Glacial Stream Ecology: Structural and Functional Assets
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Area
2.3. Physical and Chemical Habitat Variables
2.4. Invertebrate Community Structures
2.5. Egg Development in Glacial Streams
2.6. Bulk Stable Isotopes and Individual Body Mass of Chironomid Populations
2.7. Data Analysis
3. Results
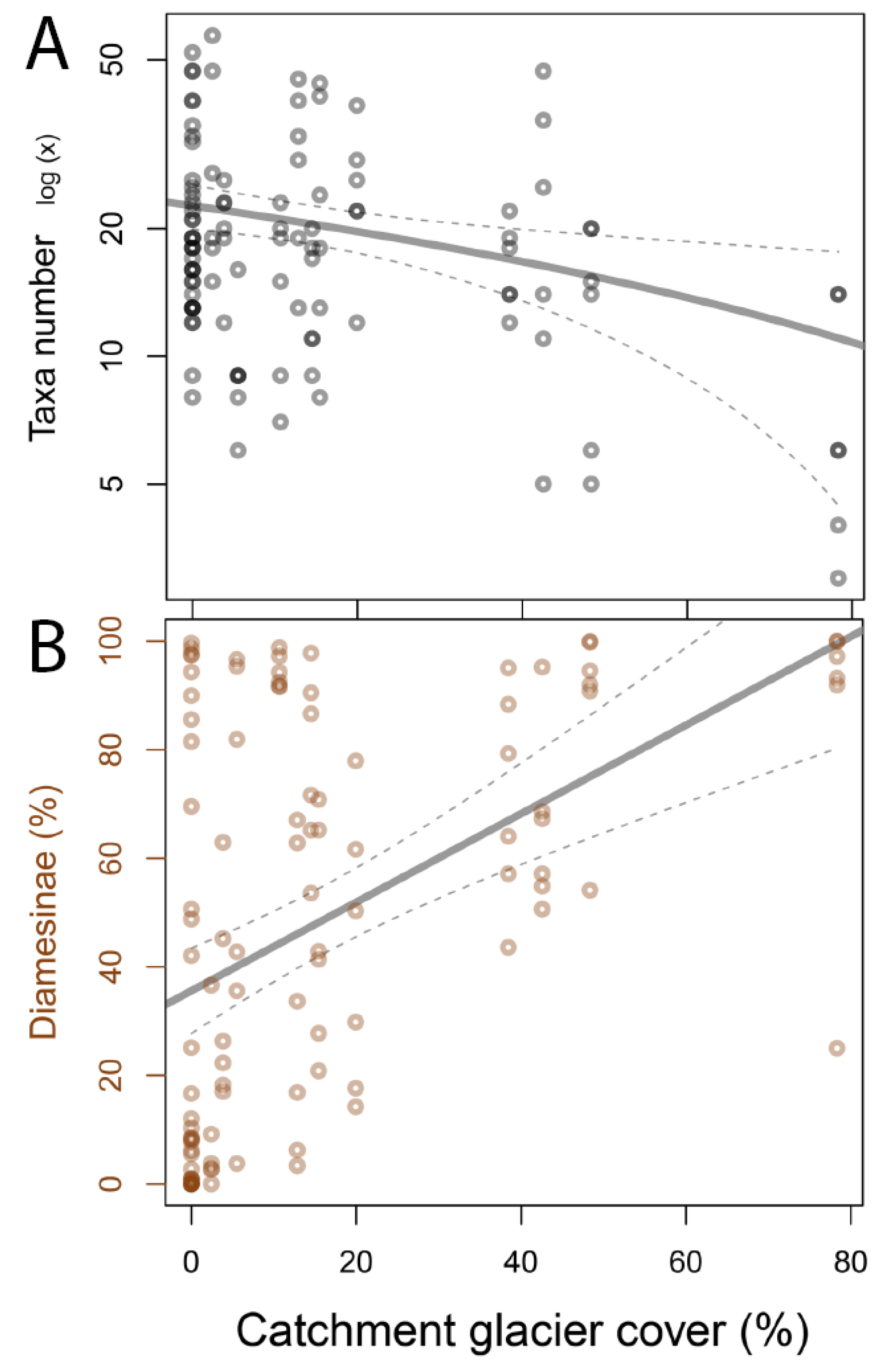
3.1. Invertebrate Taxa Number and Dominance of Diamesinae
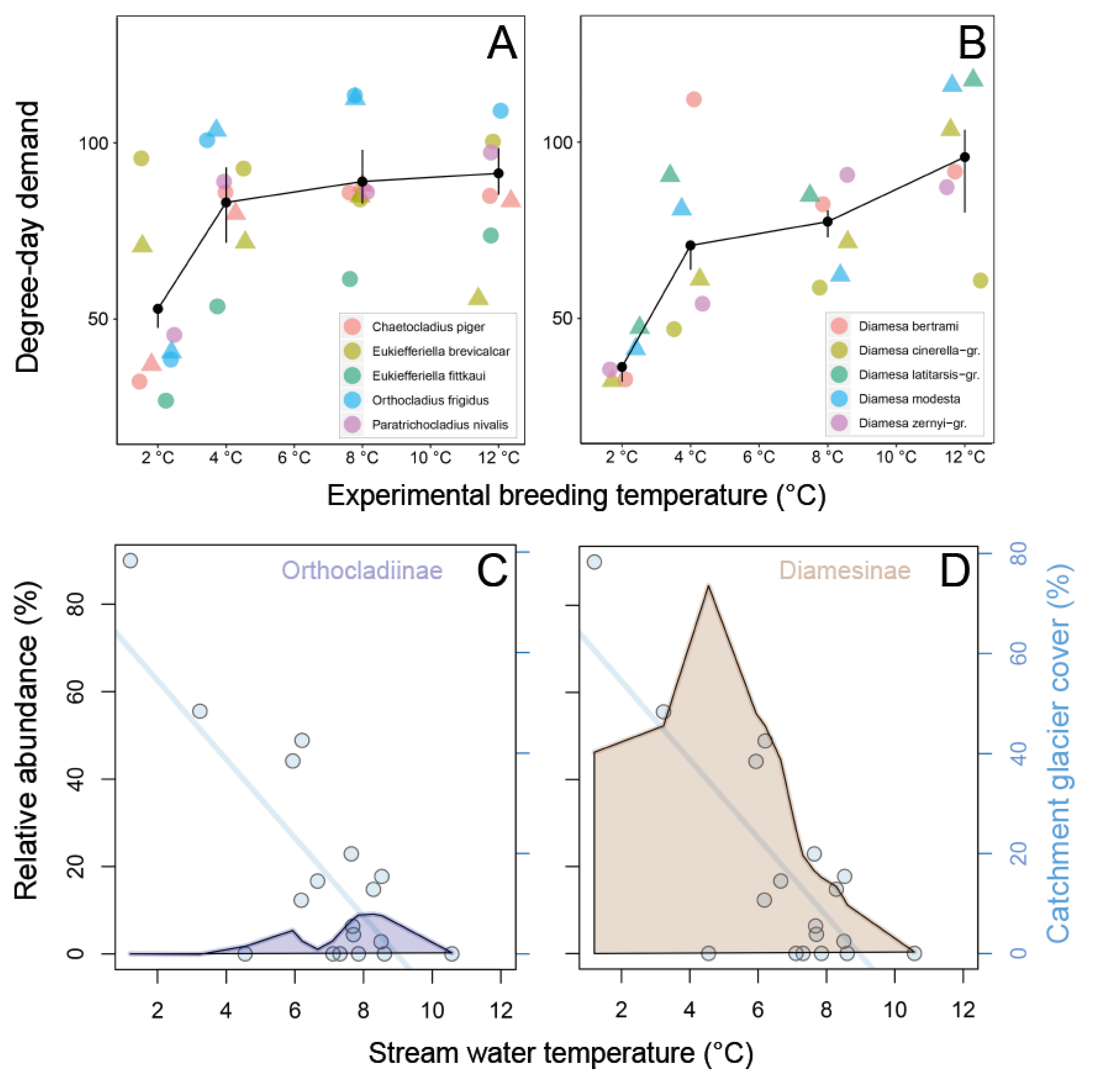
3.2. Development of Eggs and Larvae in Relation to Catchment Glacier Cover
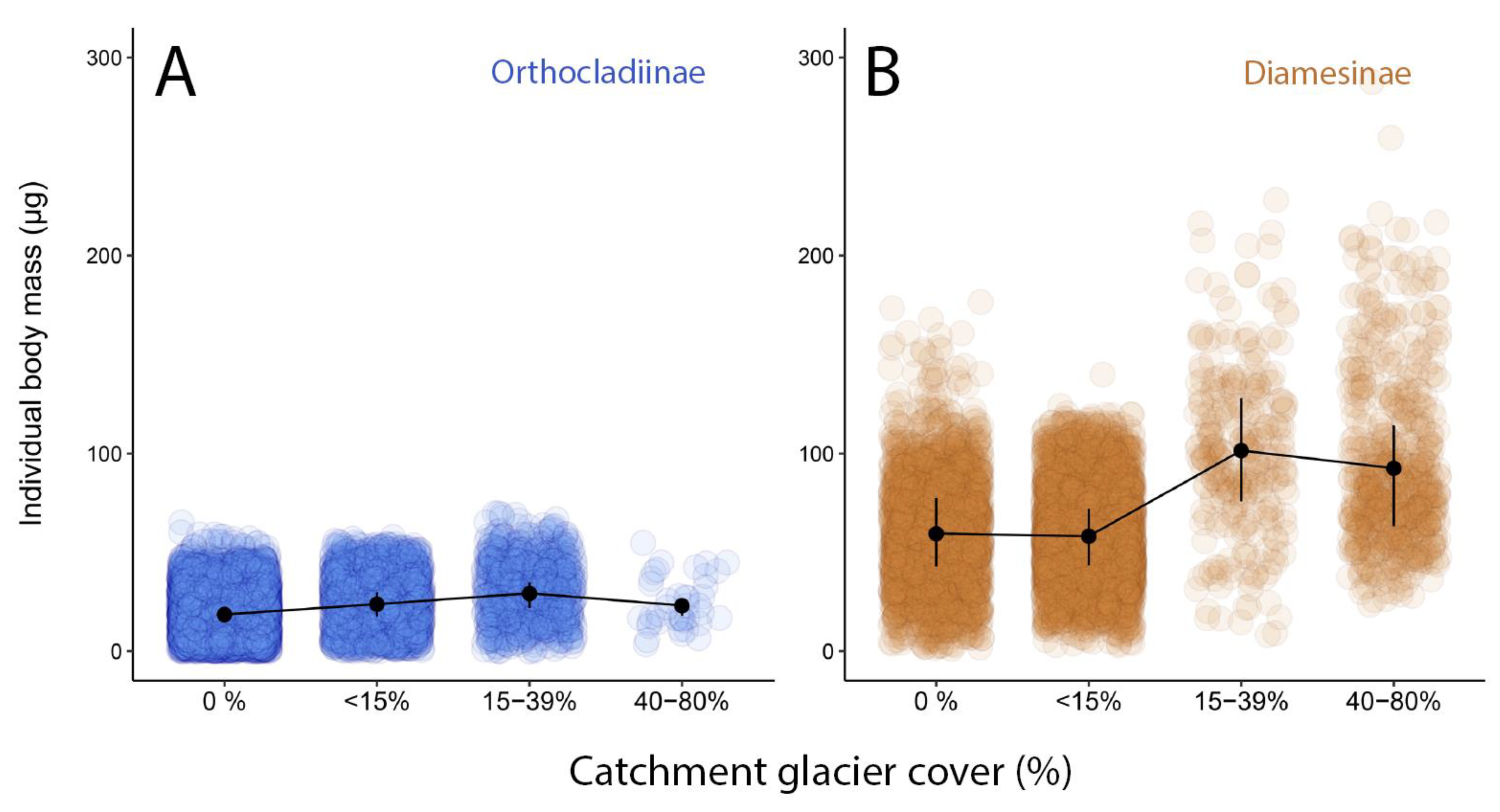
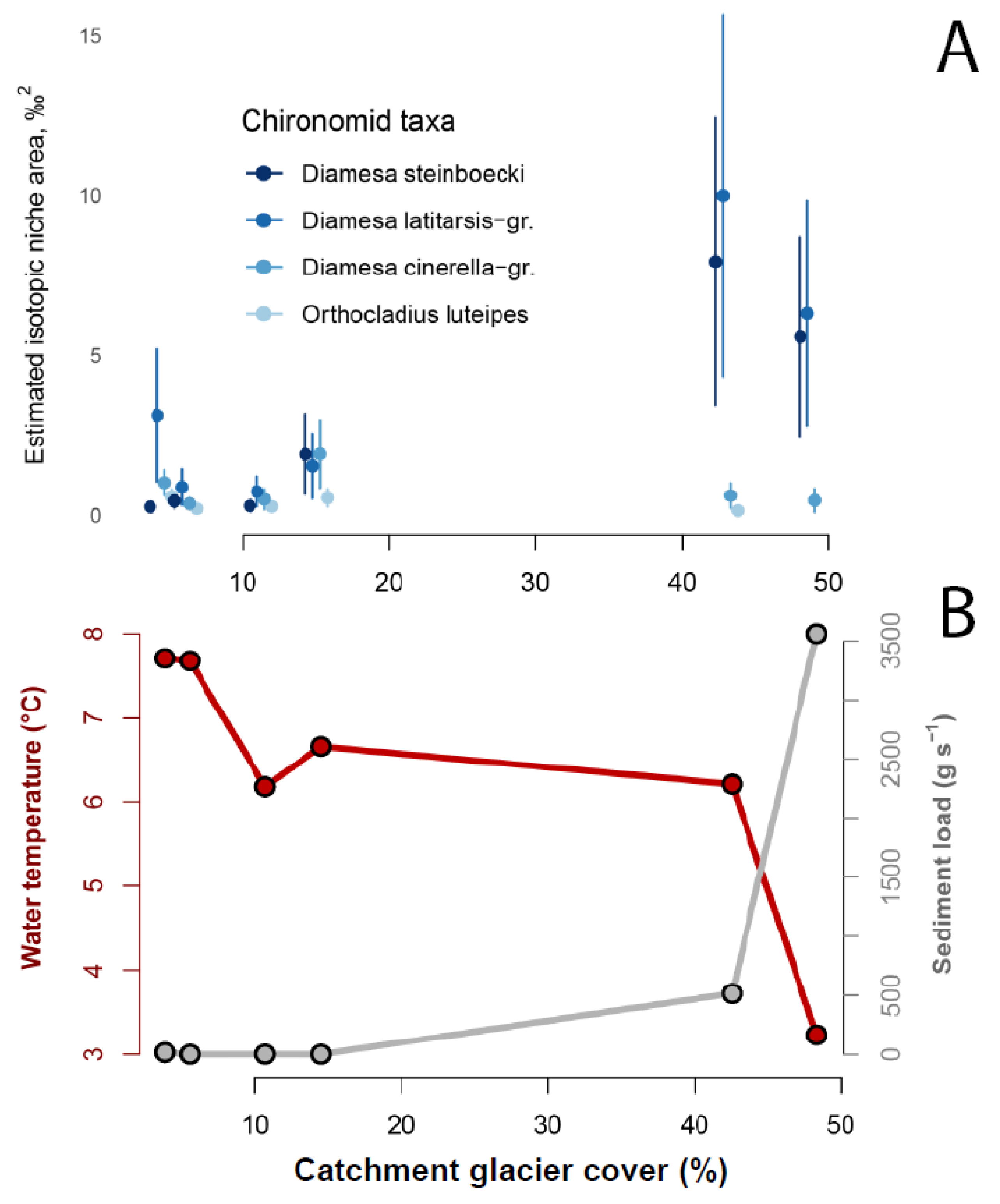
3.3. Trophic Strategies in Glacier-Fed Streams Related to Catchment Glacier Cover
4. Discussion
4.1. Invertebrate Communities in Glacier-Fed Streams Were Dominated by Diamesinae
4.2. Functional Assets in Glacial Streams
4.2.1. Fast Development of Eggs and Larvae
4.2.2. Special Trophic Strategies
4.3. Synthesis and Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Notes
