Effect of Clay Colloid Particles on Formaldehyde Transport in Unsaturated Porous Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Formaldehyde (FA)
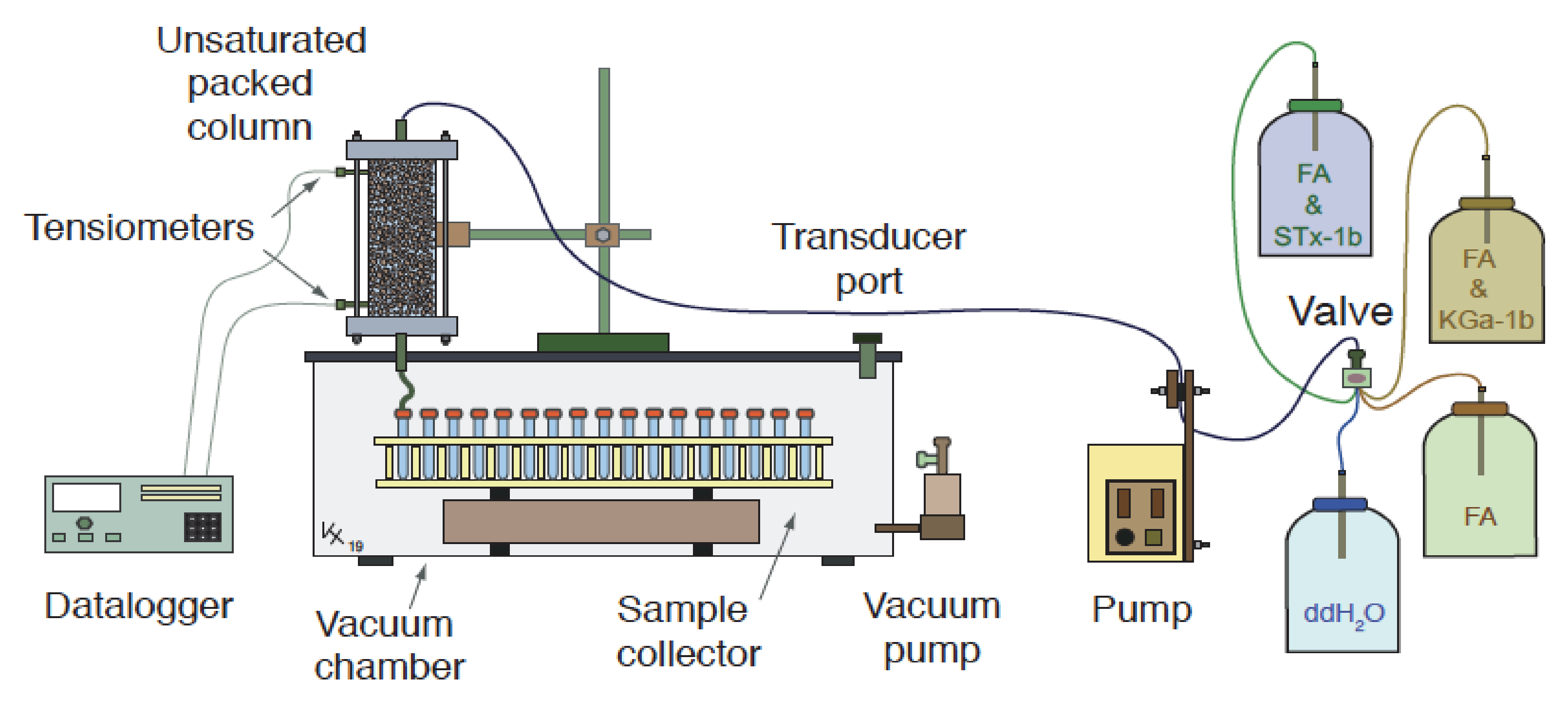
2.2. Sand Packed Columns and Colloids
2.3. Column Transport Experiments
3. Theoretical Considerations
4. Results and Discussion
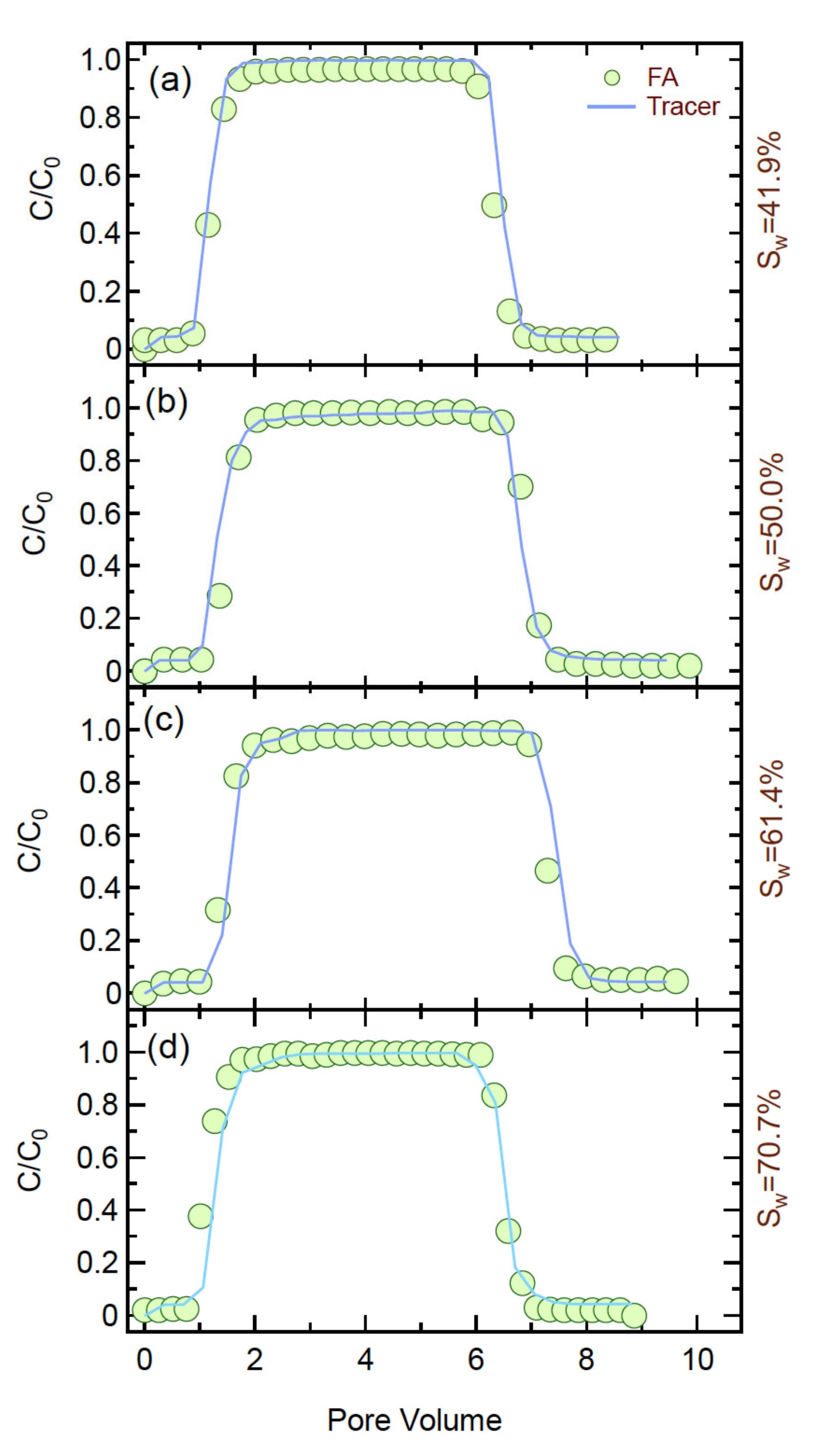
4.1. Transport Experiments
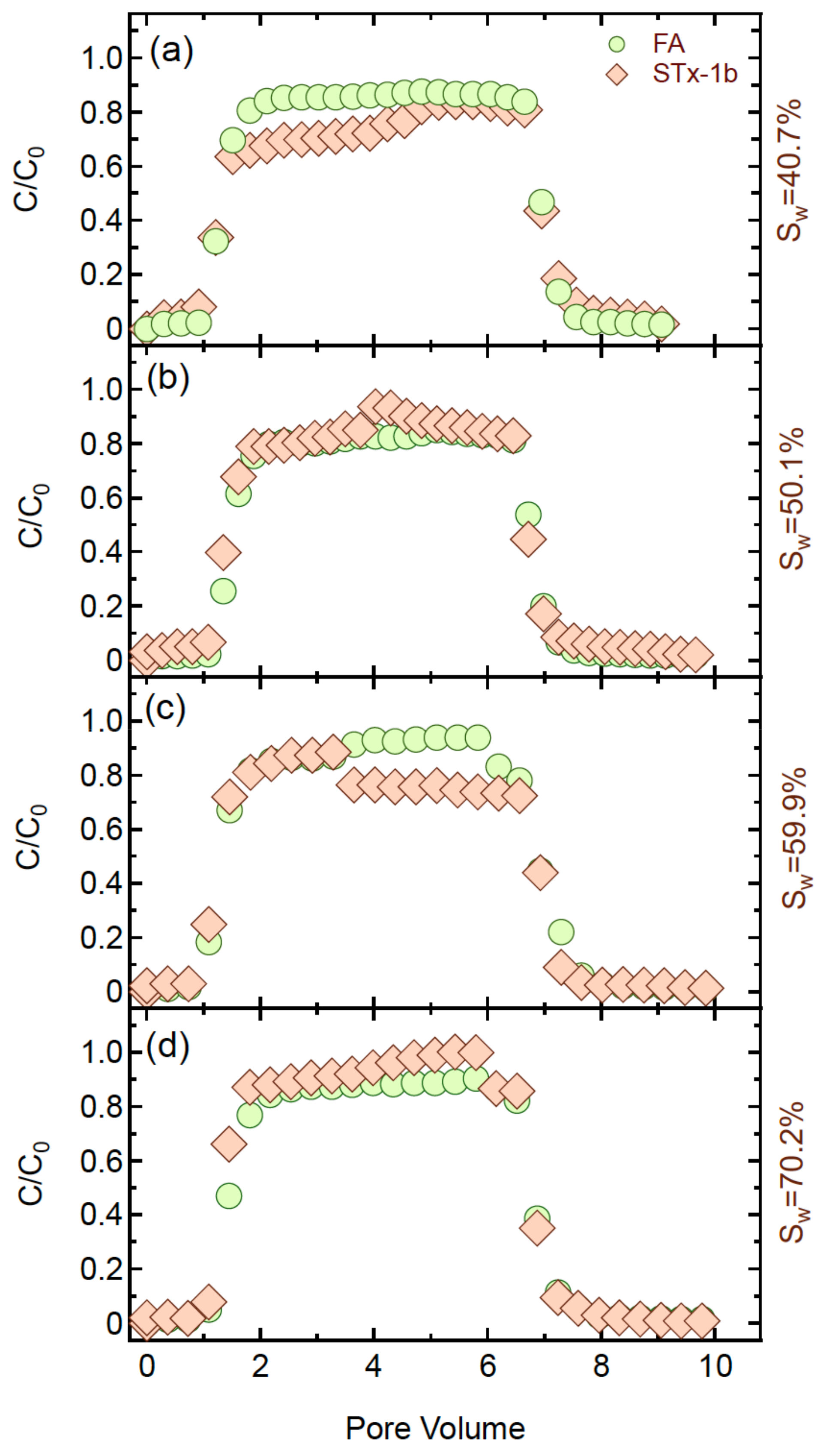
4.2. Co-Transport Experiments
4.3. Collision Efficiencies
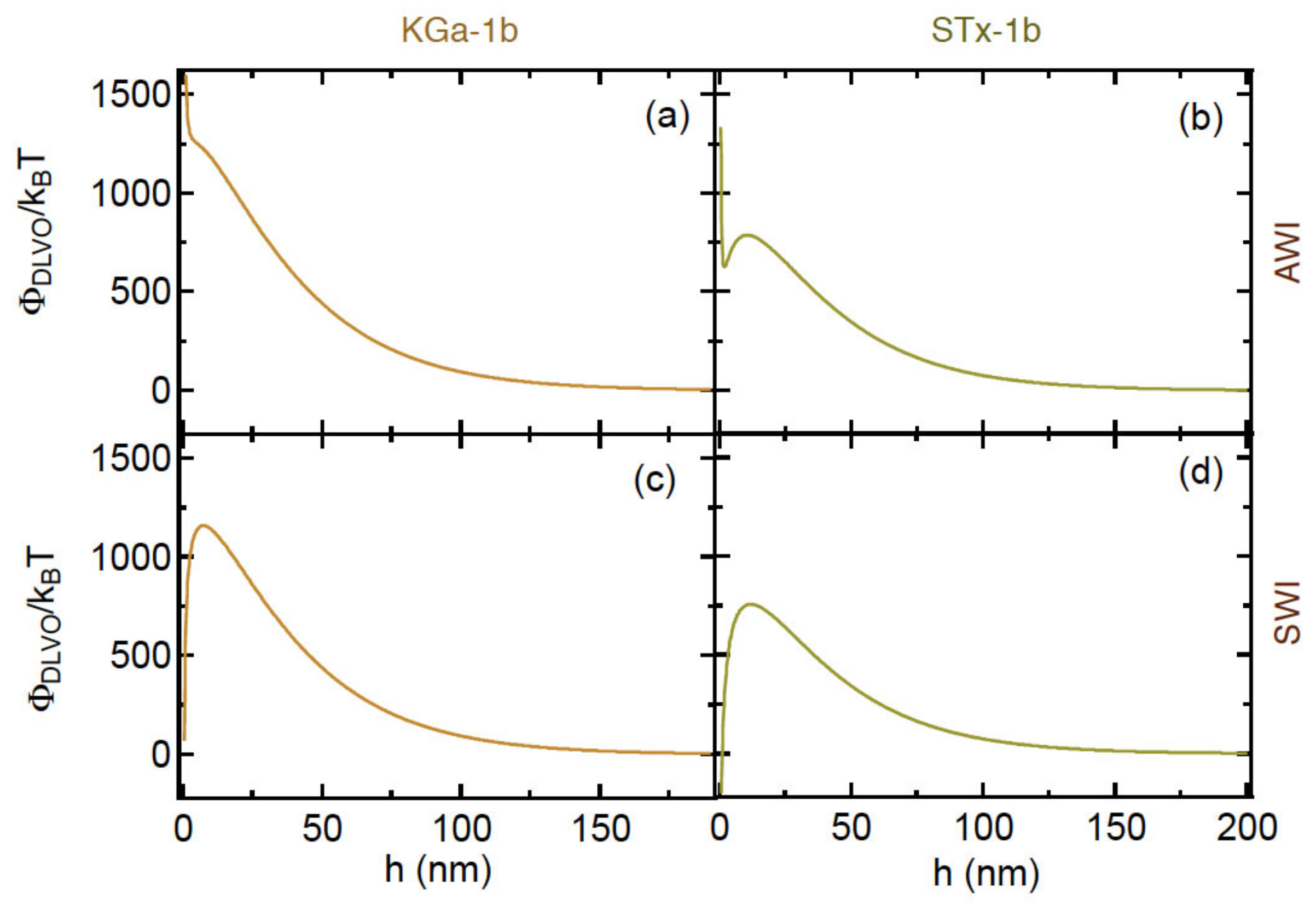
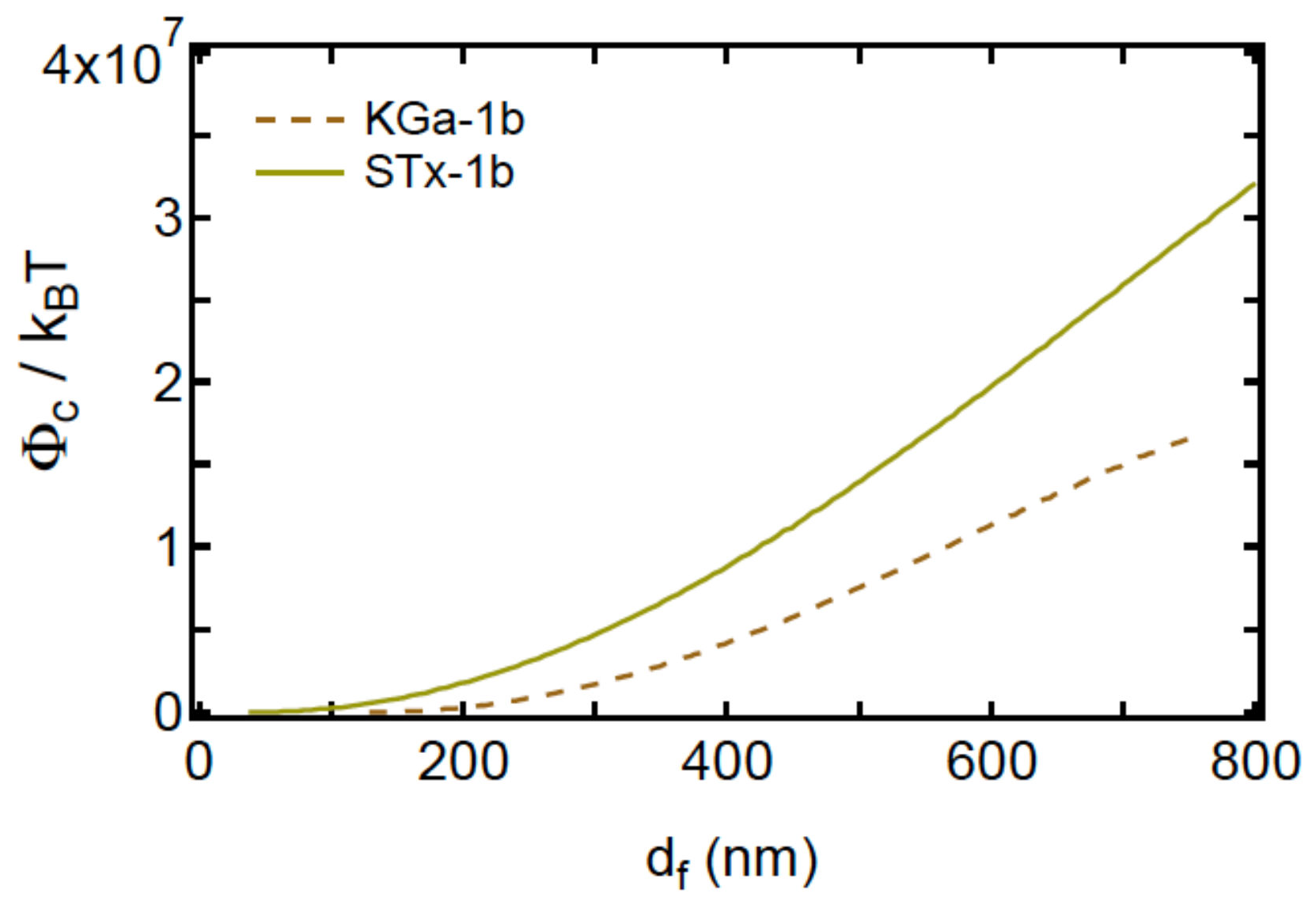
4.4. DLVO and Capillary Energy Profiles
4.5. Effect of Water Saturation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| A123 | Hamaker constant (J), M·L2/t2 |
| CFA | concentration of FA, M/L3 |
| Ccc | concentration of clay colloids, M/L3 |
| CFA-cc | concentration of FA in the presence of clay colloids, M/L3 |
| Ctr | concentration of tracer, M/L3 |
| Ciss | concentration of colloid i at steady state, M/L3 |
| Ci0 | influent colloid concentration, M/L3 |
| dc | collector diameter, L |
| df | distance that a colloid protrudes out from a thin water film, L |
| dp | average particle diameter, L |
| Fc | capillary force, M·L/t2 |
| Fpc | parallel component of capillary force, M·L/t2 |
| Fvc | vertical component of capillary force, M·L/t2 |
| Fv-tot | total vertical capillary force, M·L/t2 |
| g | gravitational acceleration, L/t2 |
| h | separation distance between two approaching surfaces, L |
| hf | water film thickness, L |
| kB | Boltzman’s constant (J/K), [M·L2/t2·T] |
| m0 | total mass in the concentration breakthrough curve, t·M/L3 |
| M1 | first normalized temporal moment, t |
| Mr | mass recovery, (–) |
| Min | mass injected in the column, M/L2 |
| rp | radius of colloidal particle, L |
| Sw | degree of saturation, (–) |
| T | temperature, K |
| U | interstitial fluid velocity, L/t |
| Greek letters | |
| aexp | experimental collision efficiency, (–) |
| β | contact angle (°) |
| single collector contact efficiency, (–) | |
| θ | porosity (voids volume to porous medium volume), (–) |
| θm | volumetric water content (liquid volume to porous medium volume), (–) |
| μw | dynamic fluid viscosity, M/L·t |
| ρp | colloidal particle density, M/L3 |
| ρf | fluid density, M/L3 |
| ΦBorn | Born potential energy (J), M·L2/t2 |
| Φc | capillary potential energy (J), M·L2/t2 |
| Φdl | electrostatic interaction energy (J), M·L2/t2 |
| ΦDLVO | DLVO potential energy (J), M·L2/t2 |
| Φmax1 | primary maximum (J), M·L2/t2 |
| Φmin1 | primary minimum (J), M·L2/t2 |
| Φmin2 | secondary minimum (J), M·L2/t2 |
