Uptake and Recovery of Gold from Simulated Hydrometallurgical Liquors by Adsorption on Pine Bark Tannin Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adsorbent
2.2. Gold Solutions
2.3. Analytic Methods
2.4. Adsorption Studies
2.4.1. Effect of the Leaching Reagent
2.4.2. Adsorption Equilibrium Isotherms
2.4.3. Kinetic Study
2.4.4. Competitive Adsorption and Selectivity
2.5. Desorption and Regeneration
3. Results and Discussion
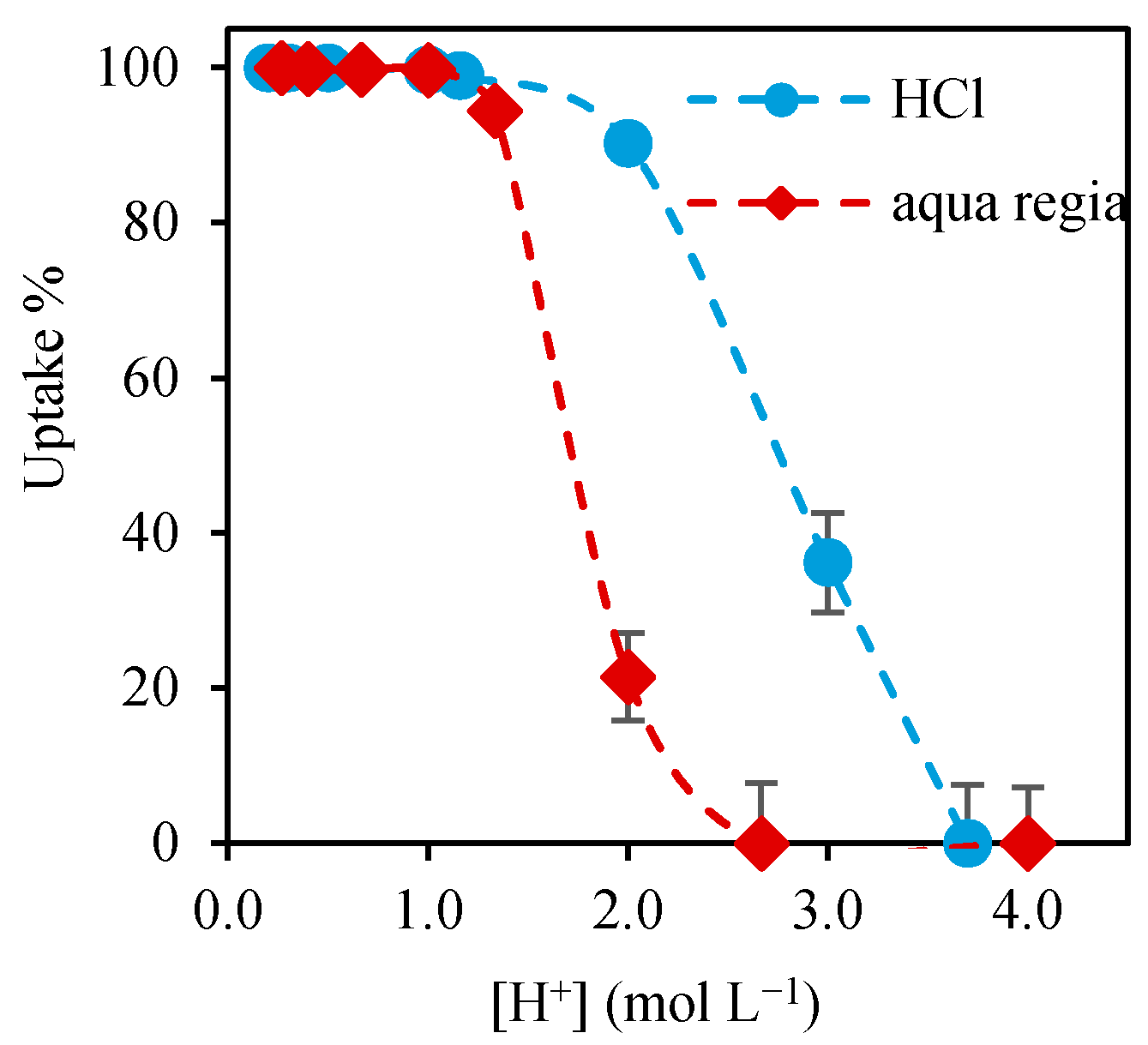
3.1. Effect of the Leaching Solution
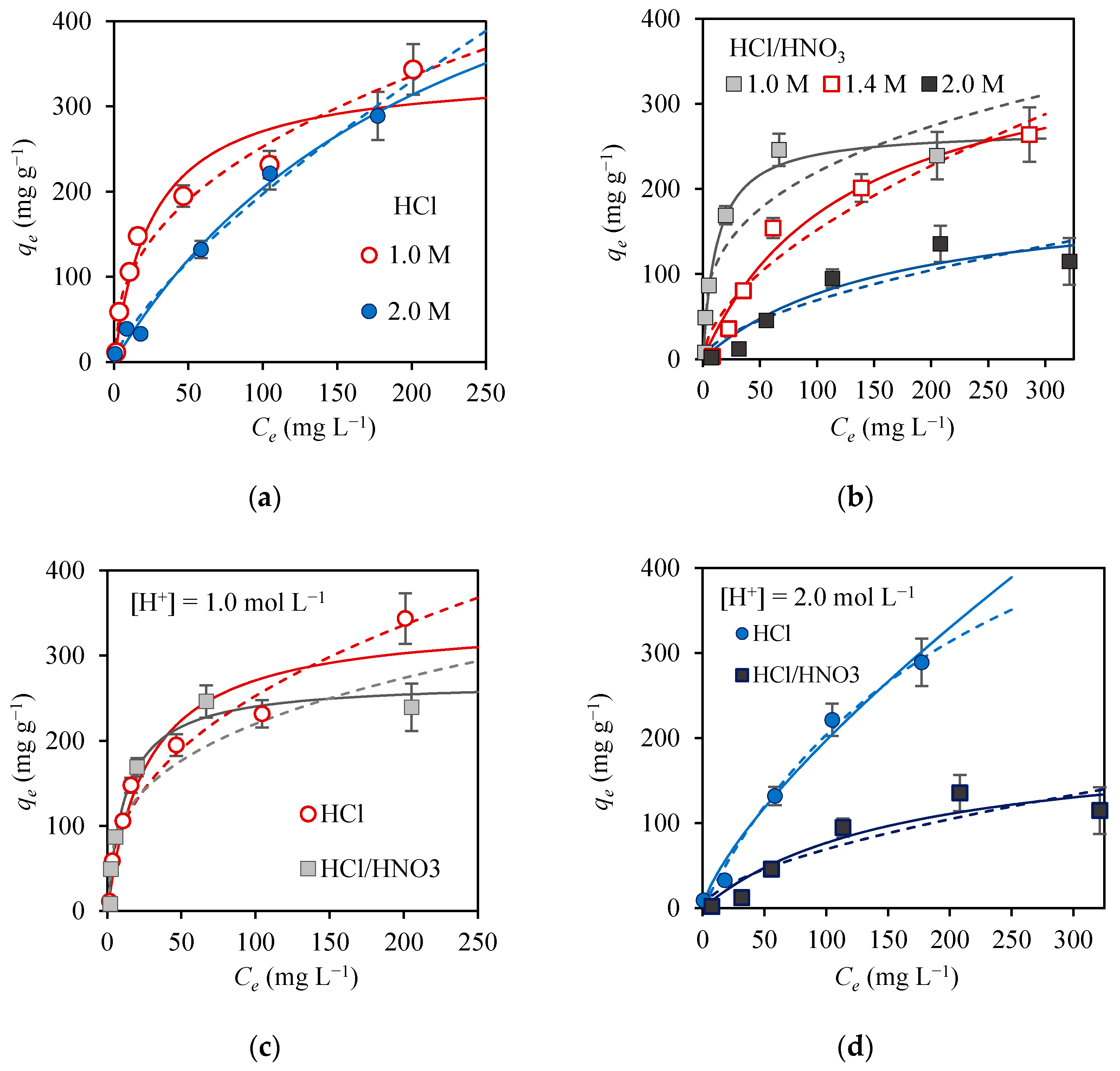
3.2. Adsorption Equilibrium Isotherms
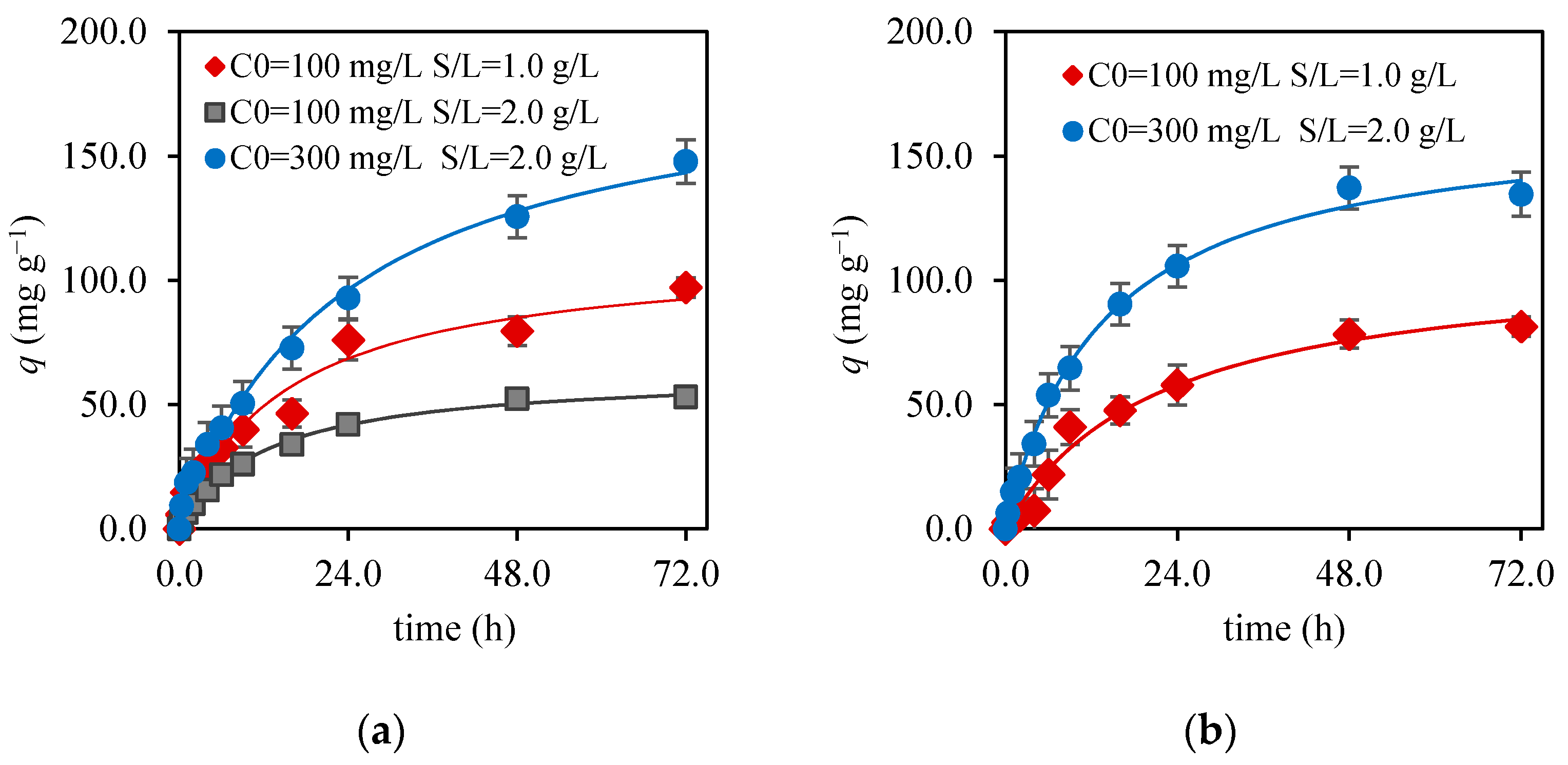
3.3. Kinetic Study
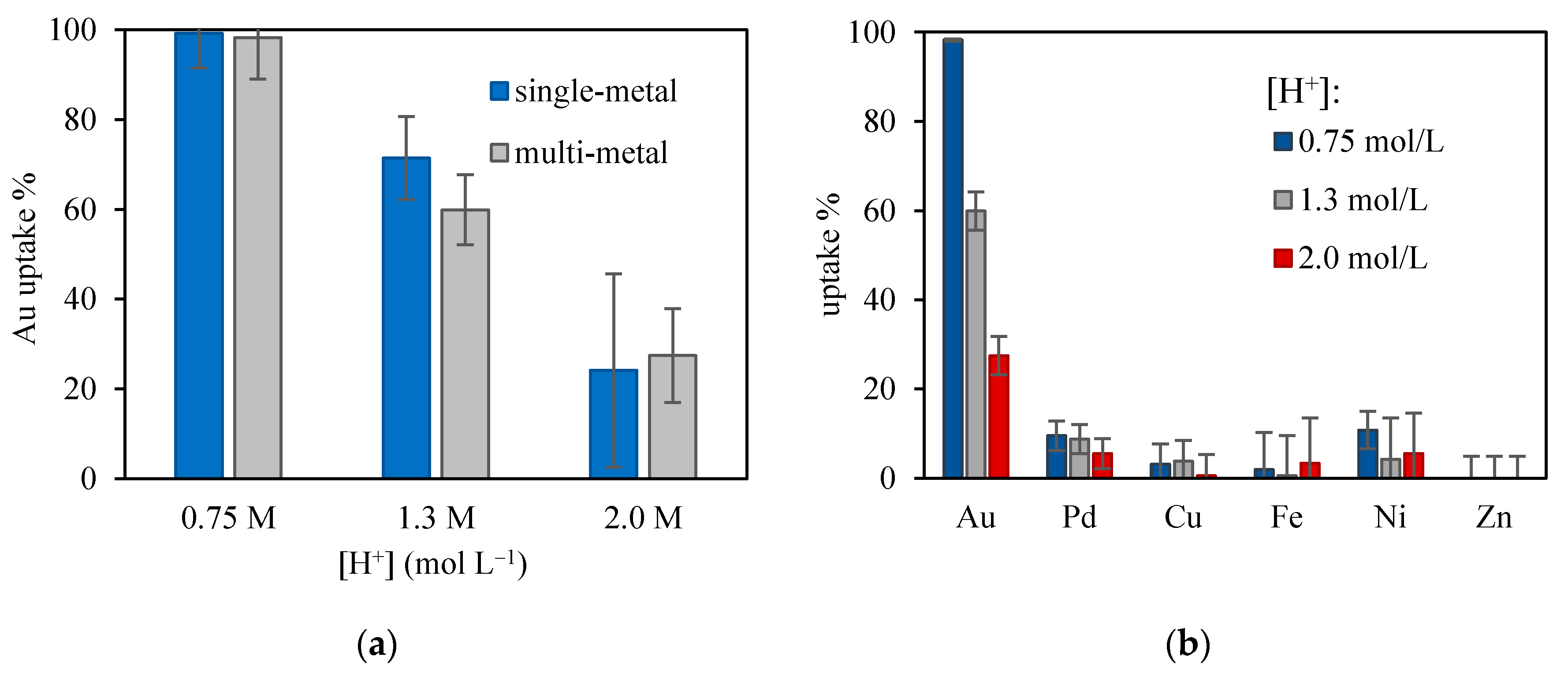
3.4. Competitive Adsorption and Selectivity
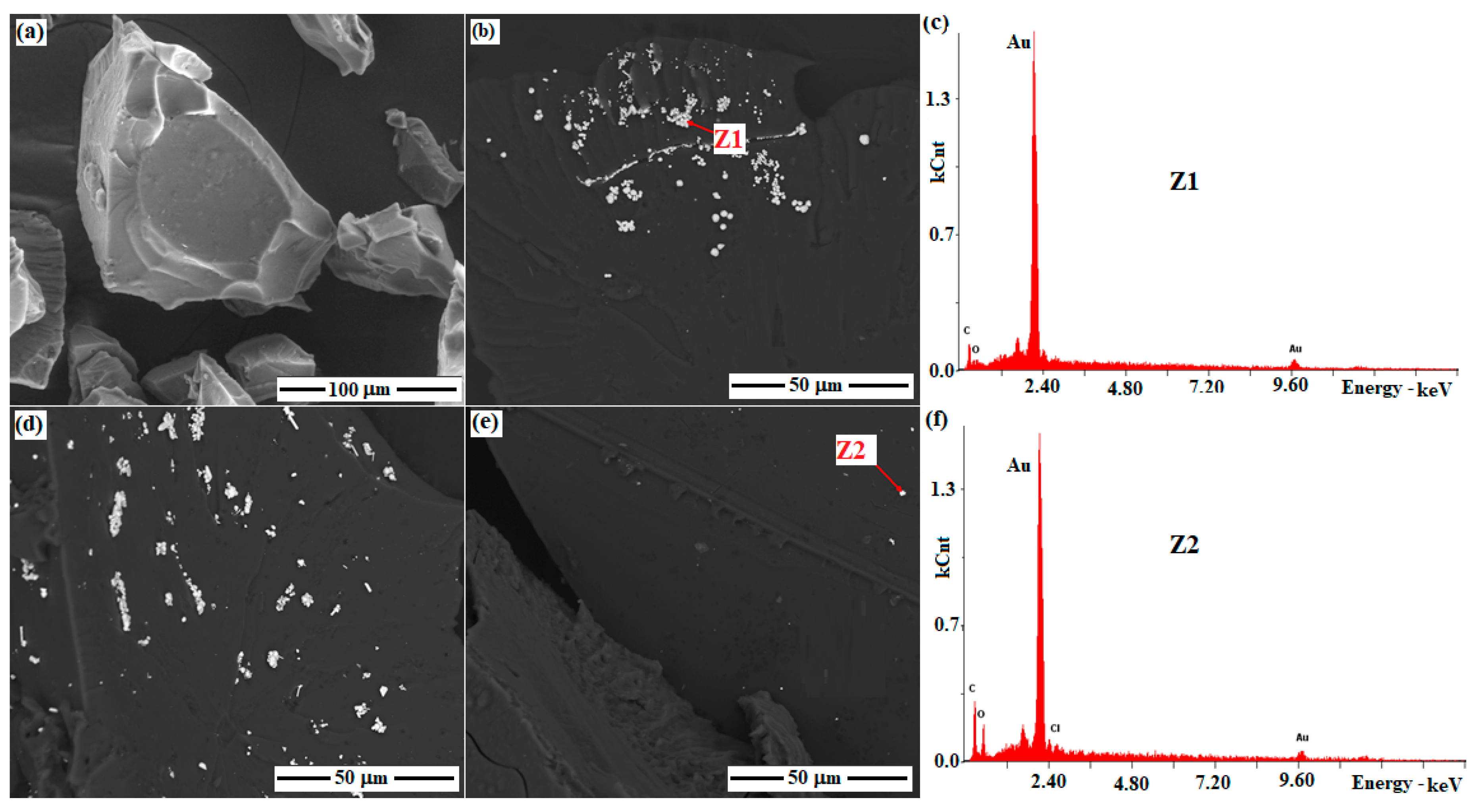
3.5. SEM and EDS Analysis
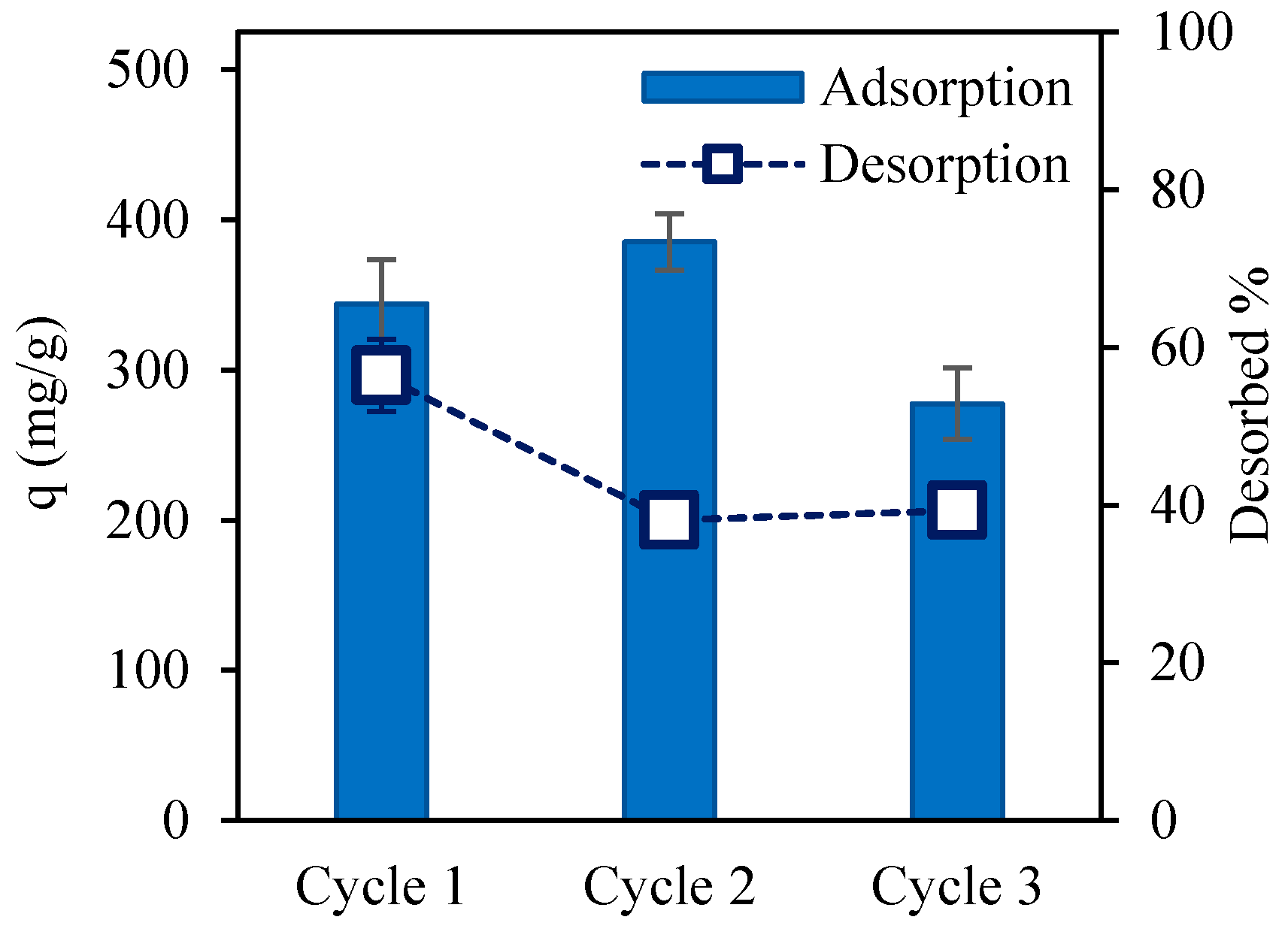
3.6. Desorption and Regeneration
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Perez, J.P.H.; Folens, K.; Leus, K.; Vanhaecke, F.; Van Der Voort, P.; Du Laing, G. Progress in hydrometallurgical technologies to recover critical raw materials and precious metals from low-concentrated streams. Resour. Conserv. Recycl. 2019, 142, 177–188. [Google Scholar] [CrossRef]
- Falahati, M.; Attar, F.; Sharifi, M.; Saboury, A.A.; Salihi, A.; Aziz, F.M.; Kostova, I.; Burda, C.; Priecel, P.; Lopez-Sanchez, J.A.; et al. Gold nanomaterials as key suppliers in biological and chemical sensing, catalysis, and medicine. Biochim. Biophys. Acta BBA Gen. Subj. 2020, 1864, 129435. [Google Scholar] [CrossRef] [PubMed]
- Forti, V.; Baldé, C.P.; Kuehr, R.; Bel, G. The Global E-waste Monitor 2020: Quantities, Flows and the Circular Economy Potential; United Nations University (UNU)/United Nations Institute for Training and Research (UNITAR)–co-hosted SCYCLE Programme, International Telecommunication Union (ITU) & International Solid Waste Association (ISWA): Bonn, Germany; Geneva, Switzerland; Rotterdam, The Netherlands, 2020. [Google Scholar]
- European Parliament and Council. Directive 2012/19/EU of the European Parliament and of the Council of 4 July 2012 on Waste Electrical and Electronic Equipment (WEEE); European Parliament and Council: Brussels, Belgium, 2012. [Google Scholar]
