Effects of Persulfate Activation with Pyrite and Zero-Valent Iron for Phthalate Acid Ester Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
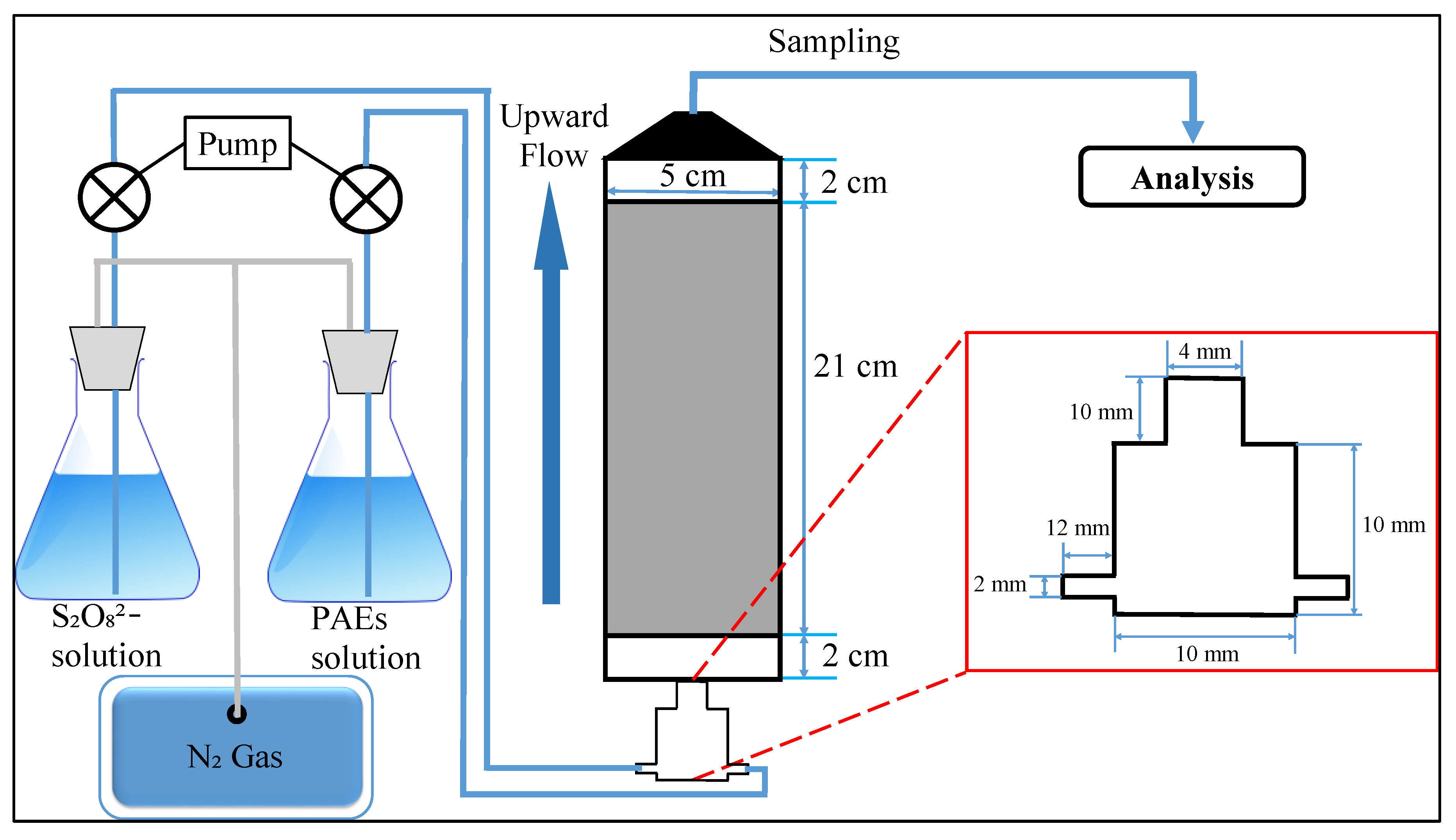
2.2. Experimental Section
2.3. Analyses and Methods
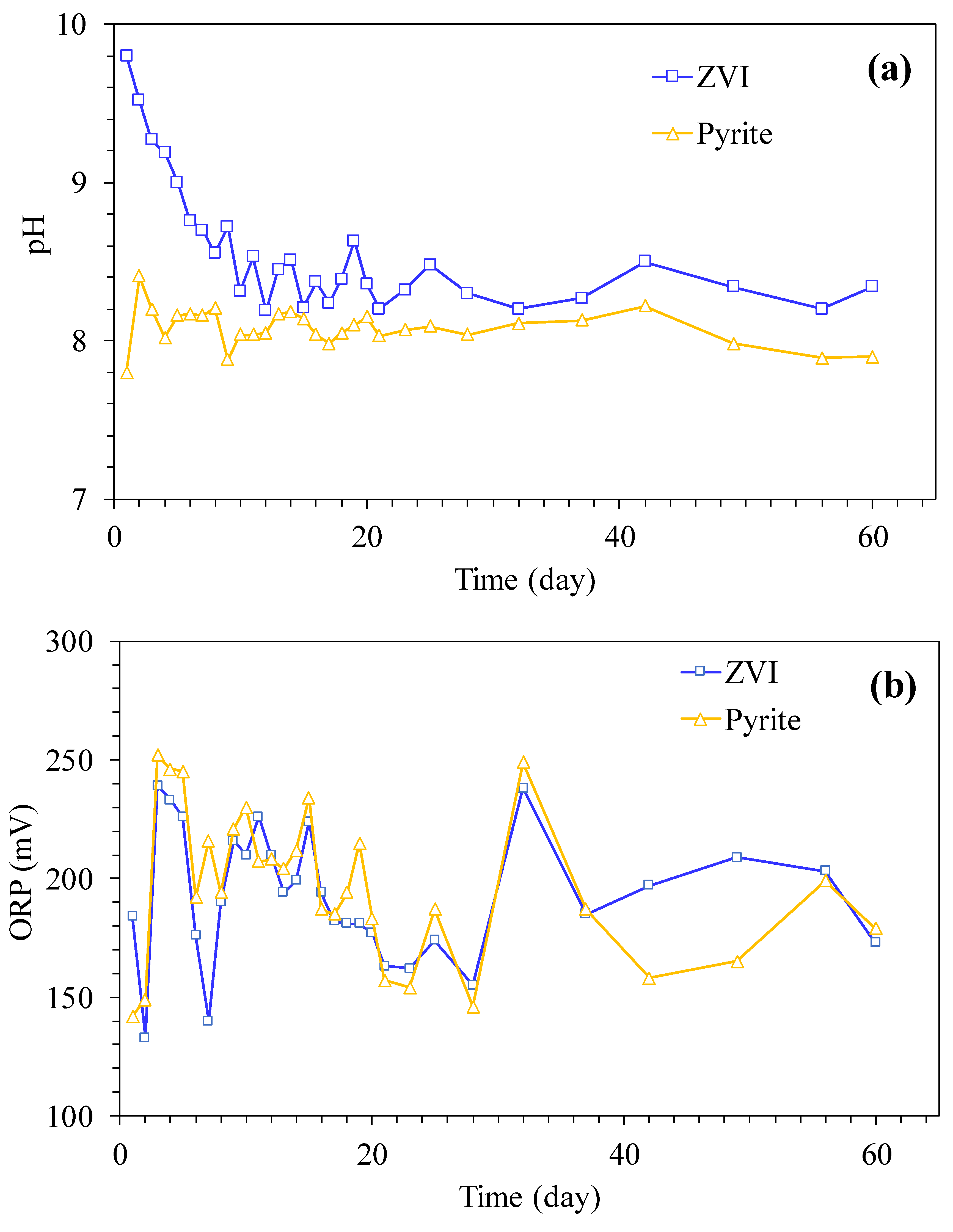
3. Results and Discussion
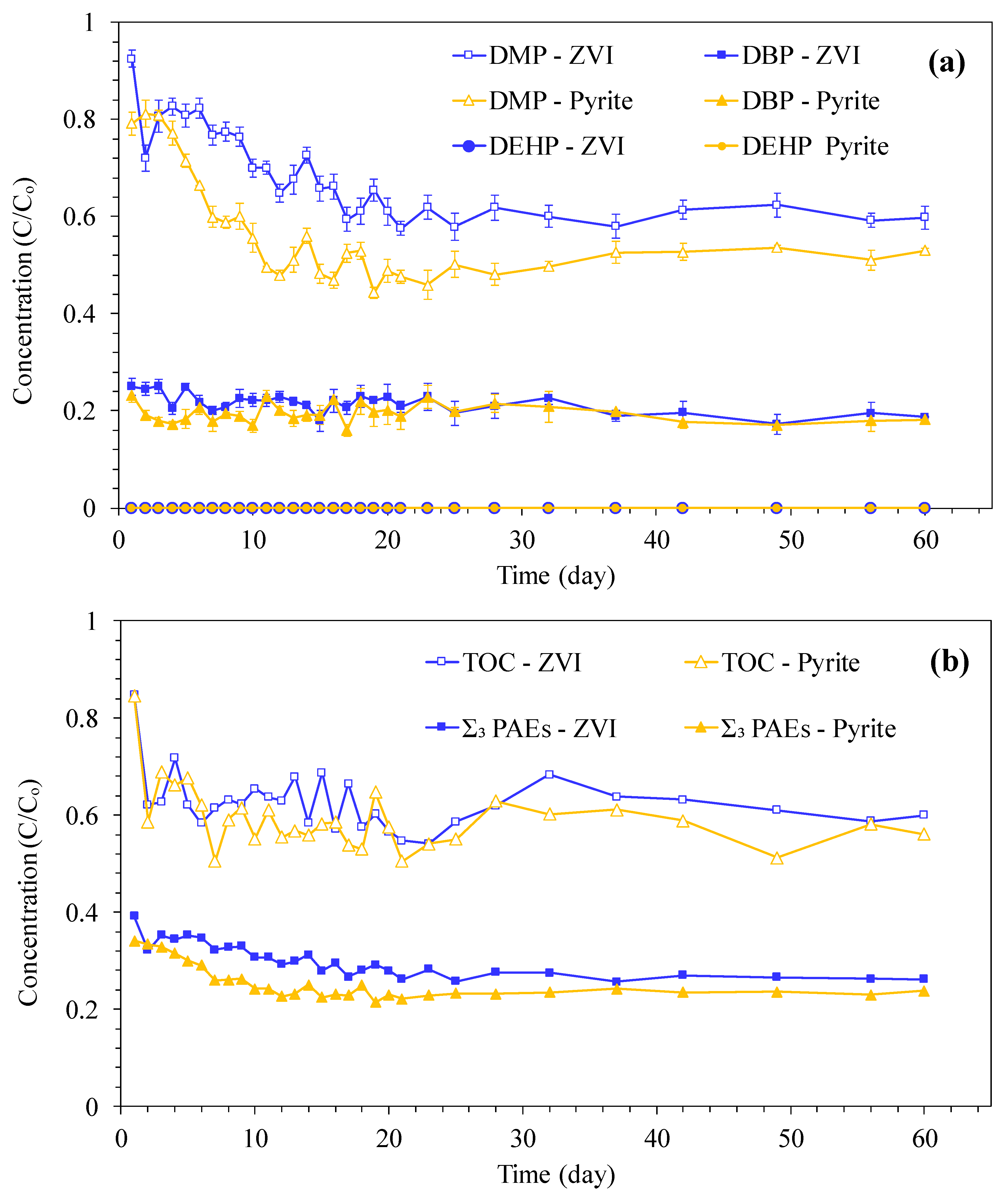
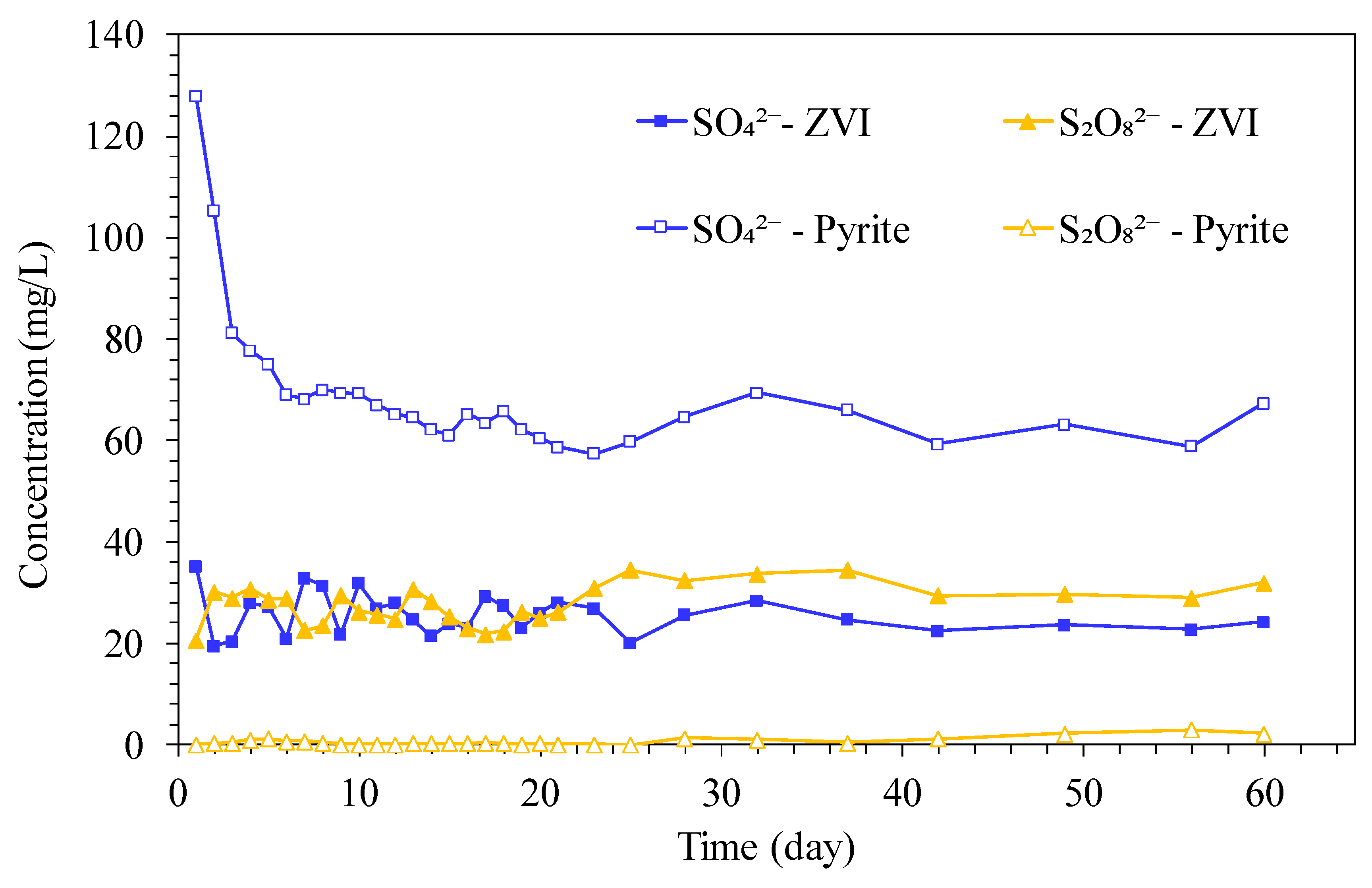
3.1. PAEs Degradation
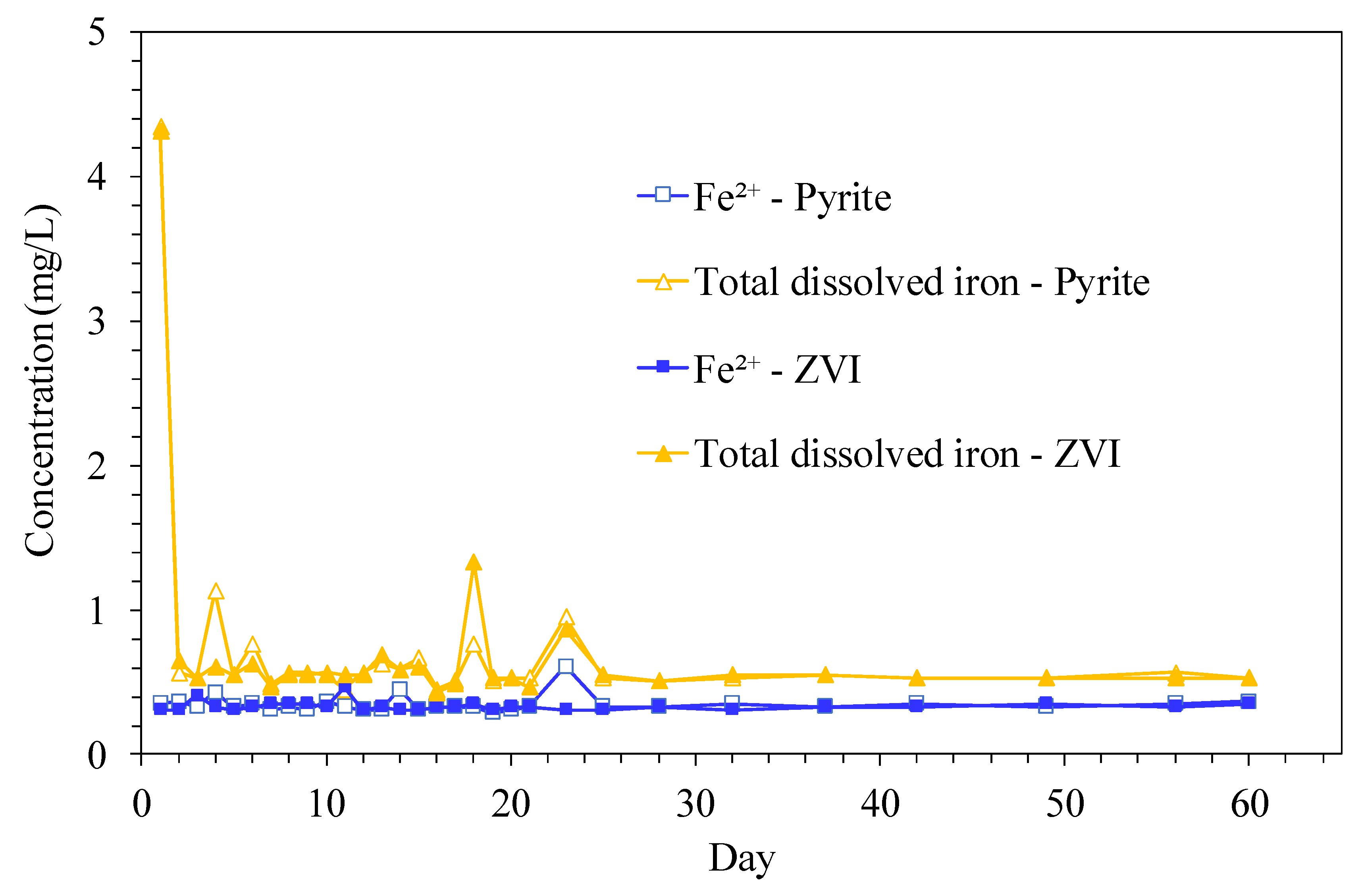
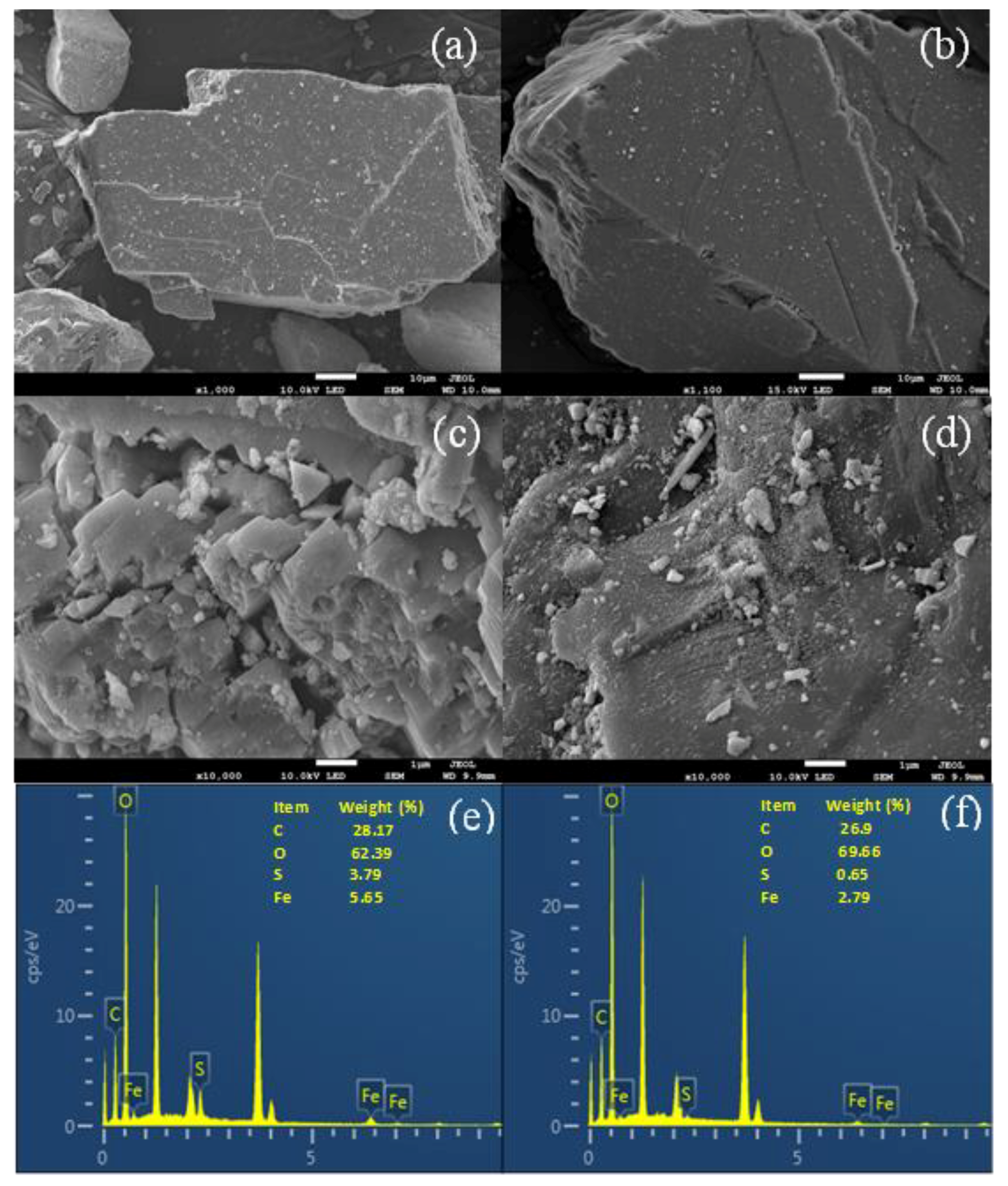
3.2. Surface Characterization of ZVI and Pyrite
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Liu, M.; Chen, H.; Hou, G. Source identification of polycyclic aromatic hydrocarbons in different ecological wetland components of the Qinkenpao Wetland in Northeast China. Ecotoxicol. Environ. Saf. 2014, 102, 160–167. [Google Scholar] [CrossRef]
- Gao, D.; Li, Z.; Wen, Z.; Ren, N. Occurrence and fate of phthalate esters in full-scale domestic wastewater treatment plants and their impact on receiving waters along the Songhua River in China. Chemosphere 2014, 95, 24–32. [Google Scholar] [CrossRef]
- Wang, J.; Luo, Y.; Teng, Y.; Ma, W.; Christie, P.; Li, Z. Soil contamination by phthalate esters in Chinese intensive vegetable production systems with different modes of use of plastic film. Environ. Pollut. 2013, 180, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Wang, F.; Zhang, L.; Shan, C.; Bai, Z.; Sun, Z.; Liu, L.; Shen, B. A comprehensive assessment of human exposure to phthalates from environmental media and food in Tianjin, China. J. Hazard. Mater. 