| ΦvdW | van der Waals potential energy (J), M·L2/t2 |
| Abbreviations | |
| AWI | air–water interface |
| DLVO | Derjaguin-Landau-Verwey-Overbeek |
| ddH2O | deionized distilled water |
| FA | Formaldehyde |
| KGa-1b | Kaolinite |
| STx-1b | Montmorillonite |
| SWI | Solid–water interface |
References
- Denovio, N.M.; Saiers, J.E.; Ryan, J.N. Colloid movement in unsaturated porous media: Recent advances and future directions. Vadose Zone J. 2004, 3, 338–351. [Google Scholar] [CrossRef]
- Sen, T.K.; Khilar, K.C. Review on subsurface colloids and colloid-associated contaminant transport in saturated porous media. Adv. Colloid Interface Sci. 2006, 119, 71–96. [Google Scholar]
- De Jonge, L.W.; Kjaergaard, C.; Moldrup, P. Colloids and colloid-facilitated transport of contaminants in soils: An introduction. Vadose Zone J. 2004, 3, 321–325. [Google Scholar] [CrossRef]
- Grolimund, D.; Borkovec, M.; Barmettler, K.; Sticher, H. Colloid-facilitated transport of strongly sorbing contaminants in natural porous media: A laboratory column study. Environ. Sci. Technol. 1996, 30, 3118–3123. [Google Scholar] [CrossRef]
- Roy, S.B.; Dzombak, D.A. Chemical factors influencing colloid-facilitated transport of contaminants in porous media. Environ. Sci. Technol. 1997, 31, 656–664. [Google Scholar] [CrossRef]
- Wikiniyadhanee, R.; Chotpantarat, S.; Ong, S.K. Effects of kaolinite colloids on Cd2+ transport through saturated sand under varying ionic strength conditions: Column experiments and modeling approaches. J. Contam. Hydrol. 2015, 182, 146–156. [Google Scholar] [CrossRef]
- Wu, J.; Shen, C.; Wang, C.; Yan, A.; Zhang, H. The failure of using equilibrium adsorption of fosthiazate onto montmorillonite clay particles to predict their cotransport in porous media as revealed by batch and column studies. J. Soils Sediments 2019, 19, 917–928. [Google Scholar] [CrossRef]
- Shen, C.; Wang, H.; Lazouskaya, V.; Du, Y.; Lu, W.; Wu, J.; Zhang, H.; Huang, Y. Cotransport of bismerthiazol and montmorillonite colloids in saturated porous media. J. Contam. Hydrol. 2015, 177–178, 18–29. [Google Scholar] [CrossRef]
- Xing, Y.; Chen, X.; Zhuang, J.; Chen, X. What happens when pharmaceuticals meet colloids. Ecotoxicology 2015, 10, 2100–2114. [Google Scholar] [CrossRef]
- Maskaoui, K.; Zhou, J.L. Colloids as a sink for certain pharmaceuticals in the aquatic environment. Environ. Sci. Pollut. Res. 2009, 17, 898–907. [Google Scholar] [CrossRef]