- Brittain, J.E.; Milner, A.M. Ecology of glacier-fed rivers: Current status and concepts. Freshw. Biol. 2001, 46, 1571–1578. [Google Scholar] [CrossRef]
- Uehlinger, U.; Malard, F.; Ward, J.V. Thermal patterns in the surface waters of a glacial river corridor (Val Roseg, Switzerland). Freshw. Biol. 2003, 48, 284–300. [Google Scholar] [CrossRef]
- Jacobsen, D.; Dangles, O. Ecology of High Altitude Waters; Oxford University Press: Oxford, UK, 2017; ISBN 9780198736868. [Google Scholar]
- Kneib, M.; Cauvy-Fraunié, S.; Escoffier, N.; Boix Canadell, M.; Horgby, Å.; Battin, T.J. Glacier retreat changes diurnal variation intensity and frequency of hydrologic variables in Alpine and Andean streams. J. Hydrol. 2020, 583, 124578. [Google Scholar] [CrossRef]
- Milner, A.M.; Brittain, J.E.; Castella, E.; Petts, G.E. Trends of macroinvertebrate community structure in glacier-fed rivers in relation to environmental conditions: A synthesis. Freshw. Biol. 2001, 46, 1833–1847. [Google Scholar] [CrossRef]
- Füreder, L.; Schütz, C.; Wallinger, M.; Burger, R. Physico-chemistry and aquatic insects of a glacier-fed and a spring-fed alpine stream. Freshw. Biol. 2001, 46, 1673–1690. [Google Scholar] [CrossRef]
- Rott, E.; Cantonati, M.; Füreder, L.; Pfister, P. Benthic algae in high altitude streams of the Alps—A neglected component of the aquatic biota. Hydrobiologia 2006, 562, 195–216. [Google Scholar] [CrossRef]
- Uehlinger, U.; Robinson, C.T.; Hieber, M.; Zah, R. The physico-chemical habitat template for periphyton in alpine glacial streams under a changing climate. Hydrobiologia 2010, 657, 107–121. [Google Scholar] [CrossRef]
- Füreder, L. Life at the Edge: Habitat Condition and Bottom Fauna of Alpine Running Waters. Int. Rev. Hydrobiol. 2007, 92, 491–513. [Google Scholar] [CrossRef]
- Zah, R.; Burgherr, P.; Bernasconi, S.M.; Uehlinger, U. Stable isotope analysis of macroinvertebrates and their food sources in a glacier stream. Freshw. Biol. 2001, 46, 871–882. [Google Scholar] [CrossRef]
- Füreder, L.; Welter, C.; Jackson, J.K. Dietary and Stable Isotope (δ 13C, δ 15N) analyses in Alpine Stream Insects. Int. Rev. Hydrobiol. 2003, 88, 314–331. [Google Scholar] [CrossRef]
- Füreder, L.; Welter, C.; Jackson, J.K. Dietary and stable isotope (δ 13C, δ 15N) analyses in alpine Ephemeroptera and Plecoptera. In Proceedings of the Research Update on Ephemeroptera & Plecoptera; Gaino, E., Ed.; University of Perugia: Perugia, Italy, 2003; pp. 39–46. [Google Scholar]
- Brown, L.E.; Khamis, K.; Wilkes, M.; Blaen, P.; Brittain, J.E.; Carrivick, J.L.; Fell, S.; Friberg, N.; Füreder, L.; Gislason, G.M.; et al. Functional diversity and community assembly of river invertebrates show globally consistent responses to decreasing glacier cover. Nat. Ecol. Evol. 2018, 2, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Niedrist, G.H.; Füreder, L. Towards a definition of environmental niches in alpine streams by employing chironomid species preferences. Hydrobiologia 2016, 781, 143–160. [Google Scholar] [CrossRef]
- Niedrist, G.H.; Füreder, L. Real-time warming of high-altitude streams: (Re)defining invertebrates’ temperature preferences. River Res. Appl.
