Insights into the Photocatalytic Bacterial Inactivation by Flower-Like Bi2WO6 under Solar or Visible Light, Through in Situ Monitoring and Determination of Reactive Oxygen Species (ROS)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Flower-Like Bi2WO6 Samples
2.2. Physical Characterization of the Bi2WO6 Flakes
2.3. Photocatalytic Antibacterial Activity on Bi2WO6 and Light Sources
3. Results
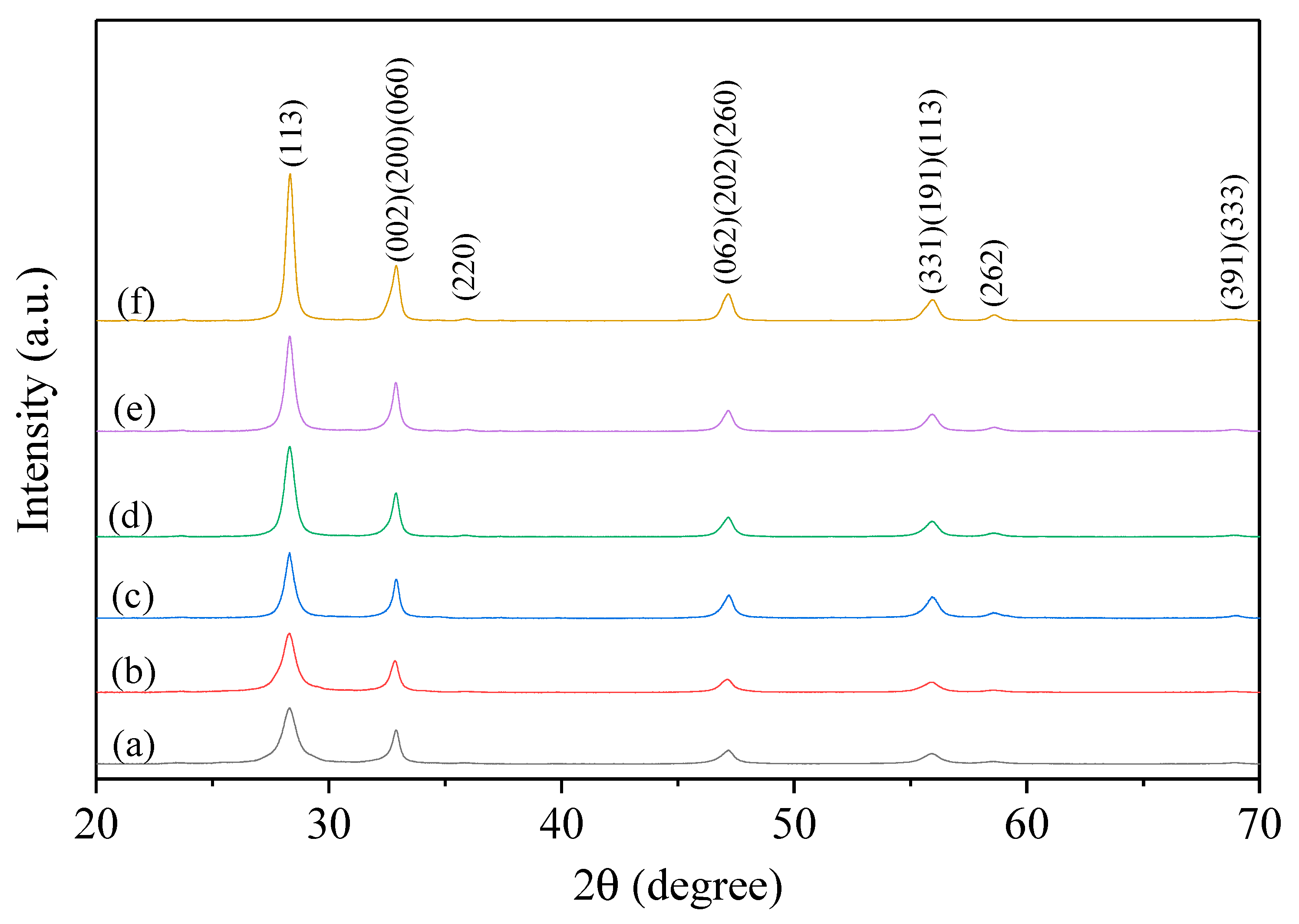
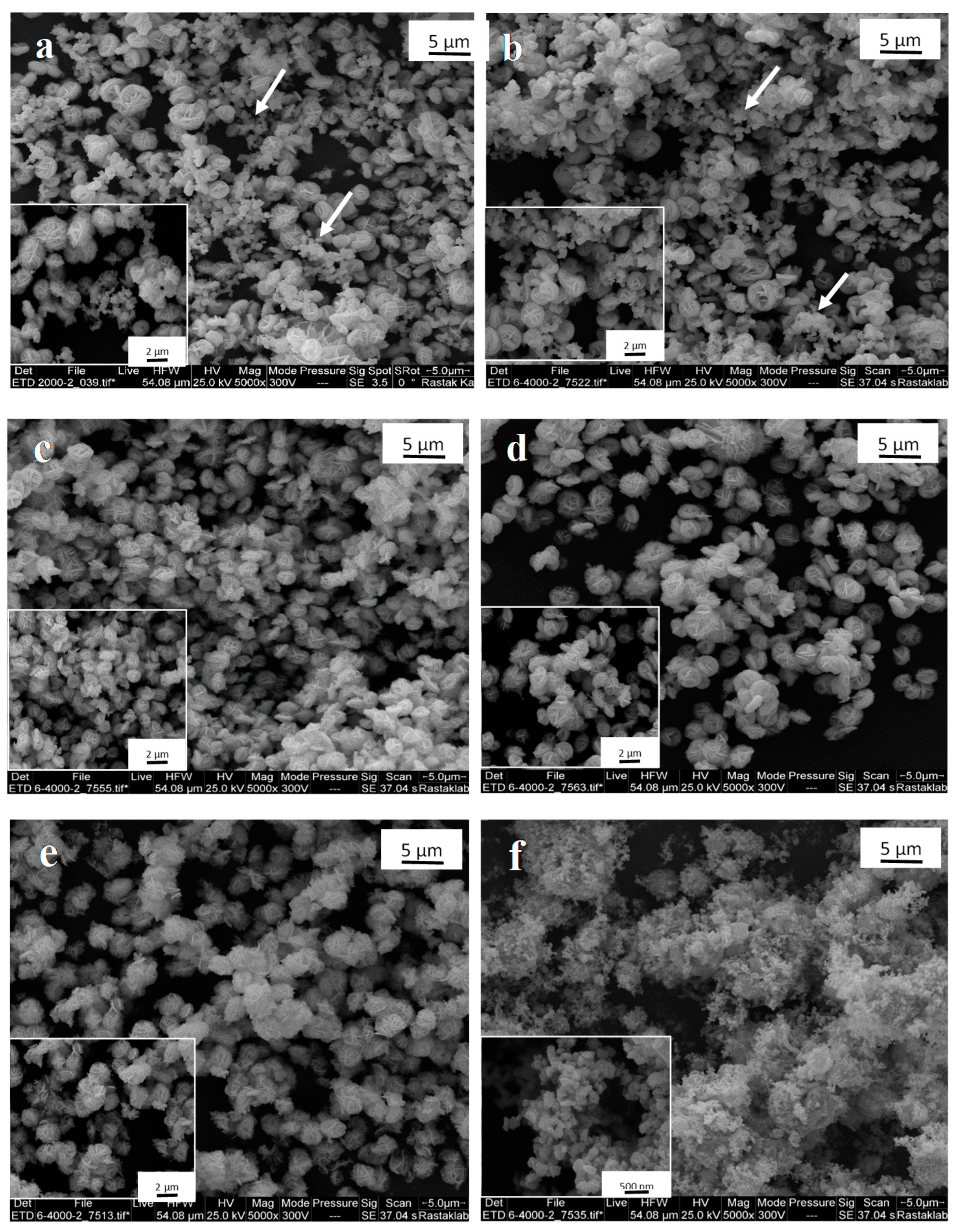
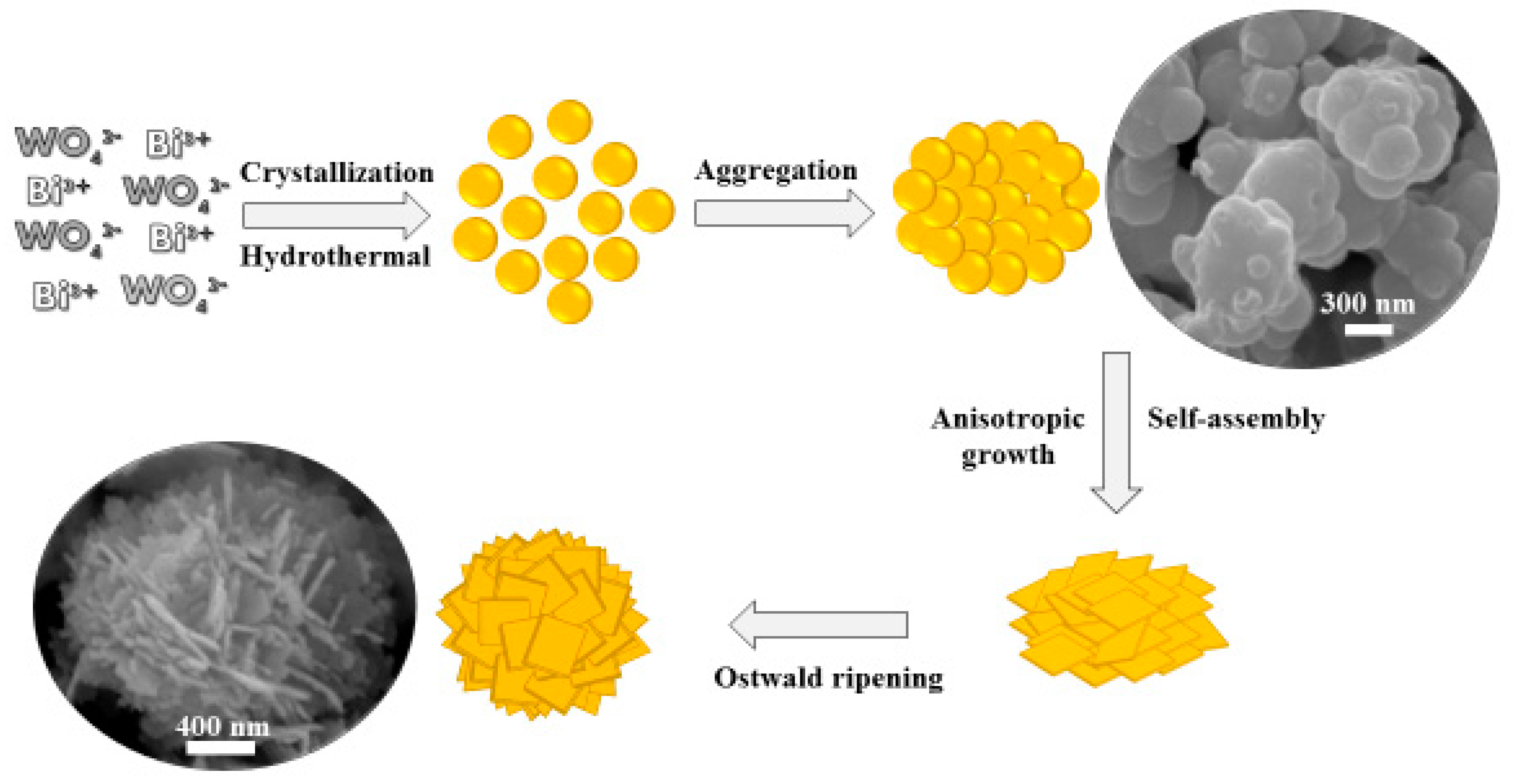
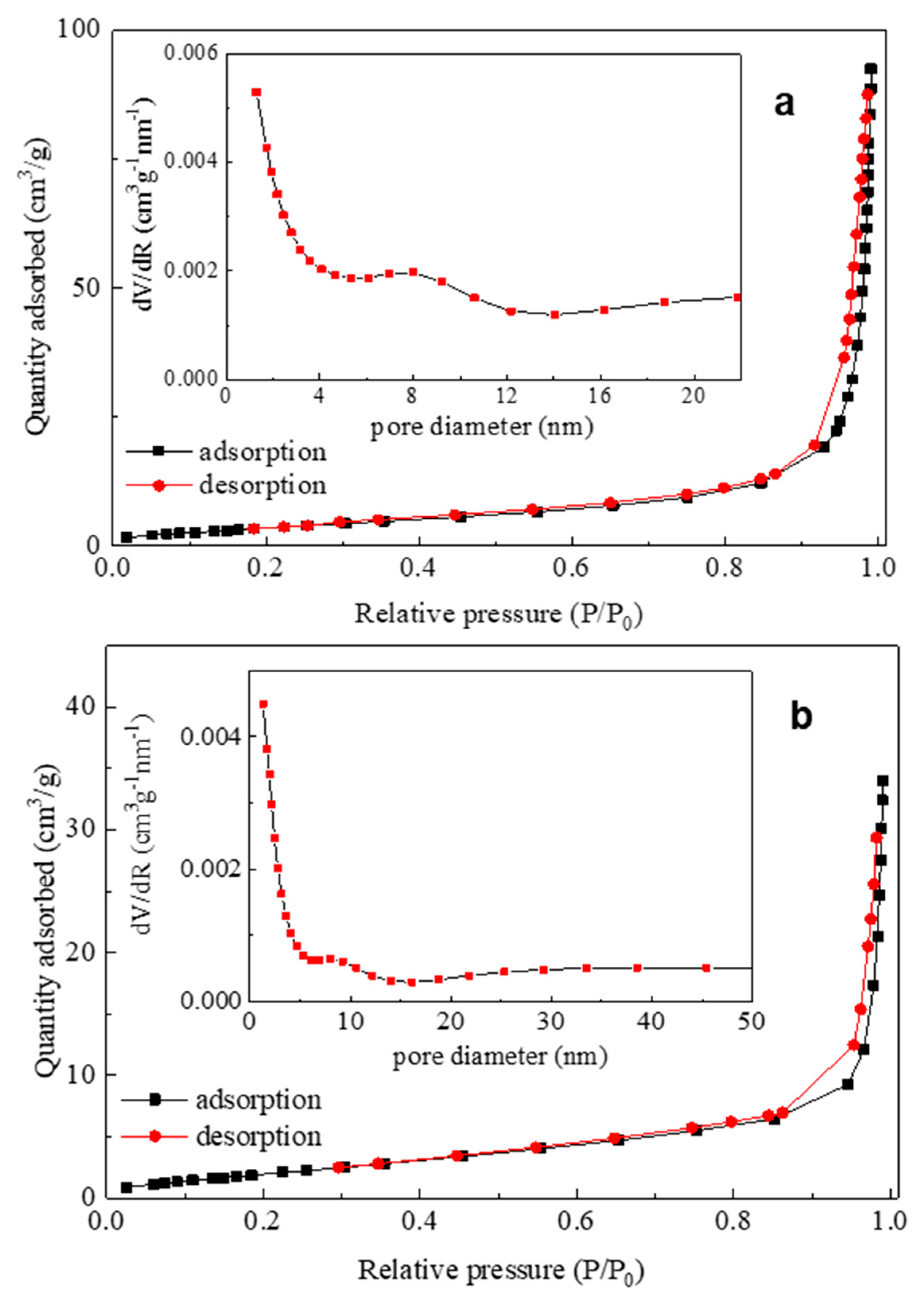
3.1. Synthesis and Characterization of Bi2WO6: X-Ray Diffraction (XRD), Scanning Electron Microscopy (SEM), X-Ray Photoelectron Spectroscopy (XPS), and SSA Determination


3.2. E. Coli Inactivation Kinetics: Effect of the Bacterial Concentration, Amount of Catalyst, Light Dose, and Applied Light Wavelength
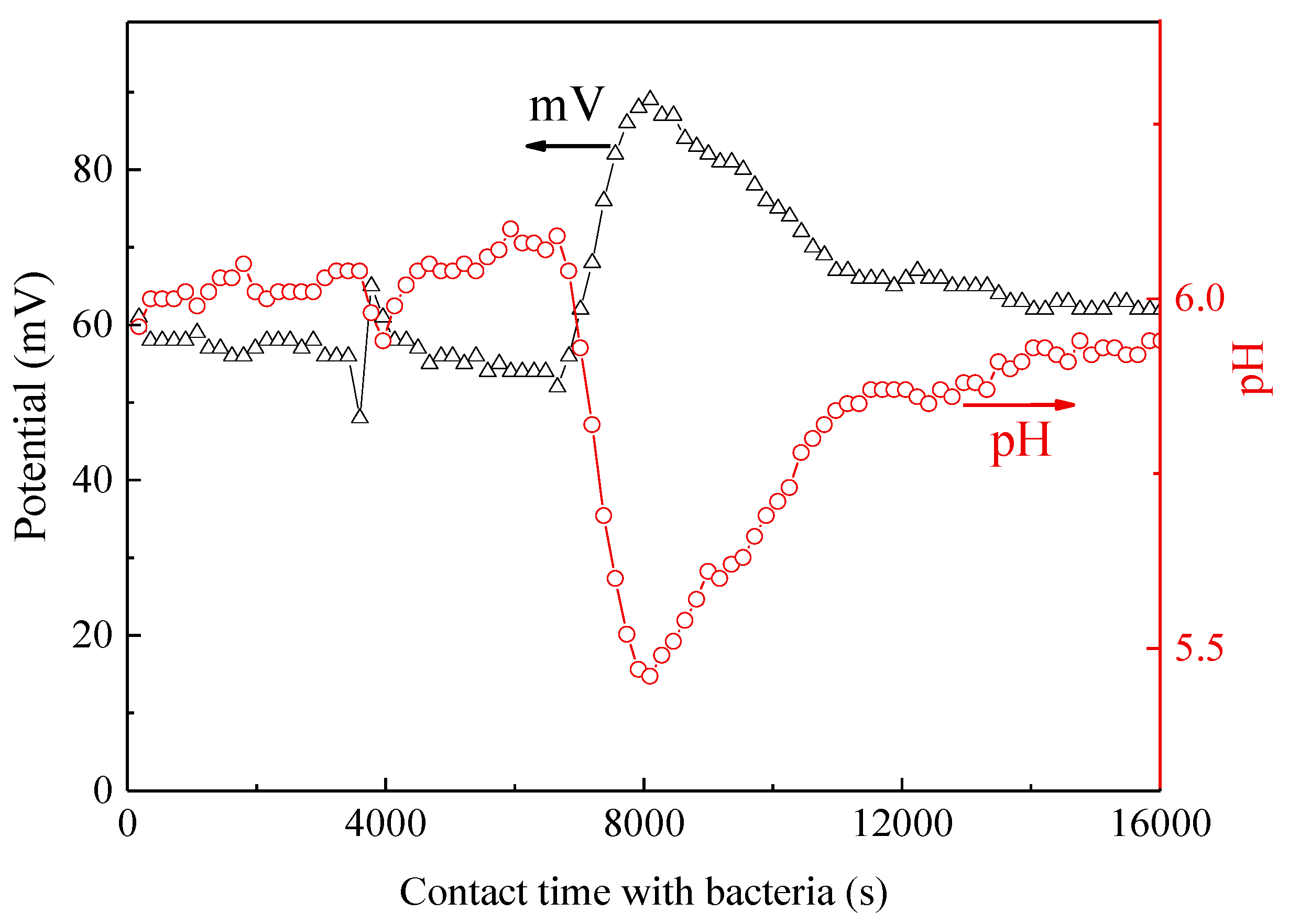
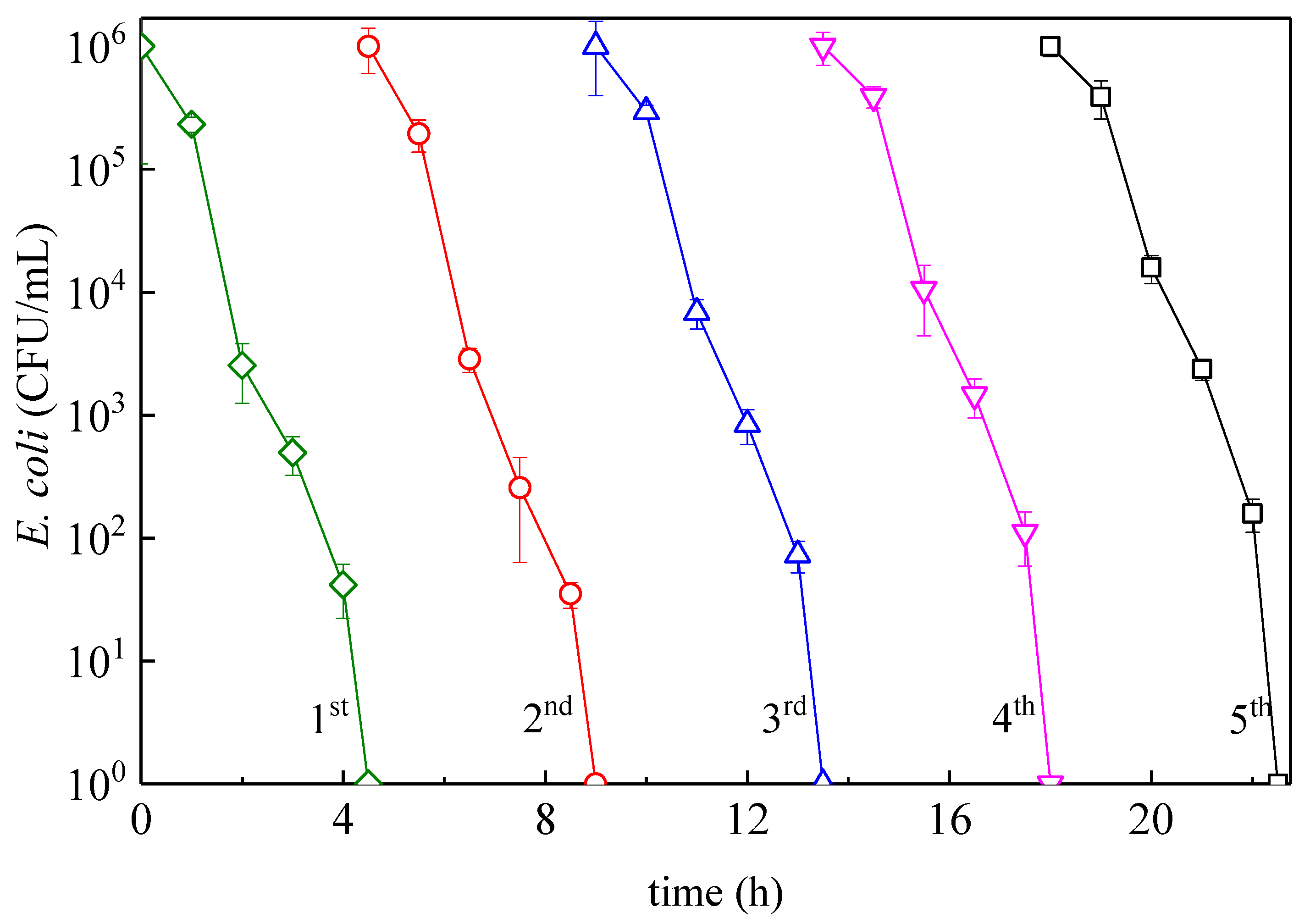
3.3. Mechanistic Interpretation: ROS-Species Involvement, Interfacial Charge Transfer, and Catalyst Reuse During Bacterial Inactivation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Giannakis, S.; Jovic, M.; Gasilova, N.; Pastor Gelabert, M.; Schindelholz, S.; Furbringer, J.M.; Girault, H.; Pulgarin, C. Iohexol degradation in wastewater and urine by UV-based Advanced Oxidation Processes (AOPs): Process modeling and by-products identification. J. Environ. Manag. 2017, 195, 174–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef]
- Giannakis, S.; Merino Gamo, A.I.; Darakas, E.; Escalas-Cañellas, A.; Pulgarin, C. Impact of different light intermittence regimes on bacteria during simulated solar treatment of secondary effluent: Implications of the inserted dark periods. Sol. Energy 2013, 98, 572–581. [Google Scholar] [CrossRef] [Green Version]
