The Effect of Submergence and Eutrophication on the Trait’s Performance of Wedelia Trilobata over Its Congener Native Wedelia Chinensis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Morphological Traits Measurement
2.2. Relative Interaction Index
2.3. Statistical Analyses
3. Results
3.1. Biomass Accumulation and Allocation
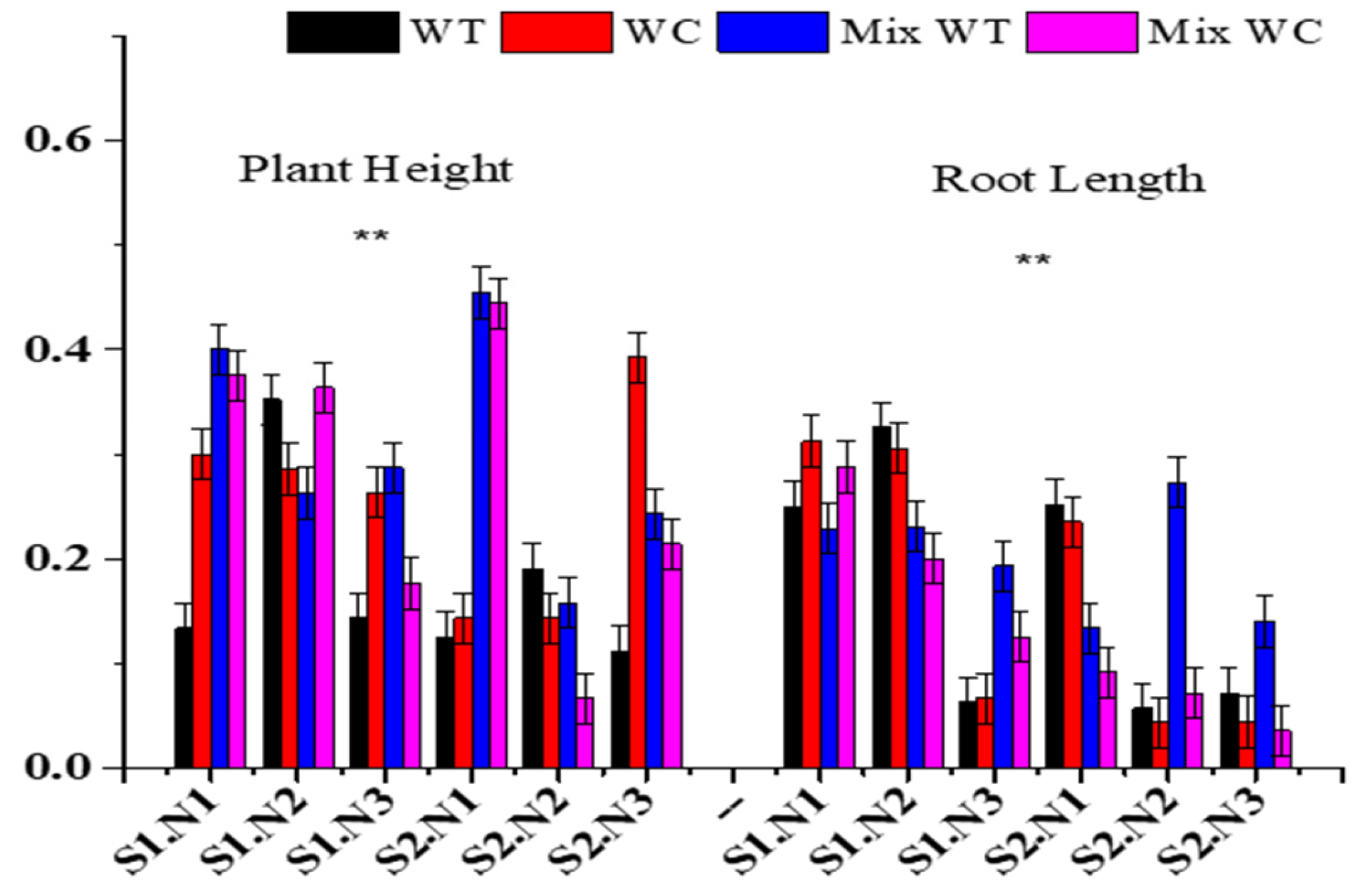
3.2. Morphological Traits
3.3. Plasticity Index
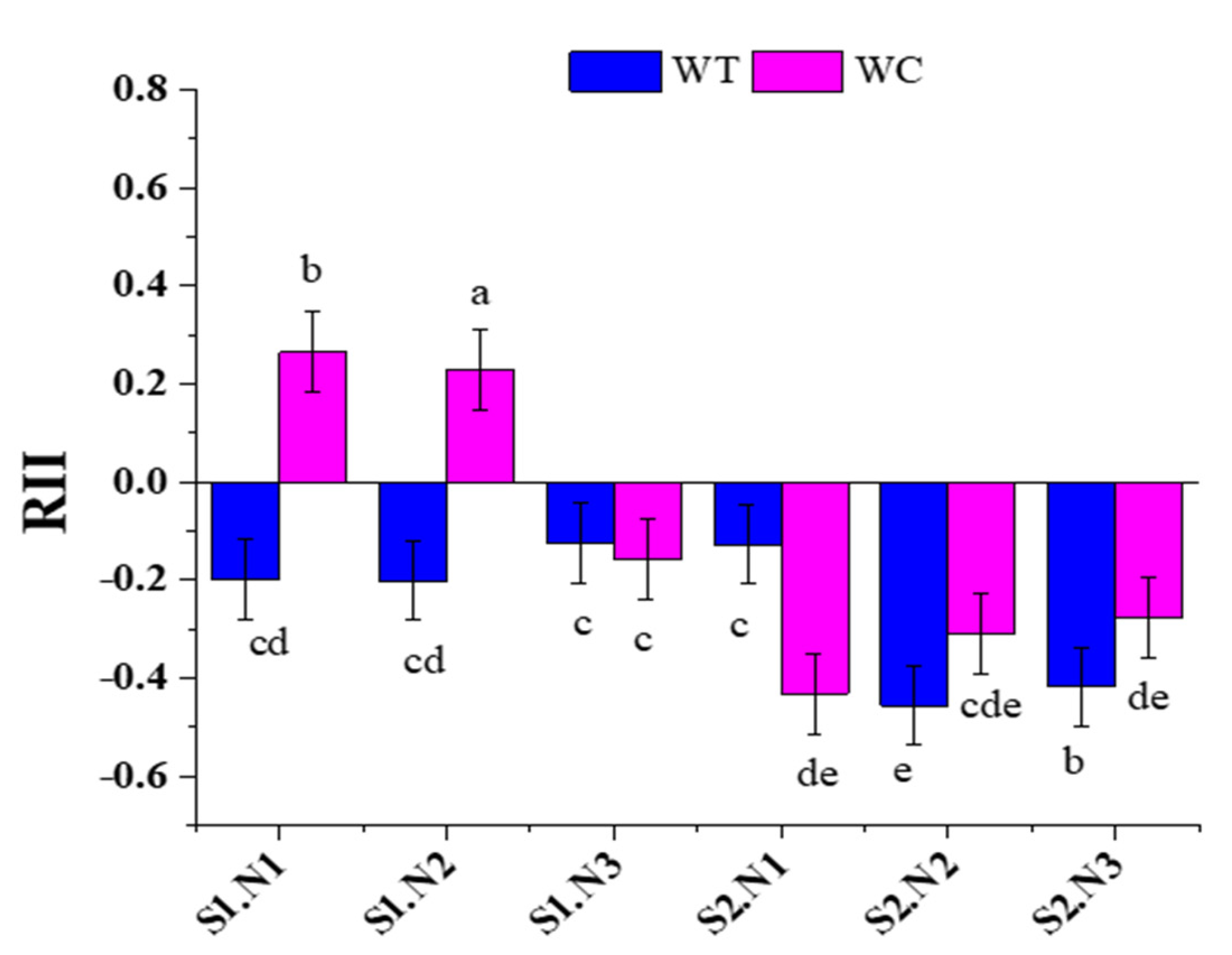
3.4. Relative Interaction Index
4. Discussion
4.1. Biomass Response under Submergence and Eutrophication
4.2. Morphological Traits under Submergence and Eutrophication
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seebens, H.; Essl, F.; Dawson, W.; Fuentes, N.; Moser, D.; Pergl, J.; Pyšek, P.; van Kleunen, M.; Weber, E.; Winter, M. Global trade will accelerate plant invasions in emerging economies under climate change. Glob. Chang. Biol. 2015, 21, 4128–4140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Kleunen, M.; Dawson, W.; Essl, F.; Pergl, J.; Winter, M.; Weber, E.; Kreft, H.; Weigelt, P.; Kartesz, J.; Nishino, M.; et al. Global exchange and accumulation of non-native plants. Nature 2015, 525, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alpert, P. The advantages and disadvantages of being introduced. Biol. Invasions 2006, 8, 1523–1534. [Google Scholar] [CrossRef]
- Guan, B.; Yu, J.; Cao, D.; Li, Y.; Han, G.; Mao, P. The ecological restoration of heavily degraded saline wetland in the Yellow River Delta. CLEAN Soil Air Water 2013, 41, 690–696. [Google Scholar] [CrossRef]
- Davis, M.A.; Grime, J.P.; Thompson, K. Fluctuating resources in plant communities: A general theory of invasibility. J. Ecol. 2000, 88, 528–534. [Google Scholar] [CrossRef] [Green Version]
- Blumenthal, D. Interrelated causes of plant invasion. Science 2005, 310, 243–244. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Yang, W.; Xia, L.; Qiao, Y.; Xiao, Y.; Cheng, X.; An, S. Nitrogen-enriched eutrophication promotes the invasion of Spartina Alterniflora in coastal China. CLEAN Soil Air Water 2015, 43, 244–250. [Google Scholar] [CrossRef]
