Biological Reduction of Organic Matter in Buji River Sediment (Shenzhen, China) with Artificial Oxygenation
Abstract
:1. Introduction
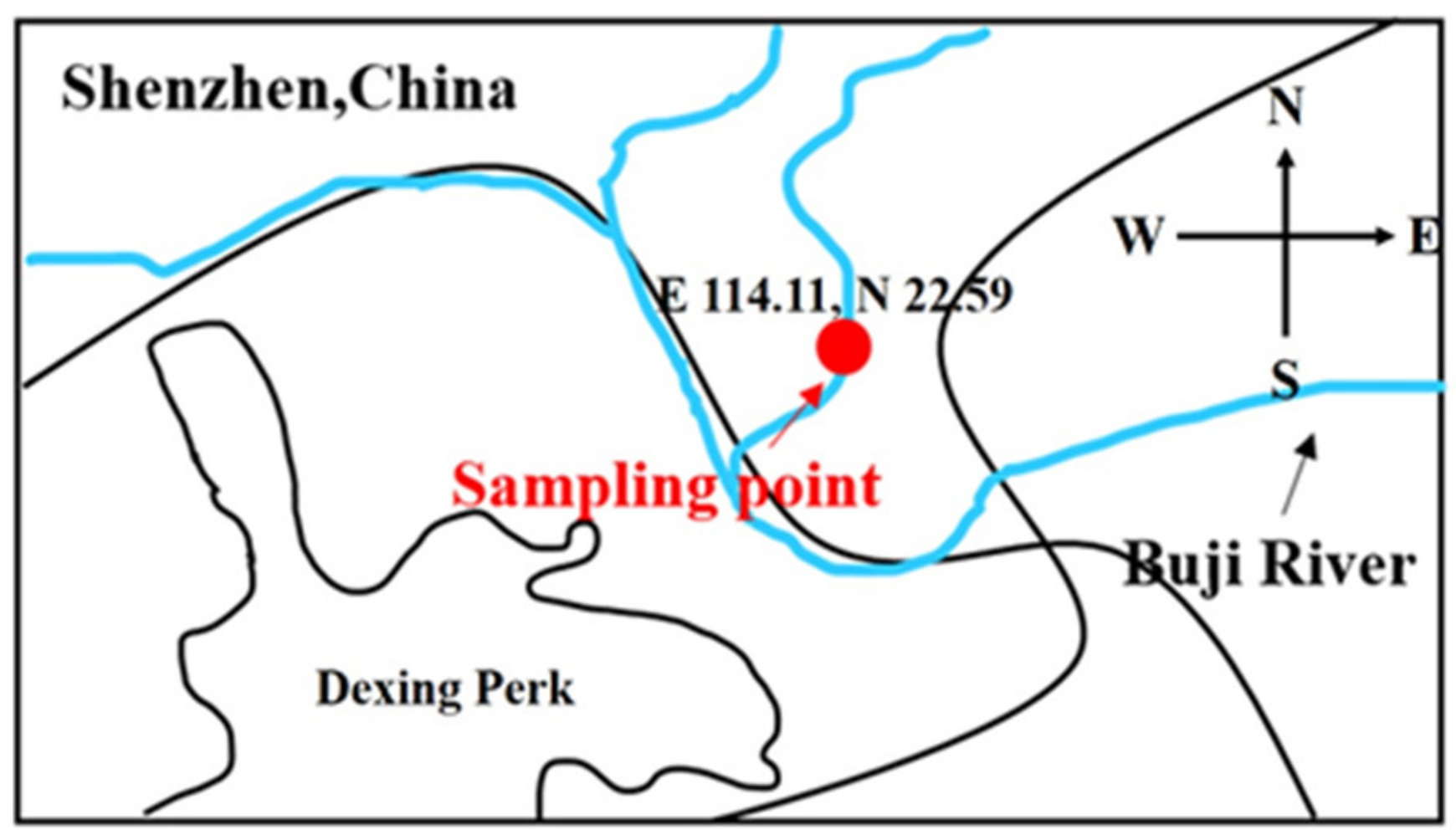
2. Materials and Methods
2.1. Chemicals and Materials
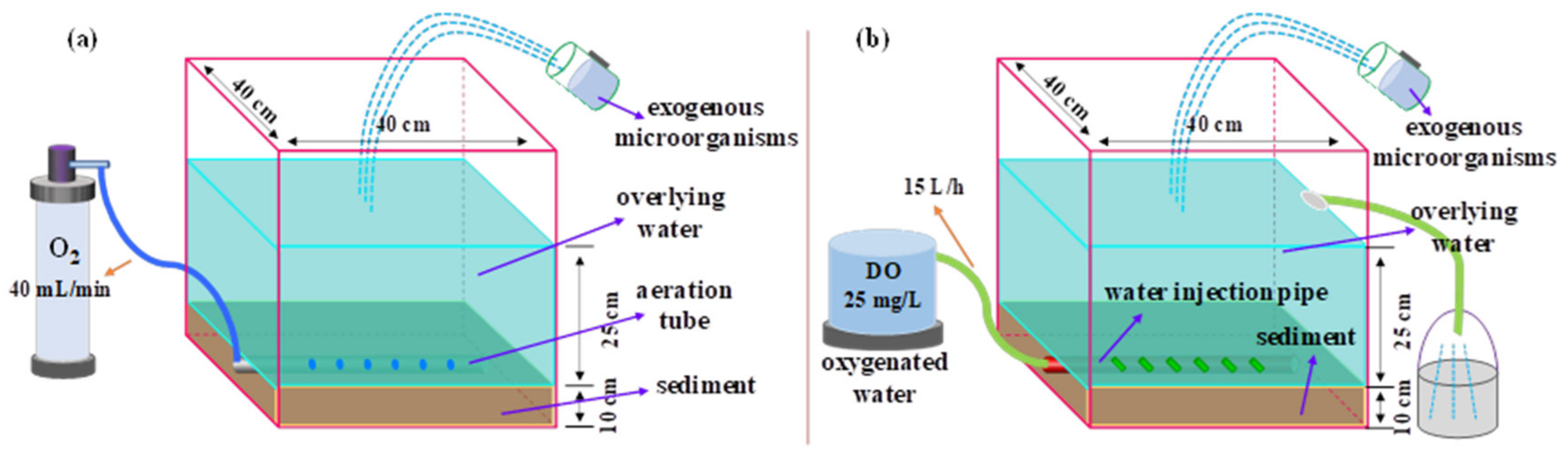
2.2. Pure Oxygen Aeration Treatment
2.3. Oxygen Enriched Water Injection Treatment
2.4. Physical-Chemical Properties of Water and Sediment
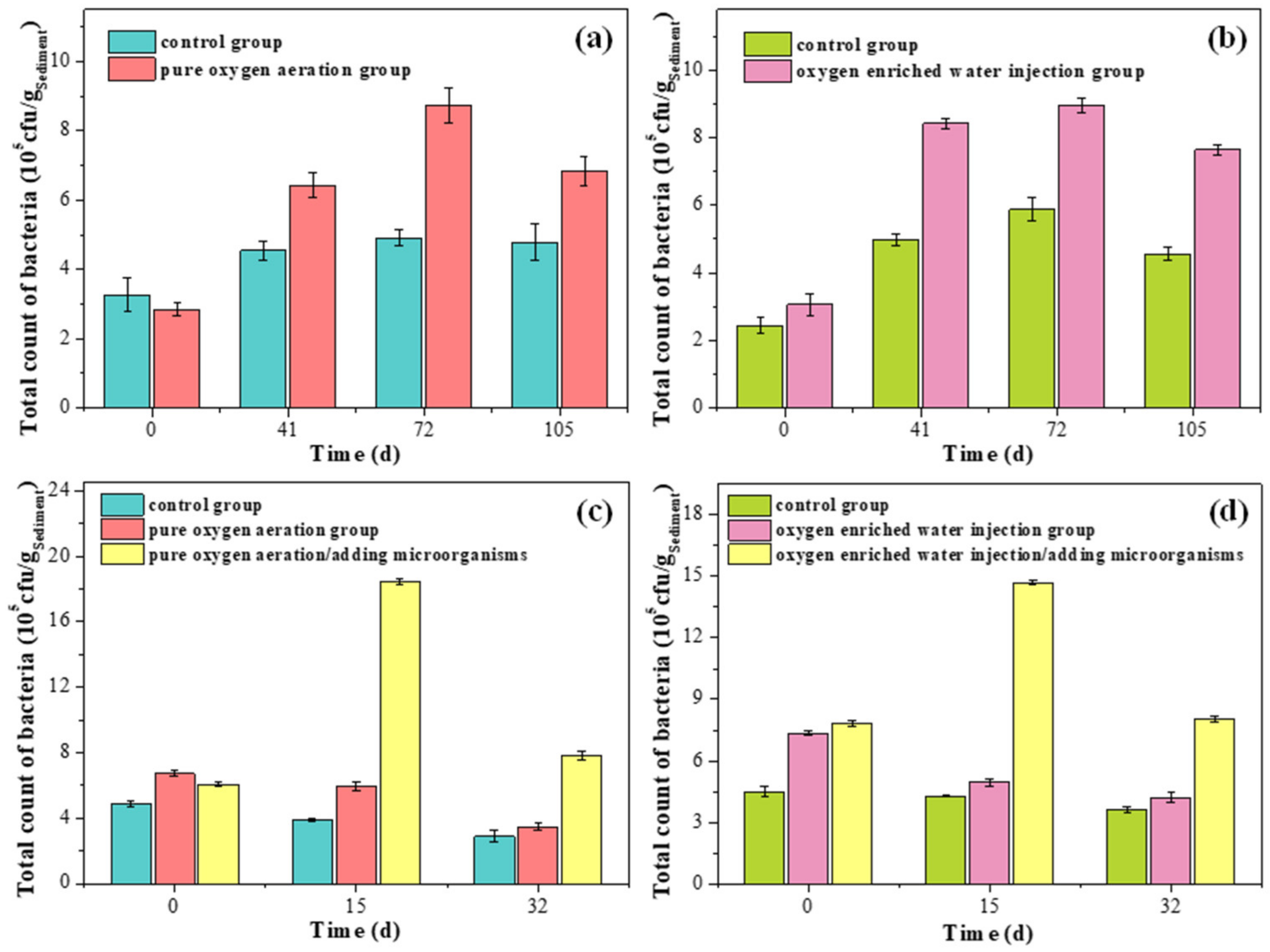
2.5. Determination of the Total Bacterial Count
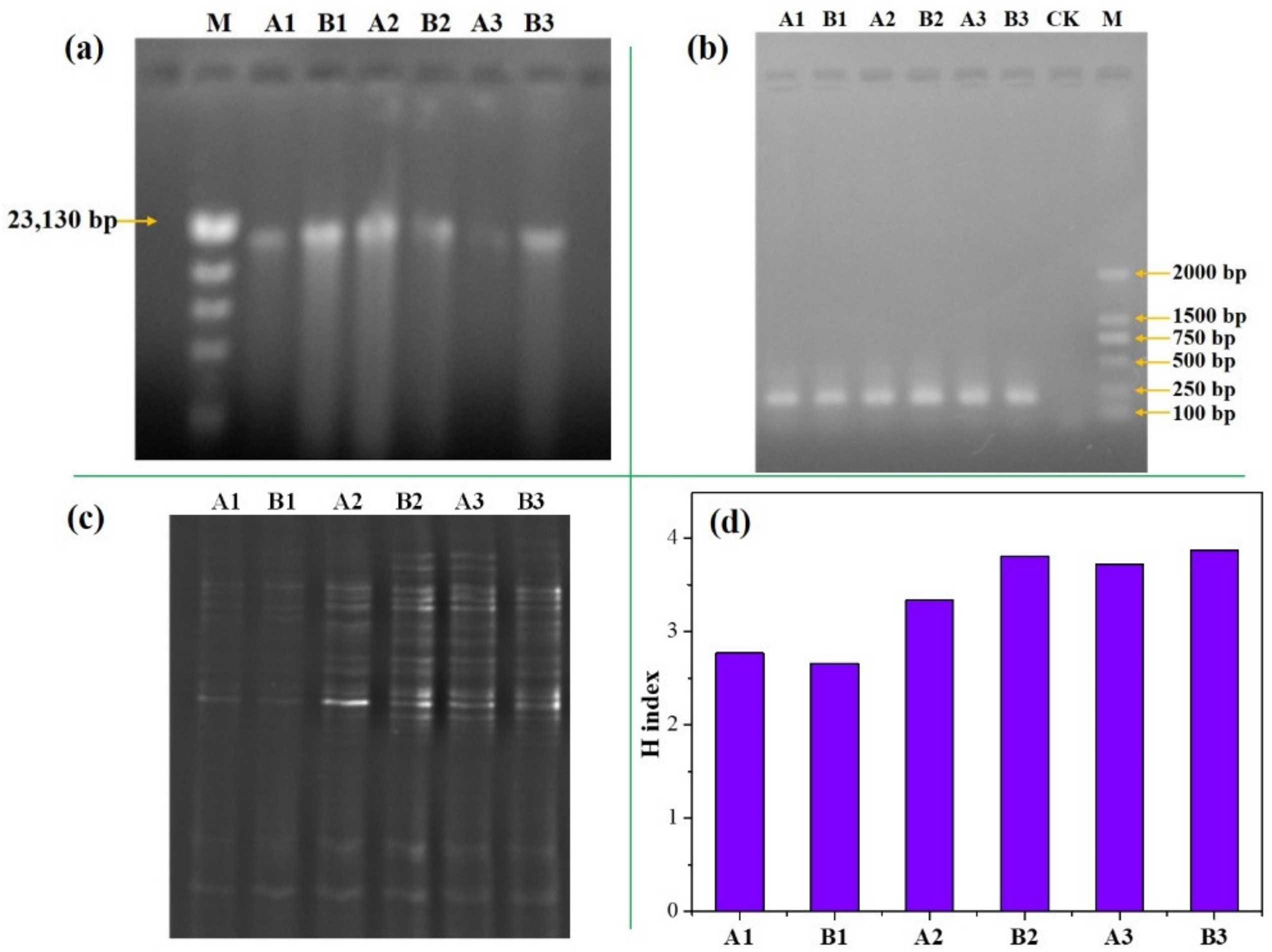
2.6. PCR-DGGE Assays
2.7. DGGE Analysis
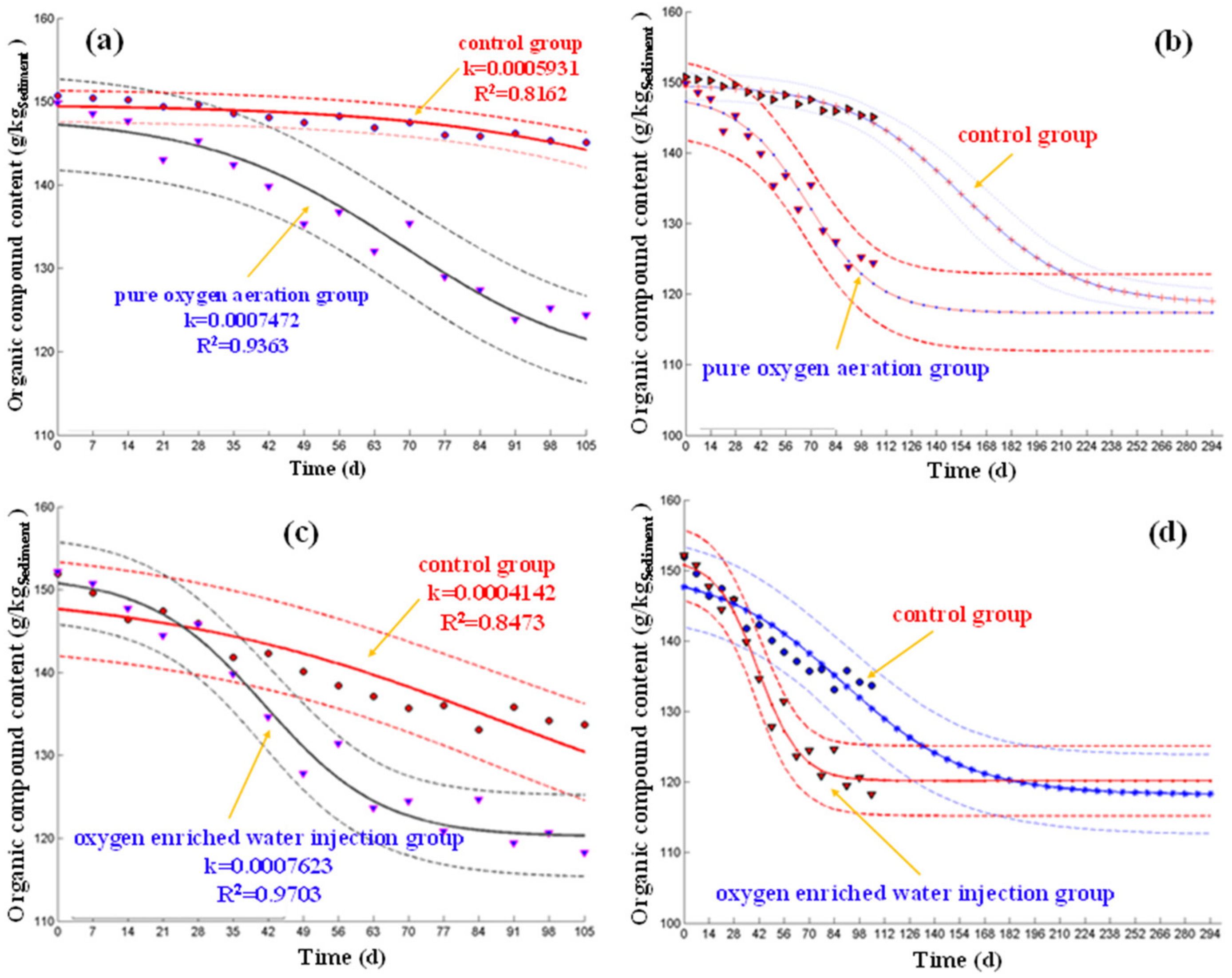
2.8. Mathematical Model
3. Results and Discussion
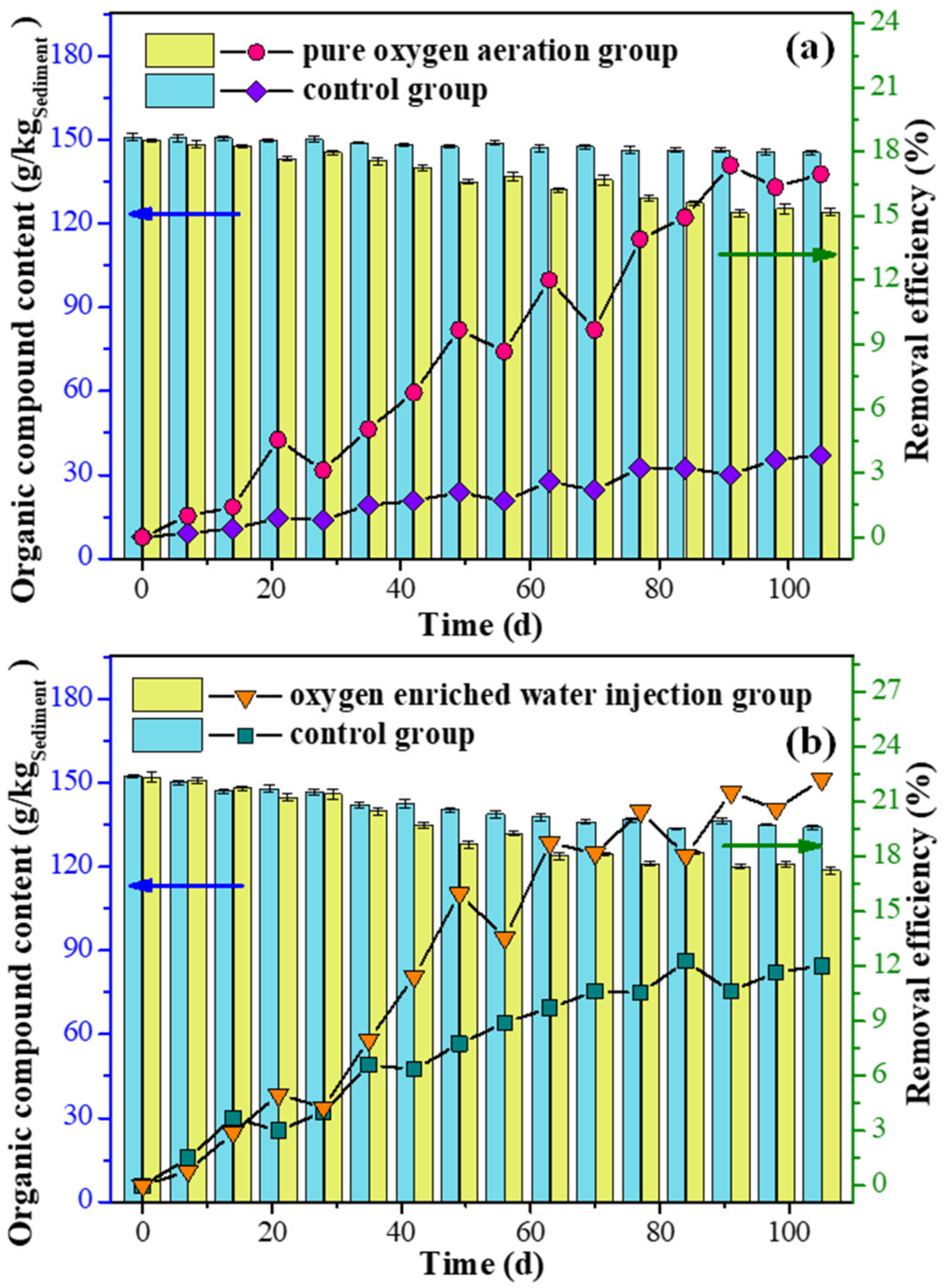
3.1. Physical-Chemical Properties of Sediment
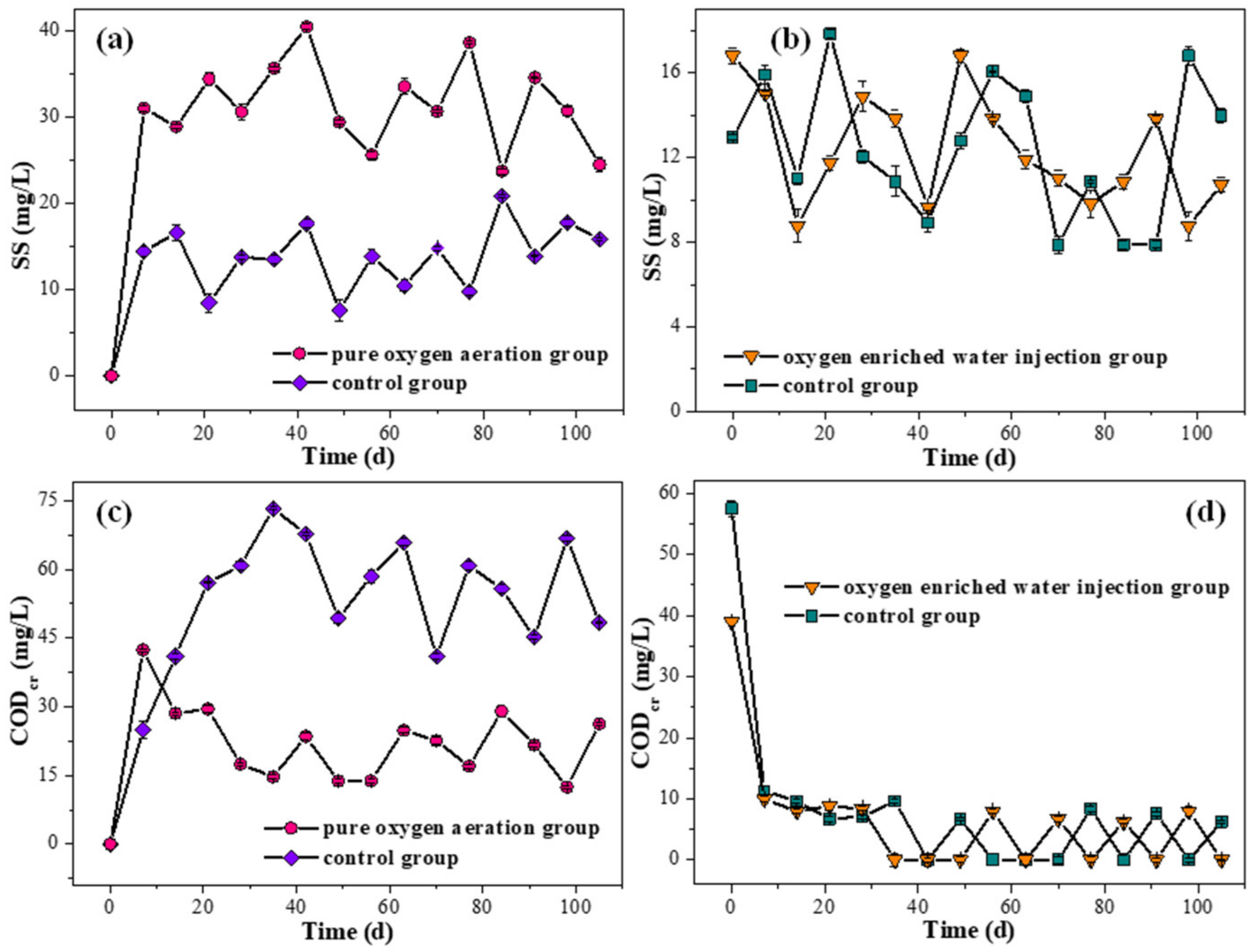
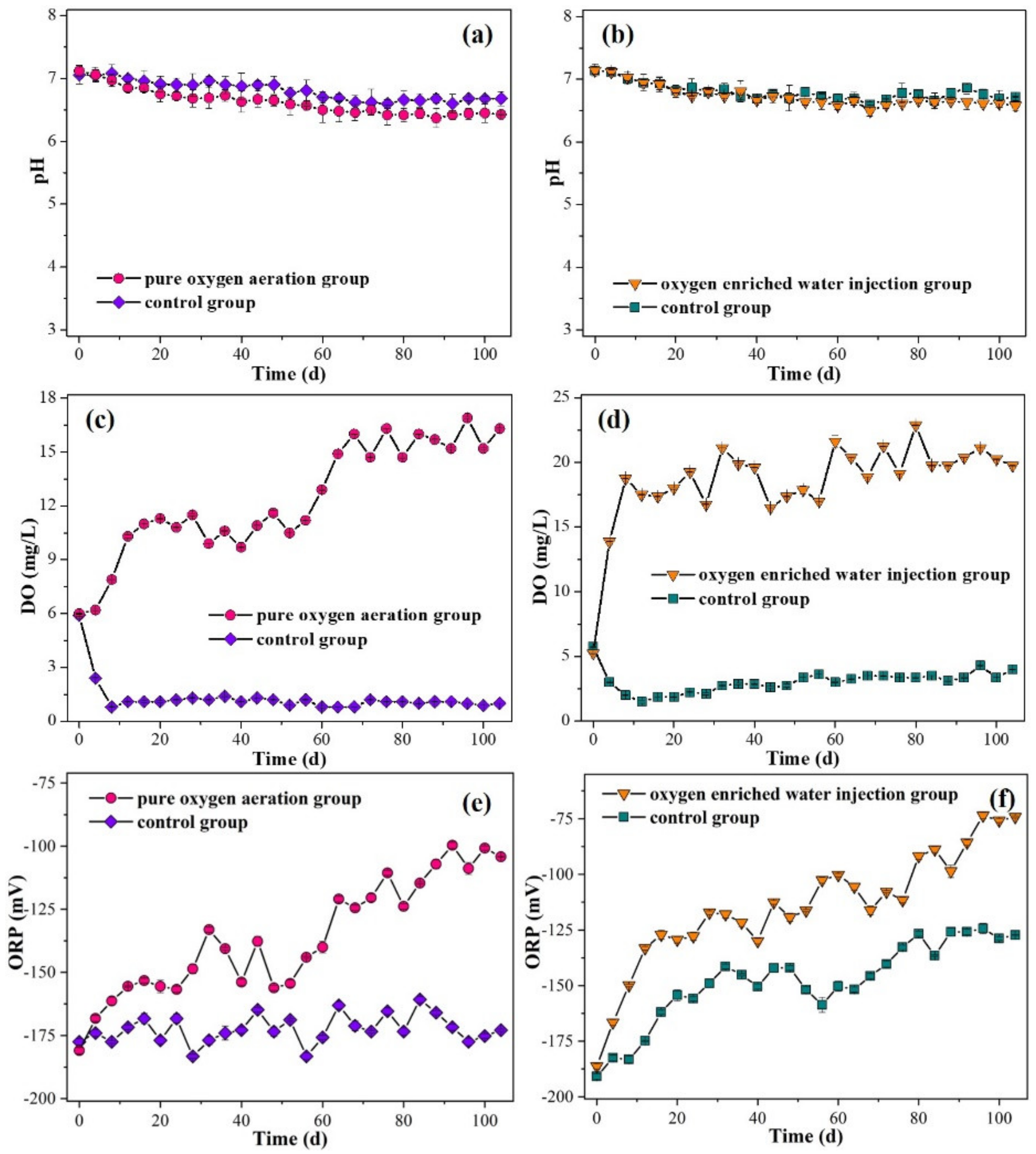
3.2. Quality of Overlying Water
3.3. Characteristic Change of Sediment
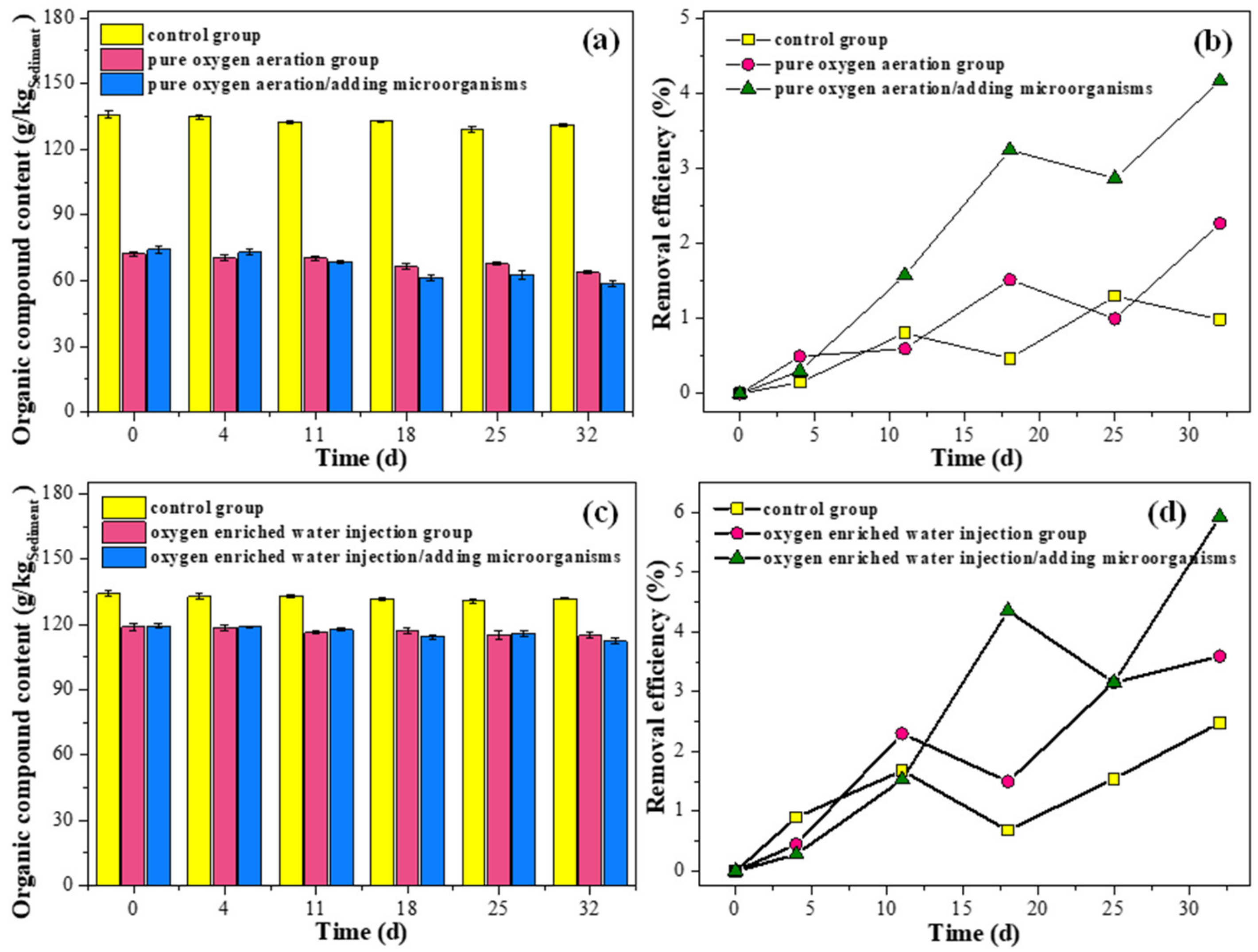
3.4. Effect of Introducing Exogenous Microorganisms
3.5. Change of Bacterial Community
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hagemann, N.; Schmidt, H.; Kägi, R.; Böhler, M.; Sigmund, G.; Maccagnan, A.; McArdell, C.S.; Bucheli, T.D. Wood-based activated biochar to eliminate organic micropollutants from biologically treated wastewater. Sci. Total Environ. 2020, 730, 138417. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chen, P. Changes in the heavy metals and petroleum hydrocarbon contents in seawater and surface sediment in the year following artificial reef construction in the Pearl River Estuary, China. Environ. Sci. Pollut. R 2020, 27, 6009–6021. [Google Scholar] [CrossRef] [PubMed]