- Giannakis, S.; Darakas, E.; Escalas-Cañellas, A.; Pulgarin, C. Environmental considerations on solar disinfection of wastewater and the subsequent bacterial (re)growth. Photochem. Photobiol. Sci. 2015, 14, 618–625. [Google Scholar] [CrossRef] [Green Version]
- Byrne, J.A.; Dunlop, P.S.M.; Hamilton, J.W.J.; Fernández-Ibáñez, P.; Polo-López, I.; Sharma, P.K.; Vennard, A.S.M. A Review of Heterogeneous Photocatalysis for Water and Surface Disinfection. Molecules 2015, 20, 5574–5615. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, L.; Della Sala, A.; Fiorentino, A.; Li Puma, G. Disinfection of urban wastewater by solar driven and UV lamp–TiO2 photocatalysis: Effect on a multi drug resistant Escherichia coli strain. Water Res. 2014, 53, 145–152. [Google Scholar] [CrossRef]
- Carré, G.; Hamon, E.; Ennahar, S.; Estner, M.; Lett, M.C.; Horvatovich, P.; Gies, J.P.; Keller, V.; Keller, N.; Andre, P. TiO2 photocatalysis damages lipids and proteins in Escherichia coli. Appl. Environ. Microbiol. 2014, 80, 2573–2581. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, K.; Dai, K.; Walker, S.L.; Huang, Q.; Yin, X.; Cai, P. Efficient photocatalytic disinfection of Escherichia coli O157:H7 using C70-TiO2hybrid under visible light irradiation. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev 2012, 13, 169. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Chen, Z.; Zhou, L.; Xu, H.; Zhu, W. Fabrication of flower-like Bi2WO6superstructures as high performance visible-light driven photocatalysts. J. Mater. Chem. 2007, 17, 2526–2532. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Chen, Z.; Wong, P.K.; Liu, J. Bi2WO6 micro/nano-structures: Synthesis, modifications and visible-light-driven photocatalytic applications. Appl. Catal. B Environ. 2011, 106, 1–13. [Google Scholar] [CrossRef]