- Chen, H.; Gao, B.; Yang, L.Y.; Ma, L.Q. Montmorillonite enhanced ciprofloxacin transport in saturated porous media with sorbed ciprofloxacin showing antibiotic activity. J. Contam Hydrol. 2015, 173, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vasiliadou, I.A.; Chrysikopoulos, C.V. Cotransport of Pseudomonas putida and kaolinite particles through water saturated columns packed with glass beads. Water Resour. Res. 2011, 47, W02543. [Google Scholar] [CrossRef] [Green Version]
- Syngouna, V.I.; Chrysikopoulos, C.V. Cotransport of clay colloids and viruses in water saturated porous media. Colloids and Surfaces A: Physicochemical and Engineering Aspects. Eng. Asp. 2013, 416, 56–65. [Google Scholar] [CrossRef]
- Syngouna, V.I.; Chrysikopoulos, C.V. Cotransport of clay colloids and viruses through water-saturated vertically oriented columns packed with glass beads: Gravity effects. Sci. Total. Environ. 2016, 545–546, 210–218. [Google Scholar] [CrossRef]
- Syngouna, V.I.; Chrysikopoulos, C.V.; Kokkinos, P.; Tselepi, M.A.; Vantarakis, A. Cotransport of human adenoviruses with clay colloids and TiO2 nanoparticles in saturated porous media: Effect of flow velocity. Sci. Total Environ. 2017, 598, 160–167. [Google Scholar] [CrossRef]
- Katzourakis, V.E.; Chrysikopoulos, C.V. Mathematical modeling of colloid and virus cotransport in porous media: Application to experimental data. Adv. Water Resour. 2014, 68, 62–73. [Google Scholar] [CrossRef]
- Molnar, I.L.; Johnson, W.P.; Gerhard, J.I.; Willson, C.S.; O’Carroll, D.M. Predicting colloid transport through saturated porous media: A critical review. Water Resour. Res. 2015, 51, 6804–6845. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhang, W.; Qin, Y.; Ma, T.; Zhou, J.; Du, S. Fe-colloid cotransport through saturated porous media under different hydrochemical and hydrodynamic conditions. Sci. Total Environ. 2019, 647, 494–506. [Google Scholar] [CrossRef]
- Knappenberger, T.; Flury, M.; Mattson, E.D.; Harsh, J.B. Does water content or flow rate control colloid transport in unsaturated porous media? Environ Sci Technol. 2014, 48, 3791–3799. [Google Scholar] [CrossRef]
- Cheng, T.; Saiers, J.E. Colloid-facilitated transport of cesium in vadose-zone sediments: The importance of flow transients. Environ. Sci. Technol. 2010, 44, 7443–7449. [Google Scholar] [CrossRef]
- Syngouna, V.I.; Chrysikopoulos, C.V. Experimental investigation of virus and clay particles cotransport in partially saturated columns packed with glass beads. J. Colloid Interf. Sci. 2015, 440, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Sirivithayapakorn, S.; Keller, A. Transport of colloids in unsaturated porous media: A pore-scale observation of processes during the dissolution of air-water interface. Water Resour. Res. 2003, 39, 1346. [Google Scholar] [CrossRef] [Green Version]