2014, 279, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, L.; Kannan, K. Phthalates and Parabens in Personal Care Products From China: Concentrations and Human Exposure. Arch. Environ. Contam. Toxicol. 2014, 66, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Alatriste-Mondragon, F.; Iranpour, R.; Ahring, B.K. Toxicity of di-(2-ethylhexyl) phthalate on the anaerobic digestion of wastewater sludge. Water Res. 2003, 37, 1260–1269. [Google Scholar] [CrossRef]
- Padhye, L.P.; Yao, H.; Kung’u, F.T.; Huang, C.-H. Year-long evaluation on the occurrence and fate of pharmaceuticals, personal care products, and endocrine disrupting chemicals in an urban drinking water treatment plant. Water Res. 2014, 51, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Toft, G.; Jonsson, B.; Lindh, C.H.; Jensen, T.K.; Hjollund, N.H.; Vested, A.; Bonde, J.P. Association between Pregnancy Loss and Urinary Phthalate Levels around the Time of Conception. Environ. Health Perspect. 2011, 120, 458–463. [Google Scholar] [CrossRef]
- Liu, X.; Shi, J.; Bo, T.; Zhang, H.; Wu, W.; Chen, Q.; Zhan, X. Occurrence of phthalic acid esters in source waters: A nationwide survey in China during the period of 2009-2012. Environ. Pollut. 2014, 184, 262–270. [Google Scholar] [CrossRef]
- Gao, X.; Li, J.; Wang, X.; Zhou, J.; Fan, B.; Li, W.; Liu, Z. Exposure and ecological risk of phthalate esters in the Taihu Lake basin, China. Ecotoxicol. Environ. Saf. 2019, 171, 564–570. [Google Scholar] [CrossRef]
