Multiannual Trend of Micro-Pollutants in Sediments and Benthic Community Response in a Mediterranean Lagoon (Sacca di Goro, Italy)
Abstract
:1. Introduction
2. Materials and Methods
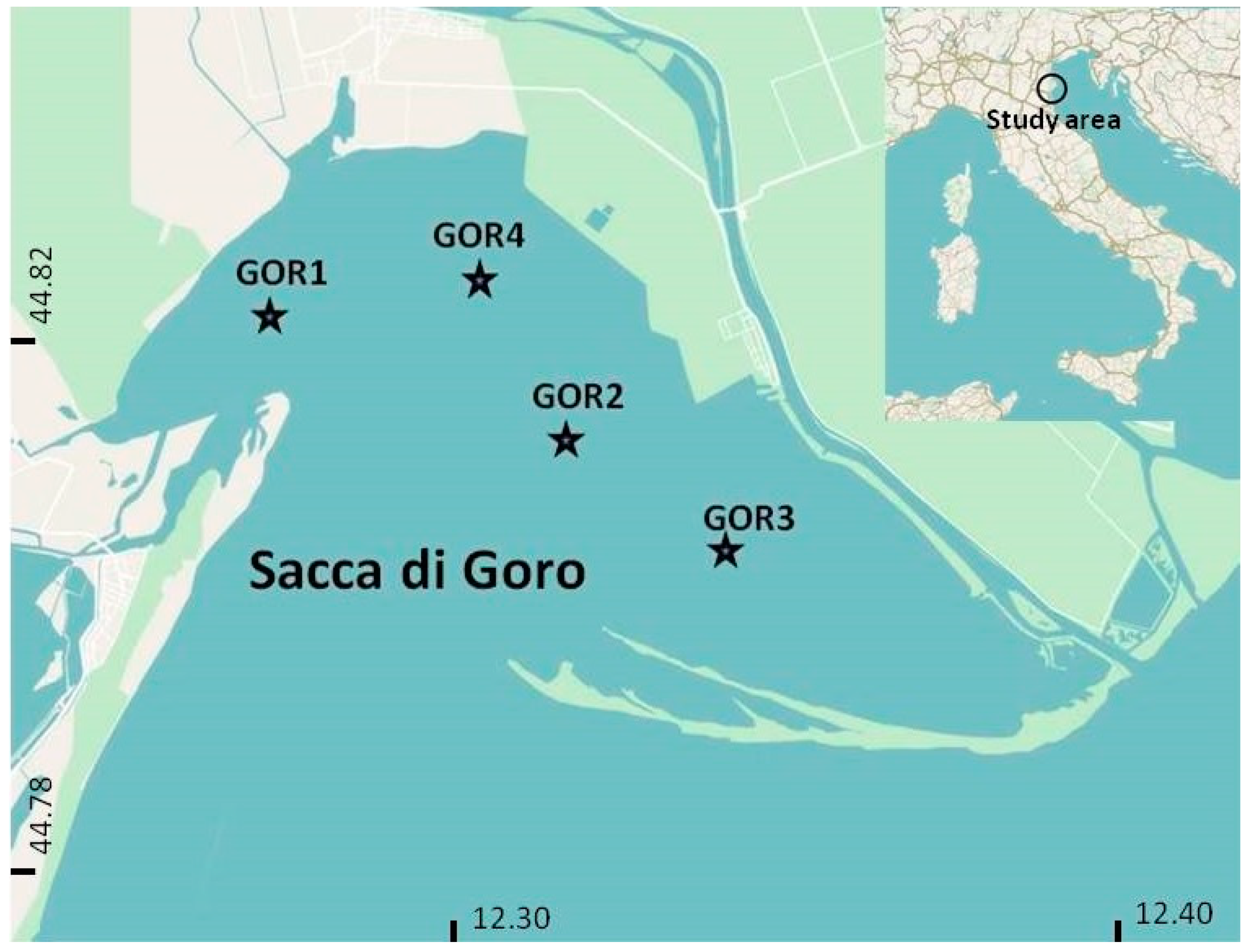
2.1. Study Area
2.2. Sample Collection and Chemical Analysis Methods
2.3. Sediment and Benthic Data Analysis
3. Results
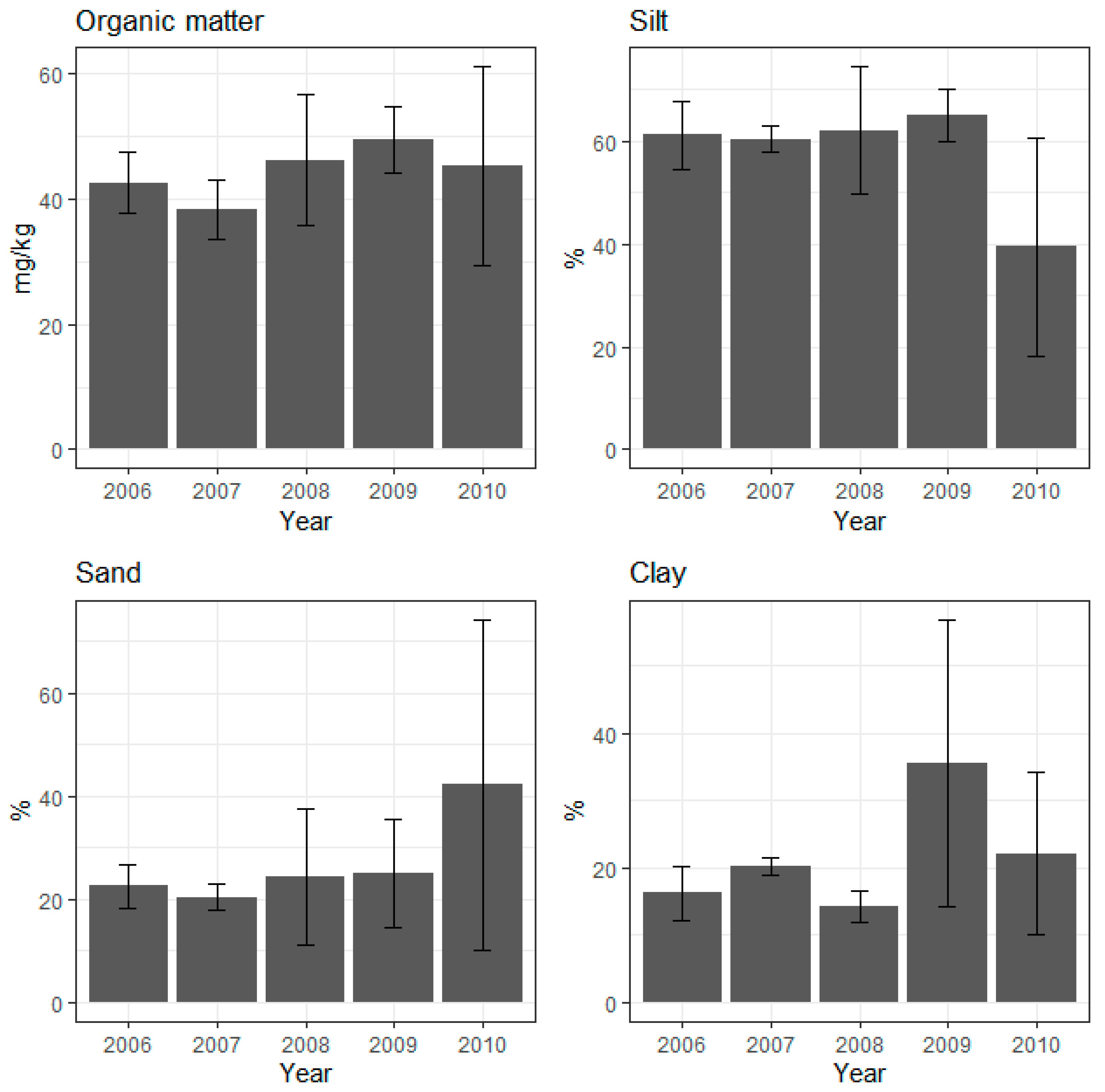
3.1. Sediment Characteristics
3.2. Trace Elements
3.3. Organochlorine Pesticides (OCPs)
3.4. Polychlorinated Dibenzodioxins (PCDDs) and Dibenzofurans (PCDFs)
3.5. Polycyclic Aromatic Hydrocarbons (PAHs)
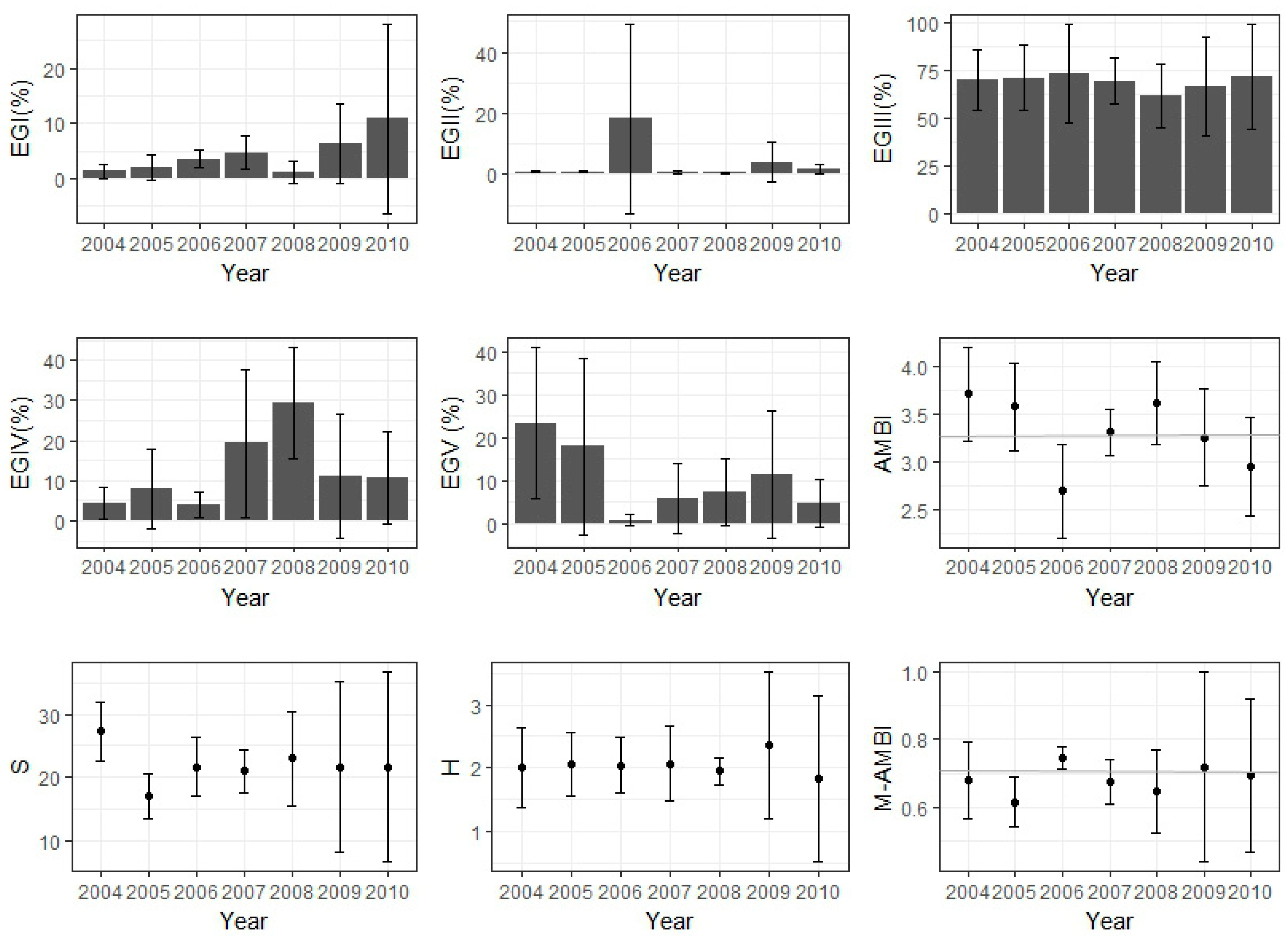
3.6. Ecological Status Based on Macrobenthic Community
3.7. Combined Trend of Abiotic and Biotic Variables
4. Discussion
4.1. Trace Elements Contamination
4.2. Organochlorine Pesticides Contamination
4.3. Polychlorinated Dibenzodioxins and Dibenzofurans Contamination
4.4. Polycyclic Aromatic Hydrocarbons Contamination
4.5. Ecological Status Based on Macrobenthic Community
4.6. Coupling Abiotic and Biotic Variables
Author Contributions
Funding
Conflicts of Interest
References
- Munari, C.; Mistri, M. Spatio-temporal pattern of community development in dredged material used for habitat enhancement: A study case in a brackish lagoon. Mar. Pollut. Bull. 2014, 89, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.; Reverdin, G.; Khodri, M.; Gastineau, G. A new record of Atlantic sea surface salinity from 1896 to 2013 reveals the signatures of climate variability and long-term trends. Geophys. Res. Lett. 2017, 44, 1866–1876. [Google Scholar] [CrossRef] [Green Version]
- European Community. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for community action in the field of water policy. Off. J. Eur. Communities 2000, 43, 1–72. [Google Scholar]
