Efficient Removal of Levofloxacin by Activated Persulfate with Magnetic CuFe2O4/MMT-k10 Nanocomposite: Characterization, Response Surface Methodology, and Degradation Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CuFe2O4/MMT-k10 and CuFe2O4
2.3. Characterization of CuFe2O4/MMT-k10
2.4. Batch Experiment
2.5. Response Surface Methodology Optimization Design
3. Results and Discussion
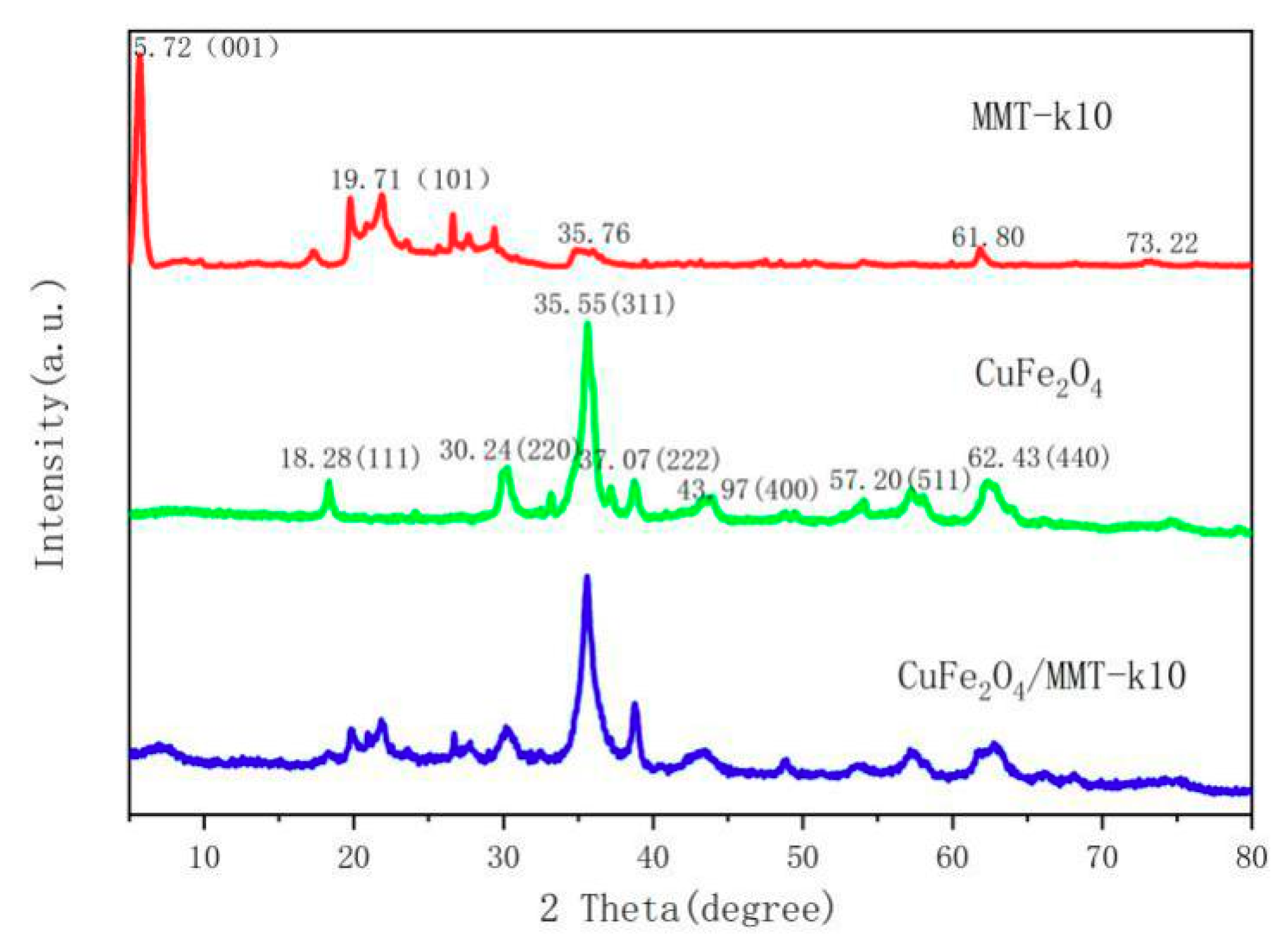
3.1. XRD Results
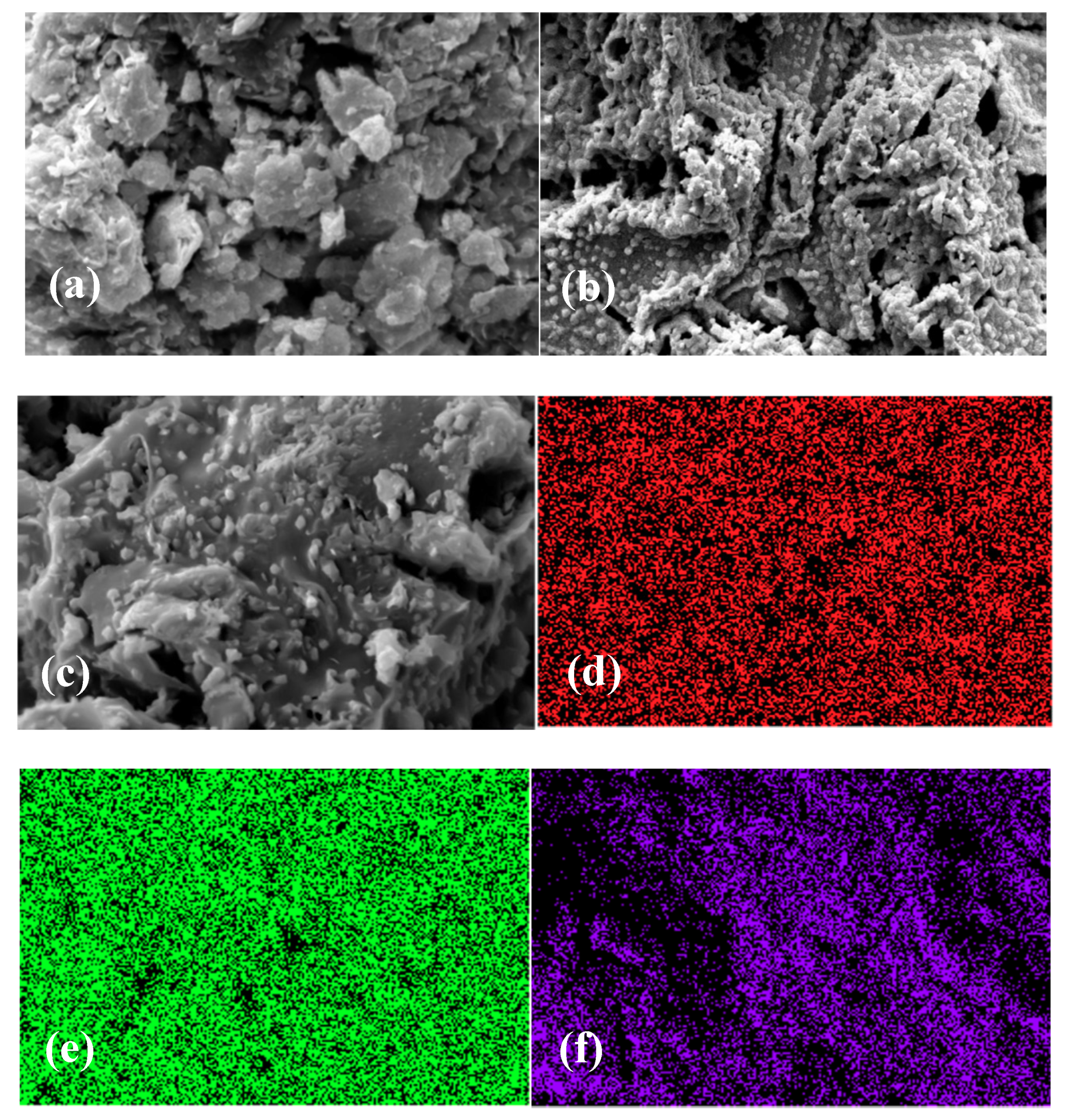
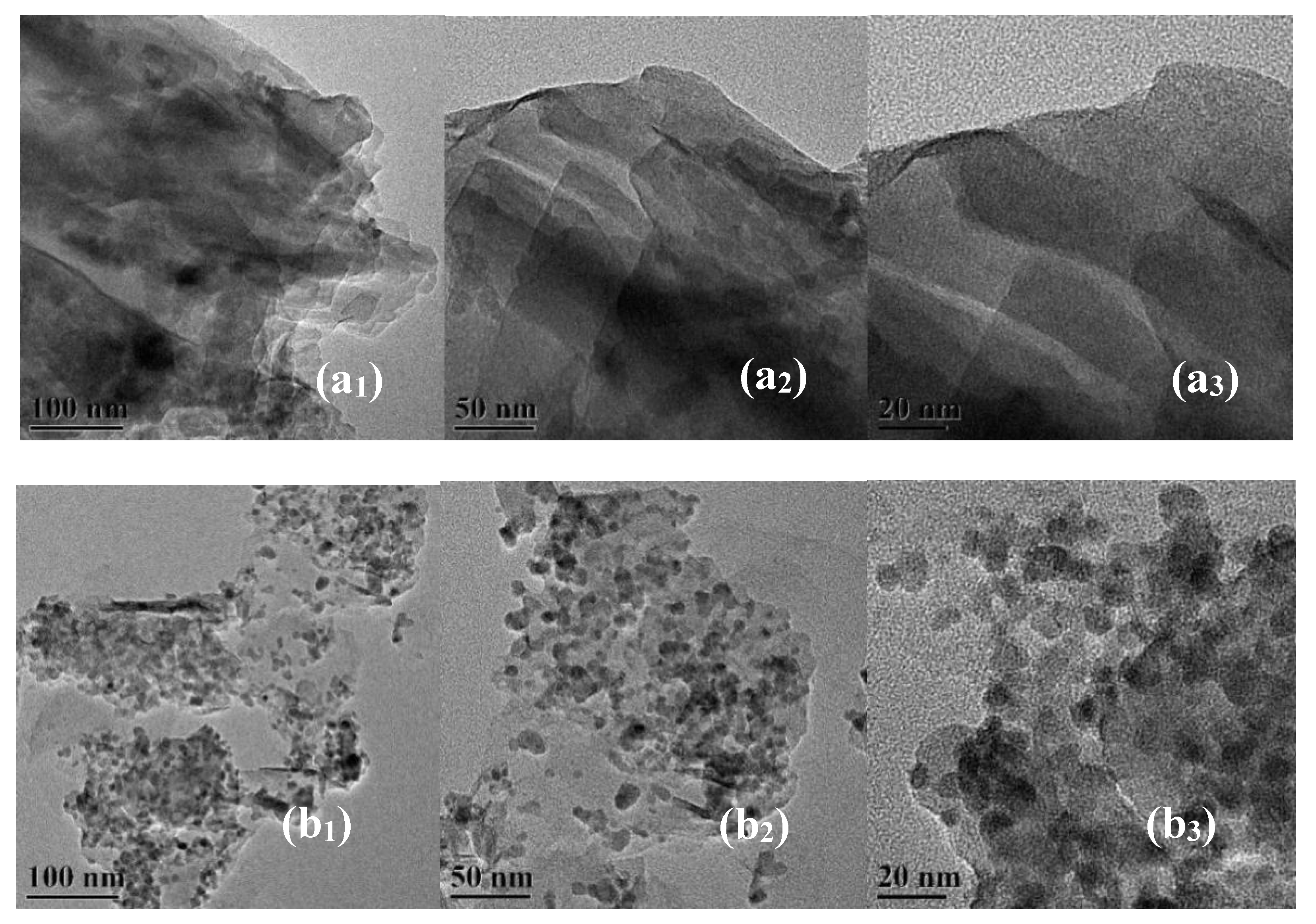
3.2. SEM and TEM Results
3.3. BET Results
3.4. VSM Results
3.5. LVF Removal
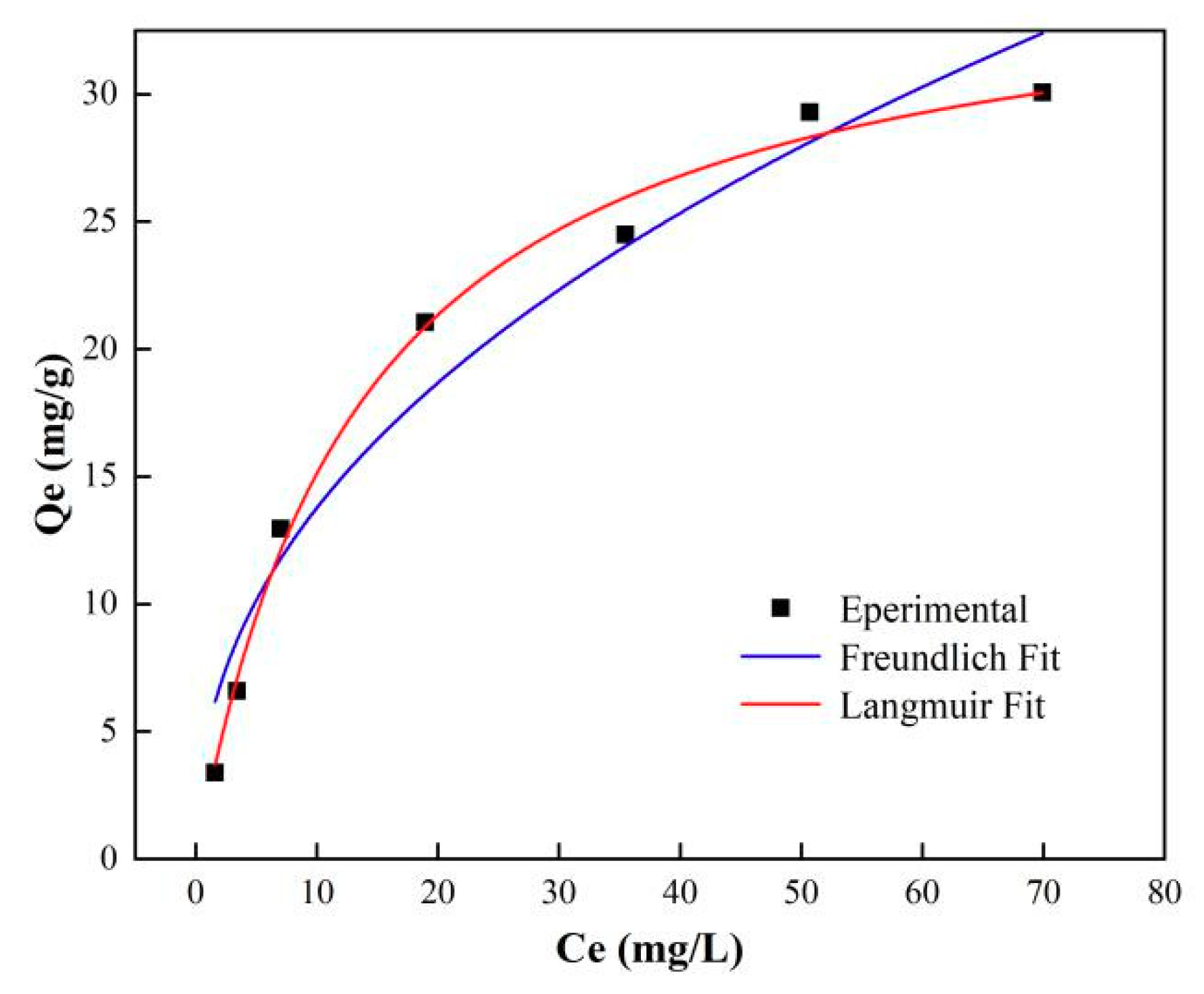
3.5.1. Adsorption Studies
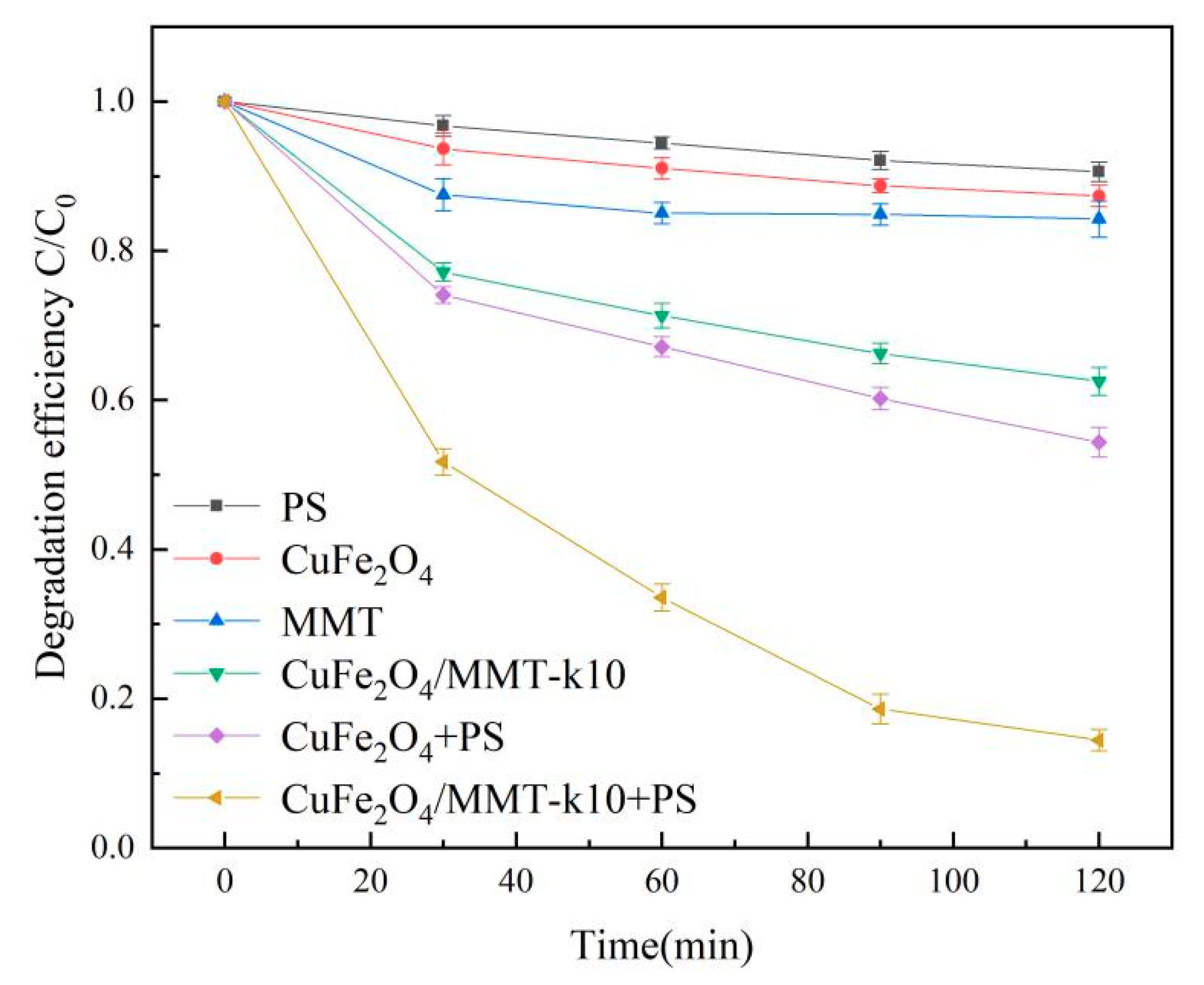
3.5.2. LVF Removal in Different Systems
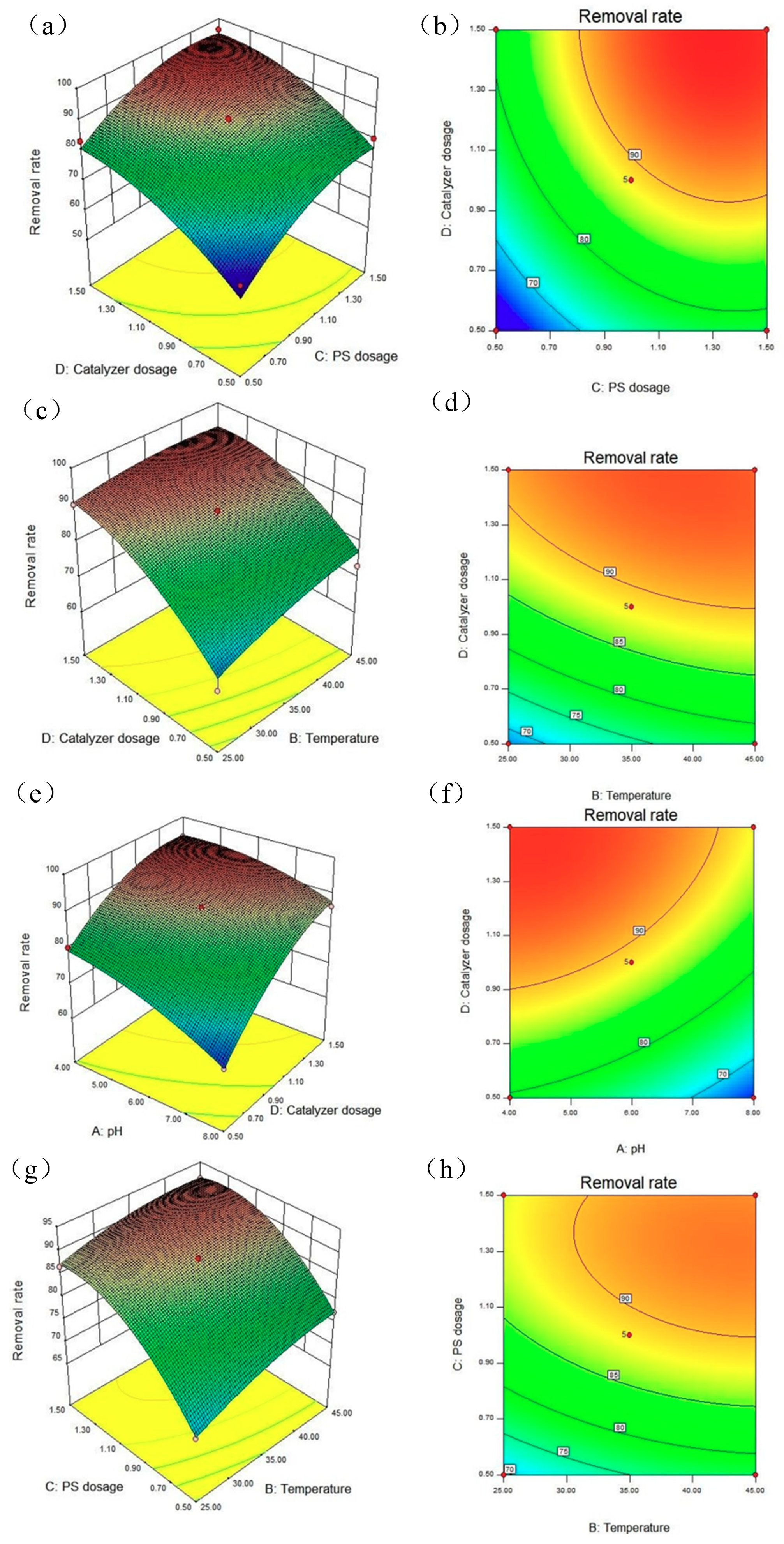
3.5.3. RSM Analysis of LVF Removal
3.6. Reusability and Stability of CuFe2O4/MMT-k10
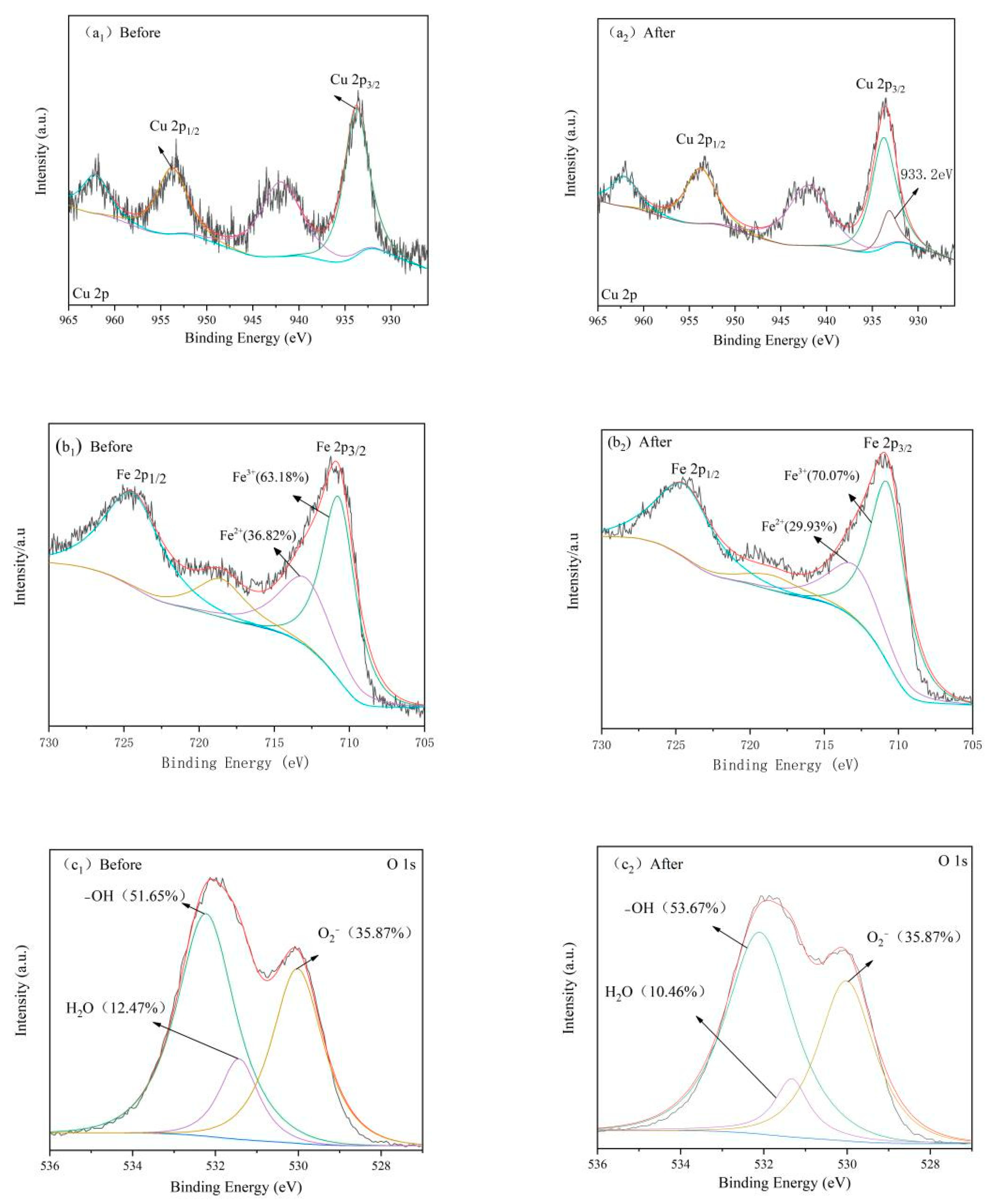
3.7. Mechanism Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Peloquin, C.A.; Phillips, P.P.J.; Mitnick, C.D.; Eisenach, K.; Patienti, R.F.; Lecc, L.; Gotuzzo, E.; Gandhi, N.R.; Butler, D.; Diacon, A.H.; et al. Increased doses lead to higher drug exposures of levofloxacin for treatment of tuberculosis. Antimicrob. Agents Chemother. 2018, 62, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Czyrski, A.; Anusiak, K.; Teżyk, A. The degradation of levofloxacin in infusions exposed to daylight with an identification of a degradation product with HPLC-MS. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Wen, X.J.; Niu, C.G.; Guo, H.; Zhang, L.; Liang, C.; Zeng, G.M. Photocatalytic degradation of levofloxacin by ternary Ag2CO3/CeO2/AgBr photocatalyst under visible-light irradiation: Degradation pathways, mineralization ability, and an accelerated interfacial charge transfer process study. J. Catal. 2018, 358, 211–223. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Y.; Zhou, D. TiO2 photocatalytic degradation of tetracycline as affected by a series of environmental factors. J. Soils Sediments 2014, 14, 1350–1358. [Google Scholar] [CrossRef]
