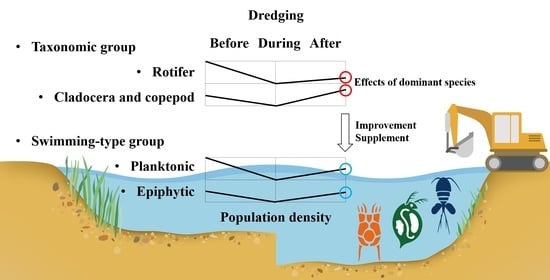
Comparison of Zooplankton Community Patterns in Relation to Sediment Disturbances by Dredging in the Guemho River, Korea
Abstract
:1. Introduction
2. Materials and Methods
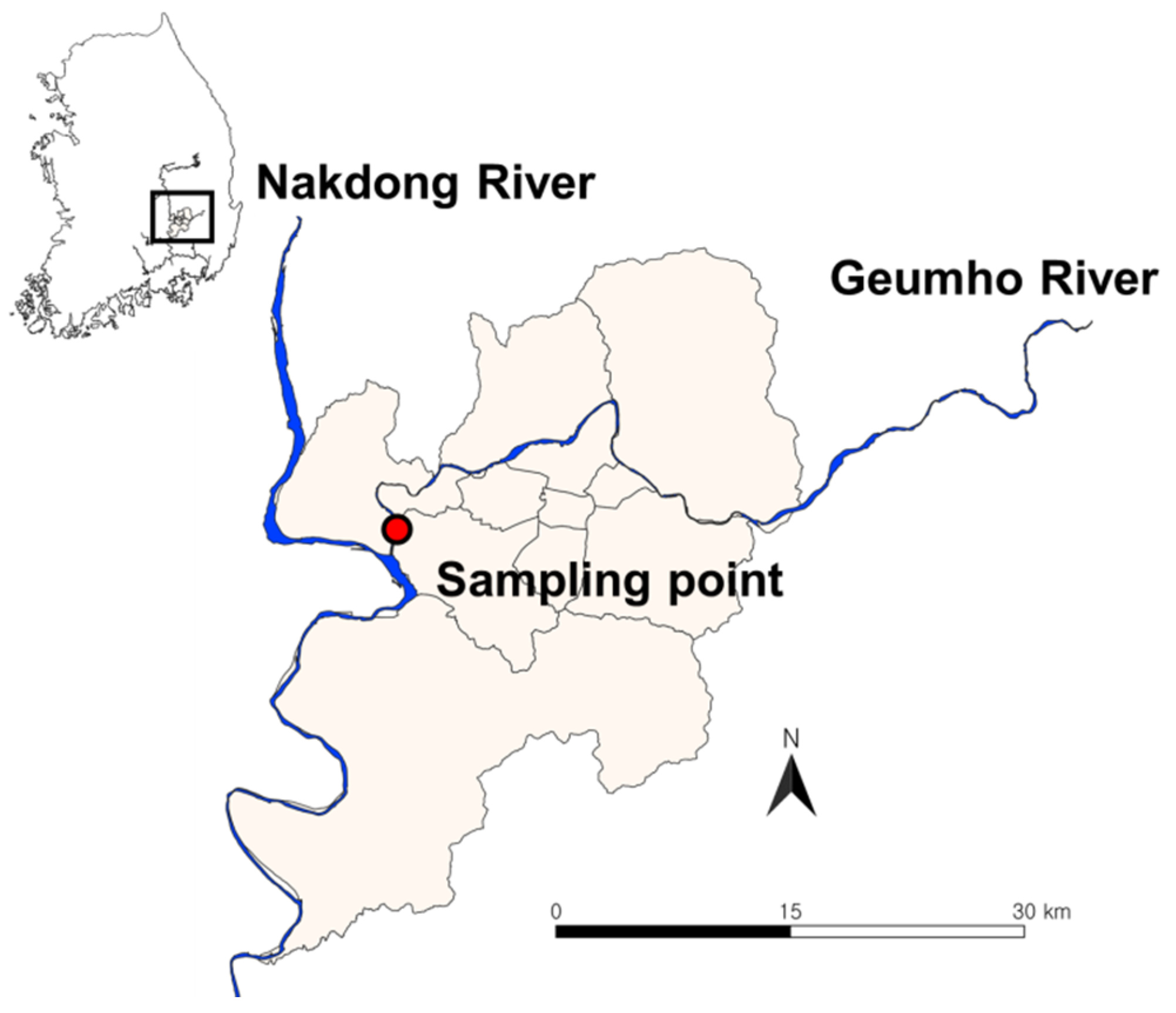
2.1. Description of the Study Site
2.2. Water Quality and Hydrological Dataset
2.3. Sampling for Zooplankton Identification
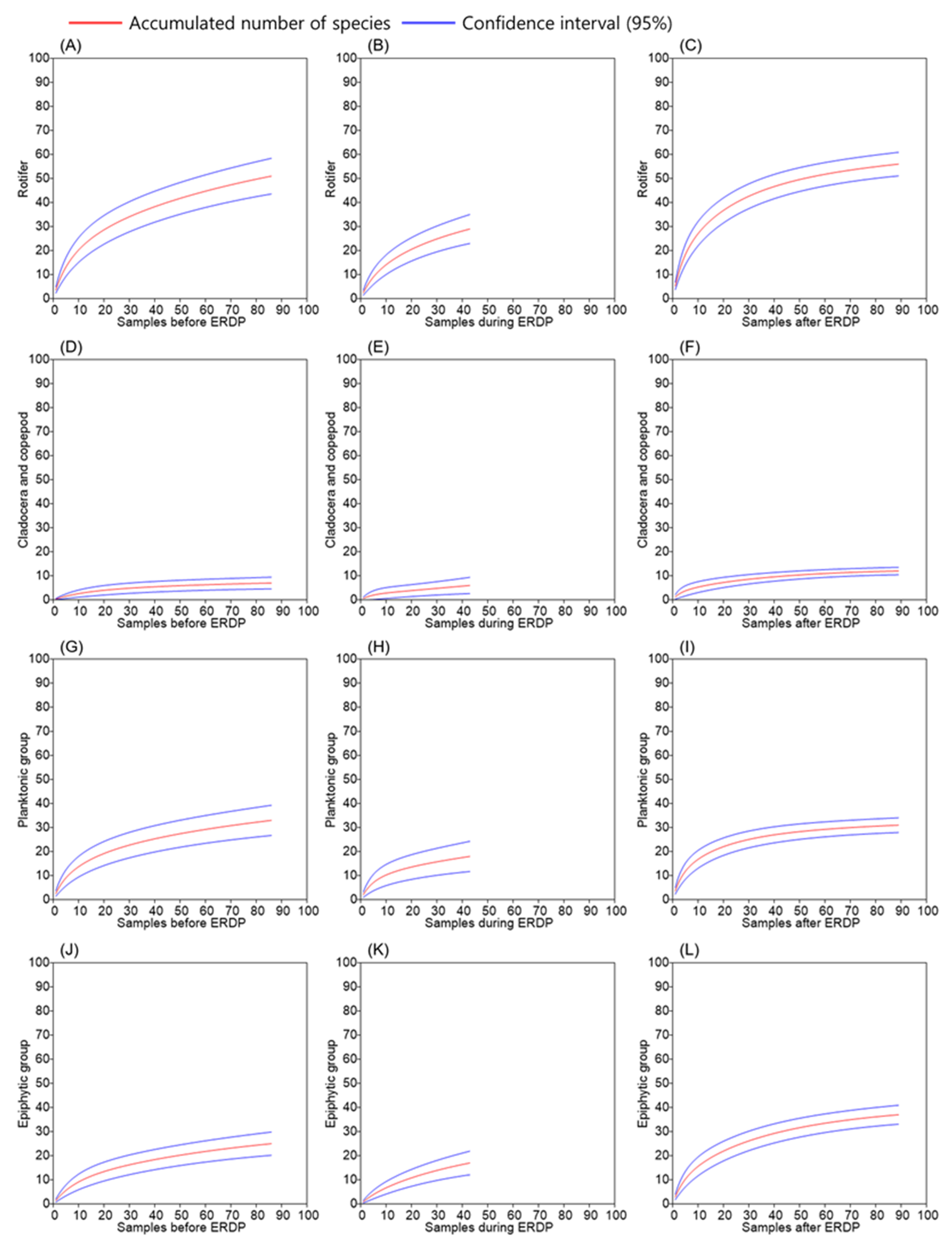
2.4. Data Analysis
3. Results
3.1. Dredging Effect on the Water Quality Parameters
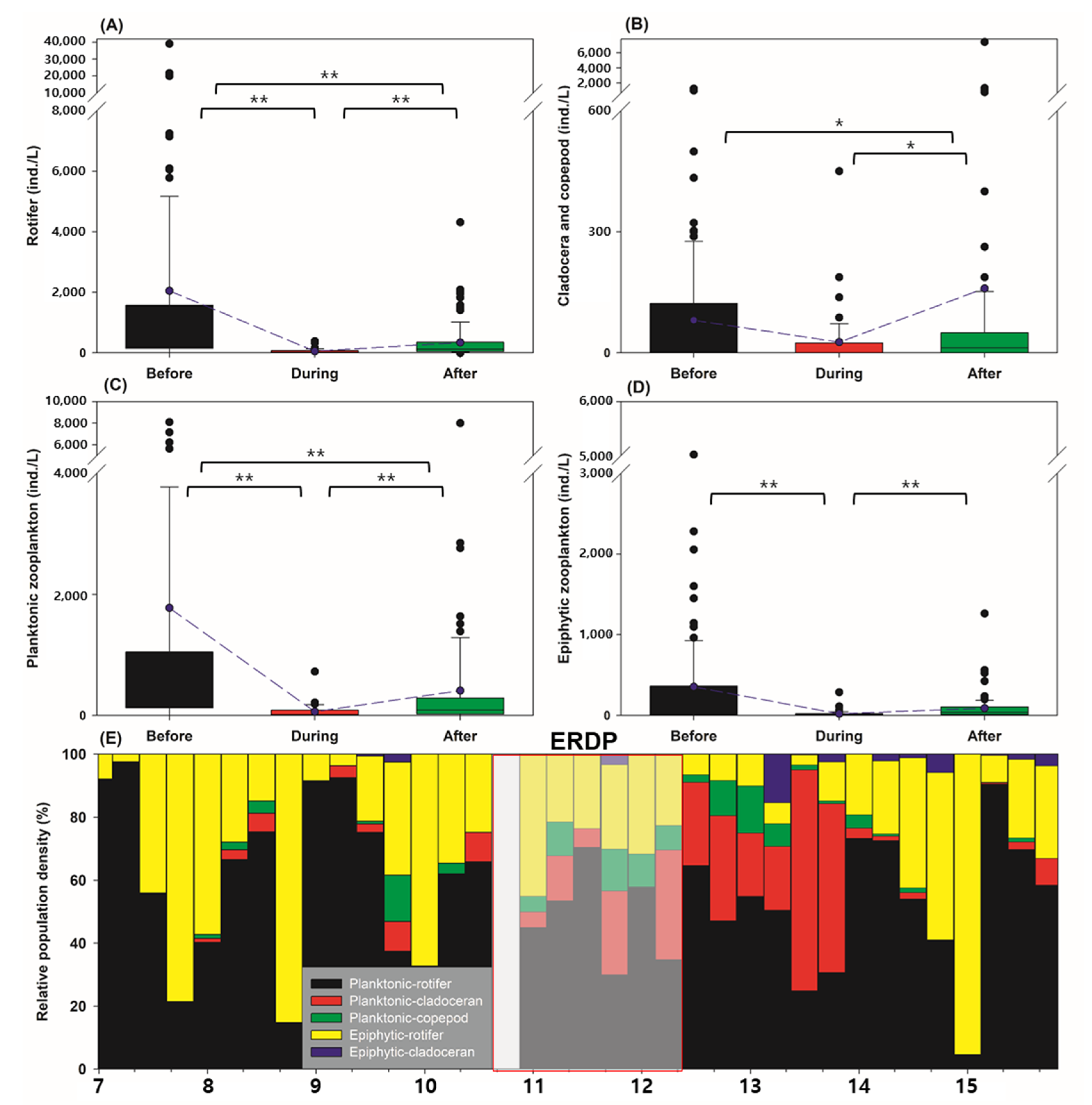
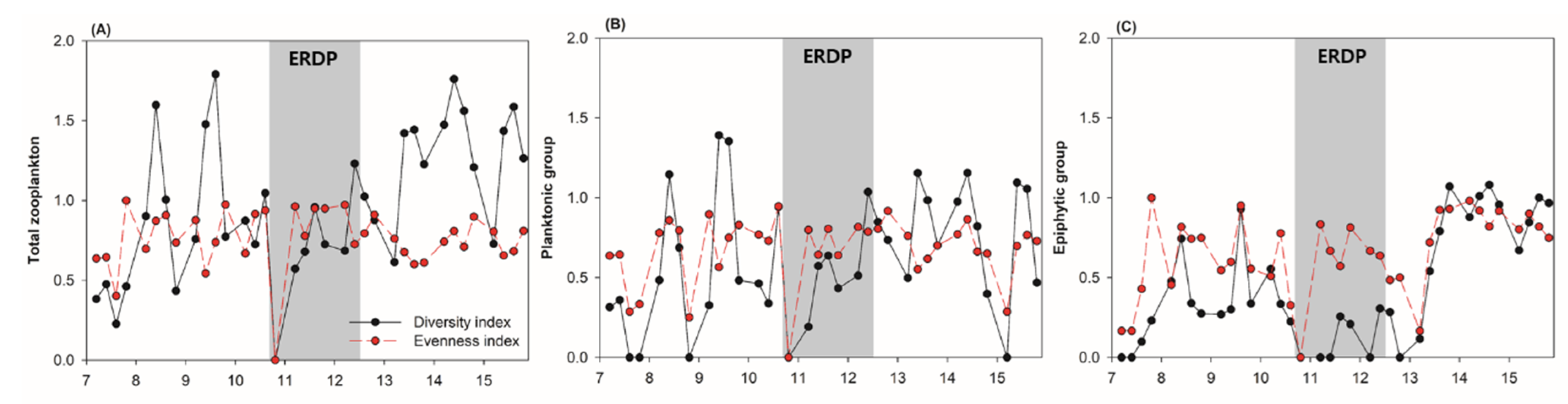
3.2. Differences between the Responses of Zooplankton Groups to Dredging
4. Discussion
4.1. Is Swimming-Type Classification Effective in Explaining the Dredging Effect?
4.2. Is the Littoral Zone Collection Representative?
4.3. What Is the Application of Zooplankton as an Aquatic Ecosystem Health Evaluation Index?
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meador, M.R.; Layher, A.O. Instream sand and gravel mining: Environmental issues and regulatory process in the United States. Fisheries 1998, 23, 6–13. [Google Scholar] [CrossRef]
- Davis, J.; Brid, J.; Finlayson, B.; Scott, R. The management of gravel extraction in alluvial rivers: A case study from the Avon River, southeastern Australia. Phys. Geogr. 2000, 21, 133–154. [Google Scholar] [CrossRef]
- Rovira, A.; Batalla, R.J.; Sala, M. Response of a river sediment budget after historical gravel mining (the lower Tordera, NE Spain). River Res. Appl. 2005, 21, 829–847. [Google Scholar] [CrossRef]
- Rempel, L.L.; Church, M. Physical and ecological response to disturbance by gravel mining in a large alluvial river. Can. J. Fish Aquat. Sci. 2009, 66, 52–71. [Google Scholar] [CrossRef]
- Freedman, J.A.; Carline, R.F.; Stauffer, J.R., Jr. Gravel dredging alters diversity and structure of riverine fish assemblages. Freshw. Biol. 2013, 58, 261–274. [Google Scholar] [CrossRef]
- Kenny, A.J.; Rees, H.L. The effects of marine gravel extraction on the macrobenthos: Early post-dredging recolonization. Mar. Pollut. Bull. 1994, 28, 442–447. [Google Scholar] [CrossRef]