- Newton, A.; Icely, J.; Cristina, S.; Brito, A.; Cardoso, A.C.; Colijn, F. An overview of ecological status, vulnerability and future perspectives of European large shallow, semi-enclosed coastal systems, lagoons and transitional waters. Estuar. Coast. Shelf Sci. 2014, 140, 95–122. [Google Scholar] [CrossRef]
- Natali, C.; Fogli, R.; Bianchini, G.; Tassinari, R.; Tessari, U. Heavy Metals Backgrounds in Sediments From the Sacca di Goro (NE, Italy). EQA-Int. J. Environ. Qual. 2016, 20, 15–26. [Google Scholar]
- Barhoumi, B.; LeMenach, K.; Devier, M.-H.; Ameur, W.B.; Etcheber, H.; Budzinski, H. Polycyclic aromatic hydrocarbons (PAHs) in surface sediments from the Bizerte Lagoon, Tunisia: Levels, sources, and toxicological significance. Environ. Monit. Assess. 2014, 186, 2653–2669. [Google Scholar] [CrossRef] [PubMed]
- Borja, A.; Barbone, E.; Basset, A.; Borgersen, G.; Brkljacic, M.; Elliott, M.; Garmendia, J.M.; Marques, J.C.; Mazik, K.; Muxika, I.; et al. Response of single benthic metrics and multi-metric methods to anthropogenic pressure gradients, in five distinct European coastal and transitional ecosystems. Mar. Pollut. Bull. 2011, 62, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Carafa, R.; Marinov, D.; Dueri, S.; Wollgast, J.; Ligthart, J.; Canuti, E.; Viaroli, P.; Zaldívar, J.M. A 3D hydrodynamic fate and transport model for herbicides in Sacca di Goro coastal lagoon (Northern Adriatic). Mar. Pollut. Bull. 2006, 52, 1231–1248. [Google Scholar] [CrossRef]
- Migani, F.; Borghesi, F.; Dinelli, E. Geochemical characterization of surface sediments from the northern Adriatic wetlands around the Po river delta. Part I: Bulk composition and relation to local background. J Geochem Explor 2015, 156, 72–88. [Google Scholar] [CrossRef]