- He, Y.; Wang, Q.; He, W.; Xu, F. The occurrence, composition and partitioning of phthalate esters (PAEs) in the water-suspended particulate matter (SPM) system of Lake Chaohu, China. Sci. Total Environ. 2019, 661, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hubner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Guerra-Rodríguez, S.; Rodríguez, E.; Singh, D.N.; Rodríguez-Chueca, J. Assessment of Sulfate Radical-Based Advanced Oxidation Processes for Water and Wastewater Treatment: A Review. Water 2018, 10, 1828. [Google Scholar] [CrossRef]
- Arellano, M.; Pazos, M.; Sanromán, M.Á. Sulfate Radicals-Based Technology as a Promising Strategy for Wastewater. Water 2019, 11, 1695. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, X.; Sun, K.; Lin, C.; Ma, J.; He, M.; Ouyang, W. Persulfate-based advanced oxidation processes (AOPs) for organic-contaminated soil remediation: A review. Chem. Eng. J. 2019, 372, 836–851. [Google Scholar] [CrossRef]
- Tsitonaki, A.; Petri, B.G.; Crimi, M.; Mosbaek, H.; Siegrist, R.L.; Bjerg, P.L. In Situ Chemical Oxidation of Contaminated Soil and Groundwater Using Persulfate: A review. Crit. Rev. Env. Sci. Technol. 2010, 40, 55–91. [Google Scholar] [CrossRef]
- Wang, Z.; Deng, D.; Yang, L. Degradation of dimethyl phthalate in solutions and soil slurries by persulfate at ambient temperature. J. Hazard. Mater. 2014, 271, 202–209. [Google Scholar] [CrossRef]
- Lin, Y.T.; Liang, C.; Chen, J.H. Feasibility study of ultraviolet activated persulfate oxidation of phenol. Chemosphere 2011, 82, 1168–1172. [Google Scholar] [CrossRef]
