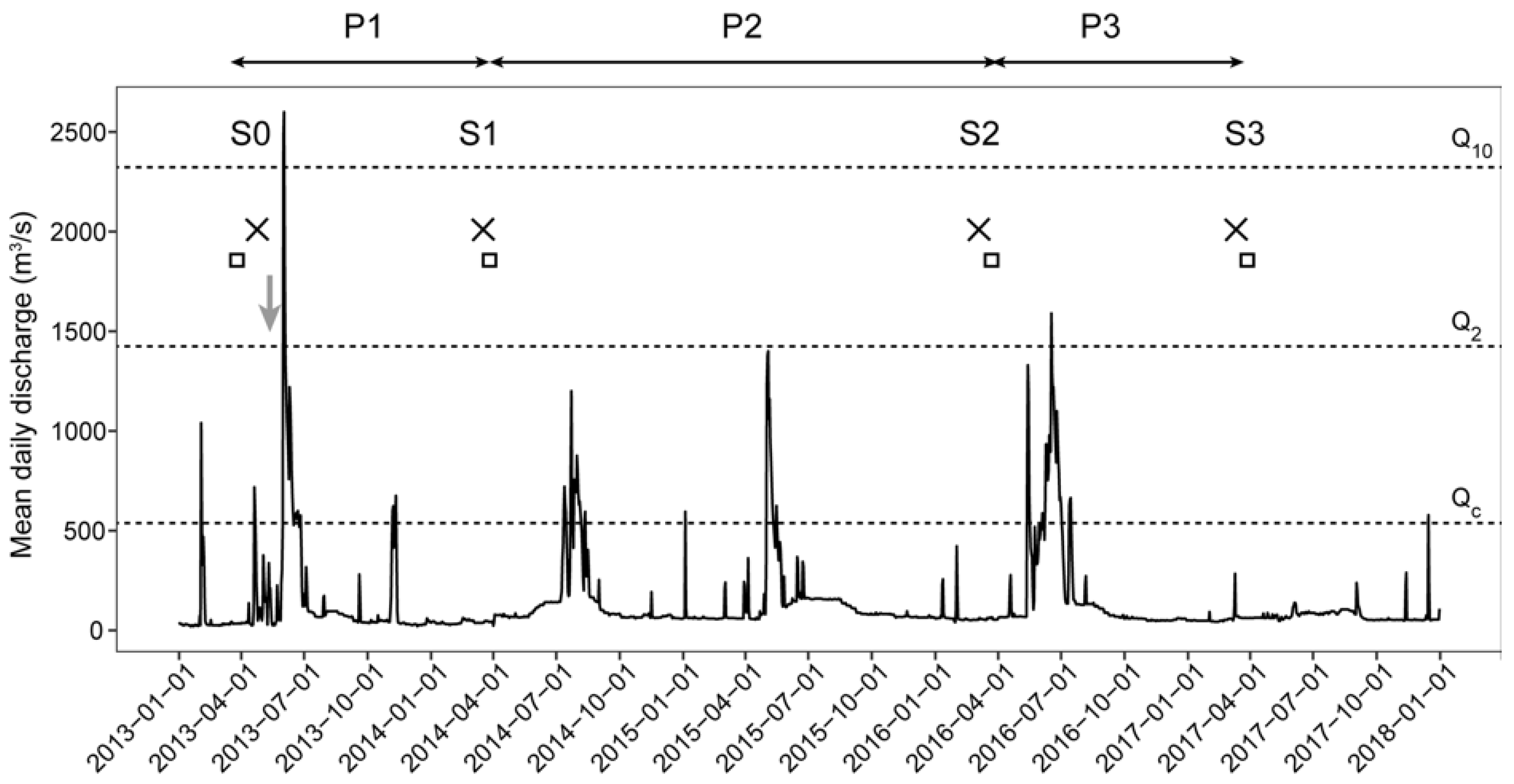
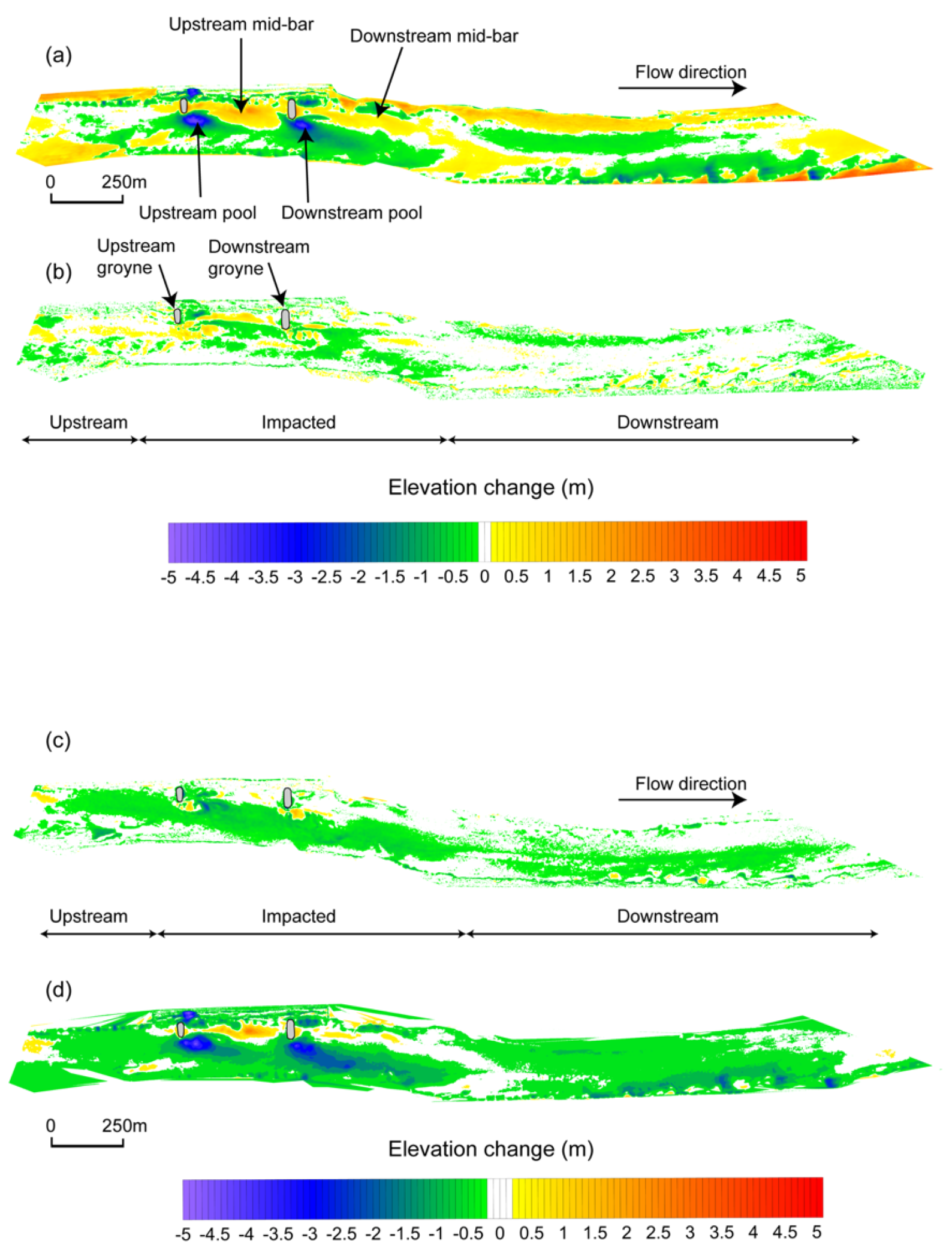
3.1. Channel Responses
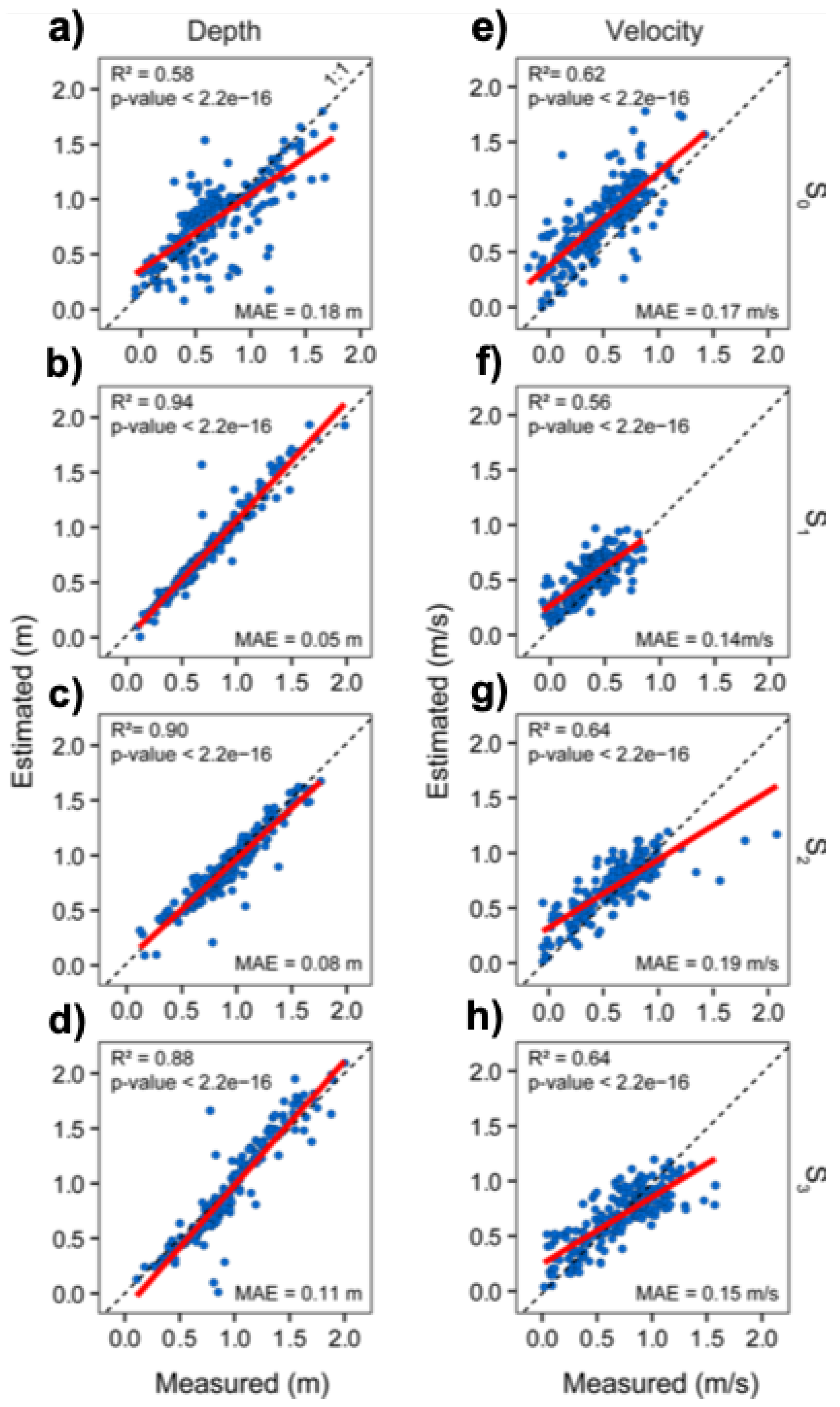
3.2. Precision of the Hydraulic Models
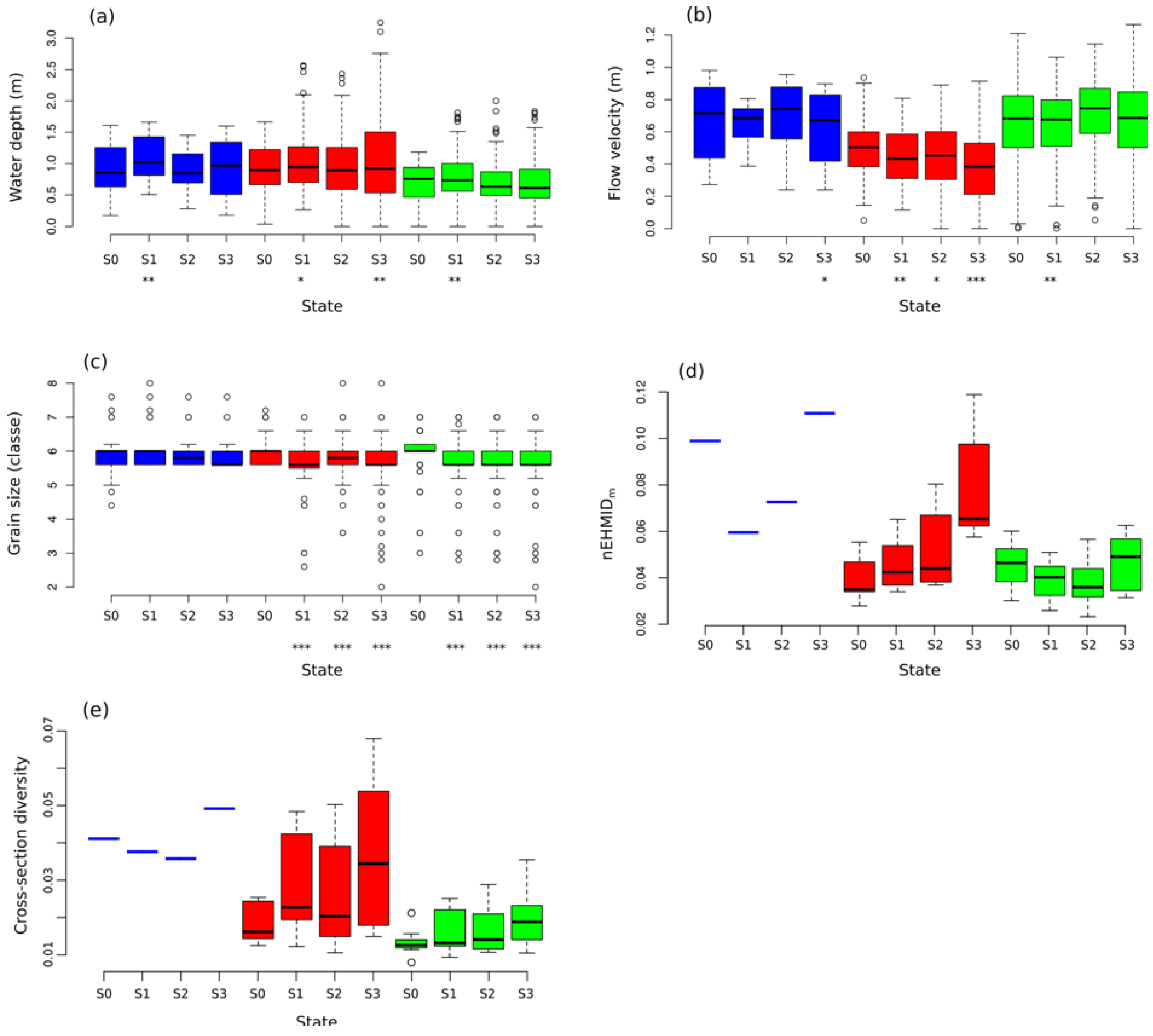
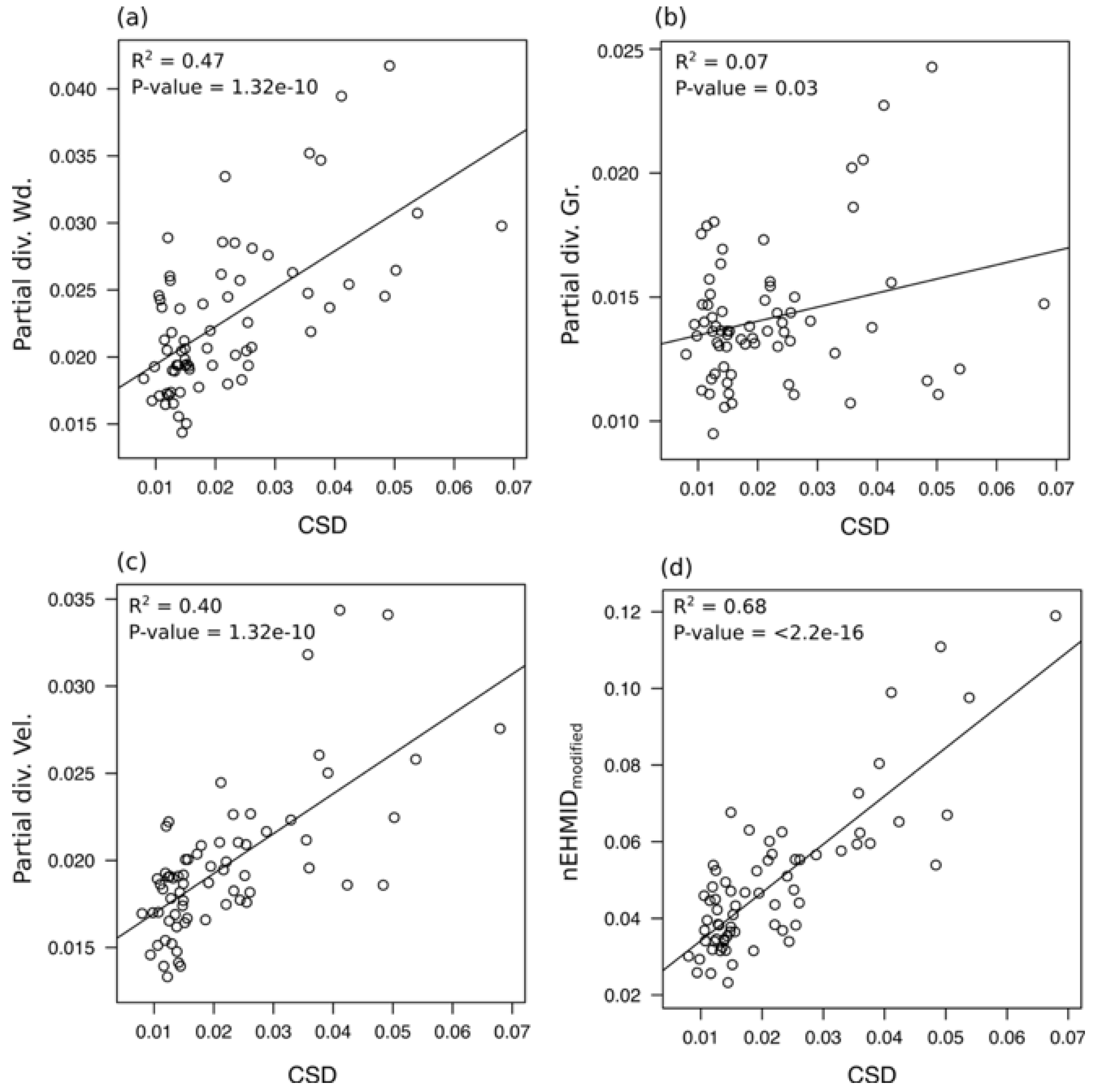
3.3. Temporal Evolution of Habitat Heterogeneity
