Short-Term Effects of Salt Stress on the Amino Acids of Phragmites australis Root Exudates in Constructed Wetlands
Abstract
1. Introduction
2. Material and Methods
2.1. Collection of Plant Samples
2.2. Salt Stress and Sample Pretreatments
2.3. Analyses
2.4. Statistical Analyses
3. Results and Discussion
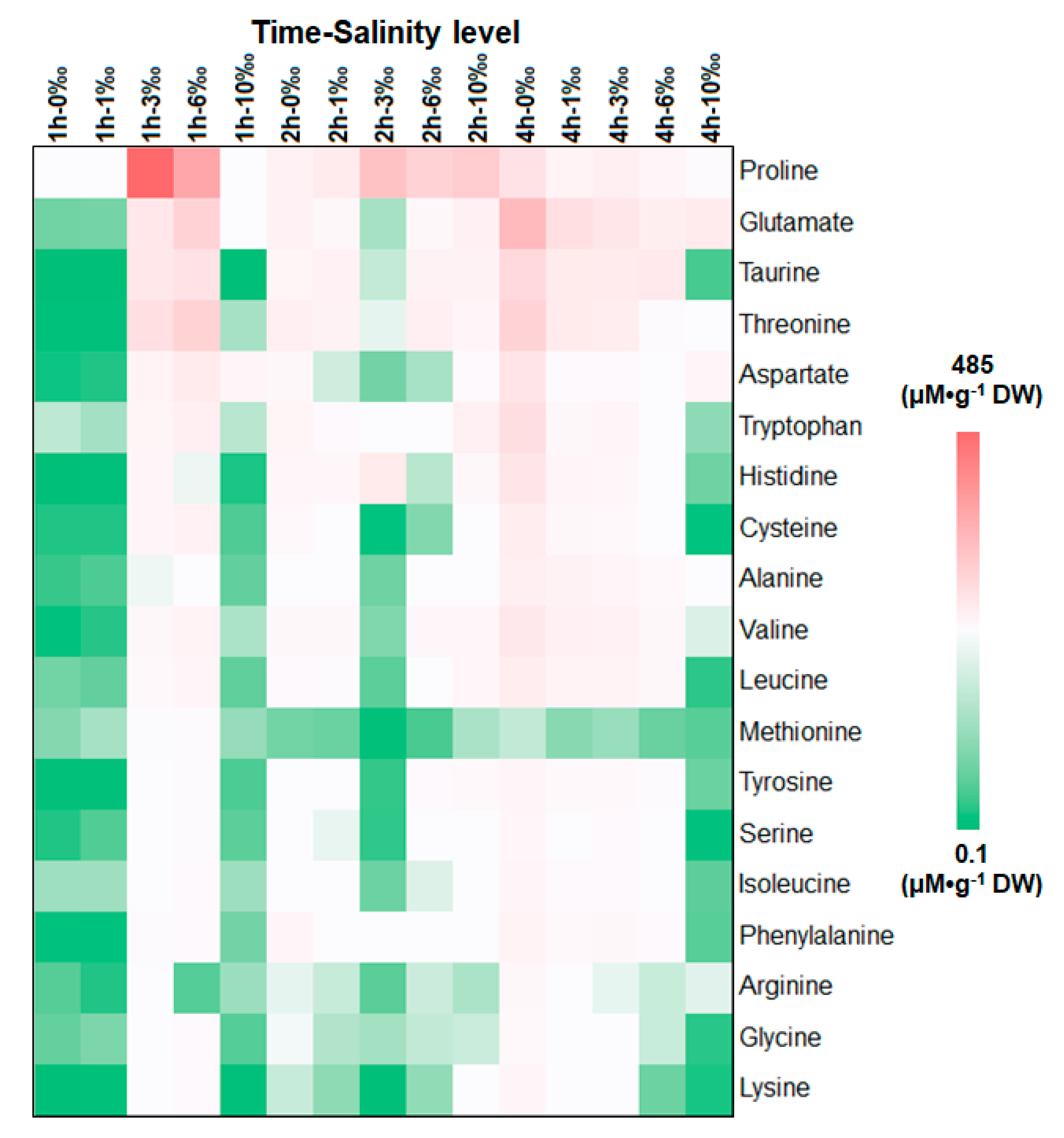
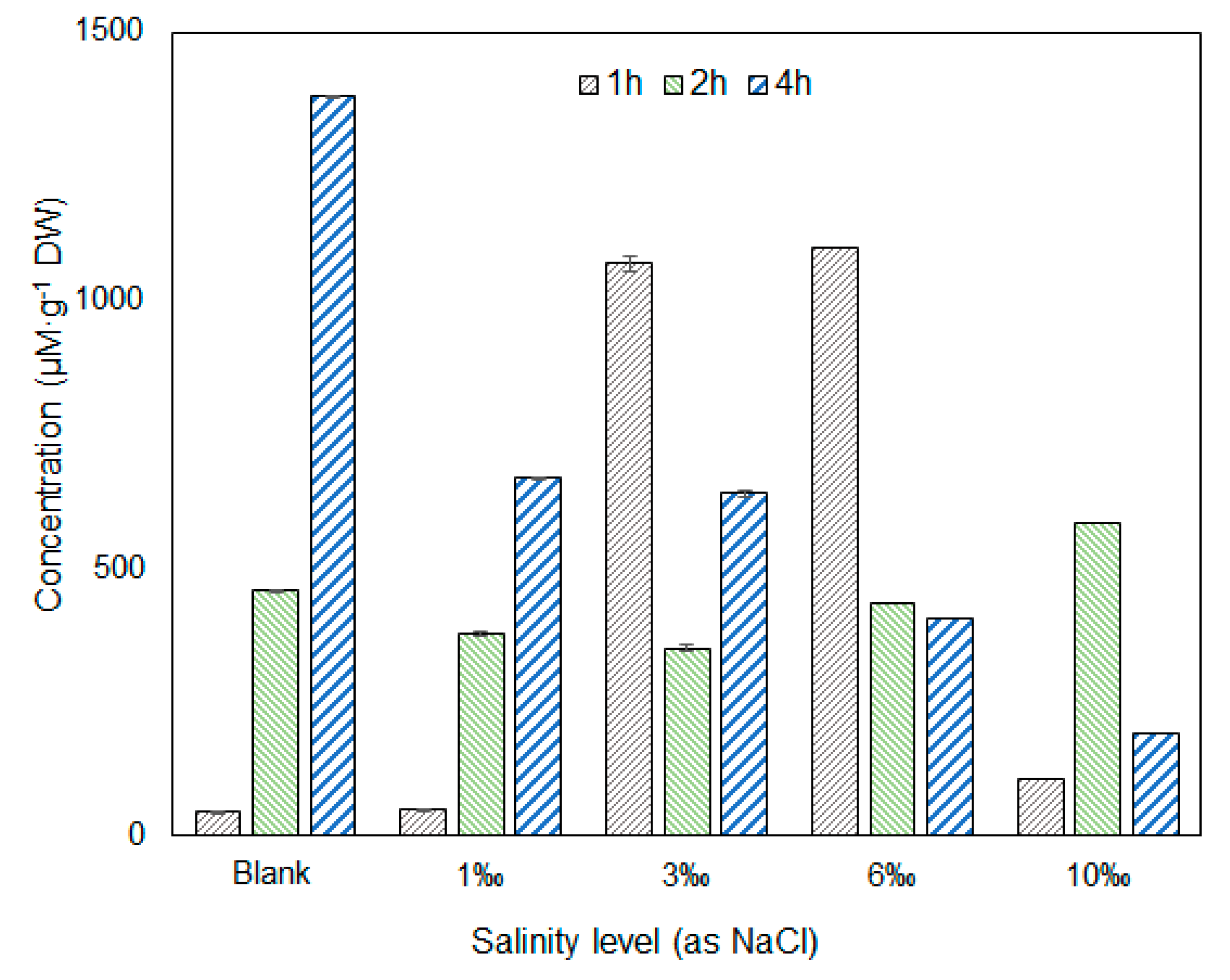
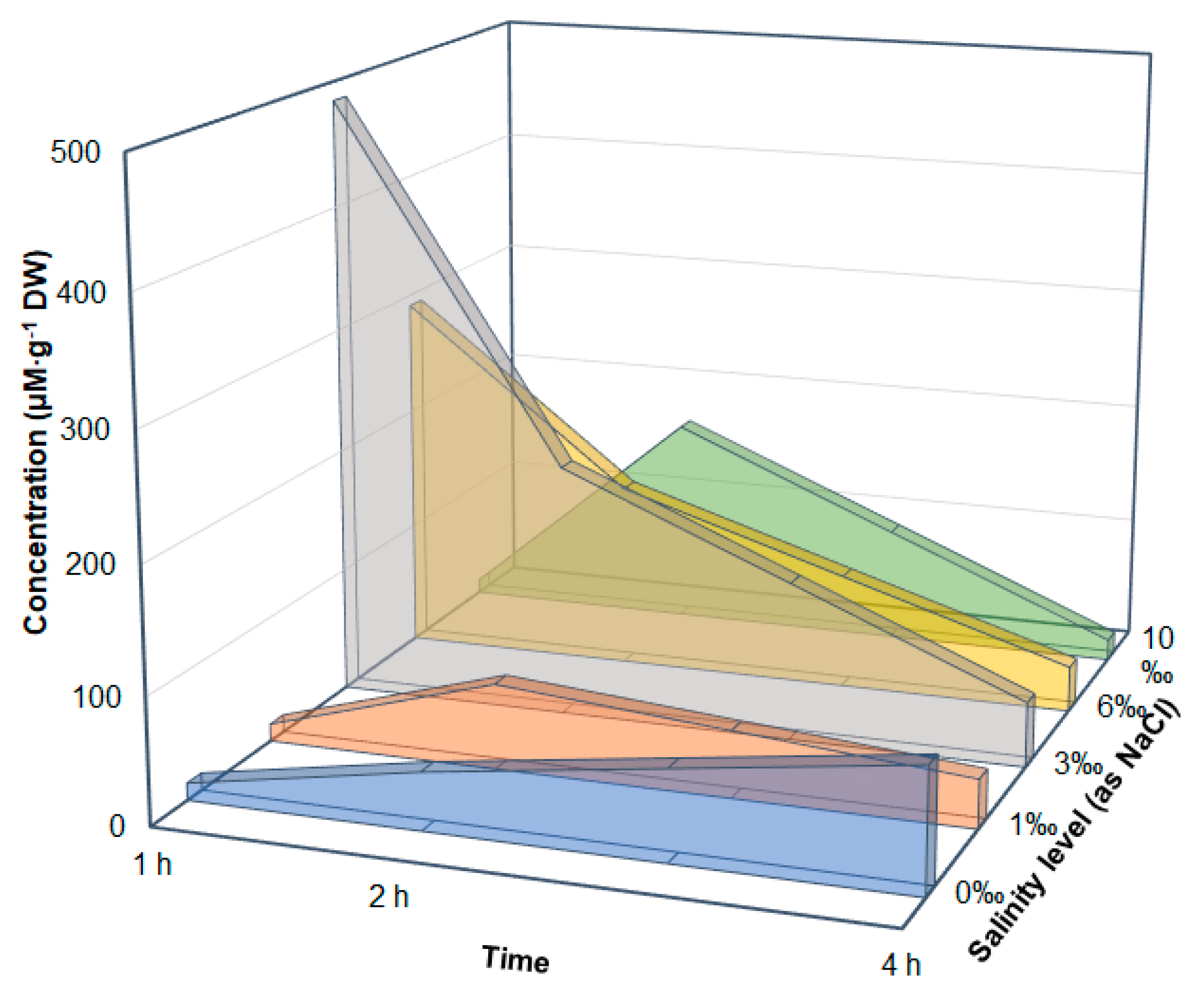
3.1. Variation in Amino Acids under Different Salt Stress Conditions
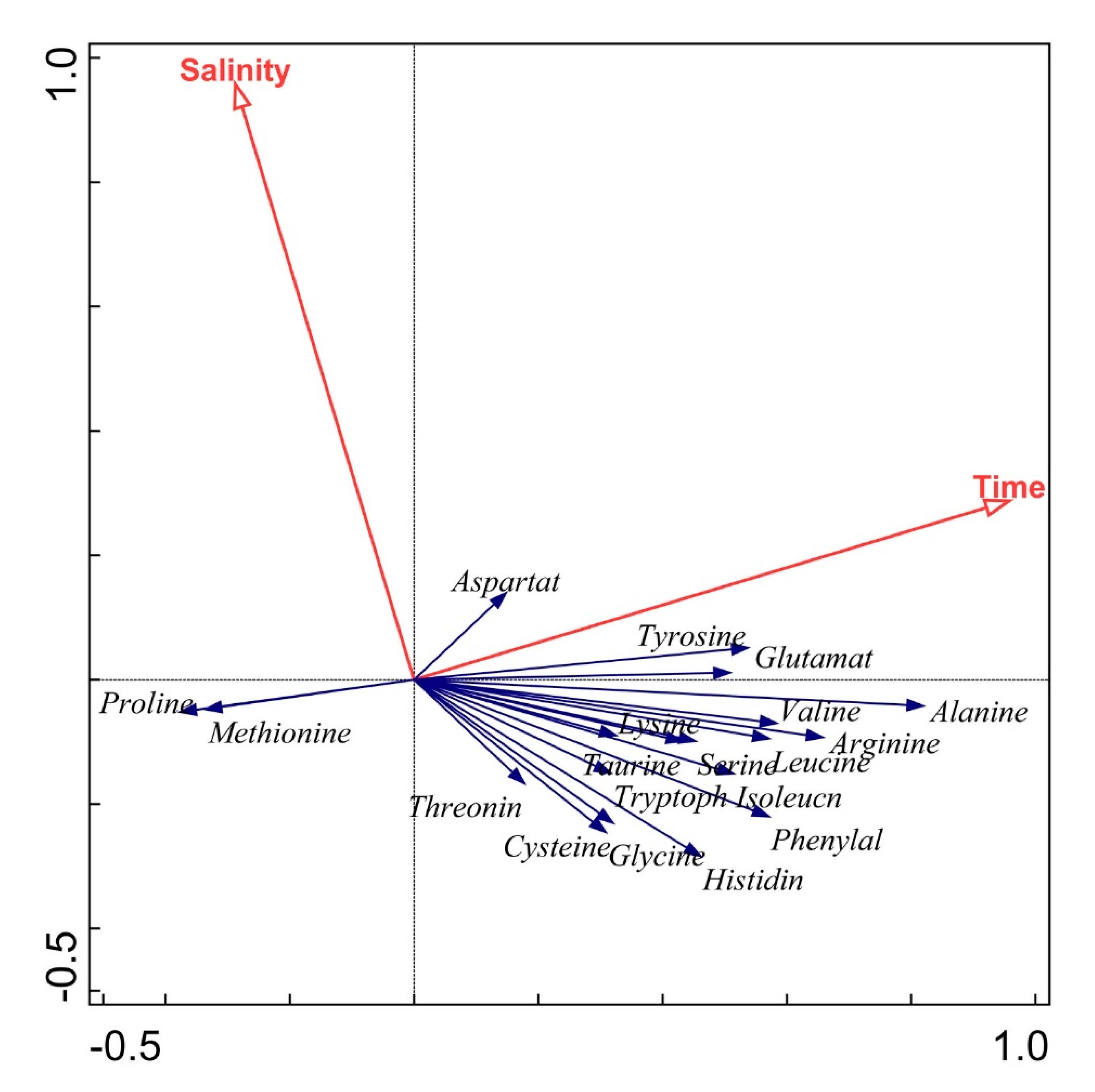
3.2. Results of the Correlation and Redundancy Analyses
3.3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, H.; Yi, J.; Zhang, J.; Zhao, Y.; Si, B.; Hill, R.L.; Cui, L.; Liu, X. Modeling of soil water and salt dynamics and its effects on root water uptake in Heihe Arid Wetland, Gansu, China. Water 2015, 7, 2382–2401. [Google Scholar] [CrossRef]
- Zhu, J.-K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Shabala, S.; Wu, H.; Bose, J. Salt stress sensing and early signalling events in plant roots: Current knowledge and hypothesis. Plant Sci. 2015, 241, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Bôto, M.; Almeida, C.M.R.; Mucha, A.P. Potential of constructed wetlands for removal of antibiotics from saline aquaculture effluents. Water 2016, 8, 465. [Google Scholar] [CrossRef]
- Gorai, M.; Ennajeh, M.; Khemira, H.; Neffati, M. Combined effect of NaCl-salinity and hypoxia on growth, photosynthesis, water relations and solute accumulation in Phragmites australis plants. Flora Morphol. Distrib. Funct. Ecol. Plants 2010, 205, 462–470. [Google Scholar] [CrossRef]