- Ghanbarian-Alavijeh, B.; Skinner, T.E.; Hunt, A.G. Saturation dependence of dispersion in porous media. Phys. Rev. E 2012, 86, 066316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flury, M.; Aramrak, S. Role of air-water interfaces in colloid transport in porous media: A review. Water Resour. Res. 2017, 53, 5247–5275. [Google Scholar] [CrossRef]
- Wan, J.; Tokunaga, T.K. Film straining of colloids in unsaturated porous media: Conceptual model and experimental testing. Environ. Sci. Technol. 1997, 31, 2413–2420. [Google Scholar] [CrossRef]
- Crist, J.T.; Zevi, Y.; McCarthy, J.F.; Throop, J.A.; Steenhuis, T.S. Transport and retention mechanisms of colloids in partially saturated porous media. Vadose Zone J. 2005, 4, 184–195. [Google Scholar] [CrossRef] [Green Version]
- Sim, Y.; Chrysikopoulos, C.V. Virus transport in unsaturated porous media. Water Resour. Res. 2000, 36, 173–179. [Google Scholar] [CrossRef]
- Baumann, T.; Fruhstorfer, P.; Klein, T.; Niessner, R. Colloid and heavy metal transport at landfill sites in direct contact with groundwater. Water Res. 2006, 40, 2776–2786. [Google Scholar] [CrossRef]
- Chotpantarat, S.; Kiatvarangkul, N. Facilitated transport of cadmium with montmorillonite KSF colloids under different pH conditions in water-saturated sand columns: Experiment and transport modeling. Water Res. 2018, 146, 216–231. [Google Scholar] [CrossRef]
- Yuan, H.; Li, G.; Yang, L.; Yan, X.; Yang, D. Development of melamine formaldehyde resin microcapsules with low formaldehyde emission suited for seed treatment. Colloids Surfaces B Biointerfaces 2015, 128, 149–154. [Google Scholar] [CrossRef]
- Nash, T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem. J. 1953, 55, 416–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fountouli, T.V.; Chrysikopoulos, C.V.; Tsanis, I.K. Effect of salinity on formaldehyde interaction with quartz sand and kaolinite colloid particles: Batch and column experiments. Environ. Earth Sci. 2019, 78, 152. [Google Scholar] [CrossRef]
- Seyfioglu, R.; Odabasi, M.; Cetin, E. Wet and dry deposition of formaldehyde in Izmir, Turkey. Sci. Total Environ. 2006, 366, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Economou, C.; Mihalopoulos, N. Formaldehyde in the rainwater in the eastern Mediterranean: Occurrence, deposition and contribution to organic carbon budget. Atmos. Environ. 2002, 36, 1337–1347. [Google Scholar] [CrossRef]
- Syngouna, V.I.; Chrysikopoulos, C.V. Transport of biocolloids in water saturated columns packed with sand: Effect of grain size and pore water velocity. J. Contam. Hydrol. 2011, 126, 301–314. [Google Scholar] [CrossRef]