- Spencer, K.L.; Dewhurst, R.E.; Penna, P. Potential impacts of water injection dredging on water quality and ecotoxicity in Limehouse Basin, River Thames, SE England, UK. Chemosphere 2006, 63, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Ryding, S.O. Lake Trehörningen restoration project. Changes in water quality after sediment dredging. Hydrobiologia 1982, 91, 549–558. [Google Scholar] [CrossRef]
- Ricciardi, A.; Rasmussen, J.B. Extinction rates of North American freshwater fauna. Conserv. Biol. 1999, 13, 1220–1222. [Google Scholar] [CrossRef]
- Rehman, M.; Yousur, A.R.; Balkhi, M.H.; Rather, M.I.; Shahi, N.; Meraj, M.; Hassan, K. Dredging induced changes in zooplankton community and water quality in Dal Lake, Kashmir, India. Afr. J. Environ. Sci. Technol. 2016, 10, 141–149. [Google Scholar]
- Zhang, S.; Zhou, Q.; Xu, D.; Lin, J.; Cheng, S.; Wu, Z. Effects of sediment dredging on water quality and zooplankton community structure in a shallow of eutrophic lake. J. Environ. Sci. 2010, 22, 218–224. [Google Scholar] [CrossRef]
- Aldridge, D.C. The impacts of dredging and weed cutting on a population of freshwater mussels (Bivalvia: Unionidae). Biol. Conserv. 2000, 95, 247–257. [Google Scholar] [CrossRef]
- Lewis, M.A.; Weber, D.E.; Stanley, R.S.; Moore, J.C. Dredging impact on an urbanized Florida bayou: Effects on benthos and algal-periphyton. Environ. Pollutt. 2001, 115, 161–171. [Google Scholar] [CrossRef]
- Jo, H.; Jeppesen, E.; Ventura, M.; Buchaca, T.; Gim, J.S.; Yoon, J.D.; Kim, D.H.; Joo, G.J. Responses of fish assemblage structure to large-scale weir construction in riverine ecosystems. Sci. Total. Environ. 2019, 657, 1334–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailly, D.; Cassemiro, F.A.; Winemiller, K.O.; Diniz-Filho, J.A.F.; Agostinho, A.A. Diversity gradients of Neotropical freshwater fish: Evidence of multiple underlying factors in human-modified systems. J. Biogeogr. 2016, 43, 1679–1689. [Google Scholar] [CrossRef]