- Srivastava, J.; Kalra, S.J.S.; Naraian, R. Environmental perspectives of Phragmites australis (Cav.) Trin. Ex. Steudel. Appl. Water Sci. 2014, 4, 193–202. [Google Scholar] [CrossRef]
- Wang, R.; Korboulewsky, N.; Prudent, P.; Domeizel, M.; Rolando, C.; Bonin, G. Feasibility of using an organic substrate in a wetland system treating sewage sludge: Impact of plant species. Bioresour. Technol. 2010, 101, 51–57. [Google Scholar] [CrossRef]
- Srivastava, J.; Gupta, A.; Chandra, H. Managing water quality with aquatic macrophytes. Rev. Environ. Sci. Bio/Technol. 2008, 7, 255–266. [Google Scholar] [CrossRef]
- Vymazal, J. Emergent plants used in free water surface constructed wetlands: A review. Ecol. Eng. 2013, 61, 582–592. [Google Scholar] [CrossRef]
- Choi, W.-J.; Ro, H.-M.; Chang, S.X. Carbon isotope composition of Phragmites australis in a constructed saline wetland. Aquat. Bot. 2005, 82, 27–38. [Google Scholar] [CrossRef]
- Liang, Y.; Zhu, H.; Bañuelos, G.; Yan, B.; Zhou, Q.; Yu, X.; Cheng, X. Constructed wetlands for saline wastewater treatment: A review. Ecol. Eng. 2017, 98, 275–285. [Google Scholar] [CrossRef]
- Hootsmans, M.J.M.; Wiegman, F. Four helophyte species growing under salt stress: Their salt of life? Aquat. Bot. 1998, 62, 81–94. [Google Scholar] [CrossRef]
- Wallace, A.; Knight, G.E.; Cowen, T.; Burnstock, G. Changes in purinergic signalling in developing and ageing rat tail artery: Importance for temperature control. Neuropharmacology 2006, 50, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Gorai, M.; Vadel, A.M.; Neffati, M.; Khemira, H. The effect of sodium chloride salinity on the growth, water status and ion content of Phragmites communis Trin. Pak. J. Biol. Sci. 2007, 10, 2225–2230. [Google Scholar] [CrossRef][Green Version]