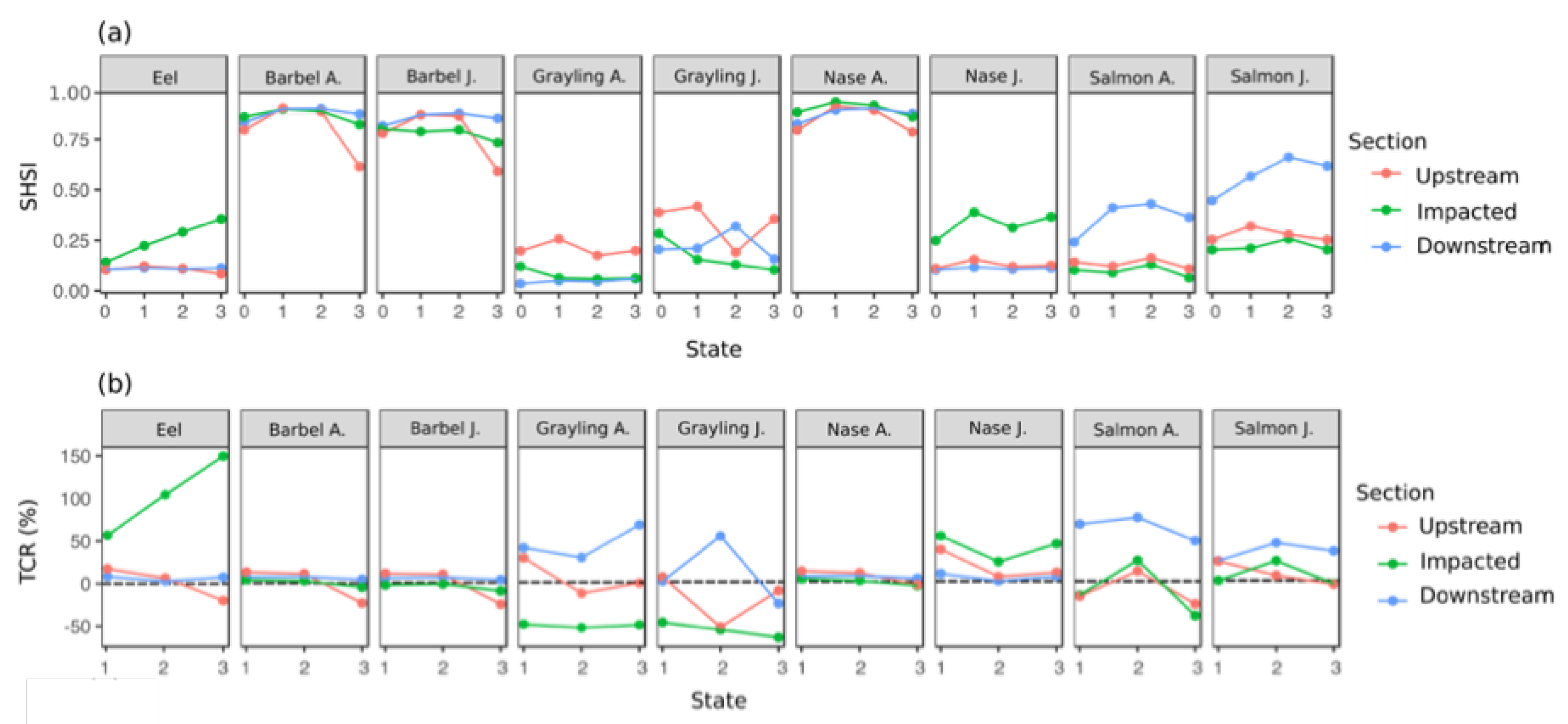
3.4. Temporal Evolution of Fish Habitat Suitability
4. Discussion
4.1. Channel Responses and Habitat Heterogeneity
4.2. Temporal Evolution of Fish Habitat Suitability
4.3. Eco-Hydraulic Modelling, a Tool for River Restoration Design
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CSD | cross-section diversity |
| GCA | grand canal d’alsace |
| HSHP | highly suitable habitat proportion |
| HSI | habitat suitability index |
| LPI | large patch index |
| nEHMIDm | normalized eco-hydro-morphological index of diversity modified |
| SHSI | synthetic habitat suitability index |
| TCR | temporal change ratio |
| WUA | weighted usable area |
References
- Alexander, J.; Wilson, R.; Green, W. A Brief History and Summary of the Effects of River Engineering and Dams on the Mississippi River System and Delta; Circular 1; U.S. Geological Survey Circular 1375: Reston, VA, USA, 2012; p. 43.