- Saison, T.; Gras, P.; Chemin, N.; Chanéac, C.; Durupthy, O.; Brezová, V.; Colbeau-Justin, C.; Jolivet, J.P. New insights into Bi2WO6 properties as a visible-light photocatalyst. J. Phys. Chem. C 2013, 117, 22656–22666. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Y. A review of controllable synthesis and enhancement of performances of bismuth tungstate visible-light-driven photocatalysts. Catal. Sci. Technol. 2012, 2, 694–706. [Google Scholar] [CrossRef]
- Chen, M. Degradation of Antibiotic Norfloxacin by Solar Light/Visible Light-Assisted Oxidation Processes in Aqueous Phase. Ph.D. Thesis, The Hong Kong Polytechnic University, Hong Kong, China, 2013. [Google Scholar]
- Shang, Y.; Cui, Y.; Shi, R.; Yang, P. Effect of acetic acid on morphology of Bi2WO6with enhanced photocatalytic activity. Mater. Sci. Semicond. Process. 2019, 89, 240–249. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, J.; Xie, Y.; Wang, M.; Ge, X. Inductive effect of poly ( vinyl pyrrolidone ) on morphology and photocatalytic performance of Bi2WO6. Appl. Surf. Sci. 2016, 368, 332–340. [Google Scholar] [CrossRef]
- Shen, R.; Jiang, C.; Xiang, Q.; Xie, J.; Li, X. Surface and interface engineering of hierarchical photocatalysts. Appl. Surf. Sci. 2018, 471, 43–87. [Google Scholar] [CrossRef]
- Li, C.; Chen, G.; Sun, J.; Rao, J.; Han, Z.; Hu, Y.; Zhou, Y. A Novel Mesoporous Single-Crystal-Like Bi2WO6with Enhanced Photocatalytic Activity for Pollutants Degradation and Oxygen Production. ACS Appl. Mater. Interfaces 2015, 7, 25716–25724. [Google Scholar] [CrossRef]
- Ren, J.; Wang, W.; Zhang, L.; Chang, J.; Hu, S. Photocatalytic inactivation of bacteria by photocatalyst Bi2 WO6 under visible light. Catal. Commun. 2009, 10, 1940–1943. [Google Scholar] [CrossRef]
- Helali, S.; Polo-López, M.I.; Fernández-Ibáñez, P.; Ohtani, B.; Amano, F.; Malato, S.; Guillard, C. Solar photocatalysis: A green technology for E. coli contaminated water disinfection. Effect of concentration and different types of suspended catalyst. J. Photochem. Photobiol. A Chem. 2014, 276, 31–40. [Google Scholar] [CrossRef]