- Harvey, B.C. Effects of suction gold dredging on fish and invertebrates in two California streams. N. Am. J. Fish Manag. 1986, 6, 401–409. [Google Scholar] [CrossRef]
- Brown, A.V.; Lyttle, M.M.; Brown, K.B. Impacts of gravel mining on gravel bed streams. Trans. Am. Fish. Soc. 1998, 127, 979–994. [Google Scholar] [CrossRef]
- Karr, J.R. Assessment of biotic integrity using fish communities. Fisheries 1981, 6, 21–27. [Google Scholar] [CrossRef]
- Won, D.H.; Jun, Y.C.; Kwon, S.J.; Hwang, S.J.; Ahn, K.G.; Lee, J.K. Development of Konan Saprobic Index using benthic macroinvertebrates and its application to biological stream environment assessment. Korean Soc. Mar. Environ. 2006, 22, 768–783. [Google Scholar]
- Pantle, R. Die biologische Uberwachung der Gewasser und die Darstellung der Ergebnisse. Gas. Und Wasserfach 1955, 96, 604. [Google Scholar]
- Woodwis, F. The biological system of stream classification used by the Trent River Board. Chem. Ind. 1964, 11, 443–447. [Google Scholar]
- Gómez, N.; Licursi, M. The Pampean Diatom Index (IDP) for assessment of rivers and streams in Argentina. Aquat. Ecol. 2001, 35, 173–181. [Google Scholar] [CrossRef]
- Miller, S.J.; Wardrop, D.H.; Mahaney, W.M.; Brooks, R.P. A plant-based index of biological integrity (IBI) for headwater wetlands in central Pennsylvania. Ecol. Indic. 2006, 6, 290–312. [Google Scholar] [CrossRef]
- Yoshida, T.; Urabe, J.; Elser, J.J. Assessment of ‘top-down’ and ‘bottom-up’ forces as determinants of rotifer distribution among lakes in Ontario, Canada. Ecol. Res. 2003, 18, 639–650. [Google Scholar] [CrossRef]
- Chang, K.H.; Doi, H.; Imai, H.; Gunji, F.; Nakano, S.I. Longitudinal changes in zooplankton distribution below a reservoir outfall with reference to river planktivory. Limnology 2008, 9, 125–133. [Google Scholar] [CrossRef]
- Baranyi, C.; Hein, T.; Holarek, C.; Keckeis, S.; Schiemer, F. Zooplankton biomass and community structure in a Danube River floodplain system: Effects of hydrology. Freshw. Biol. 2002, 47, 473–482. [Google Scholar] [CrossRef]
- Zhou, S.; Tang, T.; Wu, N.; Fu, X.; Cai, Q. Impacts of a small dam on riverine zooplankton. Intern. Rev. Hydrobiol. 2008, 93, 297–311. [Google Scholar] [CrossRef]