- Baloun, J.; Adam, V.; Trnkova, L.; Beklova, M.; Svobodova, Z.; Zeman, L.; Kizek, R. Complexes of glutathione with heavy metal ions as a new biochemical marker of aquatic environment pollution. Environ. Toxicol. Chem. 2010, 29, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Chiou, C.T.; Peters, L.J.; Freed, V.H. A physical concept of soil-water equilibria for non-ionic organic compounds. Science 1979, 206, 831–832. [Google Scholar] [CrossRef] [PubMed]
- Karickhoff, S.W.; Brown, D.S.; Scodd, T.A. Sorption of hydrophobic pollutants in natural sediments. Water Res. 1979, 13, 241–248. [Google Scholar] [CrossRef]
- Szlauer-Lukaszewska, A. Effects of river bed regulation on habitats and communities: A case study for ostracods in the Oder River, Poland. Int. Rev. Hydrobiol. 2015, 100, 69–78. [Google Scholar] [CrossRef]
- Su, J.; van Bochove, E.; Auclair, J.; Theriault, G.; Hu, C.; Li, X. Phosphorus Fluxes at the Sediment-Water Interface in a Temperate Region Agricultural Catchment. Water Air Soil Poll. 2014, 225, 1739. [Google Scholar] [CrossRef]
- Vasilyeva, G.K.; Strijakova, E.R. Bioremediation of soils and sediments contaminated by polychlorinated biphenyls. Microbiology 2007, 76, 639–653. [Google Scholar] [CrossRef]
- Yang, H.; Li, C.; Zhang, J. Determining roles of in-situ measured surface potentials of phase controlled synthesized MnO2 nanostructures for superficial adsorption. Appl. Surf. Sci. 2020, 513, 145752. [Google Scholar] [CrossRef]
- Tomaszewski, J.E.; Smithenry, D.W.; Cho, Y. Treatment and Containment of Contaminated Sediments. In Assessment and Remediation of Contaminated Sediments; Danny, R., Tomas, L., Eds.; Springer: Dordrecht, The Netherlands, 2006; Volume 73, pp. 137–178. [Google Scholar]