- Vilà, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarošík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pyšek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef]
- Funk, J.L.; Standish, R.J.; Stock, W.D.; Valladares, F. Plant functional traits of dominant native and invasive species in mediterranean-climate ecosystems. Ecology 2016, 97, 75–83. [Google Scholar] [CrossRef]
- Heberling, J.M.; Fridley, J.D. Resource-use strategies of native and invasive plants in Eastern North American forests. New Phytol. 2013, 200, 523–533. [Google Scholar] [CrossRef]
- Jia, J.; Dai, Z.; Li, F.; Liu, Y. How will global environmental changes affect the growth of alien plants? Front. Plant Sci. 2016, 7, 1623. [Google Scholar] [CrossRef] [Green Version]
- Van Kleunen, M.; Schlaepfer, D.R.; Glaettli, M.; Fischer, M. Preadapted for invasiveness: Do species traits or their plastic response to shading differ between invasive and non-invasive plant species in their native range? J. Biogeogr. 2011, 38, 1294–1304. [Google Scholar] [CrossRef] [Green Version]
- Gratani, L. Plant phenotypic plasticity in response to environmental factors. Adv. Bot. 2014. [Google Scholar] [CrossRef] [Green Version]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U.; Poot, P.; Purugganan, M.D.; Richards, C.L.; Valladares, F. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.L.; Bossdorf, O.; Muth, N.Z.; Gurevitch, J.; Pigliucci, M. Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecol. Lett. 2006, 9, 981–993. [Google Scholar] [PubMed] [Green Version]
- Liu, Y.; van Kleunen, M. Responses of common and rare aliens and natives to nutrient availability and fluctuations. J. Ecol. 2017, 105, 1111–1122. [Google Scholar] [CrossRef] [Green Version]
- Davidson, A.M.; Jennions, M.; Nicotra, A.B. Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecol. Lett. 2011, 14, 419–431. [Google Scholar] [CrossRef]