- Grill, G.; Lehner, B.; Thieme, M.; Geenen, B.; Tickner, D.; Antonelli, F.; Babu, S.; Borrelli, P.; Cheng, L.; Crochetiere, H.; et al. Mapping the world’s free-flowing rivers. Nature 2019, 569, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.R.; Infante, D.M.; Daniel, W.M.; Wehrly, K.E.; Wang, L.; Brenden, T.O. Assessment of dam effects on streams and fish assemblages of the conterminous USA. Sci. Total Environ. 2017, 586, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Jellyman, P.G.; Harding, J.S. The role of dams in altering freshwater fish communities in New Zealand. N. Z. Mar. Freshw. Res. 2012, 46, 475–489. [Google Scholar] [CrossRef] [Green Version]
- Morandi, B.; Piégay, H.; Lamouroux, N.; Vaudor, L. How is success or failure in river restoration projects evaluated ? Feedback from French restoration projects. J. Environ. Manag. 2014, 137, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, E.S.; Palmer, M.A. Restoring streams in an urbanizing world. Freashw. Biol. 2007, 52, 738–751. [Google Scholar] [CrossRef]
- Henry, C.P.; Amoros, C.; Roset, N. Restoration ecology of riverine wetlands: A 5-year post-operation survey on the Rhône River, France. Ecol. Eng. 2002, 18, 543–554. [Google Scholar] [CrossRef]
- Palmer, M.A.; Bernhardt, E.S.; Allan, J.D.; Alexander, G.; Shah, J.F.; Galat, D.L.; Hart, D.D.; Jenkinson, R.; Lave, R.; Sudduth, E. Standards for ecologically successful river restoration. J. Appl. Ecol. 2005, 42, 208–217. [Google Scholar] [CrossRef]
- Palmer, M.A.; Holly, L.M.; Bernhardt, E. River restoration, habitat heterogeneity and biodiversity: A failure of theory or practice? Freshw. Biol. 2010, 55, 205–222. [Google Scholar] [CrossRef]
- Szalkiewicz, E.; Jusik, S.; Grygoruk, M. Status of and perspectives on river restoration in Europe: 310,000 Euros per hectare of restored river. Sustainabilty 2018, 10, 129. [Google Scholar] [CrossRef] [Green Version]
- Schmutz, S.; Hein, T.; Sendzimir, J. Landmarks, Advances, and Future Challenges in Riverine Ecosystem Management; Aquatic Ecology Series; Springer: Berlin, Germany, 2018; Volume 8, pp. 563–571. [Google Scholar]
- Kondolf, G.M.; Anderson, S.; Lave, R.; Pagano, L.; Merenlender, A.; Bernhardt, E.S. Two Decades of River Restoration in California: What Can We Learn? Restor. Ecol. 2007, 15, 516–523. [Google Scholar] [CrossRef]
- Tonra, C.M.; Sager-Fradkin, K.; Morley, S.A.; Duda, J.J.; Marra, P.P. The rapid return of marine-derived nutrients to a freshwater food web following dam removal. Biol. Conserv. 2015, 192, 130–134. [Google Scholar] [CrossRef]
- Ramler, D.; Keckeis, H. Effects of large-river restoration measures on ecological fish guilds and focal species of conservation in a large European river (Danube, Austria). Sci. Total Environ. 2019, 686, 1076–1089. [Google Scholar] [CrossRef] [PubMed]
- Yossef, M.F.M. Literature Review: The Effect of Groynes on Rivers; Delft University of Technology Faculty of Civil Engineering and Geosciences Section of Hydraulic Engineering: Delft, The Netherlands, 2002; p. 58. [Google Scholar]
- Collas, F.P.; Buijse, A.D.; van den Heuvel, L.; van Kessel, N.; Schoor, M.M.; Eerden, H.; Leuven, R.S. Longitudinal training dams mitigate effects of shipping on environmental conditions and fish density in the littoral zones of the river Rhine. Sci. Total Environ. 2018, 619–620, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Shields, F.D.; Knight, S.S.; Cooper, M. Incised stream physical habitat restoation with stone weirs. Regul. Rivers Res. Manag. 1995, 10, 181–198. [Google Scholar] [CrossRef]
- Lacey, R.W.; Millar, R.G. Reach scale hydraulic assessment of instream salmonid habitat restoration. J. Am. Water Resour. Assoc. 2004, 40, 1631–1644. [Google Scholar] [CrossRef]
- Roberge, J.M.; Angelstam, P.E.R. Usefulness of the umbrella species concept as a conservation tool. Conserv. Biol. 2004, 18, 76–85. [Google Scholar] [CrossRef]
- Bain, M.B.; Jia, H. A habitat model for fish communities in large streams and small rivers. Int. J. Ecol. 2012, 2012, 8. [Google Scholar] [CrossRef] [Green Version]
- Moore, J. Fish Communities as Indicators of Environmental Quality in the West River Watershed. In Restoration of an Urban Salt Marsh An Interdisciplinary Approach; Yale University RIS Publishing: New Haven, CT, USA, 1997; pp. 178–196. [Google Scholar]
- Brazil, S.E.; Rodrigues, M.; Mattos, T.M.; Fernandes, V.H.; Martínez-capel, F.; Muñoz-Mas, R.; Araújo, F.G. Application of the physical habitat simulation for fish species to assess environmental flows in an Atlantic Forest Stream in South-eastern Brazil. Neotrop. Ichthyol. 2015, 13, 685–698. [Google Scholar] [CrossRef] [Green Version]
- Lamouroux, N.; Gore, J.A.; Lepori, F.; Statzner, B. The ecological restoration of large rivers needs science-based, predictive tools meeting public expectations: An overview of the R hône project. Freshw. Biol. 2015, 60, 1069–1084. [Google Scholar] [CrossRef]
- Papadaki, C.; Bellos, V. Evaluation of streamflow habitat relationships using habitat suitability curves and HEC-RAS. Eur. Water 2017, 58, 127–134. [Google Scholar]
- Zingraff-Hamed, A.; Noack, M.; Greulich, S.; Schwarzwälder, K.; Pauleit, S.; Wantzen, K.M. Model-based evaluation of the effects of river discharge modulations on physical fish habitat quality. Water 2018, 10, 374. [Google Scholar] [CrossRef] [Green Version]
- Wheaton, J.M.; Pasternack, G.B.; Merz, J.E. Spawning habitat rehabilitation—II. Using hypothesis development and testing in design, Mokelumne river, California, U.S.A. Int. J. River Basin Manag. 2004, 2, 21–37. [Google Scholar] [CrossRef]
- Pasternack, G.B.; Wang, C.L.; Merz, J.E. Application of a 2D hydrodynamic model to design of reach-scale spawning gravel replenishment on the Mokelumne River, California. River Res. Appl. 2004, 20, 205–225. [Google Scholar] [CrossRef]
- Bovee, K.D. A Guide to Stream Habitat Analysis Using the Instream Flow Incremental Methodology; Technical Report 82; US Fish and Wildlife Service: Fort Collins, CO, USA, 1982.
- Benjankar, R.; Tonina, D.; Mckean, J. One-dimensional and two-dimensional hydrodynamic modeling derived flow properties: Impacts on aquatic habitat quality predictions. Earth Surf. Process. Landf. 2014, 40, 340–356. [Google Scholar] [CrossRef]
- Bovee, K.D.; Lamb, B.L.; Bartholow, J.M.; Stalnaker, C.B.; Jonathan, T.; Jim, H. Stream Habitat Analysis Using the Instream Flow Incremental Methodology; Technical Report; U.S. Geological Survey, Biological Resources Division Information and Technology: Fort Collins, CO, USA, 1998.