- Zimmerman, J.R.; Ghosh, U.; Millward, R.N.; Bridges, T.S.; Luthy, R.G. Addition of carbon sorbents to reduce PCB and PAH bioavailability in marine sediments: Physicochemical tests. Environ. Sci. Technol. 2004, 38, 5458–5464. [Google Scholar] [CrossRef]
- Farhadian, M.; Vachelard, C.; Duchez, D.; Larroche, C. In situ bioremediation of monoaromatic pollutants in groundwater: A review. Bioresour. Technol. 2008, 99, 5296–5308. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, T.; Asami, H.; Watanabe, K. Impacts of bioremediation schemes on bacterial population in naphthalene-contaminated marine sediments. Biodegradation 2006, 17, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Mao, Y.; Wei, Y.; Shang, P.; Zhou, X. Bioremediation of Aquaculture Wastewater with Algal-Bacterial Biofilm Combined with the Production of Selenium Rich Biofertilizer. Water 2020, 12, 2071. [Google Scholar] [CrossRef]
- Kane, A.; Vidumsky, J.; Major, D.W.; Bauer, N.B. In-situ bioremediation of a chlorinated solvent residual source in unconsolidated sediments and bedrock using bioaugmentation. In Contaminated Soils Series; Edward, J.C., Paul, T.K., Eds.; Springer: Boston, MA, USA, 2005; Volume 9, pp. 45–55. [Google Scholar]
- Delille, D.; Delille, B.; Pelletier, E. Effectiveness of bioremediation of crude oil contaminated subantarctic intertidal sediment: The microbial response. Microb. Ecol. 2002, 44, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Bonardi, M.; Ravagnan, G.; Stirling, J.A.R.; Morucchio, C.; De Sanctis, S. Innovative treatment by bioremediation of contaminated sediments from the Venice Lagoon, Italy: The Arsenale Vecchio case study. J. Coast. Res. 2007, 895–899. [Google Scholar]