- Liu, C.S.; Shih, K.; Sun, C.X.; Wang, F. Oxidative degradation of propachlor by ferrous and copper ion activated persulfate. Sci. Total Environ. 2012, 416, 507–512. [Google Scholar] [CrossRef]
- Yang, S.; Yang, X.; Shao, X.; Niu, R.; Wang, L. Activated carbon catalyzed persulfate oxidation of Azo dye acid orange 7 at ambient temperature. J. Hazard. Mater. 2011, 186, 659–666. [Google Scholar] [CrossRef]
- Diao, Z.-H.; Liu, J.-J.; Hu, Y.-X.; Kong, L.-J.; Jiang, D.; Xu, X.-R. Comparative study of Rhodamine B degradation by the systems pyrite/H 2 O 2 and pyrite/persulfate: Reactivity, stability, products and mechanism. Sep. Purif. Technol. 2017, 184, 374–383. [Google Scholar] [CrossRef]
- Liang, H.-y.; Zhang, Y.-q.; Huang, S.-b.; Hussain, I. Oxidative degradation of p-chloroaniline by copper oxidate activated persulfate. Chem. Eng. J. 2013, 218, 384–391. [Google Scholar] [CrossRef]
- Sedlak, D.L.; Andren, A.W. Oxidation of Chlorobenzene with Fenton’s Reagent. Environ. Sci. Technol. 1991, 25, 777–782. [Google Scholar] [CrossRef]
- Li, J.; Ji, Q.; Lai, B.; Yuan, D. Degradation of p -nitrophenol by Fe0/H2O2/persulfate system: Optimization, performance and mechanisms. J. Taiwan. Ins. Chem. Eng. 2017, 80, 686–694. [Google Scholar] [CrossRef]
- Teel, A.L.; Ahmad, M.; Watts, R.J. Persulfate activation by naturally occurring trace minerals. J. Hazard. Mater. 2011, 196, 153–159. [Google Scholar] [CrossRef]
- Li, H.; Wan, J.; Ma, Y.; Huang, M.; Wang, Y.; Chen, Y. New insights into the role of zero-valent iron surface oxidation layers in persulfate oxidation of dibutyl phthalate solutions. Chem. Eng. J. 2014, 250, 137–147. [Google Scholar] [CrossRef]
