Structure and Functions of Hydrocarbon-Degrading Microbial Communities in Bioelectrochemical Systems
Abstract
1. Introduction
2. Materials and Methods
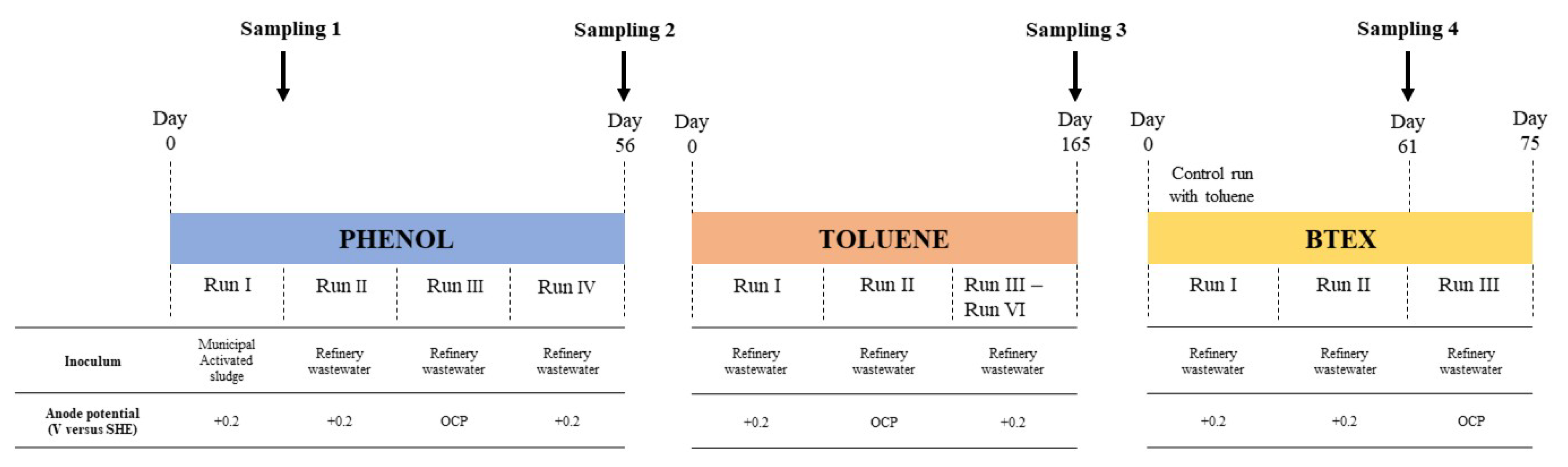
2.1. Reactor Set-Up and Operation
2.2. Experimental Procedure
2.3. DNA Extraction, Metagenome Sequencing, and Sequence Analyses
2.4. Bioinformatics Procedures
2.5. Statistical Analysis