- Hamdi El Najjar, N.; Touffet, A.; Deborde, M.; Journel, R.; Leitner, N.K.V. Levofloxacin oxidation by ozone and hydroxyl radicals: Kinetic study, transformation products and toxicity. Chemosphere 2013, 93, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Qin, W.; Fang, G.; Wang, Y.; Zhou, D. Mechanistic understanding of polychlorinated biphenyls degradation by peroxymonosulfate activated with CuFe2O4 nanoparticles: Key role of superoxide radicals. Chem. Eng. J. 2018, 348, 526–534. [Google Scholar] [CrossRef]
- Zhang, H.; Song, Y.; Nengzi, L.; Gou, J.; Li, B.; Cheng, X. Activation of persulfate by a novel magnetic CuFe2O4/Bi2O3 composite for lomefloxacin degradation. Chem. Eng. J. 2020, 379, 122362. [Google Scholar] [CrossRef]
- Santos, A.; Fernandez, J.; Rodriguez, S.; Dominguez, C.M.; Lominchar, M.A.; Lorenzo, D.; Romero, A. Abatement of chlorinated compounds in groundwater contaminated by HCH wastes using ISCO with alkali activated persulfate. Sci. Total Environ. 2018, 615, 1070–1077. [Google Scholar] [CrossRef]
- Bruton, T.A.; Sedlak, D.L. Treatment of perfluoroalkyl acids by heat-activated persulfate under conditions representative of in situ chemical oxidation. Chemosphere 2018, 206, 457–464. [Google Scholar] [CrossRef]
- Milh, H.; Schoenaers, B.; Stesmans, A.; Cabooter, D.; Dewil, R. Degradation of sulfamethoxazole by heat-activated persulfate oxidation: Elucidation of the degradation mechanism and influence of process parameters. Chem. Eng. J. 2020, 379, 122234. [Google Scholar] [CrossRef]