- Palacio-López, K.; Gianoli, E. Invasive plants do not display greater phenotypic plasticity than their native or non-invasive counterparts: A meta-analysis. Oikos 2011, 120, 1393–1401. [Google Scholar] [CrossRef]
- Liu, Y.; Oduor, A.M.O.; Zhang, Z.; Manea, A.; Tooth, I.M.; Leishman, M.R.; Xu, X.; van Kleunen, M. Do invasive alien plants benefit more from global environmental change than native plants? Glob. Chang. Biol. 2017, 23, 3363–3370. [Google Scholar] [CrossRef] [Green Version]
- Gołdyn, H. Changes in plant species diversity of aquatic ecosystems in the agricultural landscape in West Poland in the last 30 years. Biodivers. Conserv. 2010, 19, 61–80. [Google Scholar] [CrossRef]
- Parepa, M.; Fischer, M.; Bossdorf, O. Environmental variability promotes plant invasion. Nat. Commun. 2013, 4, 1604. [Google Scholar] [CrossRef] [PubMed]
- Evtimova, V.V.; Donohue, I. Quantifying ecological responses to amplified water level fluctuations in standing waters: An experimental approach. J. Appl. Ecol. 2014, 51, 1282–1291. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.; Yin, T.-F.; Liu, C.-X.; Luo, F.-L. The invasive wetland plant Alternanthera philoxeroides shows a higher tolerance to waterlogging than its native congener Alternanthera sessilis. PLoS ONE 2013, 8, 81456. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Yu, H.; Liu, C.; Yu, D.; Han, Y.; Wang, L. The effects of complete submergence on the morphological and biomass allocation response of the invasive plant Alternanthera philoxeroides. Hydrobiologia 2015, 746, 159–169. [Google Scholar] [CrossRef]
- Short, F.T.; Kosten, S.; Morgan, P.A.; Malone, S.; Moore, G.E. Impacts of climate change on submerged and emergent wetland plants. Aquat. Bot. 2016, 135, 3–17. [Google Scholar] [CrossRef]