- Lamouroux, N.; Capra, H.; Pouilly, M. Predicting habitat suitability for lotic fish: Linking statistical hydraulic models with multivariate habitat use models. Regul. Rivers Res. Manag. 1998, 14, 1–11. [Google Scholar] [CrossRef]
- Hoss, S.K.; Guyer, C.; Smith, L.L.; Schuett, G.W. Multiscale Influences of Landscape Composition and Configuration on the Spatial Ecology of Eastern Diamond-backed Rattlesnakes (Crotalus adamanteus). J. Herpetol. 2010, 44, 110–123. [Google Scholar] [CrossRef]
- Li, W.; Chen, Q.; Cai, D.; Li, R. Determination of an appropriate ecological hydrograph for a rare fish species using an improved fish habitat suitability model introducing landscape ecology index. Ecol. Model. 2015, 311, 31–38. [Google Scholar] [CrossRef]
- Hitchman, S.M.; Mather, M.E.; Smith, J.M.; Fencl, J.S. Habitat mosaics and path analysis can improve biological conservation of aquatic biodiversity in ecosystems with low-head dams. Sci. Total Environ. 2018, 619–620, 221–231. [Google Scholar] [CrossRef] [Green Version]
- Smith, E. Impact assessment using before-after-control-impact (BACI) model: Concerns and comments. Earth Surf. Process. Landf. 1993, 50, 627–637. [Google Scholar] [CrossRef]
- Lamouroux, N.; Souchon, Y. Fish habitat preferences in large streams of southern France. Freshw. Biol. 1999, 42, 673–687. [Google Scholar] [CrossRef]
- Mallet, J.P.; Lamouroux, N.; Sagnes, P.; Persat, H. Habitat preferences of European grayling in a medium size stream, the Ain river, France. J. Fish Biol. 2000, 56, 1312–1326. [Google Scholar] [CrossRef]
- Uehlinger, U.; Wantzen, K.M.; Leuven, R.S.E.W. Rivers of Europe/Klement Tockner u.a. In The Rhine River Basin; Academic Press: London, UK, 2009; pp. 199–245. [Google Scholar]
- Arnaud, F.; Schmitt, L.; Johnstone, K.; Rollet, A.J.; Piégay, H. Geomorphology Engineering impacts on the Upper Rhine channel and floodplain over two centuries. Geomorphology 2019, 330, 13–27. [Google Scholar] [CrossRef]
- Garnier, A.; Barillier, A. The Kembs project: Environmental integration of a large existing hydropower scheme. La Houille Blanche 2015, 4, 21–28. [Google Scholar] [CrossRef]
- Arnaud, F.; Piégay, H.; Béal, D.; Collery, P.; Vaudor, L.; Rollet, A.J. Monitoring gravel augmentation in a large regulated river and implications for process-based restoration. Earth Surf. Process. Landf. 2017, 42, 2147–2166. [Google Scholar] [CrossRef]
- Chardon, V.; Schmitt, L.; Piégay, H.; Arnaud, F.; Serouilou, J.; Houssier, J.; Clutier, A. Geomorphic effects of gravel augmentation on the Old Rhine River downstream from the Kembs dam. In Proceedings of the E3S Web of Conferences, Lyon-Villeurbanne, France, 5–8 September 2018. [Google Scholar] [CrossRef]
- Aelbrecht, D.; Clutier, A.; Barillier, A.; Pinte, K.; El-Kadi-Abderrezzak, K.; Die-Moran, A.; Lebert, F.; Garnier, A. Morphodynamics restoration of the Old Rhine through controlled bank erosion: Concept, laboratory modeling, and field testing and first results on a pilot site. River Flow 2014, 2014, 2397–2403. [Google Scholar]
- Schneider, M.; Noack, M.; Gebler, T. Handbook for the Habitat Simulation Model Contact Information; Schneider & Jorde Ecological Engineering GmbH: Stuttgart, Germany, 2010. [Google Scholar]
- Die Moran, A.; El Kadi Abderrezzak, K.; Mosselman, E.; Habersack, H.; Lebert, F.; Aelbrecht, D.; Laperrousaz, E. Physical model experiments for sediment supply to the old Rhine through induced bank erosion. Int. J. Sediment Res. 2013, 28, 431–447. [Google Scholar] [CrossRef]
- El Kadi Abderrezzak, K. Estimation de la Capacité Detransport Solide par Charriage Dans le VieuxRhin; Technical Report; EDF R&D LNHE: Chatou, France, 2009; p. 30. [Google Scholar]
- SAGE—Environnement S-Air Inc. Etat des Lieux HVS du Site O3 Après Travaux d’Amorce de L’érosion Maîtrisée, Compte Rendu Technique; Technical Report; SAGE: Sherbrooke, QC, Canada, 2013. [Google Scholar]
- SAGE—Environnement S-Air Inc. Etat des Lieux HVS du Site O3 Après Travaux d’Amorce de L’érosion Maîtrisée, Compte Rendu Technique; Technical Report; SAGE: Sherbrooke, QC, Canada, 2014. [Google Scholar]
- SAGE—Environnement S-Air Inc. Etat des Lieux HVS du Site O3 Après Travaux d’Amorce de L’érosion Maîtrisée, Compte Rendu Technique; Technical Report; SAGE: Sherbrooke, QC, Canada, 2016. [Google Scholar]
- SAGE—Environnement S-Air Inc. Etat des Lieux HVS du Site O3 et 01 Après Travaux d’Amorce de L’érosion Maîtrisée, Compte Rendu Technique; Technical Report; SAGE: Sherbrooke, QC, Canada, 2017. [Google Scholar]
- Malavoi, J.; Souchon, Y. Méthodologie de description et quantification des variables morphodynamiques d’un cours d’eau à fond caillouteux. Exemple d’une station sur la Filière (Haute Savoie). Rev. De Géographie Du Lyon 1989, 64, 252–259. [Google Scholar] [CrossRef]
- Ginot, V.; Souchon, Y. Evaluation de l’Habitat Physique des Poissons en Rivière; Technical Report; Cemagref, Division Biologique des Ecosystèmes Aquatiques: Lyon, France, 1998; p. 130. [Google Scholar]
- Chow, V.T. Open-Channel Hydraulics; McGraw-Hill Book Co.: New York, NY, USA, 1959; p. 680. [Google Scholar]
- Benson, M.; Dalrymphe, T. General Field and Office Procedures for Indirect Discharge Measurements; U.S. Geological Survey Techniques of Water-Resources Investigations: Washington, DC, USA, 1967; p. 30.