- Akcil, A.; Erust, C.; Gahan, C.S.; Ozgun, M.; Sahin, M.; Tuncuk, A. Precious metal recovery from waste printed circuit boards using cyanide and non-cyanide lixiviants-A review. Waste Manag. 2015, 45, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Gurung, M.; Adhikari, B.B.; Kawakita, H.; Ohto, K.; Inoue, K.; Alam, S. Recovery of gold and silver from spent mobile phones by means of acidothiourea leaching followed by adsorption using biosorbent prepared from persimmon tannin. Hydrometallurgy 2013, 133, 84–93. [Google Scholar] [CrossRef]
- Rao, M.D.; Singh, K.K.; Morrison, C.A.; Love, J.B. Challenges and opportunities in the recovery of gold from electronic waste. RSC Adv. 2020, 10, 4300–4309. [Google Scholar] [CrossRef] [Green Version]
- Kaya, M. Recovery of metals and nonmetals from electronic waste by physical and chemical recycling processes. Waste Manag. 2016, 57, 64–90. [Google Scholar] [CrossRef]
- Ventura, E.; Futuro, A.; Pinho, S.C.; Almeida, M.; Dias, J.M. Physical and thermal processing of Waste Printed Circuit Boards aiming for the recovery of gold and copper. J. Environ. Manag. 2018, 223, 297–305. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Xie, H.; Zeng, X.; Li, J. Current Status on Leaching Precious Metals from Waste Printed Circuit Boards. Procedia Environ. Sci. 2012, 16, 560–568. [Google Scholar] [CrossRef] [Green Version]
- Camelino, S.; Rao, J.; Padilla, R.L.; Lucci, R. Initial Studies about Gold Leaching from Printed Circuit Boards (PCB’s) of Waste Cell Phones. Procedia Mater. Sci. 2015, 9, 105–112. [Google Scholar] [CrossRef]
- Diaz, L.A.; Lister, T.E. Economic evaluation of an electrochemical process for the recovery of metals from electronic waste. Waste Manag. 2018, 74, 384–392. [Google Scholar] [CrossRef]
- Birich, A.; Mohamed, S.R.; Friedrich, B. Screening of Non-cyanide Leaching Reagents for Gold Recovery from Waste Electric and Electronic Equipment. J. Sustain. Met. 2018, 4, 265–275. [Google Scholar] [CrossRef]
- Korolev, I.; Kolehmainen, E.; Haapalainen, M.; Yliniemi, K.; Lundström, M. Gold Recovery from Chloride Leaching Solutions by Electrodeposition-Redox Replacement Method. In Proceedings of the 10th European Metallurgical Conference (EMC 2019), Düsseldorf, Germany, 23–26 June 2019. [Google Scholar]
- Sayiner, B.; Acarkan, N. Effect of Silver, Nickel and Copper Cyanides on Gold Adsorption on Activated Carbon in Cyanide Leach Solutions. Physicochem. Probl. Miner. Process. 2014, 50, 277–287. [Google Scholar] [CrossRef]