3. Results and Discussion
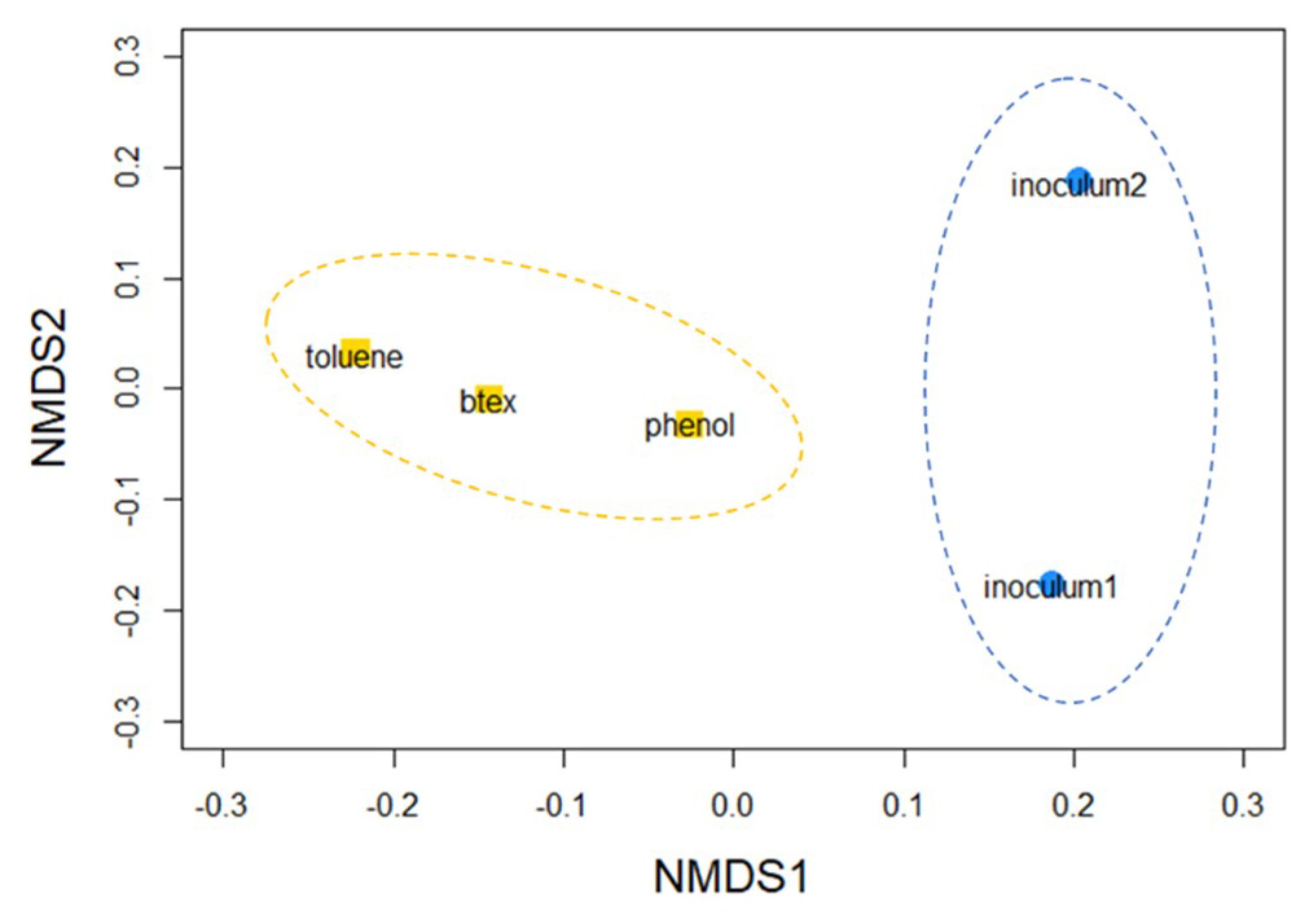
3.1. Microbial Community Composition
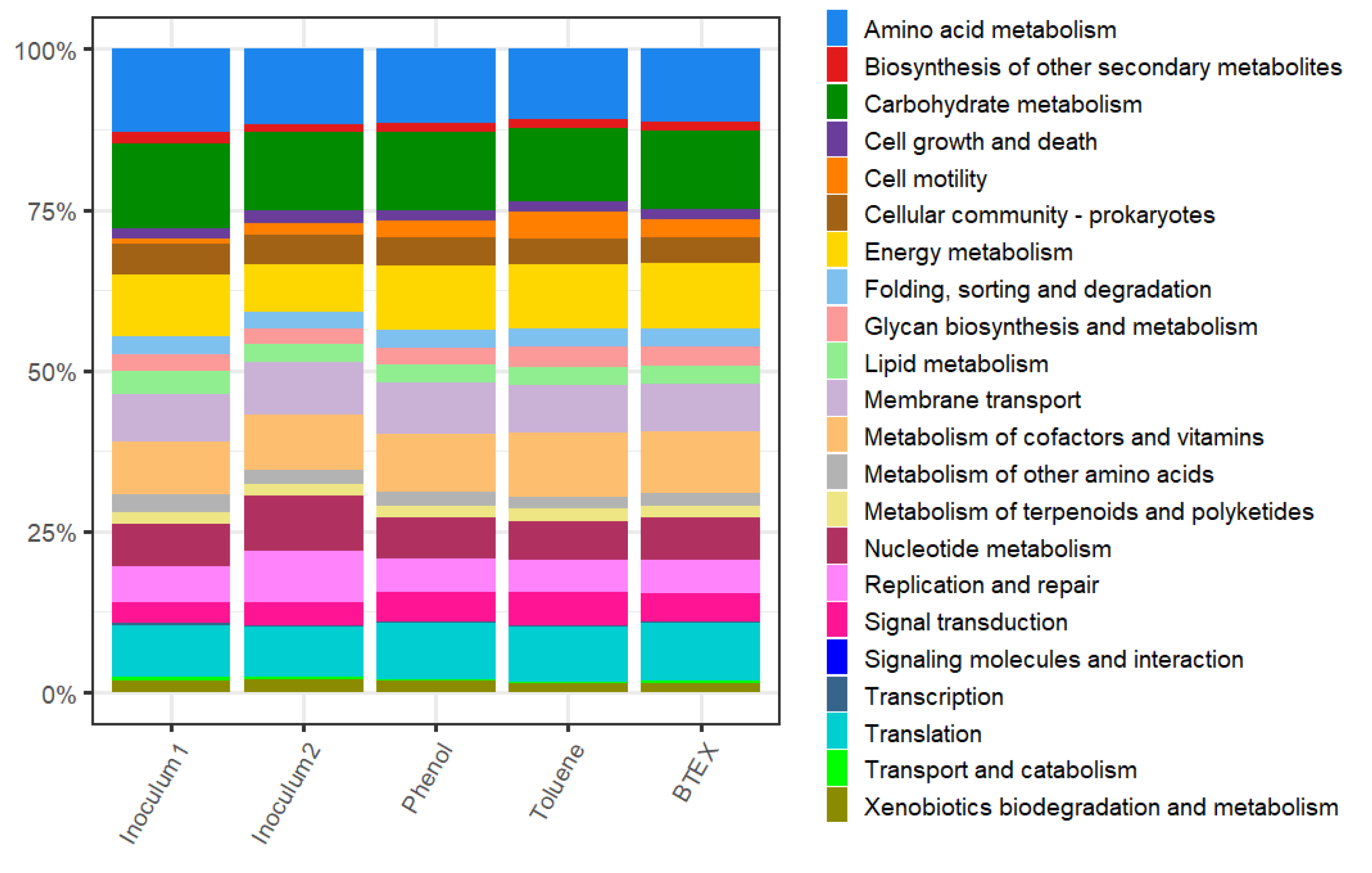
3.2. Functional Structure and Metabolic Potential of Anode Metagenomes
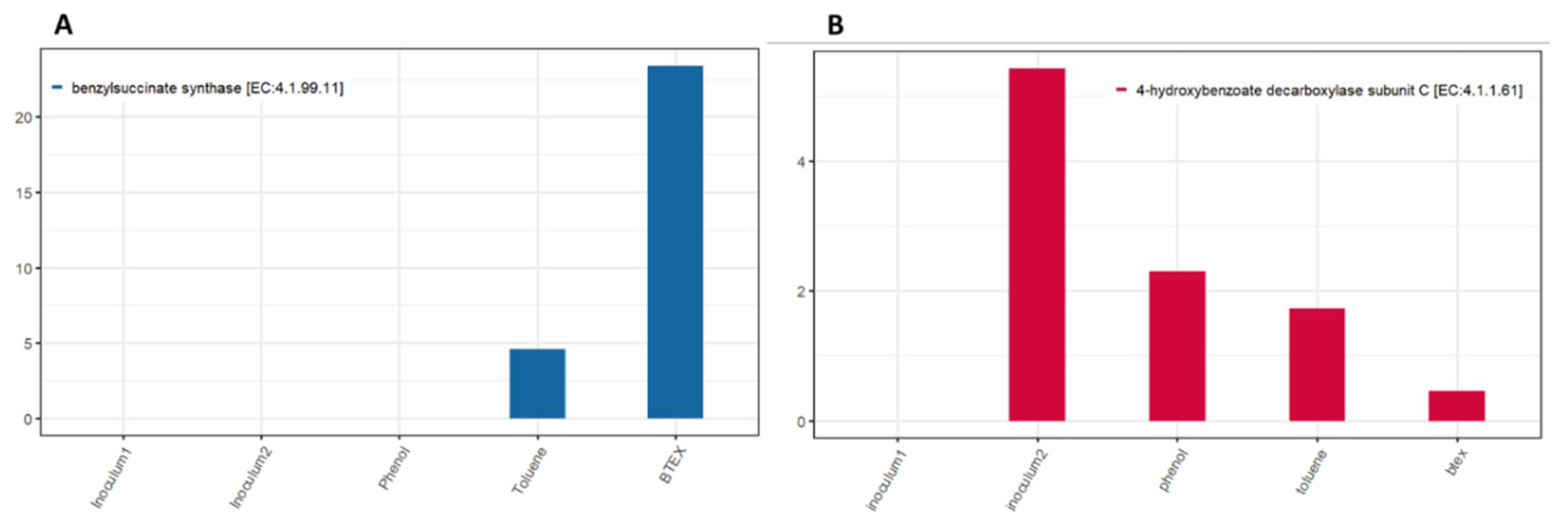
3.3. Biomarker Genes for Hydrocarbon Degradation
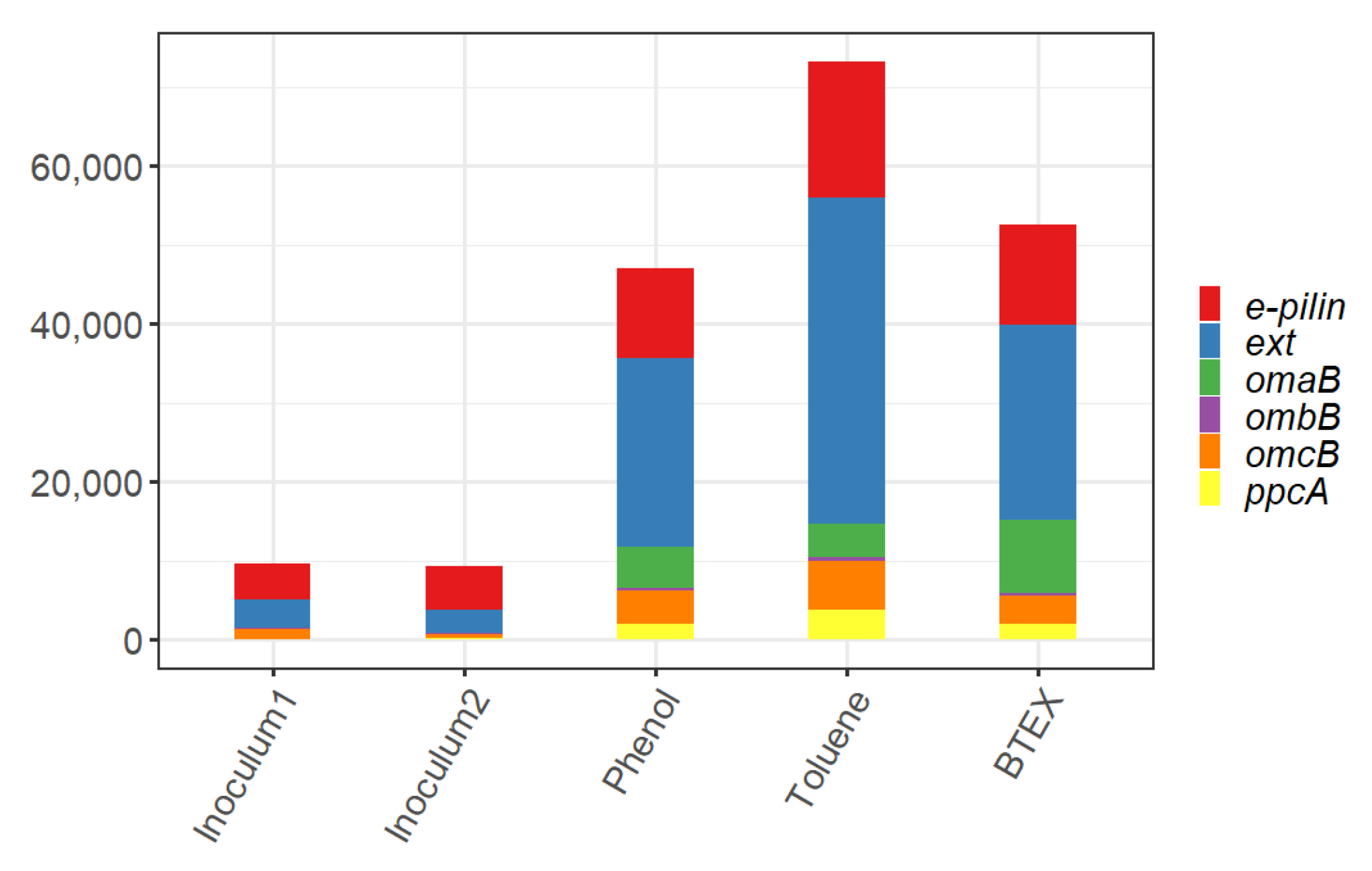
3.4. Biomarker Genes for Electrogenic Activity
3.5. Binning: Composite Genomes of Individual Populations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abha, S.; Singh, C.S. Hydrocarbon Pollution: Effects on Living Organisms, Remediation of Contaminated Environments, and Effects of Heavy Metals Co-Contamination on Bioremediation. In Introduction to Enhanced Oil Recovery (EOR) Processes and Bioremediation of Oil-Contaminated Sites; Romero-Zerón, L., Ed.; IntechOpen: Rijeka, Croatia, 2012; ISBN 978-953-51-0629-6. Available online: https://www.intechopen.com/books/introduction-to-enhanced-oil-recovery-eor-processes-and-bioremediation-of-oil-contaminated-sites/heavy-metals-interference-in-microbial-degradation-of-crude-oil-petroleum-hydrocarbons-the-challenge (accessed on 30 November 2019).