- Imran, M.A.; Tong, Y.; Ding, Y.; Liu, M.; Chen, H. Degradation of phthalic acid esters by zero-valent iron and persulfate using bacth experiments at different temperatures. Desal. Water. Treat. 2019, 150, 310–319. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, L.; Yao, J.; Herrmann, H.; Richnow, H.-H. Carbon and hydrogen isotope fractionation of phthalate esters during degradation by sulfate and hydroxyl radicals. Chem. Eng. J. 2018, 347, 111–118. [Google Scholar] [CrossRef]
- Henderson, A.D.; Demond, A.H. Permeability of iron sulfide (FeS)-based materials for groundwater remediation. Water Res. 2013, 47, 1267–1276. [Google Scholar] [CrossRef]
- Li, H.; Wan, J.; Ma, Y.; Wang, Y. Reaction pathway and oxidation mechanisms of dibutyl phthalate by persulfate activated with zero-valent iron. Sci. Total Environ. 2016, 562, 889–897. [Google Scholar] [CrossRef]
- Tay, K.S.; Rahman, N.A.; Abas, M.R.B. Fenton degradation of dialkylphthalates: Products and mechanism. Environ. Chem. Lett. 2011, 9, 539–546. [Google Scholar] [CrossRef]
- Huang, J.; Li, X.; Ma, M.; Li, D. Removal of di-(2-ethylhexyl) phthalate from aqueous solution by UV/peroxymonosulfate: Influencing factors and reaction pathways. Chem. Eng. J. 2017, 314, 182–191. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Y.; Gao, Y.; Bawa, M.; Huo, M.; Wang, X.; Zhu, S. Fast degradation of phthalate acid esters by polyoxometalate nanocatalysts through adsorption, esterolysis and oxidation. J. Hazard. Mater. 