- Murphy, P.T.; Hall, G.K.; Northcote, G.T. Lime Treatment of a Hardwater Lake to Reduce Eutrophication. Lake Reserv. Manag. 1988, 2, 51–62. [Google Scholar] [CrossRef]
- Payne, R.B.; Ghosh, U.; May, H.D.; Marshall, C.W.; Sowers, K.R. A Pilot-Scale Field Study: In Situ Treatment of PCB-Impacted Sediments with Bioamended Activated Carbon. Environ. Sci. Technol. 2019, 53, 2626–2634. [Google Scholar] [CrossRef]
- Wang, C.; He, S.; Zou, Y.; Liu, J.; Zhao, R.; Yin, X.; Zhang, H.; Li, Y. Quantitative evaluation of in -situ bioremediation of compound pollution of oil and heavy metal in sediments from the Bohai Sea, China. Mar. Pollut. Bull. 2020, 150, 110787. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, H.; Yin, W.; Shao, S.; Lv, S.; Hu, Y. Water Quality and Microbial Community Changes in an Urban River after Micro-Nano Bubble Technology in Situ Treatment. Water 2019, 11, 66. [Google Scholar] [CrossRef] [Green Version]
- Diaz, M.P.; Gringson, S.J.W.; Peppiatt, C.; Burgess, J.G. Isolation and characterization of novel hydrocarbon degrading euryhaline consortia from crude oil and mangrove sediments. Mar. Biotechnol. 2000, 2, 522–532. [Google Scholar]
- Bose, D.; Santra, M.; Sanka, V.S.P.R.; Krishnakumar, B. Bioremediation analysis of sediment-microbial fuel cells for energy recovery from microbial activity in soil. Int. J. Energy Res. 2020, 44, 98. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Wang, C.; Wu, Y.; Wang, P. Development of a novel multi-functional active membrane capping barrier for the remediation of nitrobenzene-contaminated sediment. J. Hazard. Mater. 2014, 276, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Boopathy, R. Factors limiting bioremediation technologies. Bioresour. Technol. 2000, 74, 63–67. [Google Scholar] [CrossRef]