- Varjani, S.J. Microbial degradation of petroleum hydrocarbons. Bioresour. Technol. 2017, 223, 277–286. [Google Scholar] [CrossRef]
- Daghio, M.; Aulenta, F.; Vaiopoulou, E.; Franzetti, A.; Head, I.M.; Arends, J.B.A.; Sherry, A.; Su, A.; Bestetti, G.; Rabaey, K. Electrobioremediation of oil spills. Water Res. 2017, 114, 351–370. [Google Scholar] [CrossRef]
- Espinoza Tofalos, A.; Daghio, M.; Gonzalez, M.; Papacchini, M.; Franzetti, A.; Seeger, M. Toluene degradation by Cupriavidus metallidurans CH34 in nitrate-reducing conditions and in Bioelectrochemical Systems. FEMS Microbiol. Lett. 2018, 365, 1–8. [Google Scholar]
- Rabaey, K.; Angenent, L.; Schroder, U.; Keller, J. Bioelectrochemical Systems; IWA Publishing: London, UK, 2010; ISBN 9781843392330. [Google Scholar]
- Logan, B.E.; Regan, J.M. Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol. 2006, 14, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Harnisch, F. Is there a Specific Ecological Niche for Electroactive Microorganisms? ChemElectroChem 2016, 3, 1282–1295. [Google Scholar] [CrossRef]
- Sun, J.Z.; Kingori, G.P.; Si, R.W.; Zhai, D.D.; Liao, Z.H.; Sun, D.Z.; Zheng, T.; Yong, Y.C. Microbial fuel cell-based biosensors for environmental monitoring: A review. Water Sci. Technol. 2015, 71, 801–809. [Google Scholar] [CrossRef]
- Su, L.; Jia, W.; Hou, C.; Lei, Y. Microbial biosensors: A review. Biosens. Bioelectron. 2011, 26, 1788–1799. [Google Scholar] [CrossRef]
- Wang, H.; Luo, H.; Fallgren, P.H.; Jin, S.; Ren, Z.J. Bioelectrochemical system platform for sustainable environmental remediation and energy generation. Biotechnol. Adv. 2015, 33, 317–334. [Google Scholar] [CrossRef]
- Lovley, D.R. Live wires: Direct extracellular electron exchange for bioenergy and the bioremediation of energy-related contamination. Energy Environ. Sci. 2011, 4, 4896. [Google Scholar] [CrossRef]
- Morris, J.M.; Jin, S.; Crimi, B.; Pruden, A. Microbial fuel cell in enhancing anaerobic biodegradation of diesel. Chem. Eng. J. 2009, 146, 161–167. [Google Scholar] [CrossRef]
- Viggi, C.C.; Presta, E.; Bellagamba, M.; Kaciulis, S.; Balijepalli, S.K.; Zanaroli, G.; Papini, M.P.; Rossetti, S.; Aulenta, F. The “Oil-Spill Snorkel”: An innovative bioelectrochemical approach to accelerate hydrocarbons biodegradation in marine sediments. Front. Microbiol. 2015, 6, 1–11. [Google Scholar]
- Daghio, M.; Vaiopoulou, E.; Patil, S.A.; Suárez-Suárez, A.; Head, I.M.; Franzetti, A.; Rabaey, K. Anodes stimulate anaerobic toluene degradation via sulfur cycling in marine sediments. Appl. Environ. Microbiol. 2016, 82, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Daghio, M.; Espinoza, A.; Leoni, B.; Cristiani, P.; Papacchini, M.; Jalilnejad, E.; Bestetti, G.; Franzetti, A. Bioelectrochemical BTEX removal at different voltages: Assessment of the degradation and characterization of the microbial communities. J. Hazard. Mater. 2018, 341, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Palma, E.; Daghio, M.; Franzetti, A.; Petrangeli Papini, M.; Aulenta, F. The bioelectric well: A novel approach for in situ treatment of hydrocarbon-contaminated groundwater. Microb. Biotechnol. 2018, 11, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Palma, E.; Daghio, M.; Espinoza Tofalos, A.; Franzetti, A.; Cruz Viggi, C.; Fazi, S.; Petrangeli Papini, M.; Aulenta, F. Anaerobic electrogenic oxidation of toluene in a continuous-flow bioelectrochemical reactor: Process performance, microbial community analysis, and biodegradation pathways. Environ. Sci. Water Res. Technol. 2018, 4, 2136–2145. [Google Scholar] [CrossRef]
- Palma, E.; Espinoza Tofalos, A.; Daghio, M.; Franzetti, A.; Tsiota, P.; Cruz Viggi, C.; Papini, M.P.; Aulenta, F. Bioelectrochemical treatment of groundwater containing BTEX in a continuous-flow system: Substrate interactions, microbial community analysis, and impact of sulfate as a co-contaminant. New Biotechnol. 2019, 53, 41–48. [Google Scholar] [CrossRef]
- Kouzuma, A.; Watanabe, K. Metagenomic insights into the ecology and physiology of microbes in bioelectrochemical systems. Bioresour. Technol. 2018, 255, 302–307. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, X.; Braithwaite, D.; He, Z. Phylogenetic and Metagenomic Analyses of Substrate-Dependent Bacterial Temporal Dynamics in Microbial Fuel Cells. PLoS ONE 2014, 9, e107460. [Google Scholar] [CrossRef]