- Leroux, J.D.; Bryson, A.W.; Young, B.D. A Comparison of Several Kinetic-Models for the Adsorption of Gold Cyanide onto Activated Carbon. J. S. Afr. Inst. Min. Metall. 1991, 91, 95–103. [Google Scholar]
- Dodson, J.R.; Parker, H.L.; García, A.M.; Hicken, A.; Asemave, K.; Farmer, T.J.; He, H.; Clark, J.H.; Hunt, A.J. Bio-derived materials as a green route for precious & critical metal recovery and re-use. Green Chem. 2015, 17, 1951–1965. [Google Scholar] [CrossRef]
- Bediako, J.K.; Lin, S.; Sarkar, A.K.; Zhao, Y.; Choi, J.-W.; Song, M.-H.; Wei, W.; Reddy, D.H.K.; Cho, C.-W.; Yun, Y.-S. Benignly-fabricated crosslinked polyethylenimine/calcium-alginate fibers as high-performance adsorbents for effective recovery of gold. J. Clean. Prod. 2020, 252, 119389. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, L.; Wang, W.; Yu, G.; Huang, J. Adsorptive recovery of Au(III) from aqueous solution using crosslinked polyethyleneimine resins. Chemosphere 2019, 241, 125122. [Google Scholar] [CrossRef]
- Morcali, M.H.; Zeytuncu, B.; Ozlem, E.; Aktas, S. Studies of Gold Adsorption from Chloride Media. Mater. Res. 2015, 18, 660–667. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, B.C.; Choudhary, B.C.; Borse, A.U.; Garole, D.J. Surface functionalized biomass for adsorption and recovery of gold from electronic scrap and refinery wastewater. Sep. Purif. Technol. 2018, 195, 260–270. [Google Scholar] [CrossRef]
- Bediako, J.K.; Choi, J.-W.; Song, M.-H.; Zhao, Y.; Lin, S.; Sarkar, A.K.; Cho, C.-W.; Yun, Y.-S. Recovery of gold via adsorption-incineration techniques using banana peel and its derivatives: Selectivity and mechanisms. Waste Manag. 2020, 113, 225–235. [Google Scholar] [CrossRef]
- Al-Saidi, H. The fast recovery of gold(III) ions from aqueous solutions using raw date pits: Kinetic, thermodynamic and equilibrium studies. J. Saudi Chem. Soc. 2016, 20, 615–624. [Google Scholar] [CrossRef] [Green Version]
- Fan, R.; Min, H.; Hong, X.; Yi, Q.; Liu, W.; Zhang, Q.; Luo, Z. Plant tannin immobilized Fe3O4@SiO2 microspheres: A novel and green magnetic bio-sorbent with superior adsorption capacities for gold and palladium. J. Hazard. Mater. 2019, 364, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Xie, F.; Guan, X.; Zhang, Q.; Luo, Z. Selective adsorption and recovery of Au(III) from three kinds of acidic systems by persimmon residual based bio-sorbent: A method for gold recycling from e-wastes. Bioresour. Technol. 