- Mauchamp, A.; Mésleard, F. Salt tolerance in Phragmites australis populations from coastal Mediterranean marshes. Aquat. Bot. 2001, 70, 39–52. [Google Scholar] [CrossRef]
- Fageria, N.K.; Gheyi, H.R.; Moreira, A. Nutrient bioavailability in salt affected soils. J. Plant Nutr. 2011, 34, 945–962. [Google Scholar] [CrossRef]
- Flowers, T.J.; Troke, P.F.; Yeo, A.R. The mechanism of salt tolerance in halophytes. Annu. Rev. Plant Physiol. 1977, 28, 89–121. [Google Scholar] [CrossRef]
- Dakora, F.D.; Phillips, D.A. Root exudates as mediators of mineral acquisition in low-nutrient environments. In Food Security in Nutrient-Stressed Environments: Exploiting Plants’ Genetic Capabilities; Adu-Gyamfi, J.J., Ed.; Springer: Dordrecht, The Netherlands, 2002; pp. 201–213. [Google Scholar]
- Bertin, C.; Yang, X.; Weston, L.A. The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 2003, 256, 67–83. [Google Scholar] [CrossRef]
- Carvalhais, L.C.; Dennis, P.G.; Fedoseyenko, D.; Hajirezaei, M.R.; Borriss, R.; von Wirén, N. Root exudation of sugars, amino acids, and organic acids by maize as affected by nitrogen, phosphorus, potassium, and iron deficiency. J. Plant Nutr. Soil Sci. 2011, 174, 3–11. [Google Scholar] [CrossRef]
- Maren, S.; Phan, K.L.; Liberzon, I. The contextual brain: Implications for fear conditioning, extinction and psychopathology. Nat. Rev. Neurosci. 2013, 14, 417–428. [Google Scholar] [CrossRef]