- Yamamuro, A.; Kouzuma, A.; Abe, T.; Watanabe, K. Metagenomic analyses reveal the involvement of syntrophic consortia in methanol/electricity conversion in microbial fuel cells. PLoS ONE 2014, 9, e98425. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, J.; Zhao, Q.; Wang, K.; Yu, H. Analysis of functional genomes from metagenomes: Revealing the accelerated electron transfer in microbial fuel cell with rhamnolipid addition. Bioelectrochemistry 2018, 119, 59–67. [Google Scholar] [CrossRef]
- Kiseleva, L.; Garushyants, S.K.; Ma, H.; Simpson, D.J.W.; Fedorovich, V.; Cohen, M.F.; Goryanin, I. Taxonomic and functional metagenomic analysis of anodic communities in two pilot-scale microbial fuel cells treating different industrial wastewaters. J. Integr. Bioinform. 2015, 12, 273. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.R.; Wang, Q.; Fish, J.A.; Chai, B.; McGarrell, D.M.; Sun, Y.; Brown, C.T.; Porras-Alfaro, A.; Kuske, C.R.; Tiedje, J.M. Ribosomal Database Project: Data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014, 42, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Franzetti, A.; Tagliaferri, I.; Gandolfi, I.; Bestetti, G.; Minora, U.; Mayer, C.; Azzoni, R.S.; Diolaiuti, G.; Smiraglia, C.; Ambrosini, R. Light-dependent microbial metabolisms drive carbon fluxes on glacier surfaces. ISME J. 2016, 10, 2984–2988. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Leung, H.C.M.; Yiu, S.M.; Chin, F.Y.L. IDBA-UD: A de novo assembler for single-cell and metagenomic sequencing data with highly uneven depth. Bioinformatics 2012, 28, 1420–1428. [Google Scholar] [CrossRef]
- Hyatt, D.; Chen, G.-L.; LoCascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic gene recognition and translation initiation site identification. Nat. Commun. 2010, 11, 1–11. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2014, 12, 59–60. [Google Scholar] [CrossRef]
- Huson, D.H.; Mitra, S.; Ruscheweyh, H.-J.; Weber, N.; Schuster, S.C. Integrative analysis of environmental sequences using MEGAN4. Genome Res. 2011, 21, 1552–1560. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, P.S.; Schrott, G.D.; Busalmen, J.P. A long way to the electrode: How do Geobacter cells transport their electrons? Biochem. Soc. Trans. 2012, 40, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.E.; Dang, Y.; Walker, D.J.F.; Lovley, D.R. The electrically conductive pili of Geobacter species are a recently evolved feature for extracellular electron transfer. Microb. Genom. 2016, 2, e000072. [Google Scholar] [CrossRef] [PubMed]
- Otero, F.J.; Chan, C.H.; Bond, D.R. Identification of Different Putative Outer Membrane Electron Conduits Necessary for Fe(III) Citrate, Fe(III) Oxide, Mn(IV) Oxide, or Electrode Reduction by Geobacter sulfurreducens. J. Bacteriol. 2018, 200, 1–20. [Google Scholar]
- Ueki, T.; Nevin, K.P.; Rotaru, A.E.; Wang, L.Y.; Ward, J.E.; Woodard, T.L.; Lovley, D.R. Geobacter strains expressing poorly conductive pili reveal constraints on direct interspecies electron transfer mechanisms. MBio 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Wu, Y.-W.; Tang, Y.-H.; Tringe, S.G.; Simmons, B.A.; Singer, S.W. MaxBin: An automated binning method to recover individual genomes from metagenomes using an expectation-maximization algorithm. Microbiome 2014, 2, 4904–4909. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Karp, P.D.; Paley, S.; Romero, P. The pathway tools software. Bioinformatics 2002, 18, 225–232. [Google Scholar] [CrossRef]
- Rodriguez-R, L.M.; Gunturu, S.; Harvey, W.T.; Rosselló-Mora, R.; Tiedje, J.M.; Cole, J.R.; Konstantinidis, K.T. The Microbial Genomes Atlas (MiGA) webserver: Taxonomic and gene diversity analysis of Archaea and Bacteria at the whole genome level. Nucleic Acids Res. 2018, 46, W282–W288. [Google Scholar] [CrossRef]
- Parks, D.H.; Chuvochina, M.; Waite, D.W.; Rinke, C.; Skarshewski, A.; Chaumeil, P.A.; Hugenholtz, P. A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat. Biotechnol. 2018, 36, 996. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Morishima, K. BlastKOALA and GhostKOALA: KEGG Tools for Functional Characterization of Genome and Metagenome Sequences. J. Mol. Biol. 2016, 428, 726–731. [Google Scholar] [CrossRef]