- Czerniawski, R. Zooplankton community changes between forest and meadow sections in small headwater streams, NW Poland. Biologia 2013, 68, 448–458. [Google Scholar] [CrossRef]
- Gurav, M.N.; Pejaver, M.K. Survey of rotifers to evaluate the water quality of the river Gadhi and its reservoir. Ecol. Environ. Conserv. 2013, 19, 417–423. [Google Scholar]
- Gutkowska, A.; Paturej, E.; Kowalska, E. Rotifer trophic state indices as ecosystem indicators in brackish coastal waters. Oceanologia 2013, 55, 887–899. [Google Scholar] [CrossRef]
- Gannon, J.E.; Stemberger, R.S. Zooplankton (especially crustaceans and rotifers) as indicators of water quality. Trans. Am. Microsc. Soc. 1978, 97, 16–35. [Google Scholar] [CrossRef]
- Saksena, D.N. Rotifers as indicators of water quality. Acta Hydrochim. Hydrobiol. 1987, 15, 481–485. [Google Scholar] [CrossRef]
- Pace, M.L.; Cole, J.J.; Carpenter, S.R.; Kitchell, J.F.; Hodgson, J.R.; Van de Bogert, M.C.; Bade, D.L.; Kritzberg, E.S.; Bastviken, D. Whole-lake carbon-13 additions reveal terrestrial support of aquatic food webs. Nature 2004, 427, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Water Environment Information System (Korean). Available online: http://water.nier.go.kr (accessed on 2 February 2020).
- Water Resources Management Information System (Korean). Available online: http://wamis.go.kr (accessed on 19 November 2020).
- Koste, W. Rotatoria, Die Radertiere Mitteleuropas: Uberordnung Monogononta: Ein Bestimmungswerk (German Edition), 2nd ed.; Gebruder Borntraeger: Stuttgart, German, 1978. [Google Scholar]
- Smirnov, N.N.; Timms, B.V. A revision of the Australian Cladocera (Crustacean). Rec. Aust. Mus. Suppl. 1983, 1, 1–132. [Google Scholar] [CrossRef]
- Einsle, U. Crustacea, Copepoda: Calanoida und Cyclopoida. Susswasswefauna von Mitteleuropa; Gustav Fisher Verlag: Stuttugart, German, 1993; Volume 8, part 4-1. [Google Scholar]
- Sakuma, M.; Hanazato, T.; Nakazato, R.; Haga, H. Methods for quantitative sampling of epiphytic microinvertebrates in lake vegetation. Limnology 2002, 3, 115–119. [Google Scholar] [CrossRef]