- Strange, E.; Hill, J.; Coetzee, J. Evidence for a new regime shift between floating and submerged invasive plant dominance in South Africa. Hydrobiologia 2018, 817, 349–362. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Chen, X.; Du, Y.; Wang, Y.; Wang, R. Effects of submergence and eutrophication on the morphological traits and biomass allocation of the invasive plant Alternanthera philoxeroides. J. Freshw. Ecol. 2016, 31, 341–349. [Google Scholar] [CrossRef]
- Colmer, T.; Voesenek, L. Flooding tolerance: Suites of plant traits in variable environments. Funct. Plant Biol. 2009, 36, 665–681. [Google Scholar] [CrossRef]
- Dalmolin, Â.C.; Dalmagro, H.J.; Lobo, F.D.A.; Junior, M.Z.A.; Ortíz, C.E.R.; Vourlitis, G.L. Effects of flooding and shading on growth and gas exchange of Vochysia divergens Pohl (Vochysiaceae) of invasive species in the Brazilian Pantanal. Braz. J. Plant Physiol. 2012, 24, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Luo, F.-L.; Nagel, K.A.; Scharr, H.; Zeng, B.; Schurr, U.; Matsubara, S. Recovery dynamics of growth, photosynthesis and carbohydrate accumulation after de-submergence: A comparison between two wetland plants showing escape and quiescence strategies. Ann. Bot. 2010, 107, 49–63. [Google Scholar] [CrossRef] [Green Version]
- Webb, J.A.; Wallis, E.M.; Stewardson, M.J. A systematic review of published evidence linking wetland plants to water regime components. Aquat. Bot. 2012, 103, 1–14. [Google Scholar] [CrossRef]
- Jackson, M.; Colmer, T. Response and adaptation by plants to flooding stress. Ann. Bot. 2005, 96, 501–505. [Google Scholar] [CrossRef]
- Schindler, D.W. Recent advances in the understanding and management of eutrophication. Limnol. Oceanogr. 2006, 51, 356–363. [Google Scholar] [CrossRef] [Green Version]
- O’Hare, M.T.; Baattrup-Pedersen, A.; Baumgarte, I.; Freeman, A.; Gunn, I.D.M.; Lázár, A.N.; Sinclair, R.; Wade, A.J.; Bowes, M.J. Responses of aquatic plants to eutrophication in rivers: A revised conceptual model. Front. Plant Sci. 2018, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.H.; Tilman, G.D.; Nekola, J.C. Eutrophication: Impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ. Pollut. 1999, 100, 179–196. [Google Scholar] [CrossRef]