- Hai, T.P.; Hien, T.T.; Linh, T.V.; Hien, T.D.; Thuy, T.T.N.; Thu, H.T.D.; Mai, T.T.N.; Huy, Q.N.; Byung, H.K. A Laboratory-Scale Study of the Applicability of a Halophilic Sediment Bioelectrochemical System for in situ Reclamation of Water and Sediment in Brackish Aquaculture Ponds: Establishment, Bacterial Community and Performance Evaluation. J. Microbiol. Biotechnol. 2019, 29, 1104–1116. [Google Scholar]
- Wang, Z.; Gao, M.; Wei, J.; Ma, K.; Zhang, J.; Yang, Y.; Yu, S. Extracellular polymeric substances, microbial activity and microbial community of biofilm and suspended sludge at different divalent cadmium concentrations. Bioresour. Technol. 2016, 205, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, X.; Dong, D. Study of the Rapid Measurement of COD by Laser-Induced Breakdown Spectroscopy. Spectrosc. Spect. Anal. 2019, 39, 2907–2911. [Google Scholar]
- Zhang, N.; Guo, D.; Zhu, Y.; Wang, X.; Zhu, L.; Liu, F.; Teng, Y.; Christie, P.; Li, Z.; Lu, Y. Microbial remediation of a pentachloronitrobenzene-contaminated soil under Panax notoginseng: A field experiment. Pedosphere 2020, 30, 563–569. [Google Scholar] [CrossRef]
- Bo, B.; Kim, S.; Han, N.S. Bacterial and fungal diversity in Laphet, traditional fermented tea leaves in Myanmar, analyzed by culturing, DNA amplicon-based sequencing, and PCR-DGGE methods. Int. J. Food Microbiol. 2020, 320, 108508. [Google Scholar] [CrossRef]