2014, 163, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.C.; Bacelo, H.A.M.; Boaventura, R.A.R.; Botelho, C.M.S. Tannin-Adsorbents for Water Decontamination and for the Recovery of Critical Metals: Current State and Future Perspectives. Biotechnol. J. 2019, 14, e1900060. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.T.D.G.; Moraes, L.F.; Da Silva, M.G.C.; Vieira, M.G.A. Recovery of gold through adsorption onto sericin and alginate particles chemically crosslinked by proanthocyanidins. J. Clean. Prod. 2020, 253, 119925. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.; Chen, S. Adsorption behavior of Au(III) and Pd(II) on persimmon tannin functionalized viscose fiber and the mechanism. Int. J. Biol. Macromol. 2020, 152, 1242–1251. [Google Scholar] [CrossRef]
- Yi, Q.; Fan, R.; Xie, F.; Min, H.; Zhang, Q.; Luo, Z. Selective Recovery of Au(III) and Pd(II) from Waste PCBs Using Ethylenediamine Modified Persimmon Tannin Adsorbent. Procedia Environ. Sci. 2016, 31, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Li, X.; Liang, H.; Ning, J.; Zhou, Z.; Li, G. Equilibrium, kinetics and mechanism of Au3+, Pd2+ and Ag+ ions adsorption from aqueous solutions by graphene oxide functionalized persimmon tannin. Mater. Sci. Eng. C 2017, 79, 227–236. [Google Scholar] [CrossRef]
- Bacelo, H.A.; Vieira, B.R.; Santos, S.C.; Boaventura, R.A.; Botelho, C.M. Recovery and valorization of tannins from a forest waste as an adsorbent for antimony uptake. J. Clean. Prod. 2018, 198, 1324–1335. [Google Scholar] [CrossRef]
- Sánchez-Martín, J.; Beltrán-Heredia, J.; Gibello-Pérez, P. Adsorbent biopolymers from tannin extracts for water treatment. Chem. Eng. J. 2011, 168, 1241–1247. [Google Scholar] [CrossRef]
- Bacelo, H.A.; Santos, S.C.; Botelho, C.M. Removal of arsenic from water by an iron-loaded resin prepared from Pinus pinaster bark tannins. Euro-Mediterr. J. Environ. Integr. 2020, 5, 1–17. [Google Scholar] [CrossRef]
- Gurung, M.; Adhikari, B.B.; Kawakita, H.; Ohto, K.; Inoue, K.; Alam, S. Recovery of Au(III) by using low cost adsorbent prepared from persimmon tannin extract. Chem. Eng. J. 2011, 174, 556–563. [Google Scholar] [CrossRef]