- Cauvy-Fraunié, S.; Dangles, O. A global synthesis of biodiversity responses to glacier retreat. Nat. Ecol. Evol. 2019, 3, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Niedrist, G.H.; Cantonati, M.; Füreder, L. Environmental harshness mediates the quality of periphyton and chironomid body mass in alpine streams. Freshw. Sci. 2018, 37, 519–533. [Google Scholar] [CrossRef]
- Niedrist, G.H.; Füreder, L. When the going gets tough, the tough get going: The enigma of survival strategies in harsh glacial stream environments. Freshw. Biol. 2018, 63, 1260–1272. [Google Scholar] [CrossRef]
- Schütz, S.A.; Füreder, L. Egg development and hatching success in alpine chironomids. Freshw. Biol. 2019, 64, 685–696. [Google Scholar] [CrossRef]
- QGIS Development Team QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2009.
- Jackson, A.L.; Inger, R.; Parnell, A.C.; Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER—Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 2011, 80, 595–602. [Google Scholar] [CrossRef]
- Nolte, U. Chironomid biomass determination from larval shape. Freshw. Biol. 1990, 24, 443–451. [Google Scholar] [CrossRef]
- Tod, S.P.; Schmid-Araya, J.M. Meiofauna versus macrofauna: Secondary production of invertebrates in a lowland chalk stream. Limnol. Oceanogr. 2009, 54, 450–456. [Google Scholar] [CrossRef]
- Lods-Crozet, B.; Lencioni, V.; Olafsson, J.S.; Snook, D.L.; Velle, G.; Brittain, J.E.; Castella, E.; Rossaro, B. Chironomid (Diptera: Chironomidae) communities in six European glacier-fed streams. Freshw. Biol. 2001, 46, 1791–1809. [Google Scholar] [CrossRef]
- Castella, E.; Adalsteinsson, H.; Brittain, J.E.; Gislason, G.M.; Lehmann, A.; Lencioni, V.; Lods-Crozet, B.; Maiolini, B.; Milner, A.M.; Olafsson, J.S.; et al. Macrobenthic invertebrate richness and composition along a latitudinal gradient of European glacier-fed streams. Freshw. Biol. 2001, 46, 1811–1831. [Google Scholar] [CrossRef]
- Maiolini, B.; Lencioni, V. Longitudinal distribution of macroinvertebrate assemblages in a glacially influenced stream system in the Italian Alps. Freshw. Biol. 2001, 46, 1625–1639. [Google Scholar] [CrossRef]
- Jacobsen, D.; Dangles, O. Environmental harshness and global richness patterns in glacier-fed streams. Glob. Ecol. Biogeogr. 2012, 21, 647–656. [Google Scholar] [CrossRef]
- Füreder, L. High alpine streams: Cold habitats for insect larvae. In Cold-Adapted Organisms; Margesin, R., Schinner, F., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 181–196. ISBN 978-3-662-06285-2. [Google Scholar]
- Lods-Crozet, B.; Lencioni, V.; Brittain, J.E.; Marziali, L.; Rossaro, B. Contrasting chironomid assemblages in two high Arctic streams on Svalbard. Fundam. Appl. Limnol. 2007, 170, 211–222. [Google Scholar] [CrossRef]
- Schütz, S.; Füreder, L. Unexpected patterns of chironomid larval size in an extreme environment: A highly glaciated, alpine stream. Hydrobiologia 2018, 820, 49–63. [Google Scholar] [CrossRef]
- Pritchard, G.; Harder, L.D.; Mutch, R.A. Development of aquatic insect eggs in relation to temperature and strategies for dealing with different thermal environments. Biol. J. Linn. Soc. 1996, 58, 221–244. [Google Scholar] [CrossRef]
- Lencioni, V.; Jousson, O.; Guella, G.; Bernabò, P. Cold adaptive potential of chironomids overwintering in a glacial stream. Physiol. Entomol. 2015, 40, 43–53. [Google Scholar] [CrossRef]
- Pankhurst, N.W.; Munday, P.L. Effects of climate change on fish reproduction and early life history stages. Mar. Freshw. Res. 2011, 62, 1015–1026. [Google Scholar] [CrossRef]
- Humpesch, U.H.; Elliott, J.M. Effect of Temperature on the Hatching Time of Eggs of Three Rhithrogena Spp. (Ephemeroptera) from Austrian Streams and an English Stream and River. J. Anim. Ecol. 1980, 49, 643. [Google Scholar] [CrossRef]
- Knispel, S.; Sartori, M.; Brittain, J.E. Egg development in the mayflies of a Swiss glacial floodplain. J. N. Am. Benthol. Soc. 2006, 25, 430–443. [Google Scholar] [CrossRef]
- Lillehamnur, A.; Brittain, J.E.; Saltveit, S.J.; Nielsen, P.S. Egg development, nymphal growth and life cycle strategies in Plecoptera. Ecography 1989, 12, 173–186. [Google Scholar] [CrossRef]
- Clitherow, L.R.; Carrivick, J.L.; Brown, L.E. Food Web Structure in a Harsh Glacier-Fed River. PLoS ONE 2013, 8, e60899. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.; Harrison, S. Sediments and future climate. Nat. Geosci. 2009, 2, 230. [Google Scholar] [CrossRef]
- Niedrist, G.H.; Füreder, L. Real-time warming of high-altitude streams and potential consequences within invertebrate communities. Clim. Chang. Biol.