- Saby, S.; Djafer, M.; Chen, G.H. Effect of low ORP in anoxic sludge zone on excess sludge production in oxic-settling-anoxic activated sludge process. Water Res. 2003, 37, 11–20. [Google Scholar] [CrossRef]
- Filcheva, E.; Kotsev, T.; Cholakova, Z.; Chakalov, K.; Popova, T. Content and composition of organic matter in heavy metal polluted alluvial soils from Ogosta River basin. Ecol. Future-J. Agric. Sci. For. Sci. 2011, 10, 30–39. [Google Scholar]
- Chen, J.; Lu, S.; Zhao, Y.; Wang, W.; Huang, M. Effects of overlying water aeration on phosphorus fractions and alkaline phosphatase activity in surface sediment. J. Environ. Sci. 2011, 23, 206–211. [Google Scholar] [CrossRef]
- Deng, Q.; Su, C.; Lu, X.; Chen, W.; Guan, X.; Chen, S.; Chen, M. Performance and functional microbial communities of denitrification process of a novel MFC-granular sludge coupling system. Bioresour. Technol. 2020, 306, 123173. [Google Scholar] [CrossRef] [PubMed]
- Sergienko, N.; Radjenovic, J. Manganese oxide-based porous electrodes for rapid and selective (electro) catalytic removal and recovery of sulfide from wastewater. Appl. Catal. B-Environ. 2020, 267, 118608. [Google Scholar] [CrossRef]
- Kurian, S.; Kessarkar, P.M.; Rao, V.P.; Reshma, K.; Sarkar, A.; Pattan, J.N.; Naqvi, S.W.A. Controls on organic matter distribution in oxygen minimum zone sediments from the continental slope off western India. J. Mar. Syst. 2018, 207, 103–118. [Google Scholar] [CrossRef]
- Tabla-Hernandez, J.; Hernandez-Ramirez, A.G.; Martinez-Tavera, E.; Rodriguez-Espinosa, P.F.; Mangas-Ramirez, E. Impacts on water quality by in situ induced ozone-oxygen oxidation in a polluted urban reservoir. Sci. Total Environ. 2020, 735, 139364. [Google Scholar] [CrossRef]