- Fang, Z.; Chelme-Ayala, P.; Shi, Q.; Xu, C.; Gamal El-Din, M. Degradation of naphthenic acid model compounds in aqueous solution by UV activated persulfate: Influencing factors, kinetics and reaction mechanisms. Chemosphere 2018, 211, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Li, S.; Shi, Y.; Geng, J.; Li, J.; Wu, G.; Xu, K.; Ren, H. Removal of artificial sweeteners using UV/persulfate: Radical-based degradation kinetic model in wastewater, pathways and toxicity. Water Res. 2019, 167, 115102. [Google Scholar] [CrossRef]
- Du, Y.; Ma, W.; Liu, P.; Zou, B.; Ma, J. Magnetic CoFe2O4 nanoparticles supported on titanate nanotubes (CoFe2O4/TNTs) as a novel heterogeneous catalyst for peroxymonosulfate activation and degradation of organic pollutants. J. Hazard. Mater. 2016, 308, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical generation by the interaction of transition metals with common oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.H.; Ma, J.; Ren, Y.M.; Liu, Y.L.; Xiao, J.Y.; Lin, L.; Zhang, C. Efficient degradation of atrazine by magnetic porous copper ferrite catalyzed peroxymonosulfate oxidation via the formation of hydroxyl and sulfate radicals. Water Res. 2013, 47, 5431–5438. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Lin, L.; Ma, J.; Yang, J.; Feng, J.; Fan, Z. Sulfate radicals induced from peroxymonosulfate by magnetic ferrospinel MFe2O4 (M=Co, Cu, Mn, and Zn) as heterogeneous catalysts in the water. Appl. Catal. B Environ. 2015, 165, 572–578. [Google Scholar] [CrossRef]
- Faungnawakij, K.; Viriya-Empikul, N. Catalytic behavior toward oxidative steam reforming of dimethyl ether over CuFe2O4-Al2O3 composite catalysts. Appl. Catal. A Gen. 2010, 382, 21–27. [Google Scholar] [CrossRef]
- Bai, H.; Zhao, Y.; Zhang, X.; Wang, W.; Zhang, T.; Liu, C.; Yi, H.; Song, S. Correlation of exfoliation performance with interlayer cations of montmorillonite in the preparation of two-dimensional nanosheets. J. Am. Ceram. Soc. 2019, 102, 3908–3922. [Google Scholar] [CrossRef]