- Rainbow, P.S. Trace metal bioaccumulation: Models, metabolic availability and toxicity. Environ Intl 2007, 33, 576–582. [Google Scholar] [CrossRef]
- Overmans, S.; Nordborg, M.; Rua, R.D.; Brinkman, D.L.; Negri, A.P.; Agustí, S. Phototoxic effects of PAH and UVA exposure on molecular responses and developmental success in coral larvae. Aquat. Toxicol. 2018, 198, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Corbau, C.; Munari, C.; Mistri, M.; Lovo, S.; Simeoni, U. Application of the Principles of ICZM for Restoring the Goro Lagoon. Coast. Manage. 2016, 44, 350–365. [Google Scholar] [CrossRef]
- USEPA Method 6020B: Inductively Coupled Plasma-Mass Spectrometry; USEPA, United States Environmental Protection Agency: Washington, DC, USA, 2014.
- Anastassiades, M.; Lehotay, S.; Stajnbaher, D.; Schenck, F. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- USEPA. Method 8270D: Semivolatile organic compounds by Gas Chromatography/Mass Spectrometry (GC/MS); USEPA (United States Environmental Protection Agency): Washington, DC, USA, 2007. [Google Scholar]
- Sarkar, D. Lattice: Multivariate Data Visualization with R; Springer: New York, NY, USA, 2008. [Google Scholar]
- Dinelli, E.; Ghosh, A.; Rossi, V.; Vaiani, S.C. Multiproxy reconstruction of Late Pleistocene-Holocene environmental changes in coastal successions: Microfossil and geochemical evidences from the Po Plain (Northern Italy). Stratigraphy 2012, 9, 153–167. [Google Scholar]
- Guerra, R.; Pasteris, A.; Lee, S.; Park, N.; Ok, G. Spatial patterns of metals, PCDDs/Fs, PCBs, PBDEs and chemical status of sediments from a coastal lagoon (Pialassa Baiona, NW Adriatic, Italy). Mar. Pollut. Bull. 2014, 89, 407–416. [Google Scholar] [CrossRef] [PubMed]