- Mäemets, H.; Palmik, K.; Haldna, M.; Sudnitsyna, D.; Melnik, M. Eutrophication and macrophyte species richness in the large shallow North-European Lake Peipsi. Aquat. Bot. 2010, 92, 273–280. [Google Scholar] [CrossRef]
- Song, L.; Chow, W.S.; Sun, L.; Li, C.; Peng, C. Acclimation of photosystem II to high temperature in two Wedelia species from different geographical origins: Implications for biological invasions upon global warming. J. Exp. Bot. 2010, 61, 4087–4096. [Google Scholar] [CrossRef]
- Talukdar, T.; Talukdar, D. Response of antioxidative enzymes to arsenic-induced phytotoxicity in leaves of a medicinal daisy, Wedelia chinensis Merrill. J. Nat. Sci. Biol. Med. 2013, 4, 383. [Google Scholar] [CrossRef] [Green Version]
- Dai, Z.-C.; Fu, W.; Qi, S.-S.; Zhai, D.-L.; Chen, S.-C.; Wan, L.-Y.; Huang, P.; Du, D.-L. Different responses of an invasive clonal plant Wedelia trilobata and its native congener to gibberellin: Implications for biological invasion. J. Chem. Ecol. 2016, 42, 85–94. [Google Scholar] [CrossRef]
- Talukdar, T.; Mukherjee, S.K. Comparative study of cypselas in three common species of Asteraceae. Pleione 2008, 2, 147–149. [Google Scholar]
- Chen, Y.; Liu, R.; Sun, C.; Zhang, P.; Feng, C.; Shen, Z. Spatial and temporal variations in nitrogen and phosphorous nutrients in the Yangtze River Estuary. Mar. Pollut. Bull. 2012, 64, 2083–2089. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.L. Differences in plasticity between invasive and native plants from a low resource environment. J. Ecol. 2008, 96, 1162–1173. [Google Scholar] [CrossRef]