- Amano, F.; Nogami, K.; Ohtani, B. Visible Light-Responsive Bismuth Tungstate Photocatalysts: Effects of Hierarchical Architecture on Photocatalytic Activity. J. Phys. Chem 2009, 113, 1536–1542. [Google Scholar] [CrossRef]
- Porras, J.; Giannakis, S.; Torres-Palma, R.A.; Fernandez, J.J.; Bensimon, M.; Pulgarin, C. Fe and Cu in humic acid extracts modify bacterial inactivation pathways during solar disinfection and photo-Fenton processes in water. Appl. Catal. B Environ. 2018, 235. [Google Scholar] [CrossRef]
- Marjanovic, M.; Giannakis, S.; Grandjean, D.; de Alencastro, L.F.; Pulgarin, C. Effect of ΜM Fe addition, mild heat and solar UV on sulfate radical-mediated inactivation of bacteria, viruses, and micropollutant degradation in water. Water Res. 2018, 140, 220–231. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, Y. Synthesis of Square Bi2WO6 Nanoplates as High-Activity Visible-Light-Driven Photocatalysts, Chem. Chem. Mater. 2005, 17, 3537–3545. [Google Scholar] [CrossRef]
- Liang, Y.; Shi, J.; Fang, B. Synthesis and electrochemical performance of bismuth tungsten oxides with different composition and morphology. Chem. Phys. Lett. 2019, 716, 112–118. [Google Scholar] [CrossRef]
- Guan, J.; Liu, L.; Xu, L.; Sun, Z.; Zhang, Y. Nickel flower-like nanostructures composed of nanoplates: One-pot synthesis, stepwise growth mechanism and enhanced ferromagnetic properties. CrystEngComm 2011, 13, 2636. [Google Scholar] [CrossRef]
- Donaldson, J.D.; Knifton, J.F.; Ross, S.D. The fundamental vibrational spectra of the formates of the main group elements. Spectrochim. Acta 1964, 20, 847–851. [Google Scholar] [CrossRef]