- 18 Long, E.R.; Macdonald, D.D.; Smith, S.L.; Calder, F.D. Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ. Manage. 1995, 19, 81–97. [Google Scholar] [CrossRef]
- Myles, H.; Wolfe, D.A. Nonparametric Statistical Methods; John Wiley & Sons: New York, NY, USA, 1973; pp. 139–146. [Google Scholar]
- Nemenyi, P. Distribution-free Multiple Comparisons. Biometrics 1963, 18, 263. [Google Scholar]
- Borja, A.; Franco, J.; Pérez, V. A marine biotic index to establish the ecological quality of soft-bottom benthos within European estuarine and coastal environments. Mar. Pollut. Bull. 2000, 40, 1100–1114. [Google Scholar] [CrossRef]
- Muxika, I.; Borja, A.; Bald, J. Using historical data, expert judgement and multivariate analysis in assessing reference conditions and benthic ecological status, according to the European Water Framework Directive. Mar. Pollut. Bull. 2007, 55, 16–29. [Google Scholar] [CrossRef]
- Borja, A.; Marín, S.; Muxika, I.; Pino, L.; Rodríguez, J. Is there a possibility of ranking benthic quality assessment indices to select the most responsive to different human pressures? Mar. Pollut. Bull. 2015, 97, 85–94. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Legendre, P.; Anderson, M.J. Distance-based redundancy analysis: Testing multispecies responses in multifactorial ecological experiments. Ecol. Monogr. 1999, 69, 1–24. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006. [Google Scholar]
- RDevelopmentCoreTeam “R: A Language and Environment for Statistical Computing”; R Foundation for Statistical Computing: Vienna, Austria, 2008; Available online: https://www.R-project.org/ (accessed on 12 March 2020).