- Lamarque, L.J.; Porte, A.J.; Eymeric, C.; Lasnier, J.-B.; Lortie, C.J.; Delzon, S. A test for pre-adapted phenotypic plasticity in the invasive tree Acer negundo L. PLoS ONE 2013, 8, 74239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armas, C.; Ordiales, R.; Pugnaire, F.I. Measuring plant interactions: A new comparative index. Ecology 2004, 85, 2682–2686. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.R.; Yeates, A.; Schooler, S.; Julien, M.H. Rapid response to shoot removal by the invasive wetland plant, alligator weed (Alternanthera philoxeroides). Environ. Exp. Bot. 2007, 60, 20–25. [Google Scholar] [CrossRef]
- Mommer, L.; Visser, E.J. Underwater photosynthesis in flooded terrestrial plants: A matter of leaf plasticity. Ann. Bot. 2005, 96, 581–589. [Google Scholar] [CrossRef]
- Wang, T.; Hu, J.; Gao, Y.; Yu, D.; Liu, C. Disturbance, trait similarities, and trait advantages facilitate the invasion success of Alternanthera Philoxeroides (Mart) Griseb. CLEAN Soil Air Water 2017, 45, 1600378. [Google Scholar] [CrossRef]
- Pierik, R.V.; van Aken, J.; Voesenek, L. Is elongation-induced leaf emergence beneficial for submerged Rumex species? Ann. Bot. 2008, 103, 353–357. [Google Scholar] [CrossRef] [Green Version]
- Ding, W.; Zhang, H.; Zhang, F.; Wang, L.; Cui, S. Morphology of the invasive amphiphyte Alternanthera philoxeroides under different water levels and nitrogen concentrations. Acta Biol. Crac. Bot. 2014, 56, 136–147. [Google Scholar] [CrossRef]
- Zuo, S.; Ma, Y.; Shinobu, I. Differences in ecological and allelopathic traits among Alternanthera philoxeroides populations. Weed Biol. Manag. 2012, 12, 123–130. [Google Scholar] [CrossRef]
- Sauter, M. Root responses to flooding. Curr. Opin. Plant Biol. 2013, 16, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Riis, T.; Lambertini, C.; Olesen, B.; Clayton, J.S.; Brix, H.; Sorrell, B.K. Invasion strategies in clonal aquatic plants: Are phenotypic differences caused by phenotypic plasticity or local adaptation? Ann. Bot. 2010, 106, 813–822. [Google Scholar] [CrossRef]