- Einarsson, A.; Örnólfsdóttir, E.B. Long-term changes in benthic Cladocera populations in Lake Myvatn, Iceland. Aquat. Ecol. 2004, 38, 253–262. [Google Scholar] [CrossRef] [Green Version]
- De Troch, M.; Cnudde, C.; Vyverman, W.; Vanreusel, A. Increased production of faecal pellets by the benthic harpacticoid Paramphiascella fulvofasciata: Importance of the food source. Mar. Biol. 2008, 156, 469–477. [Google Scholar] [CrossRef]
- Choi, J.Y.; La, G.H.; Kim, S.K.; Jeong, K.S.; Joo, G.J. Zooplankton community distribution in aquatic plants zone: Influence of epiphytic rotifers and cladocerans in accordance with aquatic plants cover and types. J. Ecol. Environ. 2013, 46, 86–93. [Google Scholar] [CrossRef]
- Carlson, R.E. A trophic state index for lakes. Limnol. Oceanogr. 1977, 22, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell. Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef] [Green Version]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Sullivan, B.K.; Hancock, D. Zooplankton and dredging: Research perspectives from a critical review. J. Am. Water. Resour. Assoc. 1977, 13, 461–468. [Google Scholar] [CrossRef]
- Kim, D.K.; Jeong, K.S.; Chang, K.H.; La, G.W.; Joo, G.J.; Kim, H.W. Patterning zooplankton communities in accordance with annual climatic conditions in a regulated river system (Nakdong River, South Korea). Intern. Rev. Hydrobiol. 2012, 91, 55–72. [Google Scholar] [CrossRef]
- Sládeček, V. Rotifers as indicators of water quality. Hydrobiologia 1983, 1, 169–201. [Google Scholar] [CrossRef]
- Rawer-Jost, C.; Böhmer, J.; Blank, J.; Rahmann, H. Macroinvertebrate functional feeding group methods in ecological assessment. Hydrobiologia 2000, 422, 225–232. [Google Scholar] [CrossRef]
- Thorp, J.H.; Covich, A.P. Ecology and Classification of North American Freshwater Invertebrates, 3rd ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Whiteside, M.C.; Williams, J.B. A new sampling technique for aquatic ecologists. Int. Ver. Theor. Angew. Limnol. 1975, 19, 1534–1539. [Google Scholar] [CrossRef]