- Anderegg, G.; Arnaud-Neu, F.; Delgado, R.; Felcman, J.; Popov, K. Critical evaluation of stability constants of metal complexes of complexones for biomedical and environmental applications (IUPAC Technical Report). Pure Appl. Chem. 2005, 77, 1445–1495. [Google Scholar] [CrossRef] [Green Version]
- Portanova, R.; Lajunen, L.H.J.; Tolazzi, M.; Piispanen, J. Critical evaluation of stability constants for alpha-hydroxycarboxylic acid complexes with protons and metal ions and the accompanying enthalpy changes. Part II. Aliphatic 2-hydroxycarboxylic acids (IUPAC Technical Report). Pure Appl. Chem. 2003, 75, 495–540. [Google Scholar] [CrossRef]
- Briand, G.G.; Burford, N. Coordination Complexes of Bismuth(III) Involving Organic Ligands with Pnictogen or Chalcogen Donors. Adv. Inorg. Chem. 2000, 50, 285–357. [Google Scholar]
- Tang, R.; Su, H.; Sun, Y.; Zhang, X.; Li, L.; Liu, C.; Wang, B.; Zeng, S.; Sun, D. Facile Fabrication of Bi2WO6/Ag2S Heterostructure with Enhanced Visible-Light-Driven Photocatalytic Performances. Nanoscale Res. Lett. 2016, 11, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Ge, Y.; Wang, H.; Li, B.; Yu, L.; Liang, Y.; Chen, K.; Wang, F. Sol–gel synthesis and enhanced photocatalytic activity of doped bismuth tungsten oxide composite. Mater. Res. Bull. 2016, 73, 385–393. [Google Scholar] [CrossRef]
- Li, W.; Wang, Q.; Huang, L.; Li, Y.; Xu, Y.; Song, Y.; Zhang, Q.; Xu, H.; Li, H. Synthesis and characterization of BN/Bi2WO6 composite photocatalysts with enhanced visible-light photocatalytic activity. RSC Adv. 2015, 5, 88832–88840. [Google Scholar] [CrossRef]
- Tang, R.; Su, H.; Sun, Y.; Zhang, X.; Li, L.; Liu, C.; Zeng, S.; Sun, D. Journal of Colloid and Interface Science Enhanced photocatalytic performance in Bi2WO6/SnS heterostructures: Facile synthesis, influencing factors and mechanism of the photocatalytic process. J. Colloid Interface Sci. 2016, 466, 388–399. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, G.; Gan, H.; Zhang, Y. Micro/nano-structured CaWO4/Bi2WO6 composite: Synthesis, characterization and photocatalytic properties for degradation of organic contaminants. Dalton Trans. 2012, 41, 12697. [Google Scholar] [CrossRef]