2019, 368, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.D.; Huang, C.P.; Nguyen, T.B.; Hsiung, C.F.; Wu, C.H.; Lin, Y.L.; Chen, C.W.; Hung, C.M. The degradation of phthalate esters in marine sediments by persulfate over iron-cerium oxide catalyst. Sci. Total Environ. 2019, 696, 133973. [Google Scholar] [CrossRef]
- Tomita, I.; Nakamura, Y.; Aoki, N.; Inui, N. Mutagenic/carcinogenic potential of DEHP and MEHP. Environ. Health Perspect. 1982, 45, 119–125. [Google Scholar] [CrossRef]
- Gan, L.; Zhong, Q.; Geng, A.; Wang, L.; Song, C.; Han, S.; Cui, J.; Xu, L. Cellulose derived carbon nanofiber: A promising biochar support to enhance the catalytic performance of CoFe2O4 in activating peroxymonosulfate for recycled dimethyl phthalate degradation. Sci. Total Environ. 2019, 694, 133705. [Google Scholar] [CrossRef]
- Peluffo, M.; Pardo, F.; Santos, A.; Romero, A. Use of different kinds of persulfate activation with iron for the remediation of a PAH-contaminated soil. Sci. Total Environ. 2016, 563–564, 649–656. [Google Scholar] [CrossRef]
- Xu, X.R.; Zhao, Z.Y.; Li, X.Y.; Gu, J.D. Chemical oxidative degradation of methyl tert-butyl ether in aqueous solution by Fenton’s reagent. Chemosphere 2004, 55, 73–79. [Google Scholar] [CrossRef]
- Zhang, Y.; Tran, H.P.; Du, X.; Hussain, I.; Huang, S.; Zhou, S.; Wen, W. Efficient pyrite activating persulfate process for degradation of p -chloroaniline in aqueous systems: A mechanistic study. Chem. Eng. J. 2017, 308, 1112–1119. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Z.; Feng, M.; Liu, W.; Wang, W.; Yang, Q.; Hu, Y. Degradation of 2,4-dichlorophenoxyacetic acid in water by persulfate activated with FeS (mackinawite). Chem. Eng. J. 2017, 313, 498–507. [Google Scholar] [CrossRef]