- Meharg, A.A.; Cairney, J.W.G. Ectomycorrhizas—Extending the capabilities of rhizosphere remediation? Soil Biol. Biochem. 2000, 32, 1475–1484. [Google Scholar] [CrossRef]
- Umemiya, Y.; Furuya, S. The influence of chemical. Forms on foliar-applied nitrogen absorption for peach trees. Acta Hortic. 2002, 594, 97–103. [Google Scholar]
- Fuad Mondal, M.; Asaduzzaman, M.; Kobayashi, Y.; Ban, T.; Asao, T. Recovery from autotoxicity in strawberry by supplementation of amino acids. Sci. Hortic. 2013, 164, 137–144. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Badri, D.V.; Bakker, M.G.; Sugiyama, A.; Manter, D.K.; Vivanco, J.M. Root exudation of phytochemicals in arabidopsis follows specific patterns that are developmentally programmed and correlate with soil microbial functions. PLoS ONE 2013, 8, e55731. [Google Scholar] [CrossRef]
- Kraffczyk, I.; Trolldenier, G.; Beringer, H. Soluble root exudates of maize: Influence of potassium supply and rhizosphere microorganisms. Soil Biol. Biochem. 1984, 16, 315–322. [Google Scholar] [CrossRef]
- Tegeder, M.; Rentsch, D. Uptake and partitioning of amino acids and peptides. Mol. Plant 2010, 3, 997–1011. [Google Scholar] [CrossRef]
- Tegeder, M.; Ward, J.M. Molecular evolution of plant AAP and LHT amino acid transporters. Front. Plant Sci. 2012, 3, 21. [Google Scholar] [CrossRef]
- Tegeder, M. Transporters for amino acids in plant cells: Some functions and many unknowns. Curr. Opin. Plant Biol. 2012, 15, 315–321. [Google Scholar] [CrossRef]