- DiFonzo, C.D.; Campbell, J.M. Spatial partitioning of microhabitats in littoral cladoceran communities. J. Freshw. Ecol. 1988, 4, 303–313. [Google Scholar] [CrossRef]
- Suding, K.N. The effect of spring burning on competitive ranking of prairie species. J. Veg. Sci. 2001, 12, 849–856. [Google Scholar] [CrossRef]
- Suding, K.N.; Goldberg, D.E. Do disturbances alter competitive hierarchies? Mechanisms of change following gap creation. Ecology 2001, 82, 2133–2149. [Google Scholar] [CrossRef]
- Newell, R.C.; Seiderer, L.J.; Hitchcock, D.R. The impact of dredging works in coastal waters: A review of the sensitivity to disturbance and subsequent recovery of biological resources on the sea bed. Oceanogr. Mar. Biol. Annu. Rev. 1998, 36, 127–178. [Google Scholar]
- Cabrita, M.T. Phytoplankton community indicators of changes associated with dredging in the Tagus estuary (Portugal). Environ. Pollut. 2014, 191, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Straile, D.; Geller, W. Crustacean zooplankton in Lake Constance from 1920 to 1995: Response to eutrophication and re-oligotrophication. Adv. Limnol. 1998, 53, 255–274. [Google Scholar]
- Tavernini, S.; Viaroli, P.; Rossetti, G. Zooplankton community structure and inter-annual dynamics in two sand-pit lakes with different dredging impact. Int. Rev. Hydrobiol. 2009, 94, 290–307. [Google Scholar] [CrossRef]
- Xiong, W.; Li, J.; Chen, Y.; Shan, J.; Wang, W.; Zhan, A. Determinants of community structure of zooplankton in heavily polluted river ecosystems. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandlund, O.T. The drift of zooplankton and microzoobenthos in the river Strandaelva, western Norway. Hydrobiologia 1982, 94, 33–48. [Google Scholar] [CrossRef]
- Thorp, J.H.; Delong, M.D. Dominance of autochthonous autotrophic carbon in food webs of heterotrophic rivers. Oikos 2002, 96, 543–550. [Google Scholar] [CrossRef]
| Category | Before ERDP (n = 186) | During ERDP (n = 84) | After ERDP (n = 168) |
|---|---|---|---|