- Liang, S.H.; Kao, C.M.; Kuo, Y.C.; Chen, K.F. Application of persulfate-releasing barrier to remediate MTBE and benzene contaminated groundwater. J. Hazard. Mater. 2011, 185, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.F.; Kao, C.M.; Wu, L.C.; Surampalli, R.Y.; Liang, S.H. Methyl Tert-Butyl Ether (MTBE) Degradation by Ferrous Ion-Activated Persulfate Oxidation: Feasibility and Kinetics Studies. Water Environ. Res 2009, 81, 687–694. [Google Scholar] [CrossRef]
- Bonnisselgissinger, P.; Alnot, M.; Ehrhardt, J.J.; Behra, P. Surface Oxidation of Pyrite as a Function of pH. Environ. Sci. Technol. 1998, 32, 2839–2845. [Google Scholar] [CrossRef]
- Nesbitt, H.; Muir, I. Oxidation states and speciation of secondary products on pyrite and arsenopyrite reacted with mine waste waters and air. Miner. Petrol. 2005, 62, 123–144. [Google Scholar] [CrossRef]
- Li, J.; Dou, X.; Qin, H.; Sun, Y.; Yin, D.; Guan, X. Characterization methods of zerovalent iron for water treatment and remediation. Water Res. 2019, 148, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Feng, Y.; Liu, Y.; Wu, D. Applicability study on the degradation of acetaminophen via an H2O2/PDS-based advanced oxidation process using pyrite. Chemosphere 2018, 212, 438–446. [Google Scholar] [CrossRef]
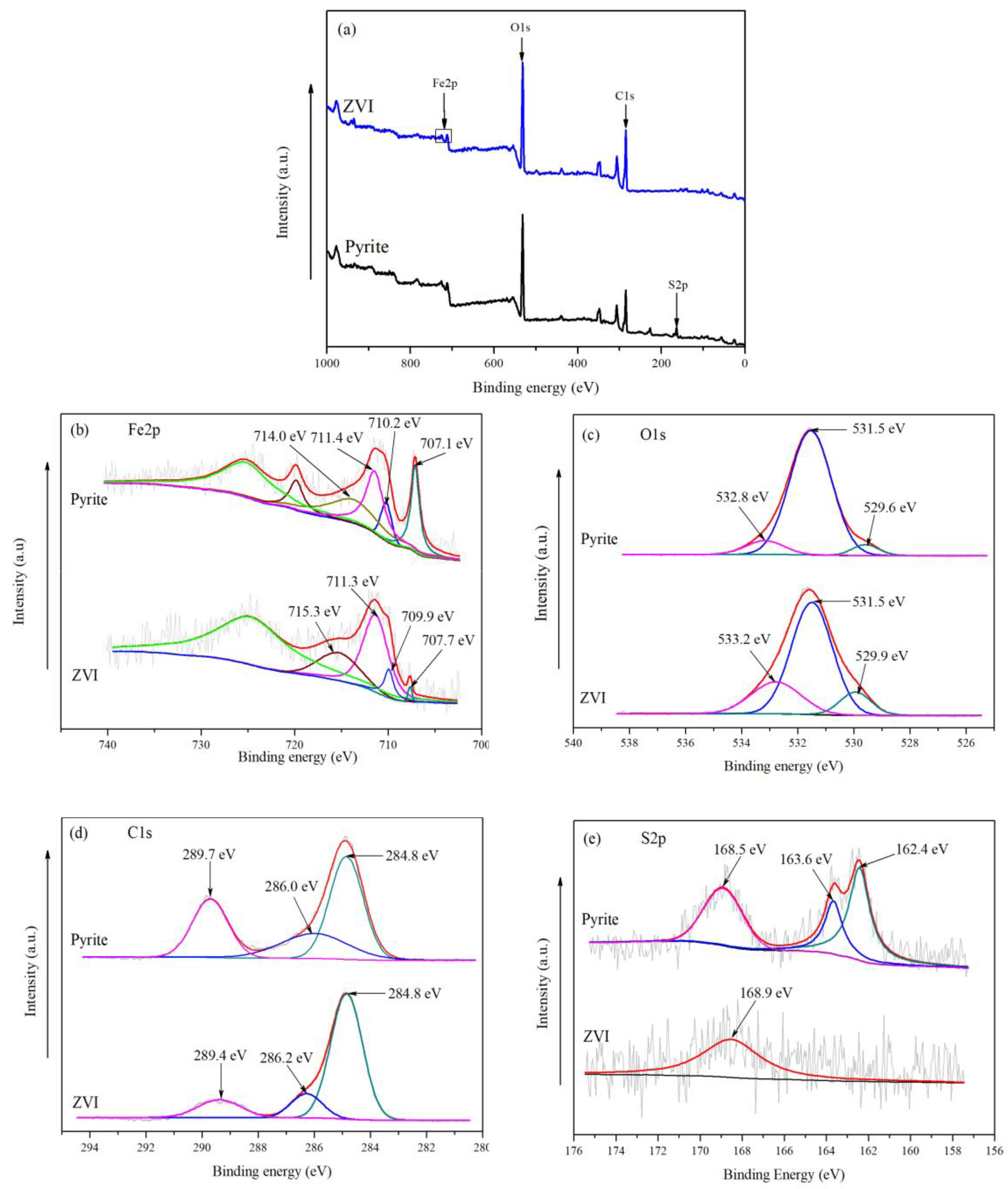
| Element | Pyrite–Persulfate System | ZVI–Persulfate System | ||||
|---|---|---|---|---|---|---|