- Pavlikova, D.; Zemanova, V.; Prochazkova, D.; Pavlik, M.; Szakova, J.; Wilhelmova, N. The long-term effect of zinc soil contamination on selected free amino acids playing an important role in plant adaptation to stress and senescence. Ecotoxicol. Environ. Saf. 2014, 100, 166–170. [Google Scholar] [CrossRef]
- Thomas, J.C.; De Armond, R.L.; Bohnert, H.J. Influence of NaCl on growth, proline, and phosphoenolpyruvate carboxylase levels in mesembryanthemum crystallinum suspension cultures. Plant Physiol. 1992, 98, 626–631. [Google Scholar] [CrossRef]
- Thomas, H.; Hardy, R. Effects of NaCl-salinity on amino acid and carbohydrate contents of Phragmites australis. Aquat. Bot. 2001, 69, 195–208. [Google Scholar] [CrossRef]
- Zhang, J.T.; Du, Y.Y.; Chen, S.Y.; Tang, H.R. Dynamic metabonomic responses of tobacco (nicotiana tabacum) plants to salt stress. J. Proteome Res. 2011, 10, 1904–1914. [Google Scholar] [PubMed]
- Fougère, F.; Le Rudulier, D.; Streeter, J.G. Effects of salt stress on amino acid, organic acid, and carbohydrate composition of roots, bacteroids, and cytosol of alfalfa (Medicago sativa L.). Plant Physiol. 1991, 96, 1228–1236. [Google Scholar] [CrossRef]
- Di Martino, C.; Delfine, S.; Pizzuto, R.; Loreto, F.; Fuggi, A. Free amino acids and glycine betaine in leaf osmoregulation of spinach responding to increasing salt stress. New Phytol. 2003, 158, 455–463. [Google Scholar]
- Yang, Z.; Xie, T.; Liu, Q. Physiological responses of Phragmites australis to the combined effects of water and salinity stress. Ecohydrology 2014, 7, 420–426. [Google Scholar] [CrossRef]