- Chick, H. An Investigation of the Laws of Disinfection. J. Hyg. (Lond) 1908, 8, 92–158. [Google Scholar] [CrossRef] [Green Version]
- Watson, H.E. A Note on the Variation of the Rate of Disinfection with Change in the Concentration of the Disinfectant. J. Hyg. (Lond) 1908, 8, 536–542. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Zhang, H.; Wei, J.; Yu, Q.; Yang, P.; Zhang, F. Preparation of point-line Bi2WO6@TiO2 nanowires composite photocatalysts with enhanced UV/visible-light-driven photocatalytic activity. Mater. Sci. Semicond. Process. 2016, 45, 51–56. [Google Scholar] [CrossRef]
- Docampo, P.; Guldin, S.; Steiner, U.; Snaith, H.J. Charge Transport Limitations in Self-Assembled TiO2 Photoanodes for Dye-Sensitized Solar Cells. J. Phys. Chem. Lett. 2013, 4, 698–703. [Google Scholar] [CrossRef]
- Kh, M.; Reza, A.; Kurny, F.G. Parameters affecting the photocatalytic degradation of dyes using TiO2: A review. Appl. Water Sci. 2017, 7, 1569–1578. [Google Scholar]
- Adhikari, S.; Banerjee, A.; Eswar, N.K.R.; Sarkar, D.; Madras, G. Photocatalytic inactivation of E. Coli by ZnO-Ag nanoparticles under solar radiation. RSC Adv. 2015, 5, 51067–51077. [Google Scholar] [CrossRef] [Green Version]
- Winkler, J. Titanium Dioxide: Production, Properties and Effective Usage; Vincentz Network: Hanover, Germany, 2013; ISBN 9783866308121. [Google Scholar]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Benabbou, A.K.; Derriche, Z.; Felix, C.; Lejeune, P.; Guillard, C. Photocatalytic inactivation of Escherischia coli: Effect of concentration of TiO2 and microorganism, nature, and intensity of UV irradiation. Appl. Catal. B Environ. 2007, 76, 257–263. [Google Scholar]