- Saman, N.; Tan, J.-W.; Mohtar, S.S.; Kong, H.; Lye, J.W.P.; Johari, K.; Hassan, H.; Mat, H. Selective biosorption of aurum(III) from aqueous solution using oil palm trunk (OPT) biosorbents: Equilibrium, kinetic and mechanism analyses. Biochem. Eng. J. 2018, 136, 78–87. [Google Scholar] [CrossRef]
- Xie, F.; Fan, Z.; Zhang, Q.; Luo, Z. Selective adsorption of Au3+from aqueous solutions using persimmon powder-formaldehyde resin. J. Appl. Polym. Sci. 2013, 130, 3937–3946. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Tien, C. Batch Adsorption Models and Model Applications. In Introduction to Adsorption; Tien, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 5; pp. 119–153. [Google Scholar] [CrossRef]
- Zur Theorie der sogenannten Adsorption gelöster Stoffe. Z. Chem. Ind. Kolloide 1907, 2, 15. [CrossRef]
- Blanchard, G.; Maunaye, M.; Martin, G. Removal of heavy metals from waters by means of natural zeolites. Water Res. 1984, 18, 1501–1507. [Google Scholar] [CrossRef]
- Ho, Y.S. Adsorption of Heavy Metals from Waste Streams by Peat. Ph.D. Thesis, The University of Birmingham, Birmingham, UK, 1995. [Google Scholar]
- Glueckauf, E. Theory of chromatography. Part 10-Formulae for diffusion into spheres and their application to chromatography. Trans. Faraday Soc. 1955, 51, 1540–1551. [Google Scholar] [CrossRef]
- Santos, S.C.; Boaventura, R. Adsorption of cationic and anionic azo dyes on sepiolite clay: Equilibrium and kinetic studies in batch mode. J. Environ. Chem. Eng. 2016, 4, 1473–1483. [Google Scholar] [CrossRef]
- Tien, C. Adsorbate Uptake and Equations Describing Adsorption Processes. In Introduction to Adsorption; Tien, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 4; pp. 87–118. [Google Scholar]
- Ogata, T.; Nakano, Y. Mechanisms of gold recovery from aqueous solutions using a novel tannin gel adsorbent synthesized from natural condensed tannin. Water Res. 2005, 39, 4281–4286. [Google Scholar] [CrossRef]
- Bergamini, M.F.; Santos, D.P.; Zanoni, M.V.B. Screen-printed carbon electrode modified with poly-L-histidine applied to gold(III) determination. J. Braz. Chem. Soc. 2009, 20, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Zhao, J.; Zhang, L.; Wang, C.; Wang, S. Efficient and Selective Adsorption of Gold Ions from Wastewater with Polyaniline Modified by Trimethyl Phosphate: Adsorption Mechanism and Application. Polymers 2019, 11, 652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokuyama, H.; Kanehara, A. Temperature swing adsorption of gold(III) ions on poly(N-isopropylacrylamide) gel. React. Funct. Polym. 2007, 67, 136–143. [Google Scholar] [CrossRef]