| * Water temperature (°C) | 17.32 ± 0.60 | 14.95 ± 0.83 | 17.94 ± 0.65 |
| * pH | 7.87 ± 0.04 | 7.92 ± 0.04 | 8.14 ± 0.04 |
| * DO (mg/L) | 10.52 ± 0.21 | 10.61 ± 0.26 | 11.66 ± 0.20 |
| * Conductivity (µS/cm) | 825.76 ± 19.21 | 719.73 ± 23.94 | 700.38 ± 14.57 |
| BOD (mg/L) | 3.66 ± 0.12 | 3.35 ± 0.16 | 3.67 ± 0.15 |
| * COD (mg/L) | 9.17 ± 0.15 | 8.52 ± 0.21 | 8.53 ± 0.16 |
| * Suspended solid (mg/L) | 11.93 ± 1.30 | 22.93 ± 2.10 | 11.43 ± 1.18 |
| Total nitrogen (mg/L) | 7.06 ± 0.14 | 6.83 ± 0.18 | 6.56 ± 0.12 |
| * Total phosphate (mg/L) | 0.62 ± 0.02 | 0.43 ± 0.01 | 0.11 ± 0.00 |
| * Chlorophyll-a (µg/L) | 51.72 ± 3.44 | 37.55 ± 3.57 | 49.70 ± 3.58 |
| * TSI (T-P) | 85.46 ± 0.31 | 81.43 ± 0.37 | 65.74 ± 0.38 |
| TSI (Chl-a) | 60.39 ± 0.64 | 56.88 ± 1.03 | 59.20 ± 0.79 |
| * Discharge (m3/s) | * 414.08 ± 11.43 (n = 1369) | * 60.88 ± 21.88 (n = 637) | * 38.83 ± 2.94 (n = 1124) |
| Habitat Character | Taxa | Species | |
|---|---|---|---|
| Planktonic | Rotifers | Anuraeopsis fissa Asplanchna spp. Brachionus angularis Brachionus budapestinesis Brachionus calyciflorus Brachionus forticula Brachionus rubens Brachionus urceolaris Brachionus sp. Conochiloides dossuarius Conochiloides spp. Conochilus spp. Euchlanis dilatata Euchlanis sp. Euchlanis triquetra Filinia longiseta | Filinia terminalis Hexarthra mira Kelicottia longispina Keratella cochlearis Keratella quadrata Keratella valga f. monstrosa Keratella valga f. tropica Keratella valga Platyias patulus Philodium roseola Ploesoma lenticulare Ploesoma tetractis Polyarthra trigla Polyarthra vulgaris Polyarthra spp. Pompholyx complanata |
| Cladocerans | Bosmina longirostris Ceriodaphnia spp. Diaphanosoma brachyurum Daphnia galeata | Daphnia spp. Moina spp. Scapholeberis kingi | |
| Copepods | copepoda | copepodid | |