- Yuan, P.; Fan, M.; Yang, D.; He, H.; Liu, D.; Yuan, A.; Zhu, J.X.; Chen, T.H. Montmorillonite-supported magnetite nanoparticles for the removal of hexavalent chromium [Cr(VI)] from aqueous solutions. J. Hazard. Mater. 2009, 166, 821–829. [Google Scholar] [CrossRef]
- Peng, X.; Tian, Y.; Liu, S.; Jia, X. Degradation of TBBPA and BPA from aqueous solution using organo-montmorillonite supported nanoscale zero-valent iron. Chem. Eng. J. 2017, 309, 717–724. [Google Scholar] [CrossRef]
- Flores, F.M.; Undabeytia, T.; Jaworski, M.; Morillo, E.; Torres Sánchez, R.M. Organo-montmorillonites as adsorbent materials for thiophanate-methyl removal: Adsorption-desorption studies and technological applications. J. Environ. Chem. Eng. 2020, 8, 103806. [Google Scholar] [CrossRef]
- Zhu, M.; Ge, F.; Zhu, R.; Wang, X.; Zheng, X. A DFT-based QSAR study of the toxicity of quaternary ammonium compounds on Chlorella vulgaris. Chemosphere 2010, 80, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Nałȩcz-Jawecki, G.; Grabińska-Sota, E.; Narkiewicz, P. The toxicity of cationic surfactants in four bioassays. Ecotoxicol. Environ. Saf. 2003, 54, 87–91. [Google Scholar] [CrossRef]
- Tandukar, M.; Oh, S.; Tezel, U.; Konstantinidis, K.T.; Pavlostathis, S.G. Long-term exposure to benzalkonium chloride disinfectants results in change of microbial community structure and increased antimicrobial resistance. Environ. Sci. Technol. 2013, 47, 9730–9738. [Google Scholar] [CrossRef]
- Li, X.; Brownawell, B.J. Quaternary ammonium compounds in urban estuarine sediment environments—A class of contaminants in need of increased attention? Environ. Sci. Technol. 2010, 44, 7561–7568. [Google Scholar] [CrossRef]
- Kumar, B.S.; Dhakshinamoorthy, A.; Pitchumani, K. ChemInform Abstract: K10 Montmorillonite Clays as Environmentally Benign Catalysts for Organic Reactions. ChemInform 2014, 4, 2378–2396. [Google Scholar] [CrossRef]