- Yu, H.; Wang, L.; Liu, C.; Fan, S. Coverage of native plants is key factor influencing the invasibility of freshwater ecosystems by exotic plants in China. Front. Plant Sci. 2018, 9, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawano, N.; Ito, O.; Sakagami, J.-I. Morphological and physiological responses of rice seedlings to complete submergence (flash flooding). Ann. Bot. 2008, 103, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Voesenek, L. Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Yang, Y.-B.; Zhu, Z.-H. Elevated nitrogen allows the weak invasive plant Galinsoga quadriradiata to become more vigorous with respect to inter-specific competition. Sci. Rep. 2018, 8, 3136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Wang, R.; Gordon, D.R.; Sun, X.; Chen, L.; Wang, Y. Predicting plant invasions following China’s water diversion project. Environ. Sci. Technol. 2017, 51, 1450–1457. [Google Scholar] [CrossRef]
- You, W.; Yu, D.; Xie, D.; Yu, L.; Xiong, W.; Han, C. Responses of the invasive aquatic plant water hyacinth to altered nutrient levels under experimental warming in China. Aquat. Bot. 2014, 119, 51–56. [Google Scholar] [CrossRef]
- Wu, H.; Ding, J. Global change sharpens the double-edged sword effect of aquatic alien plants in China and beyond. Front. Plant Sci. 2019, 10, 787. [Google Scholar] [CrossRef] [Green Version]

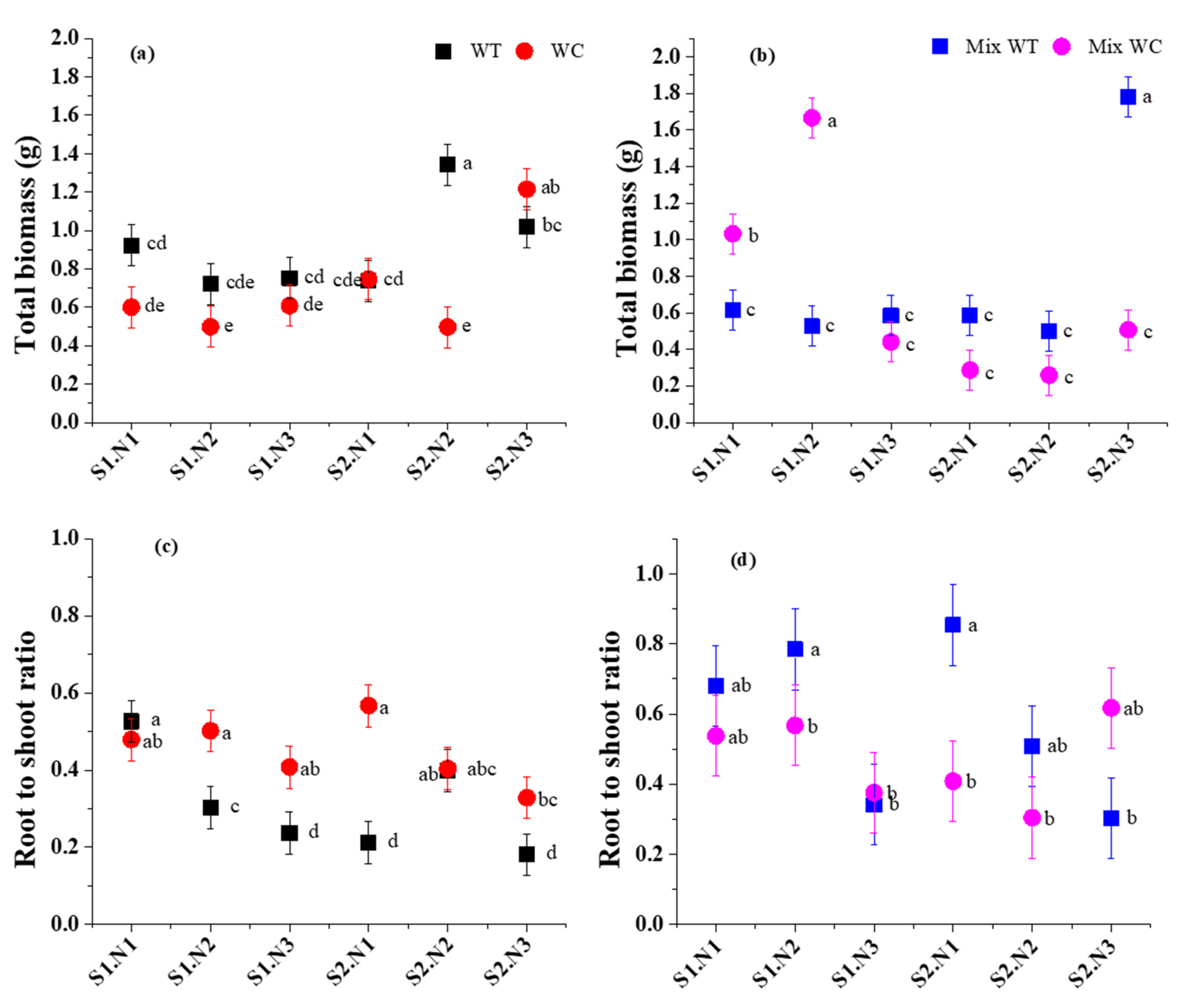
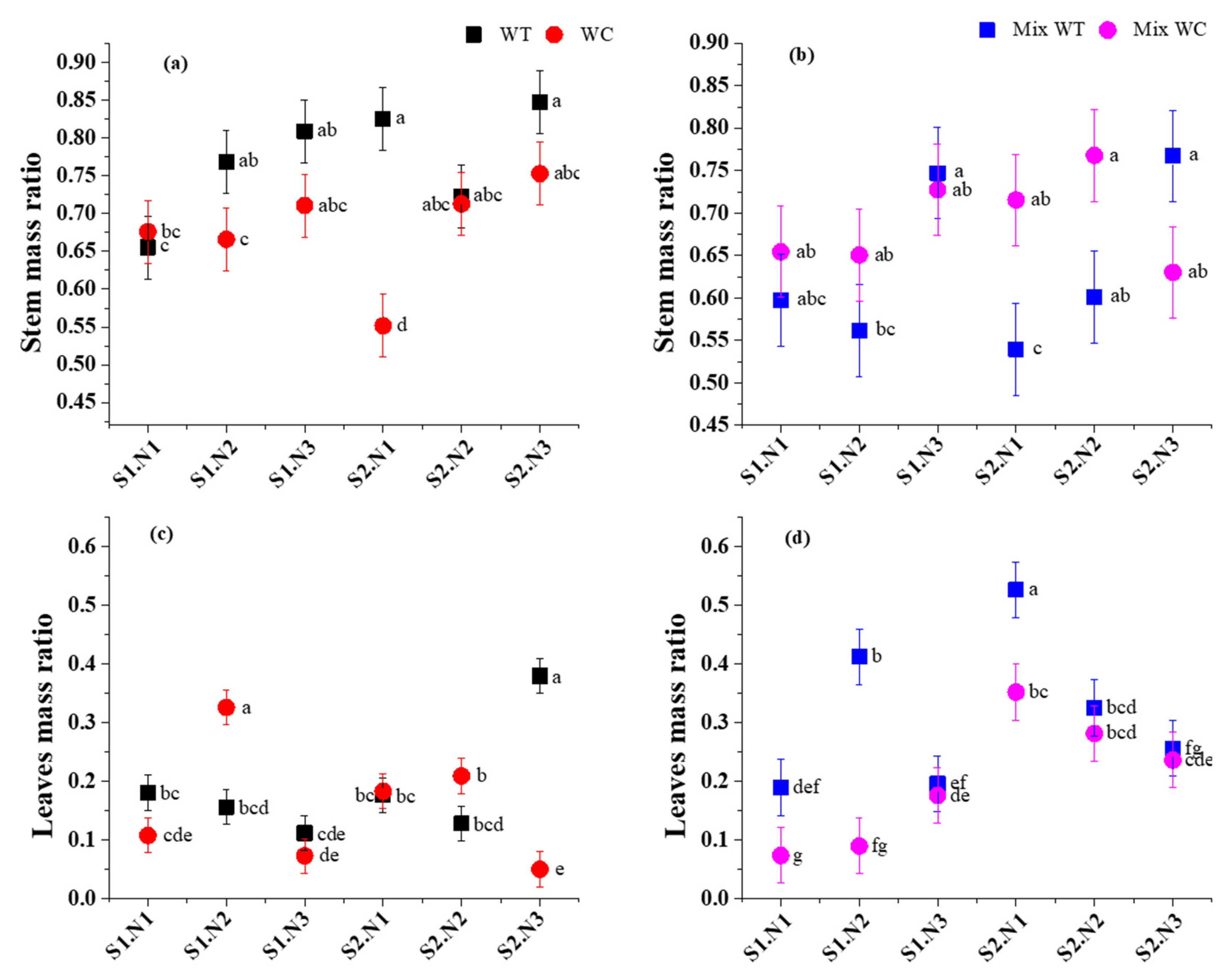
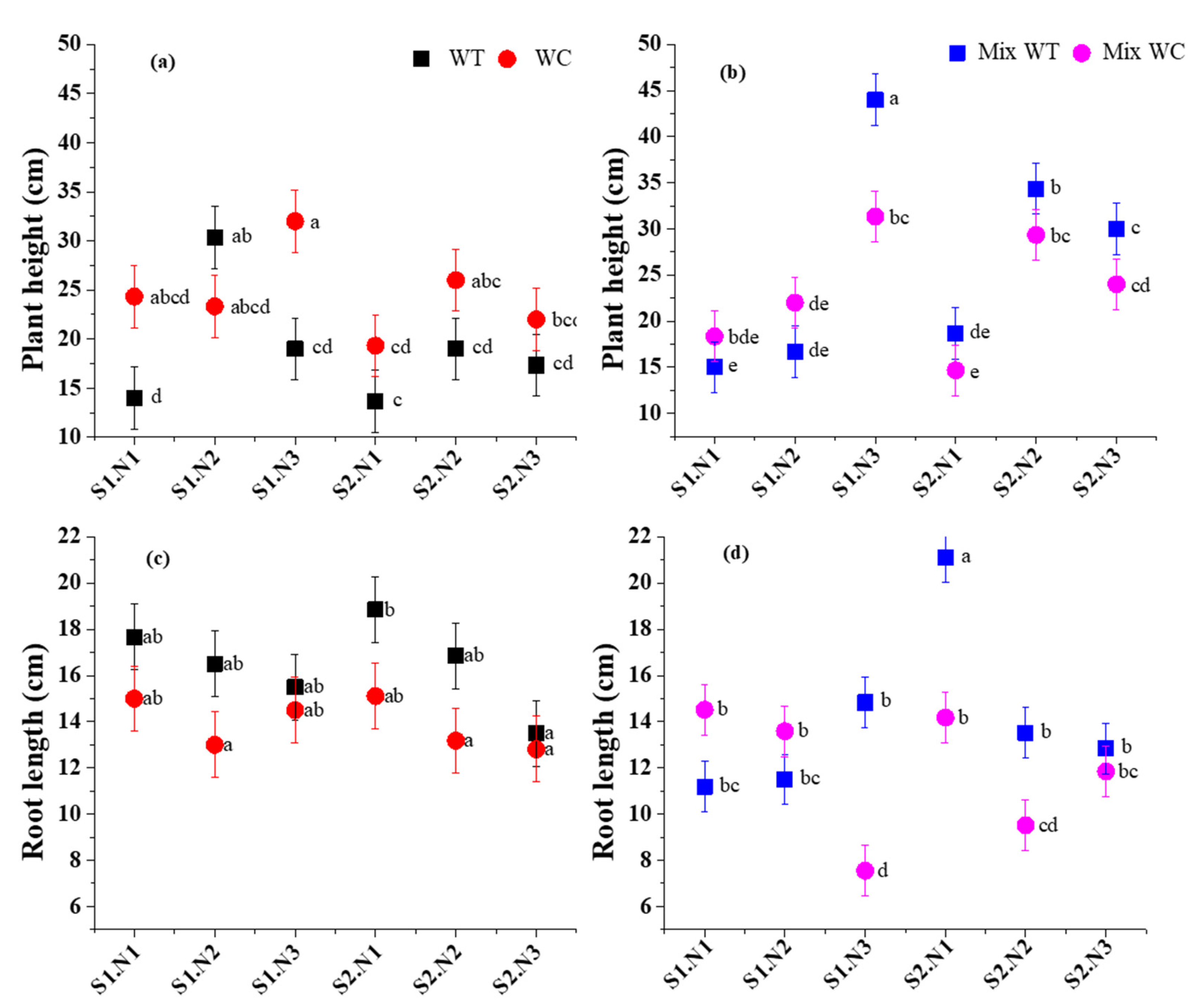
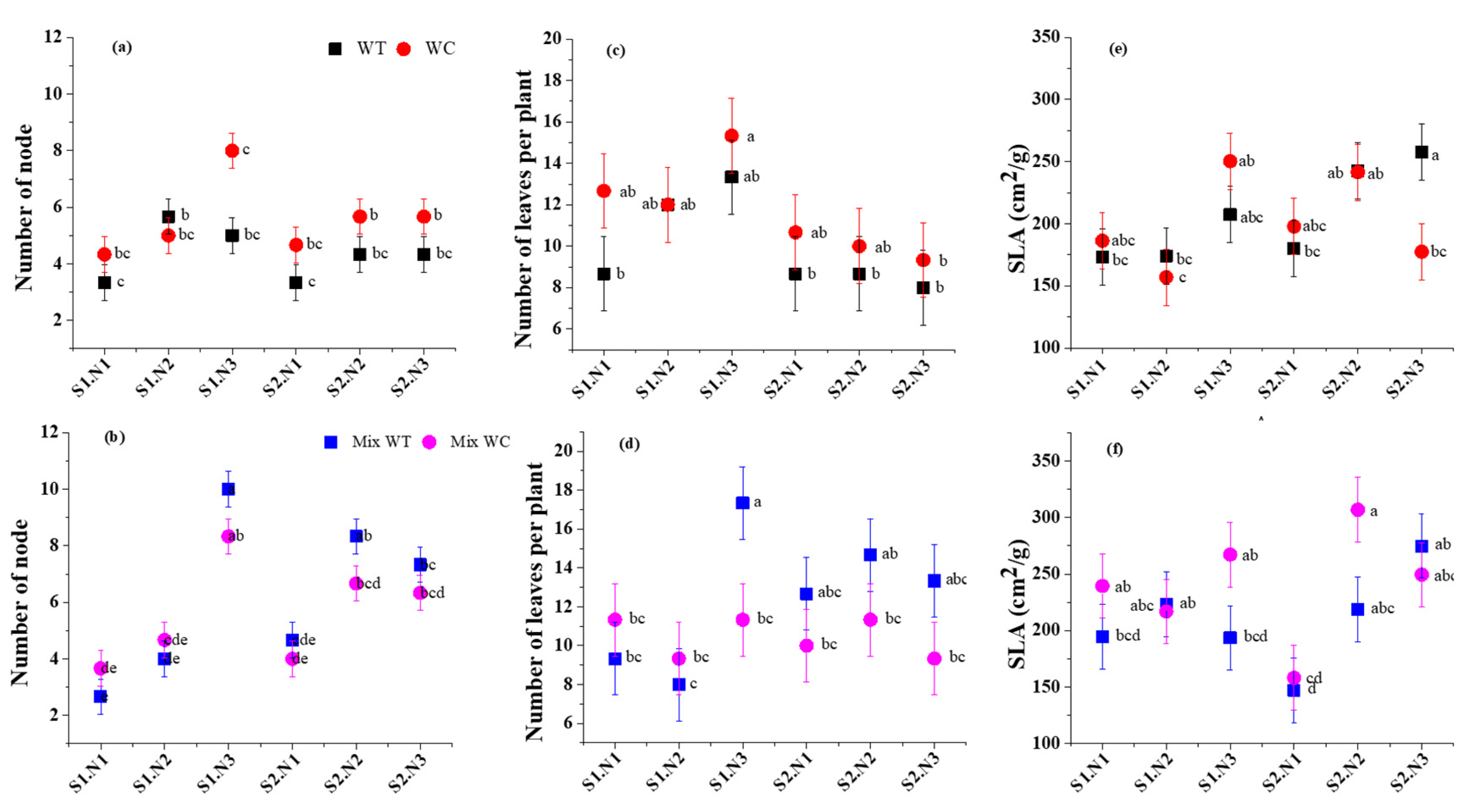
| Sources | Total Biomass | Root-to-Shoot Ratio | Stem Mass Ratio | Leaf Mass Ratio | ||||
|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | |
| S | 1.797 | 0.186 | 4.403 | <0.05 | 1.585 | 0.214 | 38.525 | <0.01 |
| N | 10.303 | <0.01 | 17.383 | <0.01 | 17.051 | <0.01 | 16.281 | <0.01 |
| C | 10.830 | <0.01 | 18.101 | <0.01 | 16.588 | <0.01 | 23.454 | <0.01 |
| S * N | 61.212 | <0.01 | 2.901 | 0.065 | 0.683 | 0.510 | 21.778 | <0.01 |
| S * C | 62.137 | <0.01 | 0.252 | 0.859 | 1.110 | 0.354 | 11.904 | <0.01 |
| N * C | 24.333 | <0.01 | 5.347 | <0.01 | 4.288 | <0.01 | 21.006 | <0.01 |