- Yang, J.; Kubota, F.; Baba, Y.; Kamiya, N.; Goto, M. Application of cellulose acetate to the selective adsorption and recovery of Au(III). Carbohydr. Polym. 2014, 111, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, D.; Kawakita, H.; Inoue, K.; Ohto, K.; Kajiyama, K. Persimmon peel gel for the selective recovery of gold. Hydrometallurgy 2007, 87, 133–139. [Google Scholar] [CrossRef]
- Malash, G.F.; El-Khaiary, M.I. Piecewise linear regression: A statistical method for the analysis of experimental adsorption data by the intraparticle-diffusion models. Chem. Eng. J. 2010, 163, 256–263. [Google Scholar] [CrossRef]
- Yao, C.; Chen, T. A new simplified method for estimating film mass transfer and surface diffusion coefficients from batch adsorption kinetic data. Chem. Eng. J. 2015, 265, 93–99. [Google Scholar] [CrossRef]
- Da Silva, J.S.; Da Rosa, M.P.; Beck, P.H.; Peres, E.C.; Dotto, G.L.; Kessler, F.; Grasel, F.D.S. Preparation of an alternative adsorbent from Acacia Mearnsii wastes through acetosolv method and its application for dye removal. J. Clean. Prod. 2018, 180, 386–394. [Google Scholar] [CrossRef]
- Mack, C.; Wilhelmi, B.; Duncan, J.; Burgess, J. Biosorption of precious metals. Biotechnol. Adv. 2007, 25, 264–271. [Google Scholar] [CrossRef]
- Wei, W.; Reddy, D.H.K.; Bediako, J.K.; Yun, Y.-S. Aliquat-336-impregnated alginate capsule as a green sorbent for selective recovery of gold from metal mixtures. Chem. Eng. J. 2016, 289, 413–422. [Google Scholar] [CrossRef]
- Li, J.; Miller, J. Reaction kinetics for gold dissolution in acid thiourea solution using formamidine disulfide as oxidant. Hydrometallurgy 2002, 63, 215–223. [Google Scholar] [CrossRef]
- Đurović, M.; Puchta, R.; Bugarčić, Ž.D.; Van Eldik, R. Studies on the reactions of [AuCl4]− with different nucleophiles in aqueous solution. Dalton Trans. 2014, 43, 8620–8632. [Google Scholar] [CrossRef] [Green Version]
| exp. | Langmuir | Freundlich | |||||||
|---|---|---|---|---|---|---|---|---|---|
| qe,m (mg g−1) | Qm (mg g−1) | KL × 103 (L mg−1) | R | SE (mg g−1) | KF (mg1−1/ng−1L1/n) | n | R | SE (mg g−1) | |
| HCl Solution | |||||||||