- Amorosi, A.; Sammartino, I. Influence of sediment provenance on background values of potentially toxic metals from near–surface sediments of Po coastal plain (Italy). Int. J. Earth Sci. 2007, 96, 389–396. [Google Scholar] [CrossRef]
- Pitacco, V.; Mistri, M.; Ferrari, C.R.; Munari, C. Heavy metals, OCPs, PAHs, and PCDD/Fs contamination in surface sediments of a coastal lagoon (Valli di Comacchio, NW Adriatic, Italy): Long term trend (2002−2013) and effect on benthic community. Mar. Pollut. Bull. 2018, 135, 1221–1229. [Google Scholar] [CrossRef]
- Arienzo, M.; Masuccio, A.; Ferrara, L. Evaluation of sediment contamination by heavy metals, organochlorinated pesticides, and polycyclic aromatic hydrocarbons in the Berre coastal lagoon (southeast France). Arch. Environ. Contam. Toxicol. 2013, 65, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Casatta, N.; Mascolo, G.; Roscioli, C.; Viganò, L. Tracing endocrine disrupting chemicals in a coastal lagoon (Sacca di Goro, Italy): Sediment contamination and bioaccumulation in Manila clams. Sci. Total Environ. 2015, 511, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Quensen, J.F.; Mueller, S.A.; Jain, M.K.; Tiedje, J.M. Reductive dechlorination of DDE to DDMU in marine sediment microcosms. Science 1998, 280, 722–724. [Google Scholar] [CrossRef] [Green Version]
- Hoke, R.; Cotter, A.; Goldenstein, T.; Kosian, P.; Ankley, G.; Phipps, G. Evaluation of equilibrium partitioning theory for predicting acute toxicity of field-collected sediments contaminated with DDT, DDE and DDD to the amphipod Hyalella azteca. Environ. Toxicol. Chem. 1994, 13, 157–166. [Google Scholar] [CrossRef]
- Swartz, R.C.; Cole, F.A.; Lamberson, J.O.; Ferraro, S.P.; Schults, D.W.; Deben, W.A.; Lee Ii, H.; Ozretich, R.J. Sediment toxicity, contamination and amphipod abundance at a DDT-and dieldrin-contaminated site in San Francisco Bay. Environ. Toxicol. Chem. 1994, 13, 949–962. [Google Scholar] [CrossRef]
- Bellucci, L.; Frignani, M.; Raccanelli, S.; Carraro, C. Polychlorinated dibenzo-p-dioxins and dibenzofurans in surficial sediments of the Venice Lagoon (Italy). Mar. Pollut. Bull. 2000, 40, 65–76. [Google Scholar] [CrossRef]
- Gómez-Lavín, S.; Gorri, D.; Irabien, Á. (Assessment of PCDD/Fs and PCBs in sediments from the Spanish northern Atlantic coast. Water Air Soil Pollut. 2011, 221, 287–299. [Google Scholar] [CrossRef]
- Danis, B.; Debacker, V.; Miranda, C.T.; Dubois, P. Levels and effects of PCDD/Fs and co-PCBs in sediments, mussels, and sea stars of the intertidal zone in the southern North Sea and the English Channel. Ecotoxicol. Environ. Saf. 2006, 65, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Dueri, S.; Marinov, D.; Fiandrino, A.; Tronczyński, J.; Zaldívar, J.M. Implementation of a 3D coupled hydrodynamic and contaminant fate model for PCDD/Fs in Thau Lagoon (France): The importance of atmospheric sources of contamination. Int. J. Env. Res. Public Health 2010, 7, 1467–1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Specchiulli, A.; Renzi, M.; Scirocco, T.; Cilenti, L.; Florio, M.; Breber, P. Comparative study based on sediment characteristics and macrobenthic communities in two Italian lagoons. Environ. Monit. Assess. 2010, 160, 237–256. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, D.; Baravelli, V.; Giannotti, K.; Donnini, F.; Fabbri, E. Bioaccumulation of cyclopenta [cd] pyrene and benzo[ghi]fluoranthene by mussels transplanted in a coastal lagoon. Chemosphere 2006, 64, 1083–1092. [Google Scholar] [CrossRef]