| S * N * C | 16.347 | <0.01 | 6.120 | <0.01 | 5.003 | <0.01 | 16.088 | <0.01 |
| Sources | Plant Height | Root Length | Number of Nodes | Number of Leaves | Specific Leaf Area | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | P | F | p | F | p | F | p | |
| S | 4.590 | <0.05 | 3.291 | 0.076 | 0.053 | 0.818 | 4.900 | <0.05 | 3.617 | 0.063 |
| N | 52.098 | <0.01 | 26.067 | <0.01 | 53.560 | <0.01 | 3.878 | <0.05 | 16.504 | <0.01 |
| C | 14.013 | <0.01 | 26.582 | <0.01 | 10.329 | <0.01 | 5.196 | <0.01 | 5.450 | <0.01 |
| S * N | 17.396 | <0.01 | 7.899 | <0.01 | 17.613 | <0.01 | 9.233 | <0.01 | 11.578 | <0.01 |
| S * C | 3.491 | <0.05 | 5.923 | <0.01 | 3.076 | <0.05 | 5.463 | <0.01 | 1.694 | 0.181 |
| N * C | 9.070 | <0.01 | 2.490 | <0.05 | 4.209 | <0.01 | 1.685 | 0.145 | 1.093 | 0.380 |
| S * N * C | 8.875 | <0.01 | 9.458 | <0.01 | 3.676 | <0.01 | 1.619 | 0.163 | 5.693 | <0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azeem, A.; Sun, J.; Javed, Q.; Jabran, K.; Du, D. The Effect of Submergence and Eutrophication on the Trait’s Performance of Wedelia Trilobata over Its Congener Native Wedelia Chinensis. Water 2020, 12, 934. https://doi.org/10.3390/w12040934
Azeem A, Sun J, Javed Q, Jabran K, Du D. The Effect of Submergence and Eutrophication on the Trait’s Performance of Wedelia Trilobata over Its Congener Native Wedelia Chinensis. Water. 2020; 12(4):934. https://doi.org/10.3390/w12040934
Chicago/Turabian StyleAzeem, Ahmad, Jianfan Sun, Qaiser Javed, Khawar Jabran, and Daolin Du. 2020. "The Effect of Submergence and Eutrophication on the Trait’s Performance of Wedelia Trilobata over Its Congener Native Wedelia Chinensis" Water 12, no. 4: 934. https://doi.org/10.3390/w12040934
APA StyleAzeem, A., Sun, J., Javed, Q., Jabran, K., & Du, D. (2020). The Effect of Submergence and Eutrophication on the Trait’s Performance of Wedelia Trilobata over Its Congener Native Wedelia Chinensis. Water, 12(4), 934. https://doi.org/10.3390/w12040934