- Aramaki, T.; Blanc-Mathieu, R.; Endo, H.; Ohkubo, K.; Kanehisa, M.; Goto, S.; Ogata, H. KofamKOALA: KEGG ortholog assignment based on profile HMM and adaptive score threshold. bioRxiv 2019, 602110. [Google Scholar] [CrossRef] [PubMed]
- Militon, C.; Jézéquel, R.; Gilbert, F.; Corsellis, Y.; Sylvi, L.; Cravo-Laureau, C.; Duran, R.; Cuny, P. Dynamics of bacterial assemblages and removal of polycyclic aromatic hydrocarbons in oil-contaminated coastal marine sediments subjected to contrasted oxygen regimes. Environ. Sci. Pollut. Res. 2015, 22, 15260–15272. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pantoja, D.; Donoso, R.; Agulló, L.; Córdova, M.; Seeger, M.; Pieper, D.H.; González, B. Genomic analysis of the potential for aromatic compounds biodegradation in Burkholderiales. Environ. Microbiol. 2012, 14, 1091–1117. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, S.; Barra, B.; Gregory Caporaso, J.; Seeger, M. From rare to dominant: A fine-tuned soil bacterial bloom during petroleum hydrocarbon bioremediation. Appl. Environ. Microbiol. 2016, 82, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Von Netzer, F.; Kuntze, K.; Vogt, C.; Richnow, H.H.; Boll, M.; Lueders, T. Functional gene markers for fumarate-adding and dearomatizing key enzymes in anaerobic aromatic hydrocarbon degradation in terrestrial environments. J. Mol. Microbiol. Biotechnol. 2016, 26, 180–194. [Google Scholar] [CrossRef]
- Acosta-González, A.; Marqués, S. Bacterial diversity in oil-polluted marine coastal sediments. Curr. Opin. Biotechnol. 2016, 38, 24–32. [Google Scholar] [CrossRef]
- Fuchs, G.; Boll, M.; Heider, J. Microbial degradation of aromatic compounds—From one strategy to four. Nat. Rev. Microbiol. 2011, 9, 803–816. [Google Scholar] [CrossRef]
- Chan, C.H.; Levar, C.E.; Jiménez-Otero, F.; Bond, D.R. Genome scale mutational analysis of Geobacter sulfurreducens reveals distinct molecular mechanisms for respiration and sensing of poised electrodes versus Fe(III) oxides. J. Bacteriol. 2017, 199, 1–18. [Google Scholar] [CrossRef]
- Acosta-Gonzalez, A.; Rossellò-Mòra, R.; Marques, S. Diversity of Benzylsuccinate Synthase-Like (bssA) Genes in Hydrocarbon-Polluted Marine Sediments Suggests Substrate- Dependent Clustering. Appl. Environ. Microbiol. 2013, 79, 3667–3676. [Google Scholar] [CrossRef]
- Heider, J.; Spormann, A.M.; Beller, H.R.; Widdel, F. Anaerobic bacterial metabolism of hydrocarbons. FEMS Microbiol. Rev. 1999, 22, 459–473. [Google Scholar] [CrossRef]
- Foght, J. Anaerobic biodegradation of aromatic hydrocarbons: Pathways and prospects. J. Mol. Microbiol. Biotechnol. 2008, 15, 93–120. [Google Scholar] [CrossRef] [PubMed]
- Tschech, A.; Fuchs, G. Anaerobic degradation of phenol via carboxylation to 4-hydroxybenzoate: In vitro study of isotope exchange between 14CO2 and 4-hydroxybenzoate. Arch. Microbiol. 1989, 152, 594–599. [Google Scholar] [CrossRef]
- Tschech, A.; Fuchs, G. Anaerobic degradation of phenol by pure cultures of newly isolated denitrifying pseudomonads. Arch. Microbiol. 1987, 148, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Lack, A.; Tommasi, I.; Aresta, M.; Fuchs, G. Catalytic properties of phenol carboxylase. Eur. J. Biochem. 1991, 197, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Breese, K.; Fuchs, G. 4-hydroxybenzoyl-CoA reductase (dehydroxylating) from the denitrifying bacterium Thauera aromatica—Prosthetic groups, electron donor, and genes of a member of the molybdenum-flavin-iron-sulfur proteins. Eur. J. Biochem. 1998, 251, 916–923. [Google Scholar] [CrossRef]
- Laempe, D.; Jahn, M.; Breese, K.; Schägger, H.; Fuchs, G. Anaerobic metabolism of 3-hydroxybenzoate by the denitrifying bacterium Thauera aromatica. J. Bacteriol. 2001, 183, 968–979. [Google Scholar] [CrossRef][Green Version]
- Vogt, C.; Kleinsteuber, S.; Richnow, H.H. Anaerobic benzene degradation by bacteria. Microb. Biotechnol. 2011, 4, 710–724. [Google Scholar] [CrossRef]
- Abu Laban, N.; Selesi, D.; Rattei, T.; Tischler, P.; Meckenstock, R.U. Identification of enzymes involved in anaerobic benzene degradation by a strictly anaerobic iron-reducing enrichment culture. Environ. Microbiol. 2010, 12, 2783–2796. [Google Scholar] [CrossRef]