- Briens, M.; Larher, F. Osmoregulation in halophytic higher plants: A comparative study of soluble carbohydrates, polyols, betaines and free proline. Plant Cell Environ. 1982, 5, 287–292. [Google Scholar]
- Zörb, C.; Herbst, R.; Forreiter, C.; Schubert, S. Short-term effects of salt exposure on the maize chloroplast protein pattern. Proteomics 2009, 9, 4209–4220. [Google Scholar]
- McFeeters, R.F.; Thompson, R.; Fleming, H. Liquid chromatographic analysis of sugars, acids, and ethanol in lactic acid vegetable fermentations. J. Assoc. Off. Anal. Chem. 1984, 67, 710–714. [Google Scholar] [CrossRef]
- Vicré, M.; Santaella, C.; Blanchet, S.; Gateau, A.; Driouich, A. Root border-like cells of Arabidopsis. Microscopical characterization and role in the interaction with rhizobacteria. Plant Physiol. 2005, 138, 998–1008. [Google Scholar] [CrossRef]
- Aghaei, K.; Ehsanpour, A.A.; Shah, A.H.; Komatsu, S. Proteome analysis of soybean hypocotyl and root under salt stress. Amino Acids 2009, 36, 91–98. [Google Scholar] [CrossRef]
- Silveira, J.A.G.; Viégas, R.d.A.; Rocha, I.M.A.d.; Moreira, A.C.d.O.M.; Moreira, R.d.A.; Oliveira, J.T.A. Proline accumulation and glutamine synthetase activity are increased by salt-induced proteolysis in cashew leaves. J. Plant Physiol. 2003, 160, 115–123. [Google Scholar] [CrossRef]
- Lesuffleur, F.; Paynel, F.; Bataillé, M.-P.; Le Deunff, E.; Cliquet, J.-B. Root amino acid exudation: Measurement of high efflux rates of glycine and serine from six different plant species. Plant Soil 2007, 294, 235–246. [Google Scholar] [CrossRef]