- Acquavita, A.; Falomo, J.; Predonzani, S.; Tamberlich, F.; Bettoso, N.; Mattassi, G. The PAH level, distribution and composition in surface sediments from a Mediterranean Lagoon: The Marano and Grado Lagoon (Northern Adriatic Sea, Italy). Mar. Pollut. Bull. 2014, 81, 234–241. [Google Scholar] [CrossRef]
- Giacalone, A.; Gianguzza, A.; Mannino, M.R.; Orecchio, S.; Piazzese, D. Polycyclic aromatic hydrocarbons in sediments of marine coastal lagoons in Messina, Italy: Extraction and GC/MS analysis, distribution and sources. Polycyc Aromat. Compd. 2004, 24, 135–149. [Google Scholar] [CrossRef]
- Culotta, L.; De Stefano, C.; Gianguzza, A.; Mannino, M.R.; Orecchio, S. The PAH composition of surface sediments from Stagnone coastal lagoon, Marsala (Italy). Mar. Chem. 2006, 99, 117–127. [Google Scholar] [CrossRef]
- Countway, R.E.; Dickhut, R.M.; Canuel, E.A. Polycyclic aromatic hydrocarbon (PAH) distributions and associations with organic matter in surface waters of the York River, VA Estuary. Org. Geochem. 2003, 34, 209–224. [Google Scholar] [CrossRef]
- Pearson, T.; Rosenberg, R. Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanogr. Mar. Biol. Ann. Rev. 1978, 16, 229–311. [Google Scholar]
- Borja, A.; Muxika, I. Guidelines for the use of AMBI (AZTI’s Marine Biotic Index) in the assessment of the benthic ecological quality. Mar. Pollut. Bull. 2005, 50, 787–789. [Google Scholar] [CrossRef]
- Elliott, M.; Quintino, V. The estuarine quality paradox, environmental homeostasis and the difficulty of detecting anthropogenic stress in naturally stressed areas. Mar. Pollut. Bull. 2007, 54, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Prato, S.; La Valle, P.; De Luca, E.; Lattanzi, L.; Migliore, G.; Morgana, J.G.; Munari, C.; Nicoletti, L.; Izzo, G.; Mistri, M. The "one-out, all-out" principle entails the risk of imposing unnecessary restoration costs: A study case in two Mediterranean coastal lakes. Mar. Pollut. Bull. 2014, 80, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Pitacco, V.; Reizopoulou, S.; Sfriso, A.; Sfriso, A.A.; Mistri, M.; Munari, C. The difficulty of disentangling natural from anthropic forcing factors makes the evaluation of ecological quality problematic: A case study from Adriatic lagoons. Mar. Environ. Res. 2019, 150, 104756. [Google Scholar] [CrossRef] [PubMed]
- Canu, D.M.; Solidoro, C.; Umgiesser, G.; Cucco, A.; Ferrarin, C. Assessing confinement in coastal lagoons. Mar. Pollut. Bull. 2012, 64, 2391–2398. [Google Scholar] [CrossRef] [PubMed]
- Jorcin, A. Temporal and spatial variability in the macrozoobenthic community along a salinity gradient in the Castillos Lagoon (Uruguay). Archiv. Hydrobiol. 1999, 146, 369–384. [Google Scholar] [CrossRef]
- Telesh, I.V.; Khlebovich, V.V. Principal processes within the estuarine salinity gradient: A review. Mar. Pollut. Bull. 2010, 61, 149–155. [Google Scholar] [CrossRef]
- Blackmore, G.; Wang, W.X. Uptake and efflux of Cd and Zn by the green mussel Perna viridis after metal preexposure. Environ. Sci. Technol. 2002, 36, 989–995. [Google Scholar] [CrossRef]