- Rakoczy, J.; Feisthauer, S.; Wasmund, K.; Bombach, P.; Neu, T.R.; Vogt, C.; Richnow, H.H. Benzene and sulfide removal from groundwater treated in a microbial fuel cell. Biotechnol. Bioeng. 2013, 110, 3104–3113. [Google Scholar] [CrossRef]
- Zhang, T.; Gannon, S.M.; Nevin, K.P.; Franks, A.E.; Lovley, D.R. Stimulating the anaerobic degradation of aromatic hydrocarbons in contaminated sediments by providing an electrode as the electron acceptor. Environ. Microbiol. 2010, 12, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Tremblay, P.L.; Chaurasia, A.K.; Smith, J.A.; Bain, T.S.; Lovley, D.R. Anaerobic benzene oxidation via phenol in Geobacter metallireducens. Appl. Environ. Microbiol. 2013, 79, 7800–7806. [Google Scholar] [CrossRef] [PubMed]
- Abed, R.M.M.; Musat, N.; Musat, F.; Mußmann, M. Structure of microbial communities and hydrocarbon-dependent sulfate reduction in the anoxic layer of a polluted microbial mat. Mar. Pollut. Bull. 2011, 62, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Ueki, T.; Zhang, T.; Malvankar, N.S.; Shrestha, P.M.; Flanagan, K.A.; Aklujkar, M.; Butler, J.E.; Giloteaux, L.; Rotaru, A.E.; et al. Geobacter: The Microbe Electric’s Physiology, Ecology, and Practical Applications; Academic Press: Amsterdam, The Netherlands, 2011; Volume 59, ISBN 9780123876614. [Google Scholar]
| Reference | Substrate | Influent Pollutant Concentration (mg L−1) | Average Pollutant Removal Rate (mg L−1 day) | Best Pollutant Removal Percentage (%) | Average Coulombic Efficiency (%) | |
|---|---|---|---|---|---|---|
| Palma et al., 2018 [16] | Phenol | 25 | 59 ± 3 | 99.5 ± 0.4 | 104 ± 4 | |
| Palma et al., 2018 [17] | Toluene | 25 | 67.2 ± 5.7 | 95.0 ± 0.5 | 79 ± 7 | |
| Palma et al., 2019 [18] | Mixture of BTEX | Benzene | 5 | 6.1 ± 0.3 | 44.8 ± 2.2 | 53.7 ± 2.1 |
| Toluene | 14 | 31.3 ± 1.5 | 82.1 ± 3.9 | |||
| Ethyl-benzene | 2 | 3.3 ± 0.1 | 60.6 ± 1.8 | |||
| Sum of o-, m-, p-xylenes | 4 | 4.5 ± 0.2 | 41.2 ± 1.8 | |||
| Name | Type of Sample | Sampling Time | Feature |
|---|---|---|---|
| Inoculum 1 | Sludge | Before inoculating | Activated sludge |
| Inoculum 2 | Wastewater | Before inoculating | Refinery wastewater |
| Phenol1 | Anodic Graphite | Sampling 1 | Graphite collected at the end of Run I, inoculated with Inoculum 1 |
| Phenol | Anodic Graphite | Sampling 2 | Graphite collected at the end of Run IV, inoculated with Inoculum 2 |
| Toluene | Anodic Graphite | Sampling 3 | Graphite collected at the end of Run VI, inoculated with Inoculum 2 |
| BTEX | Anodic Graphite | Sampling 4 | Graphite collected at the end of Run II, inoculated with Inoculum 2 |
| Reconstructed Genome (Bin) | Taxonomy | Representative of the Sample | Presence of Genes That Encode for the Degradation of Hydrocarbons | Total Number of Genes That Encode for EET | |||
|---|---|---|---|---|---|---|---|
| Toluene | Xylenes | Ethylbenzene | Phenol | ||||
| Fumarate Addition | Degradation in Denitrifying Bacteria | Degradation through 4-Hydroxybenzoate | |||||
| Bin 2 | Genus: Geobacter | Toluene, phenol, BTEX | X | 19 | |||
| Bin 107 | Family: Geobacteraceae | Phenol, Toluene | X | X | X | 26 | |
| Bin 221 | Genus: Desulfomicrobium | Inoculum 2 | X | X | X | 0 | |
| Bin 277 | Class: Actinobacteria | Inoculum 1 | X | X | 0 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinoza-Tofalos, A.; Daghio, M.; Palma, E.; Aulenta, F.; Franzetti, A. Structure and Functions of Hydrocarbon-Degrading Microbial Communities in Bioelectrochemical Systems. Water 2020, 12, 343. https://doi.org/10.3390/w12020343
Espinoza-Tofalos A, Daghio M, Palma E, Aulenta F, Franzetti A. Structure and Functions of Hydrocarbon-Degrading Microbial Communities in Bioelectrochemical Systems. Water. 2020; 12(2):343. https://doi.org/10.3390/w12020343
Chicago/Turabian StyleEspinoza-Tofalos, Anna, Matteo Daghio, Enza Palma, Federico Aulenta, and Andrea Franzetti. 2020. "Structure and Functions of Hydrocarbon-Degrading Microbial Communities in Bioelectrochemical Systems" Water 12, no. 2: 343. https://doi.org/10.3390/w12020343
APA StyleEspinoza-Tofalos, A., Daghio, M., Palma, E., Aulenta, F., & Franzetti, A. (2020). Structure and Functions of Hydrocarbon-Degrading Microbial Communities in Bioelectrochemical Systems. Water, 12(2), 343. https://doi.org/10.3390/w12020343