- Staentzel, C.; Combroux, I.; Barillier, A.; Grac, C.; Chanez, E.; Beisel, J.N. Effects of a river restoration project along the Old Rhine River (France-Germany): Response of macroinvertebrate communities. Ecol. Eng. 2019, 127, 114–124. [Google Scholar] [CrossRef]
- Gostner, W.; Alp, M.; Schleiss, A.J.; Robinson, C.T. The hydro-morphological index of diversity: A tool for describing habitat heterogeneity in river engineering projects. Hydrobiologia 2013, 712, 43–60. [Google Scholar] [CrossRef] [Green Version]
- Benjankar, R.; Tonina, D.; McKean, J.A.; Sohrabi, M.M.; Chen, Q.; Vidergar, D. Dam operations may improve aquatic habitat and offset negative effects of climate change. J. Environ. Manag. 2018, 213, 126–134. [Google Scholar] [CrossRef]
- Moir, H.J.; Gibbins, C.N.; Soulsby, C.; Youngson, A.F. Phabsim modeling of atlantic salmon spawing habitat in a upland stream: Testing the influence of habitat suitability indices on model output. River Res. Appl. 2005, 1034, 1021–1034. [Google Scholar] [CrossRef]
- Bovee, K.D. Development and Evaluation of Habitat Suitability Criteria for use in the Instream Flow Incremental Methodology; Technical Report; National Ecology Center, Division of Wildlife and Contaminant Research, Fish and Wildlife Service, US Department of the Interior: Washington, DC, USA, 1986.
- Mouton, A.M.; Schneider, M.; Depestele, J.; Goethals, P.L.; De Pauw, N. Fish habitat modeling as a tool for river management. Ecol. Eng. 2007, 29, 305–315. [Google Scholar] [CrossRef]
- Crosato, A.; Bonilla-Porras, J.; Pinkse, A.; Tiga, T.Y. River bank erosion opposite to transverse groynes. E3S Web Conf. 2018, 40, 03013. [Google Scholar] [CrossRef]
- Sukhodolov, A.; Uijttewaal, W.S.; Engelhardt, C. On the correspondence between morphological and hydrodynamical patterns of groyne fields. Earth Surf. Process. Landf. 2002, 27, 289–305. [Google Scholar] [CrossRef]
- Dietrich, W.E.; Kichner, J.W.; Ikeda, H.; Iseya, F. Sediment Supply and the Development of the Surface Layer in Gravel Bedded Rivers. Nature 1989, 340, 215–217. [Google Scholar] [CrossRef]
- Vericat, D.; Wheaton, J.M.; Brasington, J. Revisiting the morphological appoach: Opportunities and challenges with repeat high resolution topography. In Gravel-Bed Rivers: Processes and Disasters; Wiley: Hoboken, NJ, USA, 2015; Chapter 5; pp. 121–158. [Google Scholar]
- Arnaud, F.; Piégay, H.; Schmitt, L.; Rollet, A.J.; Ferrier, V.; Béal, D. Geomorphology historical geomorphic analysis (1932–2011) of a by-passed river reach in process-based restoration perspectives: The Old Rhine downstream of the Kembs diversion dam (France, Germany). Geomorphology 2015, 236, 163–177. [Google Scholar] [CrossRef]
- Chardon, V. Effets Géomorphologiques des Actions Expérimentales de Redynamisation du Rhin à l’aval de Kembs. Ph.D. Thesis, University of Strasbourg, Strasbourg, France, 2019. [Google Scholar]
- Staentzel, C.; Combroux, I.; Barillier, A.; Schmitt, L.; Chardon, V.; Garnier, A.; Beisel, J.N. Réponses des communautés biologiques à des actions de restauration de grands fleuves. La Houille Blanche 2018, 99–106. [Google Scholar] [CrossRef]
- Shahverdian, S. Chapter 3: Planning for Low-Tech Process-Based Restoration. In Low-Tech Process-Based Restoration of Riverscapes: Design Manual—Version 1.0; Utah State University Restoration Consortium: Logan, UT, USA, 2019; p. 57. [Google Scholar]
- Chardon, V.; Schmitt, L.; Houssier, J.; Piégay, H.; Clutier, A. Restoring river sediment supply and morphological diversity by bank erosion combined with groyne implementation: A test feedback on the Old Rhine (France/Germany). 2019; in preparation. [Google Scholar] [CrossRef] [Green Version]
- Chardon, V.; Schmitt, L.; Arnaud, F.; Piégay, H.; Clutier, A. Efficiency and sustainability of gravel augmentation to restore large regulated rivers: Insights from three experiments on the Rhine River (France/Germany). 2019; submitted. [Google Scholar]
- Hauer, C.; Pulg, U.; Reisinger, F.; Flödl, P. Evolution of artificial spawning sites for Atlantic salmon (Salmo Salar) Sea Trout (Salmo Trutta): Field Stud. Numer. Model. Aurland, Norway. Hydrobiologia 2020, 847, 1139–1158. [Google Scholar] [CrossRef] [Green Version]
- Schiemer, F.; Hein, T.; Reckendorfer, W. Ecohydrology, key-concept for large river restoration. Ecohydrol. Hydrobiol. 2007, 7, 101–111. [Google Scholar] [CrossRef]
- Yao, W.W.; Chen, Y.; Zhong, Y.; Zhang, W.; Fan, H. Habitat models for assessing river ecosystems and their application to the development of river restoration strategies. J. Freshw. Ecol. 2017, 32, 601–617. [Google Scholar] [CrossRef] [Green Version]
- Deacon, J.; Mize, S. Effects of Water Quality and Habitat on Composition of Fish Communities in the Upper Colorado River Basin; US Geological Survey: Denver, CO, USA, 1997.