- Regmi, C.; Joshi, B.; Ray, S.K.; Gyawali, G.; Pandey, R.P. Understanding Mechanism of Photocatalytic Microbial Decontamination of Environmental Wastewater. Front. Chem. 2018, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Zhu, Y.; Kudo, A. Nanostructured Photocatalysts; Springer International Publishing: Basel, Switzerland, 2016; ISBN 978-3-319-26077-8. [Google Scholar]
- Xu, J.; Wang, W.; Sun, S.; Wang, L. Enhancing visible-light-induced photocatalytic activity by coupling with wide-band-gap semiconductor: A case study on Bi2WO6/TiO2. Appl. Catal. B Environ. 2012, 111–112, 126–132. [Google Scholar] [CrossRef]
- Schneider, J.; Bahnemann, D.; Ye, J.; Puma, G.L.; Dionysiou, D.D. Photocatalysis; Royal Society of Chemistry: Cambridge, UK, 2016; ISBN 9781782620419. [Google Scholar]
- Wu, D.; Wang, W.; Ng, T.W.; Huang, G.; Xia, D.; Yip, H.Y.; Lee, H.K.; Li, G.; An, T.; Wong, P.K. Visible-light-driven photocatalytic bacterial inactivation and the mechanism of zinc oxysulfide under LED light irradiation. J. Mater. Chem. A 2016, 4, 1052–1059. [Google Scholar] [CrossRef]
- Sherman, I.; Gerchman, Y.; Sasson, Y.; Gnayem, H.; Mamane, H. Disinfection and Mechanistic Insights of Escherichia coli in Water by Bismuth Oxyhalide Photocatalysis. Photochem. Photobiol. 2016, 92, 826–834. [Google Scholar] [CrossRef]
- Kiwi, J.; Nadtochenko, V. New Evidence for TiO2 Photocatalysis during Bilayer Lipid Peroxidation. J. Phys. Chem. B 2004, 108, 17675–17684. [Google Scholar] [CrossRef]
- Kraeutler, B.; Bard, A.J. Heterogeneous photocatalytic decomposition of saturated carboxylic acids on titanium dioxide powder. Decarboxylative route to alkanes. J. Am. Chem. Soc. 1978, 100, 5985–5992. [Google Scholar] [CrossRef]



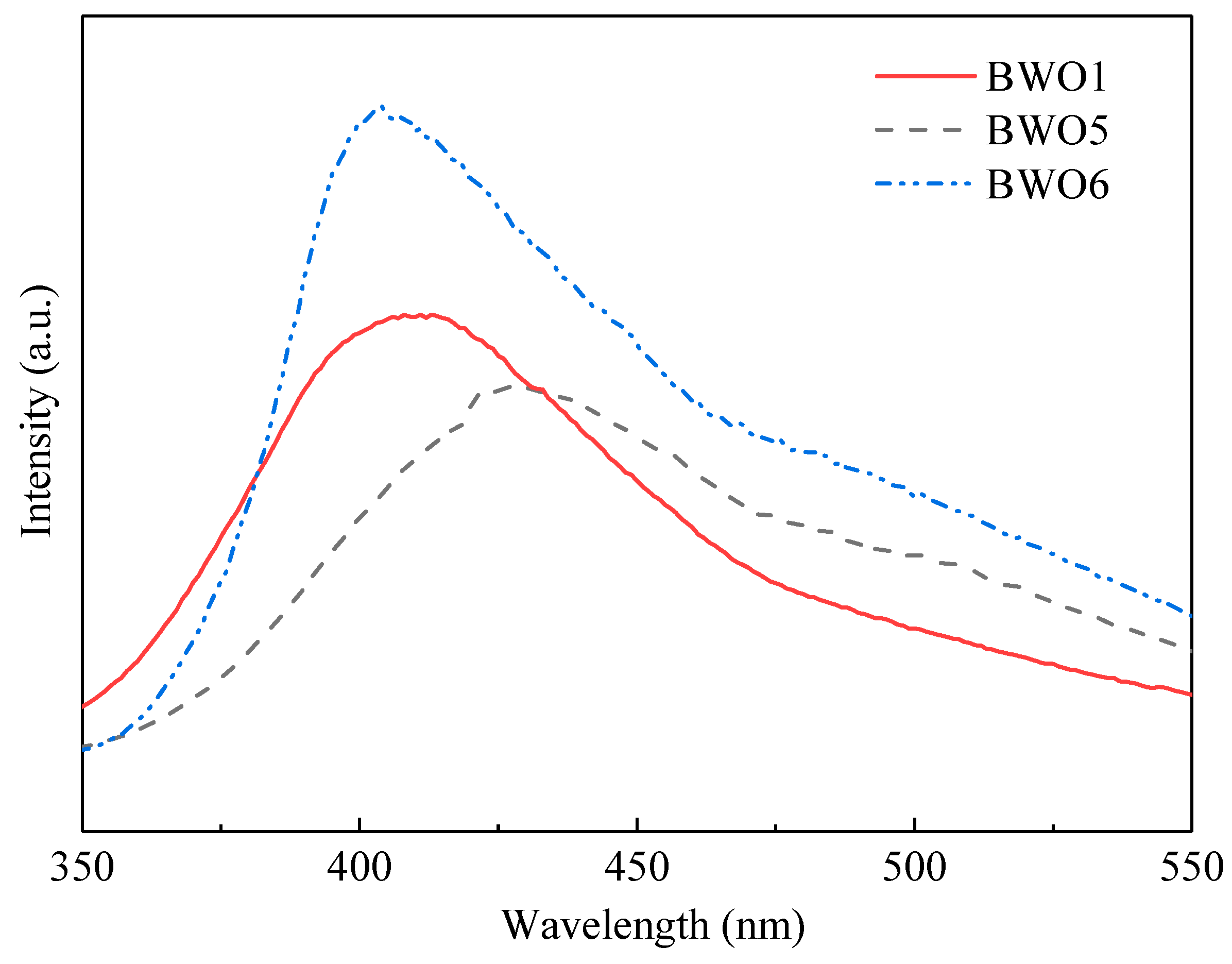