- Zhang, J.; Xue, W.; Wang, P.; Wang, T.; Liang, Y.; Zhang, Z. One-pot synthesis of 3-(furan-2-yl)-4-hydroxy-2H-chromen-2-ones using K10 montmorillonite clay as heterogeneous catalyst. Tetrahedron 2018, 74, 4712–4720. [Google Scholar] [CrossRef]
- Daud, N.K.; Hameed, B.H. Fenton-like oxidation of reactive black 5 solution using iron-Montmorillonite K10 catalyst. J. Hazard. Mater. 2010, 176, 1118–1121. [Google Scholar] [CrossRef]
- Daud, N.K.; Ahmad, M.A.; Hameed, B.H. Decolorization of Acid Red 1 dye solution by Fenton-like process using Fe-Montmorillonite K10 catalyst. Chem. Eng. J. 2010, 165, 111–116. [Google Scholar] [CrossRef]
- Salem, I.A.; El-Ghamry, H.A.; El-Ghobashy, M.A. Catalytic decolorization of Acid blue 29 dye by H2O2 and a heterogeneous catalyst. Beni-Suef Univ. J. Basic Appl. Sci. 2014, 3, 186–192. [Google Scholar] [CrossRef] [Green Version]
- Salem, I.A.; El-Ghamry, H.A.; El-Ghobashy, M.A. Application of Montmorillonite-Cu(II)ethylenediamine Catalyst for the Decolorization of Chromotrope 2R with H2O2 in Aqueous Solution; Elsevier B.V.: Amsterdam, The Netherlands, 2015; Volume 139, ISBN 0020403305. [Google Scholar]
- Ding, Y.; Zhu, L.; Wang, N.; Tang, H. Sulfate radicals induced degradation of tetrabromobisphenol A with nanoscaled magnetic CuFe2O4 as a heterogeneous catalyst of peroxymonosulfate. Appl. Catal. B Environ. 2013, 129, 153–162. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, H.; Zhang, X.; Li, B.; Guo, R.; Cheng, Q.; Cheng, X. Synthesis of magnetic CuO/MnFe2O4 nanocompisite and its high activity for degradation of levofloxacin by activation of persulfate. Chem. Eng. J. 2019, 360, 848–860. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, W.; Qiu, J.; Jin, H.; Ma, H.; Li, Z.; Cang, D. Modeling and optimization study on sulfamethoxazole degradation by electrochemically activated persulfate process. J. Clean. Prod. 2018, 197, 297–305. [Google Scholar] [CrossRef]