- Good, A.G.; Zaplachinski, S.T. The effects of drought stress on free amino acid accumulation and protein synthesis in Brassica napus. Physiol. Plant. 1994, 90, 9–14. [Google Scholar] [CrossRef]
- Delauney, A.J.; Verma, D.P.S. Proline biosynthesis and osmoregulation in plants. Plant J. 1993, 4, 215–223. [Google Scholar] [CrossRef]
- Gzik, A. Accumulation of proline and pattern of α-amino acids in sugar beet plants in response to osmotic, water and salt stress. Environ. Exp. Bot. 1996, 36, 29–38. [Google Scholar] [CrossRef]
- Khedr, A.H.A.; Abbas, M.A.; Wahid, A.A.A.; Quick, W.P.; Abogadallah, G.M. Proline induces the expression of salt-stress-responsive proteins and may improve the adaptation of Pancratium maritimum L. to salt-stress. J. Exp. Bot. 2003, 54, 2553–2562. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Boggess, S.; Stewart, C. Localization of drought effect on proline biosynthesis. Plant Physiol 1976, 57, 39. [Google Scholar]
- Dungey, N.O.; Davies, D.D. Protein Turnover in Isolated Barley Leaf Segments and the Effects of Stress. J. Exp. Bot. 1982, 33, 12–20. [Google Scholar] [CrossRef]
- Rayapati, P.J.; Stewart, C.R. Solubilization of a proline dehydrogenase from maize (Zea mays L.) mitochondria. Plant Physiol. 1991, 95, 787–791. [Google Scholar] [CrossRef]
- Sells, G.D.; Koeppe, D.E. Oxidation of proline by mitochondria isolated from water-stressed maize shoots. Plant Physiol. 1981, 68, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, G.; Naidoo, Y. Effects of salinity and nitrogen on growth, ion relations and proline accumulation in Triglochin bulbosa. Wetl. Ecol. Manag. 2001, 9, 491–497. [Google Scholar] [CrossRef]