- Niedrist, G.H.; Füreder, L. Trophic ecology of alpine stream invertebrates: Current status and future research needs. Freshw. Sci. 2017, 36, 466–478. [Google Scholar] [CrossRef]
- Hieber, M.M.; Robinson, C.T.; Rushforth, S.R.; Uehlinger, U. Algal communities associated with different alpine stream types. Arctic Antarct. Alp. Res. 2001, 33, 447–456. [Google Scholar] [CrossRef]
- Klaveness, D. Hydrurus foetidus (Chrysophyceae): An update and request for observations. Algae 2019, 34, 1–5. [Google Scholar] [CrossRef]
- Galloway, A.W.E.; Winder, M. Partitioning the Relative Importance of Phylogeny and Environmental Conditions on Phytoplankton Fatty Acids. PLoS ONE 2015, 10, e0130053. [Google Scholar] [CrossRef]
- Twining, C.W.; Brenna, J.T.; Hairston, N.G.; Flecker, A.S. Highly unsaturated fatty acids in nature: What we know and what we need to learn. Oikos 2016, 125, 749–760. [Google Scholar] [CrossRef]
- Zemp, M.; Frey, H.; Gärtner-Roer, I.; Nussbaumer, S.U.; Hoelzle, M.; Paul, F.; Haeberli, W.; Denzinger, F.; Ahlstrøm, A.P.; Anderson, B.; et al. Historically unprecedented global glacier decline in the early 21st century. J. Glaciol. 2015, 61, 745–762. [Google Scholar] [CrossRef]
- Hock, R.; Rasul, G.; Adler, C.; Cáceres, B.; Gruber, S.; Hirabayashi, Y.; Jackson, M.; Kääb, A.; Kang, S.; Kutuzov, S.; et al. High Mountain Areas. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.-O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., Okem, A., et al., Eds.; IPCC: Genf, Switzerland, 2019; p. 72. [Google Scholar]
- Ward, J.V.; Uehlinger, U. Ecology of a Glacial Flood Plain; Springer: Dordrecht, The Netherlands, 2003; ISBN 978-1-4020-1792-6. [Google Scholar]
- Füreder, L.; Schütz, C.; Burger, R.; Wallinger, M. Seasonal abundance and community structure of Chironomidae in two contrasting high alpine streams. SIL Proc. 2000, 27, 1596–1601. [Google Scholar] [CrossRef]
- Schütz, C.; Wallinger, M.; Burger, R.; Füreder, L. Effects of snow cover on the benthic fauna in a glacier-fed stream. Freshw. Biol. 2001, 46, 1691–1704. [Google Scholar] [CrossRef]
- Martyniuk, N.; Modenutti, B.; Balseiro, E.G. Seasonal variability in glacial influence affects macroinvertebrate assemblages in North-Andean Patagonian glacier-fed streams. Inl. Waters 2019, 9, 522–533. [Google Scholar] [CrossRef]
- Tockner, W. Tockner Biodiversity: Towards a unifying theme for river ecology. Freshw. Biol. 2001, 46, 807–819. [Google Scholar]