- Karimifard, S.; Alavi Moghaddam, M.R. Application of response surface methodology in physicochemical removal of dyes from wastewater: A critical review. Sci. Total Environ. 2018, 640–641, 772–797. [Google Scholar] [CrossRef]
- Xiao, J.; Ge, D.; Yuan, H.; Zhu, N. Waste activated sludge conditioning in a new Fe2+/persulfate/tannic acid process: Effectiveness and optimization study to enhance dewaterability. J. Environ. Chem. Eng. 2020, 8, 103785. [Google Scholar] [CrossRef]
- Zolgharnein, J.; Shahmoradi, A.; Ghasemi, J.B. Comparative study of Box-Behnken, central composite, and Doehlert matrix for multivariate optimization of Pb (II) adsorption onto Robinia tree leaves. J. Chemom. 2013, 27, 12–20. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Li, T.; Ai, B.; Yang, S.; Fu, J.; He, Q.; Yu, G.; Deng, S. Highly efficient removal of enrofloxacin by magnetic montmorillonite via adsorption and persulfate oxidation. Chem. Eng. J. 2019, 360, 1119–1127. [Google Scholar] [CrossRef]
- Al-Rawas, A.D.; Widatallah, H.M.; Al-Omari, I.A.; Johnson, C.; Elzain, M.E.; Gismelseed, A.M.; Al-Taie, S.; Yousif, A.A. The influence of mechanical milling and subsequent calcination on the formation of nanocrystalline CuFe2O4. AIP Conf. Proc. 2005, 765, 277–281. [Google Scholar] [CrossRef]
- Kumar, S.; Nair, R.R.; Pillai, P.B.; Gupta, S.N.; Iyengar, M.A.R.; Sood, A.K. Graphene oxide-MnFe2O4 magnetic nanohybrids for efficient removal of lead and arsenic from water. ACS Appl. Mater. Interfaces 2014, 6, 17426–17436. [Google Scholar] [CrossRef] [PubMed]