- Mari, A.H.; Rajpar, I.; Hassan, Z.; Tunio, S.; Ahmad, S. Ions accumulation, proline content and juice quality ff sugar beet genotypes as affected by water salinity. J. Anim. Plant Sci. 2018, 28, 1405–1412. [Google Scholar]
- Tal, M.; Katz, A.; Heikin, H.; Dehan, K. Salt tolerance in the wild relatives of the cultivated tomato: Proline accumulation in lycopersicon esculentum Mill., L. peruvianum Mill. and solanum pennelli Cor. treated with Nacl and polyethylene glycole. New Phytol. 1979, 82, 349–355. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, E.; Wei, X.; Ding, A.; Zheng, L.; Wu, X.; Anderson, B. Short-Term Effects of Salt Stress on the Amino Acids of Phragmites australis Root Exudates in Constructed Wetlands. Water 2020, 12, 569. https://doi.org/10.3390/w12020569
Xie E, Wei X, Ding A, Zheng L, Wu X, Anderson B. Short-Term Effects of Salt Stress on the Amino Acids of Phragmites australis Root Exudates in Constructed Wetlands. Water. 2020; 12(2):569. https://doi.org/10.3390/w12020569
Chicago/Turabian StyleXie, En, Xuejing Wei, Aizhong Ding, Lei Zheng, Xiaona Wu, and Bruce Anderson. 2020. "Short-Term Effects of Salt Stress on the Amino Acids of Phragmites australis Root Exudates in Constructed Wetlands" Water 12, no. 2: 569. https://doi.org/10.3390/w12020569
APA StyleXie, E., Wei, X., Ding, A., Zheng, L., Wu, X., & Anderson, B. (2020). Short-Term Effects of Salt Stress on the Amino Acids of Phragmites australis Root Exudates in Constructed Wetlands. Water, 12(2), 569. https://doi.org/10.3390/w12020569