- Scotti, A.; Jacobsen, D.; Tappeiner, U.; Bottarin, R. Spatial and temporal variation of benthic macroinvertebrate assemblages during the glacial melt season in an Italian glacier-fed stream. Hydrobiologia 2019, 827, 123–139. [Google Scholar] [CrossRef]
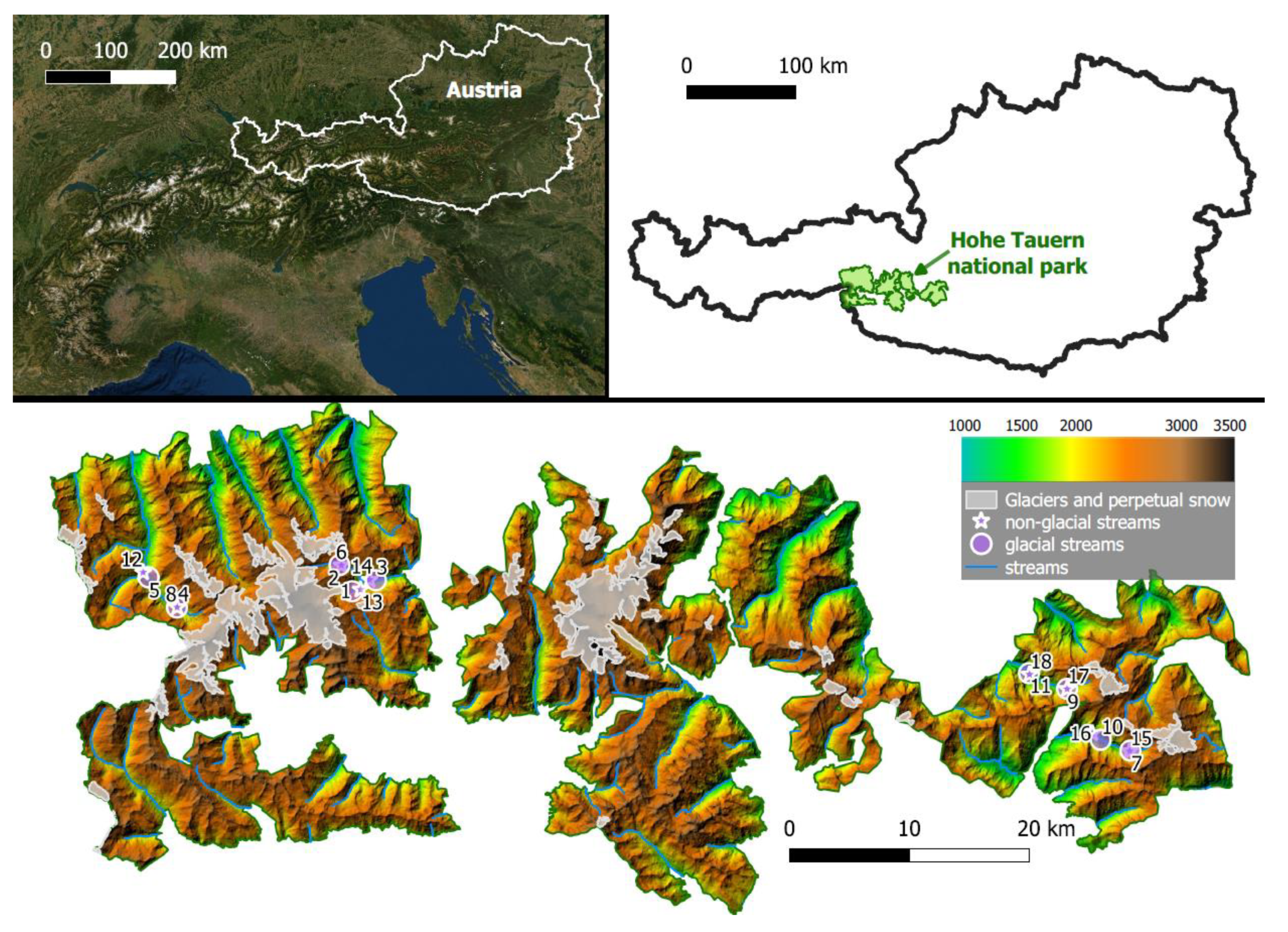
| # Site | Coordinates WGS 84 | Catchment Glacier Cover (%) | maxT | Transported Solids (mg/s) | Taxa Richness | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 47°6′50.7″N, 12°25′5.8″E | 78.3 | 1.42 | ± | 0.1 | 215.2 | ± | 17.4 | 8 | ± | 5 |
| 2 | 47°8′0.2″N, 12°24′11.8″E | 48.4 | 4.97 | ± | 1.11 | 3559.0 | ± | 142.6 | 13 | ± | 7 |
| 3 | 47°7′22.4″N, 12°26′37.7″E | 42.6 | 7.75 | ± | 0.84 | 515.2 | ± | 46.6 | 23 | ± | 16 |
| 4 | 47°5′50.8″N, 12°13′34.2″E | 38.4 | 7.68 | ± | 1.62 | 11.9 | ± | 0.9 | 17 | ± | 4 |
| 5 | 47°7′9.4″N, 12°11′37.2″E | 19.9 | 9.68 | ± | 1.62 | 16.8 | ± | 0.9 | 25 | ± | 9 |
| 6 | 47°7′59.8″N, 12°24′19.2″E | 15.4 | 12.99 | ± | 3.23 | 0.1 | ± | 0.2 | 25 | ± | 15 |
| 7 | 47°0′38.7″N, 13°16′40.8″E | 14.5 | 9.17 | ± | 1.89 | 2.1 | ± | 0.3 | 14 | ± | 5 |
| 8 | 47°5′51.3″N, 12°13′35.2″E | 12.8 | 11.24 | ± | 2.30 | 1.2 | ± | 0.3 | 30 | ± | 12 |
| 9 | 47°3′21.4″N, 13°12′25.7″E | 10.7 | 7.54 | ± | 1.46 | 2.8 | ± | 0.3 | 16 | ± | 6 |
| 10 | 47°1′5.9″N, 13°14′43.2″E | 5.5 | 9.23 | ± | 1.20 | 1.9 | ± | 0.3 | 10 | ± | 3 |
| 11 | 47°4′0.9″N, 13°9′53.6″E | 3.8 | 8.66 | ± | 0.92 | 16.8 | ± | 1.4 | 21 | ± | 5 |
| 12 | 47°7′22.1″N, 12°11′15.0″E | 2.4 | 10.58 | ± | 1.48 | 1.9 | ± | 0.3 | 31 | ± | 17 |
| 13 | 47°6′56.1″N, 12°25′38.4″E | 0.0 | 9.59 | ± | 1.16 | 0.0 | ± | 0.2 | 35 | ± | 14 |
| 14 | 47°7′23.1″N, 12°26′12.6″E | 0.0 | 9.38 | ± | 1.50 | 1.1 | ± | 0.3 | 32 | ± | 10 |
| 15 | 47°0′36.8″N, 13°16′38.4″E | 0.0 | 9.07 | ± | 1.09 | 0.1 | ± | 0.2 | 15 | ± | 4 |
| 16 | 47°1′22.5″N, 13°14′9.3″E | 0.0 | 13.08 | ± | 1.66 | 1.2 | ± | 0.3 | 15 | ± | 5 |
| 17 | 47°3′20.7″N, 13°12′25.1″E | 0.0 | 4.80 | ± | 0.52 | 0.2 | ± | 0.2 | 16 | ± | 2 |
| 18 | 47°3′57.7″N, 13°9′55.7″E | 0.0 | 10.25 | ± | 1.20 | 1.6 | ± | 0.3 | 22 | ± | 7 |
| Asset | Evidence | References |
|---|---|---|
| Structure | ||
| Dominance of chironomidae | Chironomidae usually represent most of the benthic invertebrate community | [5,6,25] |
| Ratio Diamesinae/Orthocladiinae | The proportion of Diamesinae in benthic habitats is high and positively related to glacier cover/glacial influence | e.g., [14,26] |