| Peak Position | FWHM | Assignment | Peak Position | FWHM | Assignment | |
| Fe2p | 707.1 | 1.06 | Fe(II)-S | 707.7 | 0.5 | |
| 710.2 | 1.36 | 709.9 | 1.12 | |||
| 711.4 | 6.16 | 711.3 | 3.15 | |||
| O1s | 529.6 | 1.75 | Oxide | 529.9 | 1.72 | Oxide |
| 531.5 | 1.13 | Hydroxide | 531.5 | 1.31 | Hydroxide | |
| 532.8 | 1.61 | Absorbate | 533.2 | 2.08 | Absorbate | |
| C1s | 284.8 | 1.40 | C–C/C–H | 284.8 | 1.34 | C–C/C–H |
| 286.0 | 2.79 | C–O | 286.2 | 1.31 | C–O | |
| 289.7 | 1.49 | 289.4 | 1.80 | |||
| S2p | 162.4 | 1.18 | – | – | – | |
| 163.6 | 2.00 | – | – | – | ||
| 168.5 | 1.15 | 168.9 | 3.29 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imran, M.A.; Tong, Y.; Hu, Q.; Liu, M.; Chen, H. Effects of Persulfate Activation with Pyrite and Zero-Valent Iron for Phthalate Acid Ester Degradation. Water 2020, 12, 354. https://doi.org/10.3390/w12020354
Imran MA, Tong Y, Hu Q, Liu M, Chen H. Effects of Persulfate Activation with Pyrite and Zero-Valent Iron for Phthalate Acid Ester Degradation. Water. 2020; 12(2):354. https://doi.org/10.3390/w12020354
Chicago/Turabian StyleImran, Muhammad A., Yuzhen Tong, Qing Hu, Mingzhu Liu, and Honghan Chen. 2020. "Effects of Persulfate Activation with Pyrite and Zero-Valent Iron for Phthalate Acid Ester Degradation" Water 12, no. 2: 354. https://doi.org/10.3390/w12020354
APA StyleImran, M. A., Tong, Y., Hu, Q., Liu, M., & Chen, H. (2020). Effects of Persulfate Activation with Pyrite and Zero-Valent Iron for Phthalate Acid Ester Degradation. Water, 12(2), 354. https://doi.org/10.3390/w12020354