- Jonidi Jafari, A.; Kakavandi, B.; Jaafarzadeh, N.; Rezaei Kalantary, R.; Ahmadi, M.; Akbar Babaei, A. Fenton-like catalytic oxidation of tetracycline by AC@Fe3O4 as a heterogeneous persulfate activator: Adsorption and degradation studies. J. Ind. Eng. Chem. 2017, 45, 323–333. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lo, S.L.; Kuo, J.; Huang, C.P. Promoted degradation of perfluorooctanic acid by persulfate when adding activated carbon. J. Hazard. Mater. 2013, 261, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, Z.; Xiang, Y.; Ling, L.; Fang, J.; Shang, C.; Dionysiou, D.D. Bromate formation in bromide-containing water through the cobalt-mediated activation of peroxymonosulfate. Water Res. 2015, 83, 132–140. [Google Scholar] [CrossRef]
- Wang, Y.R.; Chu, W. Degradation of 2,4,5-trichlorophenoxyacetic acid by a novel Electro-Fe(II)/Oxone process using iron sheet as the sacrificial anode. Water Res. 2011, 45, 3883–3889. [Google Scholar] [CrossRef]
- Liang, C.; Su, H.W. Identification of sulfate and hydroxyl radicals in thermally activated persulfate. Ind. Eng. Chem. Res. 2009, 48, 5558–5562. [Google Scholar] [CrossRef]
- Chen, X.Y.; Ma, C.; Li, X.X.; Chen, P.; Fang, J.G. Hierarchical Bi2CuO4 microspheres: Hydrothermal synthesis and catalytic performance in wet oxidation of methylene blue. Catal. Commun. 2009, 10, 1020–1024. [Google Scholar] [CrossRef]
- Xu, Y.; Ai, J.; Zhang, H. The mechanism of degradation of bisphenol A using the magnetically separable CuFe2O4/peroxymonosulfate heterogeneous oxidation process. J. Hazard. Mater. 2016, 309, 87–96. [Google Scholar] [CrossRef]
- Li, J.; Ren, Y.; Ji, F.; Lai, B. Heterogeneous catalytic oxidation for the degradation of p-nitrophenol in aqueous solution by persulfate activated with CuFe2O4 magnetic nano-particles. Chem. Eng. J. 2017, 324, 63–73. [Google Scholar] [CrossRef]
- Wu, J.; Cagnetta, G.; Wang, B.; Cui, Y.; Deng, S.; Wang, Y.; Huang, J.; Yu, G. Efficient degradation of carbamazepine by organo-montmorillonite supported nCoFe2O4-activated peroxymonosulfate process. Chem. Eng. J. 2019, 368, 824–836. [Google Scholar] [CrossRef]