- Fountouli, T.V.; Chrysikopoulos, C.V. Adsorption and thermodynamics of pharmaceuticals, acyclovir and fluconazole, onto quartz sand under static and dynamic conditions. Environ. Eng. Sci. 2018, 35, 909–917. [Google Scholar] [CrossRef]
- Chrysikopoulos, C.V.; Sotirelis, N.P.; Kallithrakas-Kontos, N.G. Cotransport of graphene oxide nanoparticles and kaolinite colloids in porous media. Transp. Porous Media 2017, 119, 181–204. [Google Scholar] [CrossRef]
- Rong, X.; Huanga, Q.; He, X.; Chen, H.; Cai, P.; Liang, W. Interaction of Pseudomonas putida with kaolinite and montmorillonite: A combination study by equilibrium adsorption, ITC, SEM and FTIR. Colloids Surf. B 2008, 64, 49–55. [Google Scholar] [CrossRef]
- Xu, S.; Qi, J.; Chen, X.; Lazouskay, V.; Zhuang, J.; Jin, Y. Coupled effect of extended DLVO and capillary interactions on the retention and transport of colloids through unsaturated porous media. Sci. Total Environ. 2016, 573, 564–572. [Google Scholar] [CrossRef]
- El-Farhan, Y.H.; Denovio, N.M.; Herman, J.S.; Hornberger, G.M. Mobilization and transport of soil particles during infiltration experiments in an agricultural field, Shenandoah Valley, Virginia. Environ. Sci. Technol. 2000, 34, 3555–3559. [Google Scholar] [CrossRef]
- Anders, R.; Chrysikopoulos, C.V. Transport of viruses through saturated and unsaturated columns packed with sand. Transp. Porous Media 2009, 76, 121–138. [Google Scholar] [CrossRef]
- Mitropoulou, P.N.; Syngouna, V.I.; Chrysikopoulos, C.V. Transport of colloids in unsaturated packed columns: Role of ionic strength and sand grain size. J. Chem. Eng. 2013, 232, 237–248. [Google Scholar] [CrossRef]
- Lewis, J.; Sjöstrom, J. Optimizing the experimental design of soil columns in saturated and unsaturated transport experiments. J. Contam. Hydrol. 2010, 115, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chrysikopoulos, C.V. Artificial tracers for geothermal reservoir studies. Environ. Geology 1993, 22, 60–70. [Google Scholar]
- James, S.C.; Chrysikopoulos, C.V. Monodisperse and polydisperse colloid transport in water-saturated fractures with various orientations: Gravity effects. Adv. Water Resour. 2011, 34, 1249–1255. [Google Scholar] [CrossRef]
- Chrysikopoulos, C.V.; Katzourakis, V.E. Colloid particle size-dependent dispersivity. Water Resour. Res. 2015, 51, 4668–4683. [Google Scholar] [CrossRef]
- Saiers, J.E.; Lenhart, J.J. Colloid mobilization and transport within unsaturated porous media under transient-flow conditions. Water Resour. Res. 2003, 39, 1019. [Google Scholar] [CrossRef] [Green Version]