| 1.0 mol L−1 H+ | 344 ± 30 | 343 ± 38 | 38 ± 13 | 0.97 | 31.0 | 38 ± 7 | 2.4 ± 0.3 | 0.98 | 23.7 |
| 2.0 mol L−1 H+ | 289 ± 28 | 675 ± 152 | 4 ± 2 | 1.00 | 12.6 | 7 ± 2 | 1.4 ± 0.1 | 0.99 | 15.7 |
| Aqua Regia Solution | |||||||||
| 1.0 mol L−1 H+ | 246 ± 19 | 270 ± 19 | 81 ± 21 | 0.99 | 19.3 | 51 ± 21 | 3 ± 2 | 0.91 | 47.2 |
| 1.4 mol L−1 H+ | 264 ± 32 | 386 ± 62 | 8 ± 3 | 0.98 | 20.9 | 10 ± 6 | 1.7 ± 0.3 | 0.96 | 31.5 |
| 2.0 mol L−1 H+ | 136 ± 21 | 200 ± 65 | 6 ± 4 | 0.95 | 19.3 | 5 ± 4 | 1.7 ± 0.5 | 0.92 | 24.8 |
| Adsorbent | T (K) | pH or [H+] | Ce (mg L−1) | qe,m (mg g−1) | Qm (mg g−1) | Ref. |
|---|---|---|---|---|---|---|
| Commercial resin IRA400 | pH 2 | 0–215 | 902.3 | [19] | ||
| Commercial resin Lewatit TP214 | 298 | pH 6.1 | 35–225 | 108.7 | [20] | |
| Polyaniline modified by TMP | 298 | pH 4 | 0–300 | 881 | 883 | [48] |
| Crosslinked PEI resins | pH 2 | 0–210 | 943.5 | [19] | ||
| NIPA gel | 323 | 1 mol L−1 | 0–790 | 125.5 | [49] | |
| Activated rice husk | 298 | pH 6.1 | 50–260 | 93.46 | [20] | |
| PEI-alginate fibers | 298 | 0.1 mol L−1 | 0–2000 | 1240 | 1404 | [18] |
| GA-PEI-alginate fibers | 298 | 0.1 mol/L | 0–1500 | 2325 | 2182 | [18] |
| Cellulose acetate fibers | 298 | 2 mol L−1 | 0–800 | 110 | [50] | |
| Raw date pits | 298 | 0.5 mol L−1 | 0–35 | 78 | 61 | [23] |
| Banana peel | 298 | pH 1 | 0–1200 | 370.18 | 377.2 | [22] |
| Banana peel (lipid extraction) | 298 | pH 1 | 0–1000 | 475.48 | 448.4 | [22] |
| Oil palm trunk (dewaxed) | 303 | pH 2 | 0–120 | 91.47 | 95.16 | [35] |
| PEI-modified L. speciosa leaves | 298 | pH 1 | 0–200 | 282 | 286 | [21] |
| Persimmon resin | 303 | pH 2 | 150–351 | ≈965 | 1905 | [36] |
| Persimmon peel gel | 303 | 0.1 mol L−1 | 0–11 × 103 | 1.8 × 103 | [51] | |
| Crosslinked persimmon tannin gel | 303 | 0.1 mol L−1 | 0–2.4 × 103 | 1517 | [34] | |
| TEPA-persimmon tannin gel | 303 | 0.1 mol L−1 | 0–1.2 × 103 | 1168 | [6] | |
| EDA-modified persimmon tannin | 303 | 0.1 mol L−1 | 0–1.5 × 103 | 1550.4 | [29] | |
| persimmon tannin onto Fe3O4@SiO2 microspheres | 298 | pH 5 | (0.2–1.8) × 103 | 860 | 917.4 | [24] |
| persimmon tannin functionalized viscose fiber | 298 | pH 2 | 0–120 | 528 | 536 | [28] |
| Sericin and alginate particles chemically crosslinked by proanthocyanidins | 298 | pH 2.5–3 | 0–140 | 196.1 | 188.4 | [27] |
| Pine bark tannin resin | 298 | 1 mol L−1 | 0–200 | 344 | 343 | This work |