- Karimipourfard, D.; Eslamloueyan, R.; Mehranbod, N. Novel heterogeneous degradation of mature landfill leachate using persulfate and magnetic CuFe2O4/RGO nanocatalyst. Process Saf. Environ. Prot. 2019, 131, 212–222. [Google Scholar] [CrossRef]
- Dong, X.; Ren, B.; Sun, Z.; Li, C.; Zhang, X.; Kong, M.; Zheng, S.; Dionysiou, D.D. Monodispersed CuFe2O4 nanoparticles anchored on natural kaolinite as highly efficient peroxymonosulfate catalyst for bisphenol A degradation. Appl. Catal. B Environ. 2019, 253, 206–217. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Huang, M.; Wang, S.; Mao, X.; Hu, Y.; Chen, X. Efficient Removal of Levofloxacin by Activated Persulfate with Magnetic CuFe2O4/MMT-k10 Nanocomposite: Characterization, Response Surface Methodology, and Degradation Mechanism. Water 2020, 12, 3583. https://doi.org/10.3390/w12123583
Yang J, Huang M, Wang S, Mao X, Hu Y, Chen X. Efficient Removal of Levofloxacin by Activated Persulfate with Magnetic CuFe2O4/MMT-k10 Nanocomposite: Characterization, Response Surface Methodology, and Degradation Mechanism. Water. 2020; 12(12):3583. https://doi.org/10.3390/w12123583
Chicago/Turabian StyleYang, Junying, Minye Huang, Shengsen Wang, Xiaoyun Mao, Yueming Hu, and Xian Chen. 2020. "Efficient Removal of Levofloxacin by Activated Persulfate with Magnetic CuFe2O4/MMT-k10 Nanocomposite: Characterization, Response Surface Methodology, and Degradation Mechanism" Water 12, no. 12: 3583. https://doi.org/10.3390/w12123583
APA StyleYang, J., Huang, M., Wang, S., Mao, X., Hu, Y., & Chen, X. (2020). Efficient Removal of Levofloxacin by Activated Persulfate with Magnetic CuFe2O4/MMT-k10 Nanocomposite: Characterization, Response Surface Methodology, and Degradation Mechanism. Water, 12(12), 3583. https://doi.org/10.3390/w12123583