- Tufenkji, N.; Elimelech, M. Correlation equation for predicting single-collector efficiency in physicochemical filtration in saturated porous media. Environ. Sci. Technol. 2004, 38, 529–536. [Google Scholar] [CrossRef]
- Van Olphen, H.; Fripiat, J.J. Data Handbook for Clay Minerals and Other Non-Metallic Minerals; Pergamon Press: Oxford, UK, 1979. [Google Scholar]
- Chrysikopoulos, C.V.; Syngouna, V.I. Attachment of bacteriophages MS2 and ΦX174 onto kaolinite and montmorillonite: Extended-DLVO interactions. Colloids Surfaces B Biointerfaces 2012, 92, 74–83. [Google Scholar] [CrossRef]
- Verwey, E.J.W.; Overbeek, J.T.G. Theory of the Stability of Lyophobic Colloids: The Interaction of Sol Particles Having an Electric Double Layer; Elsevier: Amsterdam, The Netherlands, 1948. [Google Scholar]
- Hogg, R.; Healy, T.W.; Fuerstenau, D.W. Mutual coagulation of colloidal dispersions. Trans. Faraday Soc. 1966, 62, 1638–1651. [Google Scholar] [CrossRef]
- Loveland, J.P.; Ryan, J.N.; Amy, G.L.; Harvey, R.W. The reversibility of virus attachment to mineral surfaces. Colloids Surfaces A Physicochem. Eng. Asp. 1996, 107, 205–221. [Google Scholar] [CrossRef]
- Gregory, J. Approximate expressions for retarded van der Waals interaction. J. Colloid Interface Sci. 1981, 83, 138–145. [Google Scholar] [CrossRef]
- Ruckenstein, E.; Prieve, D.C. Adsorption and desorption of particles and their chromatographic separation. AIChE J. 1976, 22, 276–283. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces 2011, 3rd ed.; Academic Press: London, UK, 2011. [Google Scholar]
- Novich, B.E.; Ring, T.A. Colloid stability of clays using photon correlation spectroscopy. Clays Clay Miner. 1984, 32, 400–406. [Google Scholar] [CrossRef]
- Shang, J.; Flury, M.; Chen, G.; Zhuang, J. Impact of flow rate, water content, and capillary forces on in situ colloid mobilization during infiltration in unsaturated sediments. Water Resour. Res. 2008, 44, W06411. [Google Scholar] [CrossRef]
- Gao, B.; Steenhuis, T.S.; Zevi, Y.; Morales, V.L.; Nieber, J.L.; Richards, B.K.; McCarthy, J.F.; Parlange, J.-Y. Capillary retention of colloids in unsaturated porous media. Water Resour. Res. 2008, 44, W04504. [Google Scholar] [CrossRef] [Green Version]
- Bai, H.; Cochet, N.; Pauss, A.; Lamy, E. DLVO, hydrophobic, capillary and hydrodynamic forces acting on bacteria at solid-air-water interfaces: Their relative impact on bacteria deposition mechanisms in unsaturated porous media. Colloids Surf. B Biointerfaces 2017, 150, 41–49. [Google Scholar] [CrossRef]