| Distinct succession of invertebrate families | Depending on the habitat template, invertebrate families successively colonize glacier-fed streams along their longitudinal dimension | e.g., [5] |
| Diversity and abundance | Diversity rises and falls along a hump shape harsh-benign gradient, whereas abundance increases with decreasing harshness. | [1,47] |
| Seasonal patterns | Significant difference in community structure in winter samples | [48,49] |
| Spatial and temporal heterogeneity | Dominance of certain taxonomic groups in the invertebrate community varies considerably between habitat types, along the longitudinal succession of streams, and between different seasons. | [6,14,50,51,52] |
| Function | ||
| Cold-hardiness | Cold river specialists (Diamesinae) possess cold hardiness mechanisms that allows winter-activity and survival at sub-zero temperatures | [32] |
| Feeding plasticity | Populations of same glacial river specialist species have variable feeding niches, depending on the harshness of the habitat | [11,18] |
| Food exploitation | Specificity in food items | [40] |
| Body mass variability | The body mass of same glacial river specialist species is positively related with habitat harshness, whereas other species have consistent body-mass | [17,30] |
| Trait variability | Relative abundance and variability of species traits are related to degree of glacial influence on particular stream habitats | [9,13] |
| Life-cycle adaptation | Alpine stream chironomid species (and especially for glacial river specialists) have lower degree-day demand and faster development as larvae at colder water temperatures | [17,19,30] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Füreder, L.; Niedrist, G.H. Glacial Stream Ecology: Structural and Functional Assets. Water 2020, 12, 376. https://doi.org/10.3390/w12020376
Füreder L, Niedrist GH. Glacial Stream Ecology: Structural and Functional Assets. Water. 2020; 12(2):376. https://doi.org/10.3390/w12020376
Chicago/Turabian StyleFüreder, Leopold, and Georg H. Niedrist. 2020. "Glacial Stream Ecology: Structural and Functional Assets" Water 12, no. 2: 376. https://doi.org/10.3390/w12020376
APA StyleFüreder, L., & Niedrist, G. H. (2020). Glacial Stream Ecology: Structural and Functional Assets. Water, 12(2), 376. https://doi.org/10.3390/w12020376