| Epiphytic | Rotifers | Brachionus quadridentatus Cephalodella spp. Colurella uncinata icuspidata Colurella spp. Dissotrocha acureata Lecane aculeata Lecane flexilis Lecane haliclysta Lecane leontina Lecane luna Lecane nana Lecane sp. Lepadella acumnata Lepadella oblonga Lepadella quadricarinata Lepadella sp. Monostyla bulla Monostyla closterocerca Monostyla crenata | Monostyla hamata Monostyla lunaris Monostyla pygmaea Monostyla sp. Mytilina ventralis Notholca labis Philodina spp. Scaridium longicauda Synchaeta spp. Testudinella elliptica Testudinella sp. Trichocerca capucina Trichocerca elongate Trichocerca longiseta Trichocerca pusilla Trichocerca sp. Trichotria tetractys Trichocerca tenuior Trichotria tetractys Trichotria truncata |
| Cladocerans | Alona gutta Alona rectangular Alona sp. | Chydorus sphaericus Pleuroxus spp. | |
| Copepods | - | ||
| Category | Before ERDP | During ERDP | After ERDP | |||
|---|---|---|---|---|---|---|
| Species | PD | Species | PD | Species | PD | |
| 1st | P. vulgaris | 692.8 ± 328.8 | B. longristris | 18.9 ± 10.9 | B. longristris | 123.9 ± 75.0 |
| 2nd | B. calyciflorus | 375.5 ± 166.5 | P. vulgaris | 9.6 ± 2.6 | P. vulgaris. | 89.9 ± 48.5 |
| 3rd | B. rubens | 127.0 ± 33.7 | B. calyciflorus | 6.1 ± 2.2 | K. cochlearis | 45.1 ± 22.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, E.-J.; Kim, D.-K.; Jung, E.-S.; Heo, Y.-J.; Joo, G.-J.; Kim, H.-W. Comparison of Zooplankton Community Patterns in Relation to Sediment Disturbances by Dredging in the Guemho River, Korea. Water 2020, 12, 3434. https://doi.org/10.3390/w12123434
Ko E-J, Kim D-K, Jung E-S, Heo Y-J, Joo G-J, Kim H-W. Comparison of Zooplankton Community Patterns in Relation to Sediment Disturbances by Dredging in the Guemho River, Korea. Water. 2020; 12(12):3434. https://doi.org/10.3390/w12123434
Chicago/Turabian StyleKo, Eui-Jeong, Dong-Kyun Kim, Eun-Song Jung, Yu-Ji Heo, Gea-Jae Joo, and Hyun-Woo Kim. 2020. "Comparison of Zooplankton Community Patterns in Relation to Sediment Disturbances by Dredging in the Guemho River, Korea" Water 12, no. 12: 3434. https://doi.org/10.3390/w12123434
APA StyleKo, E.-J., Kim, D.-K., Jung, E.-S., Heo, Y.-J., Joo, G.-J., & Kim, H.-W. (2020). Comparison of Zooplankton Community Patterns in Relation to Sediment Disturbances by Dredging in the Guemho River, Korea. Water, 12(12), 3434. https://doi.org/10.3390/w12123434