- Wu, W. Baseline studies of the clay minerals society source clays: Colloid and surface phenomena. Clays Clay Miner. 2001, 49, 446–452. [Google Scholar] [CrossRef]
- Abdel-Salam, A.; Chrysikopoulos, C. Modeling of colloid and colloid-facilitated contaminant transport in a two-dimensional fracture with spatially variable aperture. Transport Porous Media 1995, 20, 197–221. [Google Scholar] [CrossRef]
- Abdel-Salam, A.; Chrysikopoulos, C. Analysis of a model for contaminant transport in fractured media in the presence of colloids. J. Hydrol. 1995, 165, 261–281. [Google Scholar] [CrossRef]
- Katzourakis, V.E.; Chrysikopoulos, C.V. Modeling dense-colloid and virus cotransport in three-dimensional porous media. J. Contam. Hydrol. 2015, 181, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Won, J.; Wirth, X.; Burns, S.E. An experimental study of cotransport of heavy metals with kaolinite colloids. J. Hazard. Mater. 2019, 373, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Pang, L. Microbial removal rates in subsurface media estimated from published studies of field experiments and large intact soil cores. J. Environ. Qual. 2009, 38, 1531–1559. [Google Scholar] [CrossRef] [PubMed]
- Walshe, G.E.; Pang, L.; Flury, M.; Close, M.E.; Flintoft, M. Effects of pH, ionic strength, dissolved organic matter, and flow rate on the co-transport of MS2 bacteriophages with kaolinite in gravel aquifer media. Water Res. 2010, 44, 1255–1269. [Google Scholar] [CrossRef]
- Grasso, D.; Subramaniam, K.; Butkus, M.; Strevett, K.; Bergendahl, J. A review of non-DLVO interactions in environmental colloidal systems. Rev. Environ. Sci. Biotechnol. 2002, 1, 17–38. [Google Scholar] [CrossRef]
- Bradford, S.A.; Torkzaban, S. Colloid transport and retention in unsaturated porous media: A review of interface-, collector-, and pore-scale processes and models. Vadose Zone J. 2008, 7, 667–681. [Google Scholar] [CrossRef] [Green Version]
- Zevi, Y.; Dathe, A.; McCarthy, J.F.; Richards, B.K.; Steenhuis, T.S. Distribution of colloid particles onto interfaces in partially saturated sand. Environ. Sci. Technol. 2005, 39, 18. [Google Scholar] [CrossRef]
- Gao, B.; Saiers, J.E.; Ryan, J.N. Deposition and mobilization of clay colloids in unsaturated porous media. Water Resour. Res. 2004, 40, W08602. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Gao, B.; Wu, L.; Morales, V.L.; Yang, L.; Zhou, Z.; Wang, H. Deposition and transport of graphene oxide in saturated and unsaturated porous media. Chem. Eng. J. 2013, 229, 444–449. [Google Scholar] [CrossRef] [Green Version]

| Zeta Potentials (mV) | |
|---|---|
| KGa-1b | −32.7 ± 2.6 |
| STx-1b | −25.6 ± 4.6 |