- Relyea, R.A. A cocktail of contaminants: How mixtures of pesticides at low concentrations affect aquatic communities. Oecologia 2008, 159, 363–376. [Google Scholar] [CrossRef]
- Li, M.; Yang, W.; Sun, T.; Jin, Y. Potential ecological risk of heavy metal contamination in sediments and macrobenthos in coastal wetlands induced by freshwater releases: A case study in the Yellow River Delta, China. Mar. Pollut. Bull. 2016, 103, 227–239. [Google Scholar] [CrossRef] [Green Version]
- Ventura-Lima, J.; Sandrini, J.Z.; Cravo, M.F.; Piedras, F.R.; Moraes, T.B.; Fattorini, D. Toxicological responses in Laeonereis acuta (Annelida, Polychaeta) after arsenic exposure. Environ. Int. 2007, 33, 559–564. [Google Scholar] [CrossRef]
- Depledge, M.H.; Galloway, T.S. Healthy animals, healthy ecosystems. Front Ecol Environ 2005, 3, 251–258. [Google Scholar] [CrossRef]
- Yang, W.; Li, X.; Pei, J.; Sun, T.; Shao, D.; Bai, J. Bioavailability of trace metals in sediments of a recovering freshwater coastal wetland in China’s Yellow River Delta, and risk assessment for the macrobenthic community. Chemosphere 2017, 189, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Blackmore, G.; Wang, W.X. Effects of aqueous and dietary preexposure and resulting body burden on silver biokinetics in the green mussel Perna viridis. Environ. Sci. Technol. 2003, 37, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Paul-Pont, I.; De Montaudouin, X.; Gonzalez, P.; Soudant, P.; Baudrimont, M. How life history contributes to stress response in the Manila clam Ruditapes philippinarum. Environ. Sci. Pollut. Res. 2010, 17, 987–998. [Google Scholar] [CrossRef]
- Boisson, F.; Hartl, M.G.; Fowler, S.W.; Amiard-Triquet, C. Influence of chronic exposure to silver and mercury in the field on the bioaccumulation potential of the bivalve Macoma balthica. Mar. Environ. Res. 1998, 45, 325–340. [Google Scholar] [CrossRef]
- Ross, K.; Cooper, N.; Bidwell, J.R.; Elder, J. Genetic diversity and metal tolerance of two marine species: A comparison between populations from contaminated and reference sites. Mar. Pollut Bull. 2002, 44, 671–679. [Google Scholar] [CrossRef]


| Ni (mg/kg) | Cr (mg/kg) | Hg (mg/kg) | Pb (mg/kg) | Cd (mg/kg) | As (mg/kg) | |
|---|---|---|---|---|---|---|
| 2004 | 95.5 ± 7.8 | 80.6 ± 8.9 | 0.0 ± 0.0 | 29.6 ± 7.2 | 0.4 ± 0.1 | 10.0 ± 3.3 |
| 2005 | 83.9 ± 2.4 | 77.4 ± 9.5 | 0.1 ± 0.2 | 28.8 ± 10.1 | 0.3 ± 0.2 | 9.3 ± 1.2 |
| 2006 | 110.3 ± 14.2 | 149.9 ± 17.7 | 0.2 ± 0.1 | 36.0 ± 14.6 | 0.4 ± 0.2 | 9.6 ± 3.5 |
| 2007 | 113.9 ± 7.2 | 157.5 ± 8.3 | 0.1 ± 0.1 | 43.5 ± 13.9 | 0.5 ± 0.2 | 9.7 ± 3.6 |
| 2008 | 93.4 ± 7.5 | 143.6 ± 13.1 | 0.3 ± 0.1 | 33.6 ± 8.4 | 0.4 ± 0.1 | 6.7 ± 1.5 |
| 2009 | 100.1 ± 16.0 | 129.6 ± 28.4 | 0.2 ± 0.1 | 32.0 ± 10.2 | 0.4 ± 0.1 | 8.3 ± 2.8 |
| 2010 | 106.7 ± 10.3 | 137.2 ± 14.6 | 0.1 ± 0.1 | 28.6 ± 13.9 | 0.4 ± 0.1 | 9.2 ± 3.5 |
| Max | 113.9 ± 7.2 | 157.5 ± 8.3 | 0.0 ± 0.0 | 43.5 ± 13.9 | 0.5 ± 0.2 | 10.0 ± 3.3 |
| Min | 83.9 ± 2.4 | 77.4 ± 9.6 | 0.3 ± 0.1 | 28.6 ± 13.9 | 0.3 ± 0.2 | 6.7 ± 1.5 |
| EQS (mg/kg) | 30 | 50 | 0.3 | 30 | 0.3 | 12 |
| >EQS | 100% | 100% | 14% | 57% | 100% | 0% |
| Ref (mg/kg) | 97 | 158 | 0.12 | 24 | - | - |
| >Ref | 71% | 0% | 43% | 100% | - | - |
| ERL (mg/kg) | 20.9 | 81 | 0.15 | 46.7 | 1.2 | 8.2 |