- Song, T.; Yan, Z.; Zhao, Z.; Jiang, H. Removal of organic matter in freshwater sediment by microbial fuel cells at various external resistances. J. Chem. Technol. Biotechnol. 2010, 85, 1489–1493. [Google Scholar] [CrossRef]
- Tao, K.; Liu, X.; Chen, X.; Hu, X.; Cao, L.; Yuan, X. Biodegradation of crude oil by a defined co-culture of indigenous bacterial consortium and exogenous Bacillus subtilis. Bioresour. Technol. 2017, 224, 327–332. [Google Scholar] [CrossRef]
- Perez, M.C.; Alvarez-Hornos, F.J.; San-Valero, P.; Gabaldon, C.; Martinez-Soria, V. Evolution of bacterial community in a full-scale biotrickling filter by fluorescence in situ hybridization (FISH). Procedia Eng. 2012, 42, 666–671. [Google Scholar] [CrossRef]
- Chen, Q.; Ni, J.; Ma, T.; Liu, T.; Zheng, M. Bioaugmentation treatment of municipal wastewater with heterotrophic-aerobic nitrogen removal bacteria in a pilot-scale SBR. Bioresour. Technol. 2015, 183, 25–32. [Google Scholar] [CrossRef]
- Fayiah, M.; Dong, S.; Li, Y.; Xu, Y.; Gao, X.; Li, S.; Shen, H.; Xiao, J.; Yang, Y.; Wessell, K. The relationships between plant diversity, plant cover, plant biomass and soil fertility vary with grassland type on Qinghai-Tibetan Plateau. Agric. Ecosyst. Environ. 2019, 286, 106659. [Google Scholar] [CrossRef]

| Primer Names | Primer Sequences | Reaction Conditions |
|---|---|---|
| 341f | 5′-CGCCCGCCGCGCGCGGCGGGCGGGG CGGGGGCACGGGGGCCCTACGGGAGG CAGCAG-3′ | Denaturation at 94 °C for 10 min; 30 standard cycles: denaturation at 94 °C for 1 min, annealing at 55 °C for 1 min, extension at 72 °C for 1 min; extension at 72 °C for 10 min after the end of the cycle. |