| KGa-1b & FA | −36.8 ± 5.8 |
| STx-1b & FA | −37.8 ± 3.0 |
| Experiment | Flow Rate (mL/min) | Sw (%) | θm (-) | θ (-) | U (cm/min) | Mr (%) | M1(i)/M1(tr) (-) | aexp (-) | (C/C0)max (-) |
|---|---|---|---|---|---|---|---|---|---|
| Formaldehyde (FA) | 1 | 41.9 | 0.18 | 0.43 | 0.44 | 84.2 | 0.95 | – | 0.97 |
| FA | 1.5 | 50.0 | 0.22 | 0.44 | 0.65 | 89.0 | 1.04 | – | 0.98 |
| FA | 2 | 61.4 | 0.28 | 0.45 | 0.84 | 91.3 | 1.06 | – | 0.99 |
| FA | 3 | 70.7 | 0.31 | 0.44 | 1.29 | 85.9 | 1.02 | – | 1.00 |
| FA-(KGa-1b) | 1 | 40.9 | 0.18 | 0.44 | 0.43 | 67.6–44.3 | (0.76)–(0.50) | 0.129 | (0.75)–(0.59) |
| FA-(KGa-1b) | 1.5 | 52.4 | 0.23 | 0.43 | 0.66 | 62.6–66.8 | (0.74)–(0.79) | 0.021 | (0.73)–(0.94) |
| FA-(KGa-1b) | 2 | 59.0 | 0.25 | 0.42 | 0.89 | 64.7–64.1 | (0.76)–(0.75) | 0.065 | (0.78)-(0.84) |
| FA-(KGa-1b) | 3 | 70.0 | 0.30 | 0.43 | 1.31 | 72.4–82.8 | (0.86)–(0.98) | 0.009 | (0.85)–(0.98) |
| FA-(STx-1b) | 1 | 40.7 | 0.17 | 0.41 | 0.46 | 74.8–68.5 | (0.85)–(0.77) | 0.039 | (0.88) –(0.84) |
| FA-(STx-1b) | 1.5 | 50.1 | 0.21 | 0.41 | 0.68 | 69.7–74.1 | (0.82)–(0.87) | 0.035 | (0.85)–(0.89) |
| FA-(STx-1b) | 2 | 59.9 | 0.24 | 0.41 | 0.93 | 78.7–71.3 | (0.92)–(0.83) | 0.044 | (0.94)–(0.89) |
| FA-(STx-1b) | 3 | 70.2 | 0.29 | 0.41 | 1.38 | 74.5–80.2 | (0.89)–(0.95) | 0.001 | (0.90)–(1.00) |
| Tracer | 1 | 40.8 | 0.17 | 0.42 | 0.45 | 88.5 | – | ||
| Tracer | 1.5 | 49.5 | 0.21 | 0.42 | 0.67 | 84.8 | – | ||
| Tracer | 2 | 59.6 | 0.25 | 0.42 | 0.89 | 85.7 | – | ||
| Tracer | 3 | 70.0 | 0.30 | 0.42 | 1.34 | 84.1 | – |
| Interacting Pair | Φmax1 (kBT) | Φmin1 (kBT) | Φmin2 (kBT) |
|---|---|---|---|
| (KGa-1b)-AWI | 1278.9 | na | na |
| (STx-1b)-AWI | 788.2 | na | na |
| (KGa-1b)-SWI | 1160.9 | na | −0.004 |
| (STx-1b)-SWI | 758.1 | −958.5 | −0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fountouli, T.V.; Chrysikopoulos, C.V. Effect of Clay Colloid Particles on Formaldehyde Transport in Unsaturated Porous Media. Water 2020, 12, 3541. https://doi.org/10.3390/w12123541
Fountouli TV, Chrysikopoulos CV. Effect of Clay Colloid Particles on Formaldehyde Transport in Unsaturated Porous Media. Water. 2020; 12(12):3541. https://doi.org/10.3390/w12123541
Chicago/Turabian StyleFountouli, Theodosia V., and Constantinos V. Chrysikopoulos. 2020. "Effect of Clay Colloid Particles on Formaldehyde Transport in Unsaturated Porous Media" Water 12, no. 12: 3541. https://doi.org/10.3390/w12123541
APA StyleFountouli, T. V., & Chrysikopoulos, C. V. (2020). Effect of Clay Colloid Particles on Formaldehyde Transport in Unsaturated Porous Media. Water, 12(12), 3541. https://doi.org/10.3390/w12123541