| Pine bark tannin resin | 298 | 1 mol L−1 (HCl/HNO3) | 0–200 | 246 | 270 | This work |
| Pseudo-First Order Model | Pseudo-Second Order Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C0 (mg L−1) | S/L (g L−1) | qe (mg g−1) | k1 × 102 (h−1) | R | SE (mg g−1) | qe (mg g−1) | k2 × 104 (g mg−1 h−1) | R | SE (mg g−1) |
| 1.0 mol L−1 HCl | |||||||||
| 100 | 1.0 | 91 ± 6 | 6 ± 1 | 0.98 | 7.2 | 112 ± 9 | 6 ± 2 | 0.98 | 6.2 |
| 100 | 2.0 | 52 ± 2 | 7.7 ± 0.6 | 0.99 | 2.2 | 63 ± 2 | 1.3 ± 0.1 | 1.00 | 1.4 |
| 300 | 2.0 | 147 ± 8 | 4.7 ± 0.6 | 0.99 | 7.5 | 190 ± 12 | 2.3 ± 0.5 | 0.99 | 6.0 |
| 1.4 mol L−1 H+ Aqua Regia Solution | |||||||||
| 100 | 1.0 | 84 ± 4 | 5.3 ± 0.7 | 0.99 | 4.5 | 109 ± 9 | 4 ± 1 | 0.99 | 4.8 |
| 300 | 2.0 | 136 ± 3 | 7.2 ± 0.5 | 1.0 | 4.2 | 167 ± 5 | 4.4 ± 0.5 | 1.0 | 3.8 |
| C0 (mg L−1) | S/L (g L−1) | R | kLDF (h−1) | Dh (m2 s−1) |
|---|---|---|---|---|
| 1.0 mol L−1 HCl | ||||
| 100 | 1.0 | 0.98 | 0.076 | 3.6 × 10−14 |
| 100 | 2.0 | 0.98 | 0.051 | 2.5 × 10−14 |
| 300 | 2.0 | 0.99 | 0.215 | 1.1 × 10−13 |
| 1.4 mol L−1 H+ Aqua Regia Solution | ||||
| 100 | 1.0 | 1.00 | 0.021 | 1.0 × 10−14 |
| 300 | 2.0 | 0.99 | 0.087 | 4.3 × 10−14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torrinha, M.B.Q.L.F.; Bacelo, H.A.M.; Santos, S.C.R.; Boaventura, R.A.R.; Botelho, C.M.S. Uptake and Recovery of Gold from Simulated Hydrometallurgical Liquors by Adsorption on Pine Bark Tannin Resin. Water 2020, 12, 3456. https://doi.org/10.3390/w12123456
Torrinha MBQLF, Bacelo HAM, Santos SCR, Boaventura RAR, Botelho CMS. Uptake and Recovery of Gold from Simulated Hydrometallurgical Liquors by Adsorption on Pine Bark Tannin Resin. Water. 2020; 12(12):3456. https://doi.org/10.3390/w12123456
Chicago/Turabian StyleTorrinha, Maria Beatriz Q. L. F., Hugo A. M. Bacelo, Sílvia C. R. Santos, Rui A. R. Boaventura, and Cidália M. S. Botelho. 2020. "Uptake and Recovery of Gold from Simulated Hydrometallurgical Liquors by Adsorption on Pine Bark Tannin Resin" Water 12, no. 12: 3456. https://doi.org/10.3390/w12123456
APA StyleTorrinha, M. B. Q. L. F., Bacelo, H. A. M., Santos, S. C. R., Boaventura, R. A. R., & Botelho, C. M. S. (2020). Uptake and Recovery of Gold from Simulated Hydrometallurgical Liquors by Adsorption on Pine Bark Tannin Resin. Water, 12(12), 3456. https://doi.org/10.3390/w12123456