| R518 | 5′-ATTACCGCGGCTGCTGG-3′ |
| Sampling Point | pH | Water Content | ORP (mV) | Organic Matter Content (g/kg) |
|---|---|---|---|---|
| A1-1 | 7.32 | 67.1% | −165 | 153.3 |
| A1-2 | 7.18 | 64.2% | −179 | 160.2 |
| A1-3 | 7.23 | 74.7% | −163 | 145.8 |
| B1-1 | 7.20 | 80.0% | −168 | 149.3 |
| B1-2 | 7.09 | 68.8% | −187 | 173.7 |
| B1-3 | 7.16 | 70.3% | −172 | 137.7 |
| C1-1 | 7.35 | 64.0% | −159 | 150.5 |
| C1-2 | 7.17 | 66.1% | −181 | 173.2 |
| C1-3 | 7.28 | 71.4% | −172 | 170.7 |
| average value | 7.22 | 69.6% | −172 | 157.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che, L.; Jin, W.; Zhou, X.; Cao, C.; Han, W.; Qin, C.; Tu, R.; Chen, Y.; Feng, X.; Wang, Q. Biological Reduction of Organic Matter in Buji River Sediment (Shenzhen, China) with Artificial Oxygenation. Water 2020, 12, 3592. https://doi.org/10.3390/w12123592
Che L, Jin W, Zhou X, Cao C, Han W, Qin C, Tu R, Chen Y, Feng X, Wang Q. Biological Reduction of Organic Matter in Buji River Sediment (Shenzhen, China) with Artificial Oxygenation. Water. 2020; 12(12):3592. https://doi.org/10.3390/w12123592
Chicago/Turabian StyleChe, Lin, Wenbiao Jin, Xu Zhou, Chenbo Cao, Wei Han, Changlei Qin, Renjie Tu, Yidi Chen, Xiaochi Feng, and Qilin Wang. 2020. "Biological Reduction of Organic Matter in Buji River Sediment (Shenzhen, China) with Artificial Oxygenation" Water 12, no. 12: 3592. https://doi.org/10.3390/w12123592
APA StyleChe, L., Jin, W., Zhou, X., Cao, C., Han, W., Qin, C., Tu, R., Chen, Y., Feng, X., & Wang, Q. (2020). Biological Reduction of Organic Matter in Buji River Sediment (Shenzhen, China) with Artificial Oxygenation. Water, 12(12), 3592. https://doi.org/10.3390/w12123592