- Lessard, J.L.; Hayes, D.B. Effects of elevated water temperature on fish and macroinvertebrate communities below small dams. River Res. Appl. 2003, 19, 721–732. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W. The river continuum concept TL-37(1). Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Hughes, L. Biological consequences of global warming: Is the signal already apparent? Trends Ecol. Evol. 2000, 15, 56–61. [Google Scholar] [CrossRef]
- Kuczynski, L.; Chevalier, M.; Kuczynski, L.; Chevalier, M.; Laffaille, P.; Legrand, M. Indirect effect of temperature on fish population abundances through phenological changes Indirect effect of temperature on fish population abundances through phenological changes. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Comte, L.; Grenouillet, G. Do stream fish track climate change? Assessing distribution shifts in recent decades. Ecography 2013, 36, 1236–1246. [Google Scholar] [CrossRef]
- Boavida, I.; Santos, M.; Pinheiro, N.; Ferreira, M.T. Fish habitat availability simulations using different morphological variables. Limnetica 2011, 30, 393–404. [Google Scholar]
- Rosenfeld, J. Assessing the Habitat Requirements of Stream Fishes: An Overview and Evaluation of Different Approaches. Trans. Am. Fish. Soc. 2004, 132, 953–968. [Google Scholar] [CrossRef]
- Rosenfeld, J.S.; Leiter, T.; Lindner, G.; Rothman, L. Food abundance and fish density alters habitat selection, growth, and habitat suitability curves for juvenile coho salmon (Oncorhynchus Kisutch). Can. J. Fish. Aquat. Sci. 2005, 62, 1691–1701. [Google Scholar] [CrossRef] [Green Version]
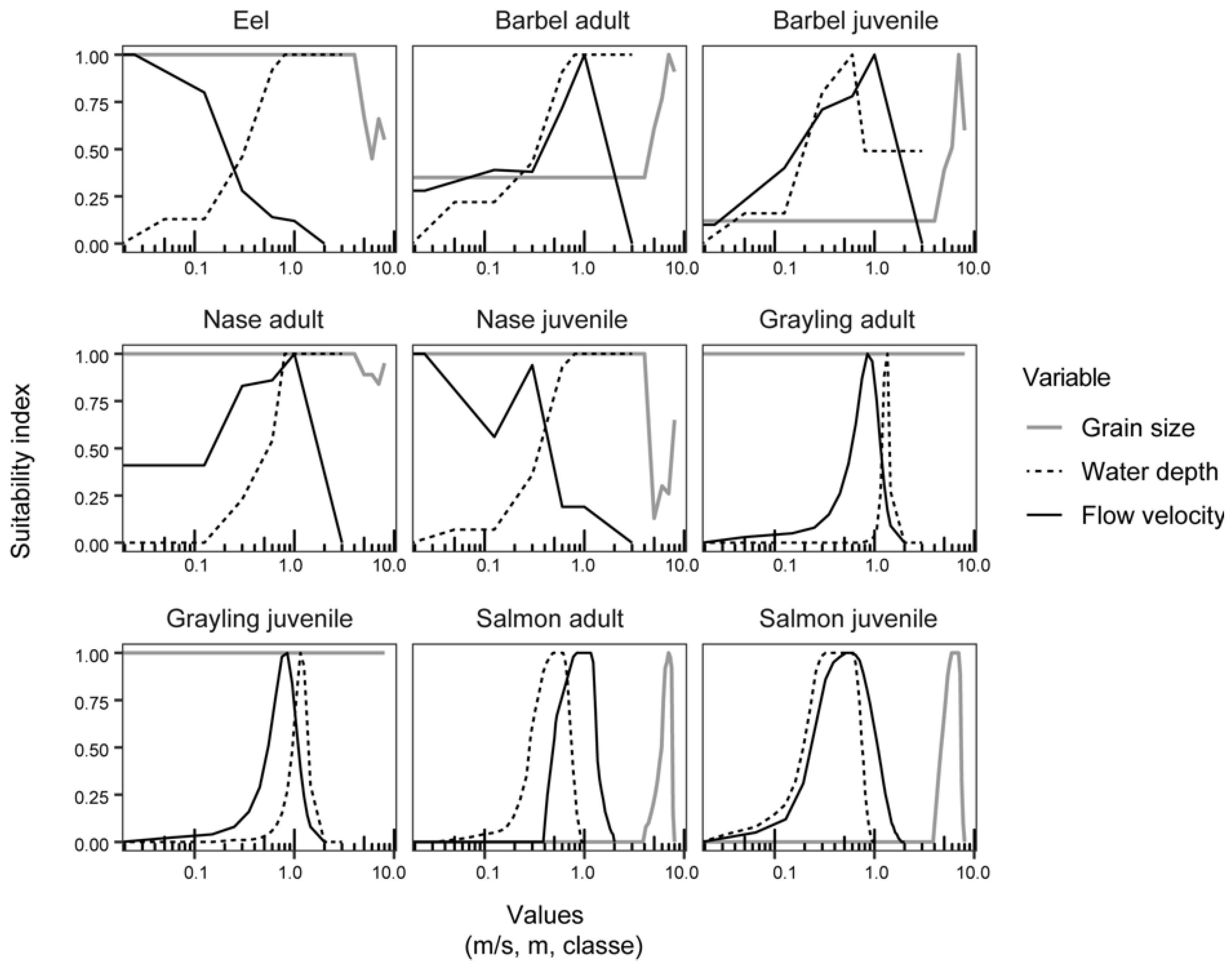
| Common and Scientific Names | Life Stages | Source | Studied Reaches in the Fish Surveys |
|---|---|---|---|
| Eel (Anguilla anguilla) | [36] | Rhône river (a); Ain river; Ardèche river; Drôme river; Loire river; Garonne river | |
| Barbel (Barbus barbus) | Adult and juvenile | [36] | Rhône river (a); Ain river; Ardèche river; Drôme river; Loire river; Garonne river |
| Nase (Chondrostoma nasus) | Adult and juvenile | [36] | Rhône river (a); Ain river; Ardèche river; Drôme river; Loire river; Garonne river; |
| Grayling (Thymallus thymallus) | Adult and juvenile | [37] | Ain river |
| Salmon (Salmo salar) | Adult and juvenile | Expert curves revised in 1997 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chardon, V.; Schmitt, L.; Piégay, H.; Beisel, J.-N.; Staentzel, C.; Barillier, A.; Clutier, A. Effects of Transverse Groynes on Meso-Habitat Suitability for Native Fish Species on a Regulated By-Passed Large River: A Case Study along the Rhine River. Water 2020, 12, 987. https://doi.org/10.3390/w12040987
Chardon V, Schmitt L, Piégay H, Beisel J-N, Staentzel C, Barillier A, Clutier A. Effects of Transverse Groynes on Meso-Habitat Suitability for Native Fish Species on a Regulated By-Passed Large River: A Case Study along the Rhine River. Water. 2020; 12(4):987. https://doi.org/10.3390/w12040987
Chicago/Turabian StyleChardon, Valentin, Laurent Schmitt, Hervé Piégay, Jean-Nicolas Beisel, Cybill Staentzel, Agnès Barillier, and Anne Clutier. 2020. "Effects of Transverse Groynes on Meso-Habitat Suitability for Native Fish Species on a Regulated By-Passed Large River: A Case Study along the Rhine River" Water 12, no. 4: 987. https://doi.org/10.3390/w12040987