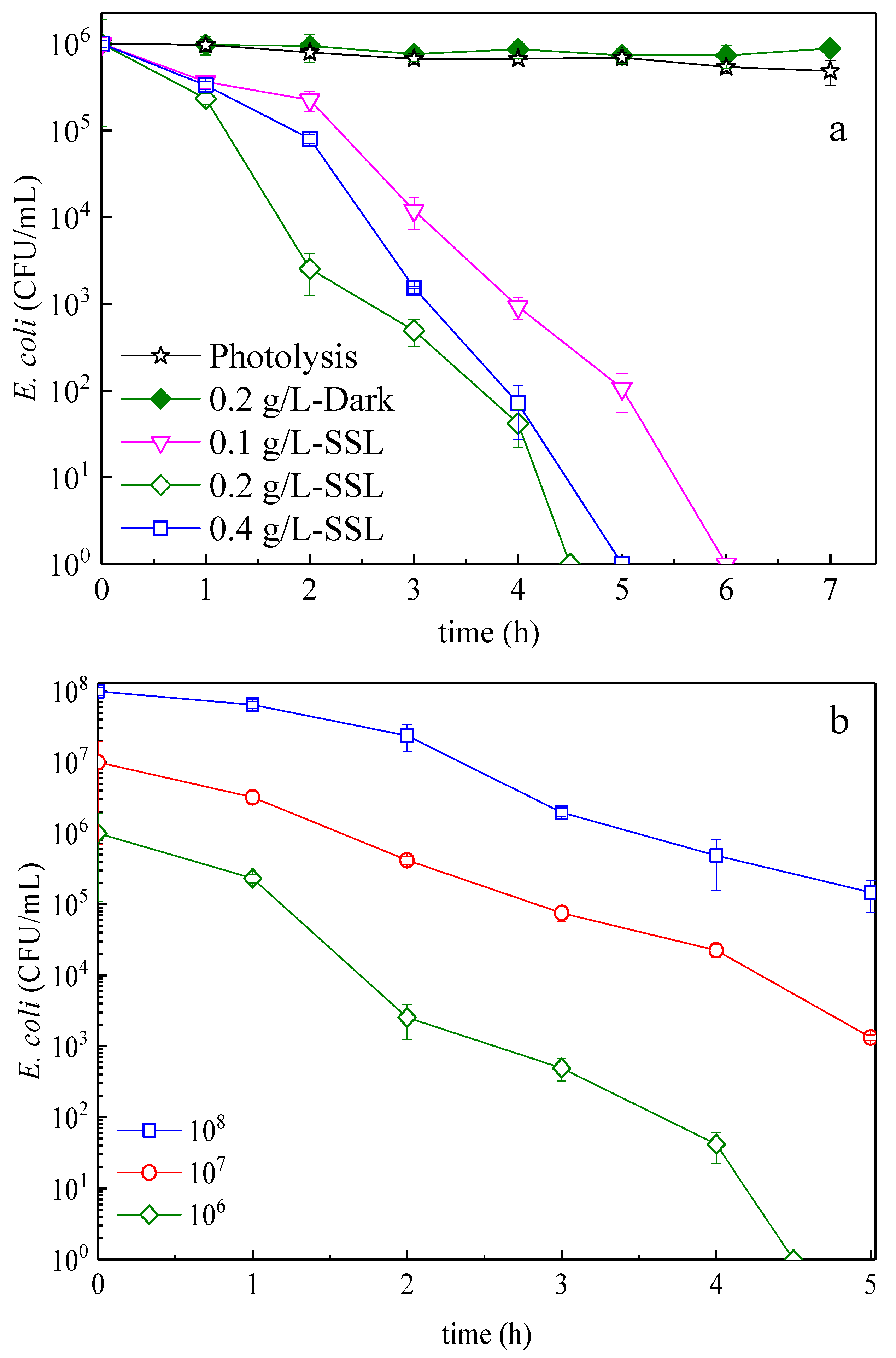
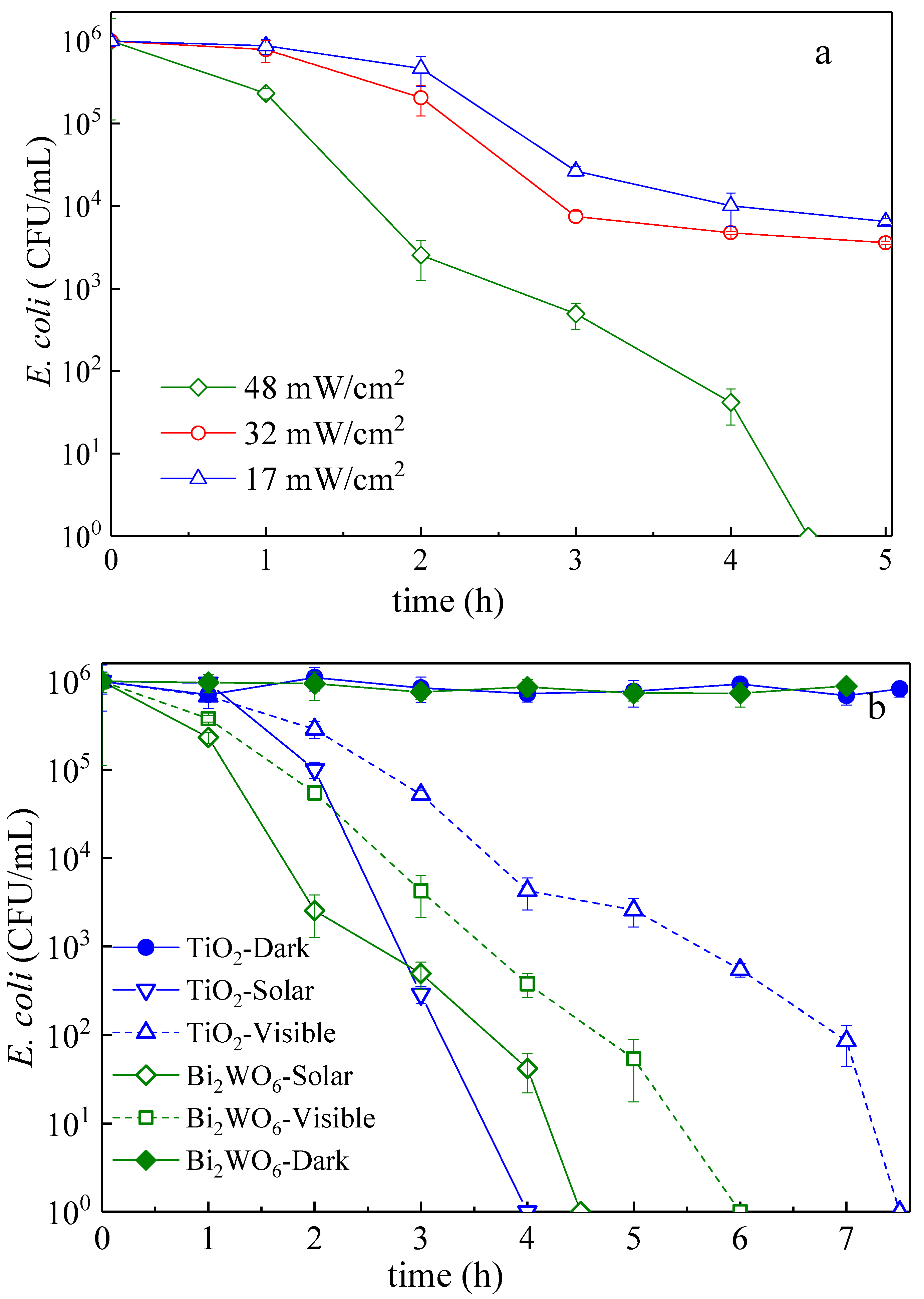
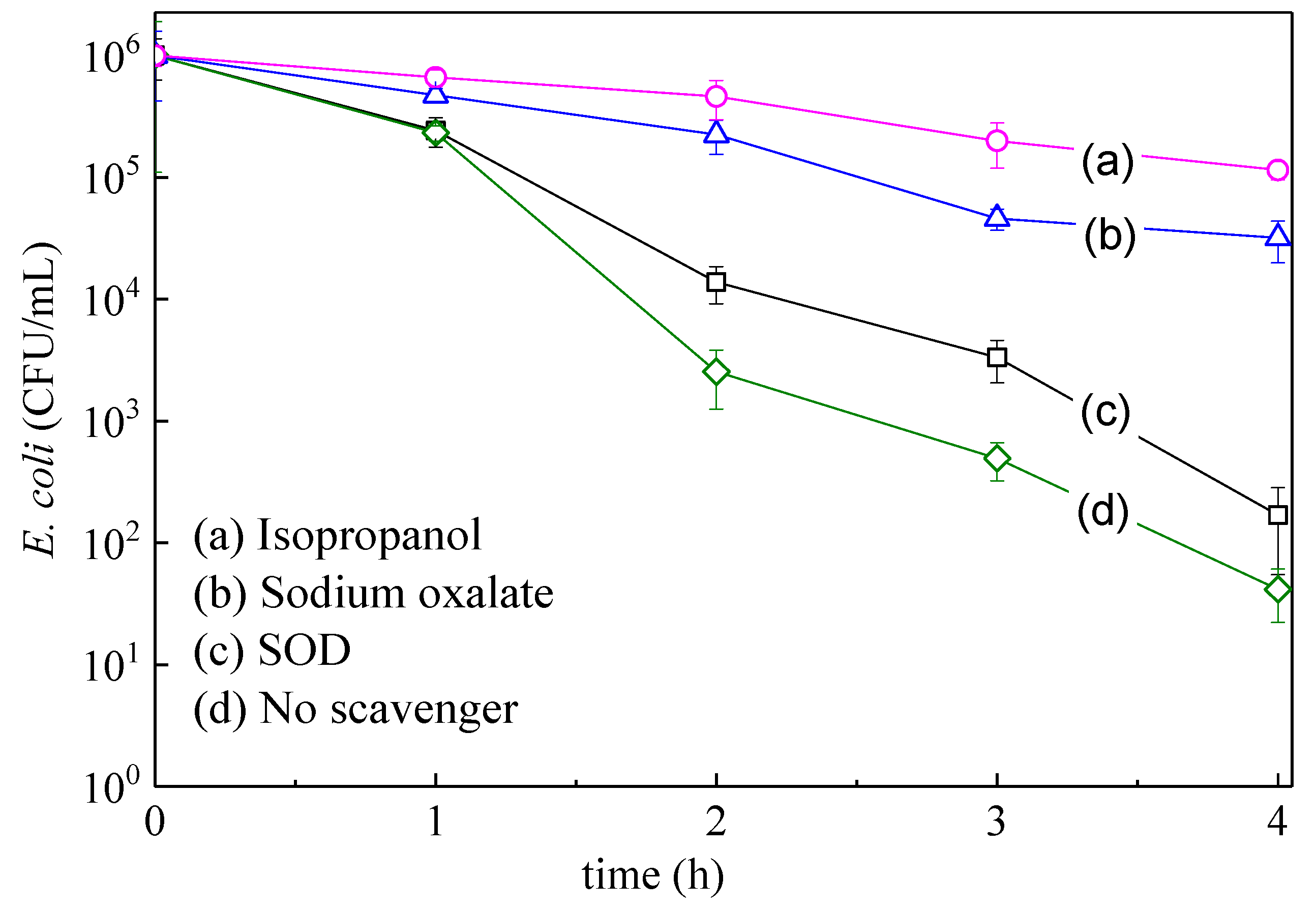

| Samples | Reaction Time (h) | Reaction Temperature (°C) |
|---|---|---|
| BWO1 | 12 | 160 |
| BWO2 | 18 | 160 |
| BWO3 | 24 | 160 |
| BWO4 | 24 | 180 |
| BWO5 | 24 | 200 |
| BWO6 | 24 | 200 (pH = 10) |
| Samples | Crystallite Size (nm) |
|---|---|
| BWO1 | 9 |
| BWO2 | 10 |
| BWO3 | 17 |
| BWO4 | 20 |
| BWO5 | 22 |
| BWO6 | 31 |
| Samples | Surface Areas (m2 g−1) | Total Pore Volumes (cm3 g−1) |
|---|---|---|
| BWO5 | 14.475 | 0.142 |
| BWO6 | 6.87 | 0.0532 |
| Cycle Number | kapp (min-1) |
|---|---|
| First | 0.0488 ± 0.005 |
| Second | 0.0494 ± 0.004 |
| Third | 0.0484 ± 0.005 |
| Fourth | 0.0480 ± 0.006 |
| Fifth | 0.0471 + 0.007 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karbasi, M.; Karimzadeh, F.; Raeissi, K.; Rtimi, S.; Kiwi, J.; Giannakis, S.; Pulgarin, C. Insights into the Photocatalytic Bacterial Inactivation by Flower-Like Bi2WO6 under Solar or Visible Light, Through in Situ Monitoring and Determination of Reactive Oxygen Species (ROS). Water 2020, 12, 1099. https://doi.org/10.3390/w12041099
Karbasi M, Karimzadeh F, Raeissi K, Rtimi S, Kiwi J, Giannakis S, Pulgarin C. Insights into the Photocatalytic Bacterial Inactivation by Flower-Like Bi2WO6 under Solar or Visible Light, Through in Situ Monitoring and Determination of Reactive Oxygen Species (ROS). Water. 2020; 12(4):1099. https://doi.org/10.3390/w12041099
Chicago/Turabian StyleKarbasi, Minoo, Fathallah Karimzadeh, Keyvan Raeissi, Sami Rtimi, John Kiwi, Stefanos Giannakis, and Cesar Pulgarin. 2020. "Insights into the Photocatalytic Bacterial Inactivation by Flower-Like Bi2WO6 under Solar or Visible Light, Through in Situ Monitoring and Determination of Reactive Oxygen Species (ROS)" Water 12, no. 4: 1099. https://doi.org/10.3390/w12041099