| >ERL | 1 | 0.71 | 0.43 | 0 | 0 | 0.86 |
| ERM (mg/kg) | 51.6 | 370 | 0.71 | 218 | 9.6 | 70 |
| >ERM | 100% | 0% | 0% | 0% | 0% | 0% |
| Hexachlorobenzene | DDE | DDD | DDT | Simazine | Terbuthylazine | Alaclor | ΣOCPs | |
|---|---|---|---|---|---|---|---|---|
| 2004 | <0.1 | 0.06 ± 0.13 | 0.03 ± 0.05 | <0.1 | <0.1 | <0.1 | <0.1 | 0.09 ± 0.18 |
| 2005 | 0.15 ± 0.30 | 1.04 ± 1.29 | 0.40 ± 0.80 | <0.1 | <0.1 | <0.1 | <0.1 | 1.59 ± 1.88 |
| 2006 | <0.1 | 1.41 ± 1.21 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | 1.41 ± 1.21 |
| 2007 | 0.09 ± 0.07 | 1.91 ± 1.04 | 0.28 ± 0.49 | 0.08 ± 0.23 | 0.15 ± 0.42 | 1.25 ± 1.67 | 0.38 ± 0.60 | 4.14 ± 2.44 |
| 2008 | <0.1 | 1.09 ± 0.44 | 1.35 ± 2.20 | <0.1 | <0.1 | <0.1 | <0.1 | 2.44 ± 2.19 |
| 2009 | <0.1 | 1.51 ± 0.75 | 0.35 ± 0.60 | <0.1 | <0.1 | <0.1 | <0.1 | 1.86 ± 1.29 |
| 2010 | <0.1 | 2.47 ± 1.34 | 0.69 ± 0.88 | <0.1 | <0.1 | <0.1 | <0.1 | 3.16 ± 2.07 |
| EQS | 0.4 | 1.8 | 0.8 | 1 | ||||
| ERL | 2.2 | 1.58 | ||||||
| ERM | 27 | 46.1 |
| H7CDD | O8CDD | H7CDF | O8CDF | ΣPCDD/Fs | ΣTEQ | ||
|---|---|---|---|---|---|---|---|
| 2007 | Mean ± SD | 19.58 ± 22.60 | 123.91 ± 91.51 | 13.30 ± 7.53 | 21.67 ± 16.71 | 178.46 | 0.47 |
| TEQ | 0.20 | 0.12 | 0.13 | 0.02 | |||
| 2008 | Mean ± SD | 18.83 ± 16.89 | 89.14 ± 82.67 | 6.66 ± 4.78 | 22.14 ± 22.92 | 136.76 | 0.37 |
| TEQ | 0.19 | 0.09 | 0.07 | 0.02 | |||
| 2009 | Mean ± SD | 12.74 ± 9.41 | 88.40 ± 58.45 | 8.59 ± 6.43 | 18.41 ± 13.68 | 128.15 | 0.32 |
| TEQ | 0.13 | 0.09 | 0.09 | 0.02 | |||
| 2010 | Mean ± SD | 17.23 ± 11.10 | 94.18 ± 72.88 | 8.58 ± 4.79 | 21.15 ± 10.62 | 141.13 | 0.37 |
| TEQ | 0.17 | 0.09 | 0.09 | 0.02 |
| Ni | Cr | As | Pb | PCDFs | OM | DDE | Clay | M-AMBI | AMBI | H | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ni | |||||||||||
| Cr | 0.56 | ||||||||||
| As | 0.69 | NS | |||||||||
| Hg | −0.54 | NS | NS | ||||||||
| Pb | NS | 0.62 | NS | ||||||||
| PCDFs | NS | NS | NS | 0.7 | |||||||
| PCDDs | NS | NS | NS | 0.9 | 0.89 | ||||||
| OM | −0.61 | −0.58 | NS | NS | NS | ||||||
| DDE | 0.69 | NS | 0.89 | NS | NS | −0.54 | |||||
| Sand | NS | NS | NS | NS | NS | 0.55 | NS | ||||
| Silt | 0.55 | NS | NS | NS | NS | NS | NS | ||||
| AMBI | NS | NS | NS | NS | NS | NS | NS | −0.69 | −0.59 | ||
| H | NS | NS | NS | NS | NS | NS | NS | NS | 0.89 | −0.64 | |
| S | NS | NS | −0.67 | NS | NS | NS | −0.62 | NS | 0.71 | NS | NS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitacco, V.; Mistri, M.; Ferrari, C.R.; Sfriso, A.; Sfriso, A.A.; Munari, C. Multiannual Trend of Micro-Pollutants in Sediments and Benthic Community Response in a Mediterranean Lagoon (Sacca di Goro, Italy). Water 2020, 12, 1074. https://doi.org/10.3390/w12041074
Pitacco V, Mistri M, Ferrari CR, Sfriso A, Sfriso AA, Munari C. Multiannual Trend of Micro-Pollutants in Sediments and Benthic Community Response in a Mediterranean Lagoon (Sacca di Goro, Italy). Water. 2020; 12(4):1074. https://doi.org/10.3390/w12041074
Chicago/Turabian StylePitacco, Valentina, Michele Mistri, Carla Rita Ferrari, Adriano Sfriso, Andrea Augusto Sfriso, and Cristina Munari. 2020. "Multiannual Trend of Micro-Pollutants in Sediments and Benthic Community Response in a Mediterranean Lagoon (Sacca di Goro, Italy)" Water 12, no. 4: 1074. https://doi.org/10.3390/w12041074






