Rootstock Effects on Water Relations of Young Almond Trees (cv. Soleta) When Subjected to Water Stress and Rehydration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Conditions
2.2. Treatments and Experimental Design
2.3. Growth and Plant Water Measurements
2.4. Statistical Analyses of Data
3. Results
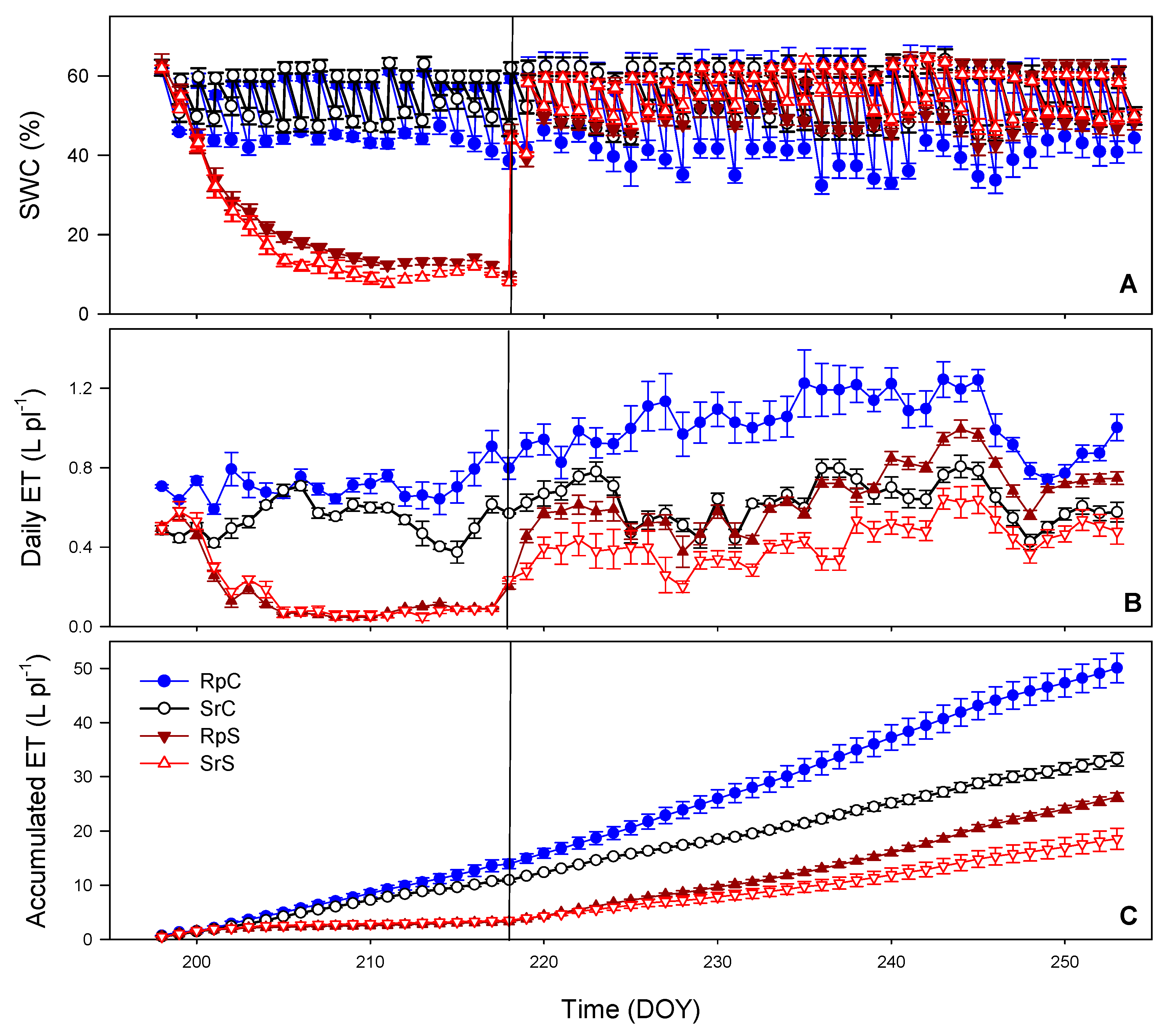
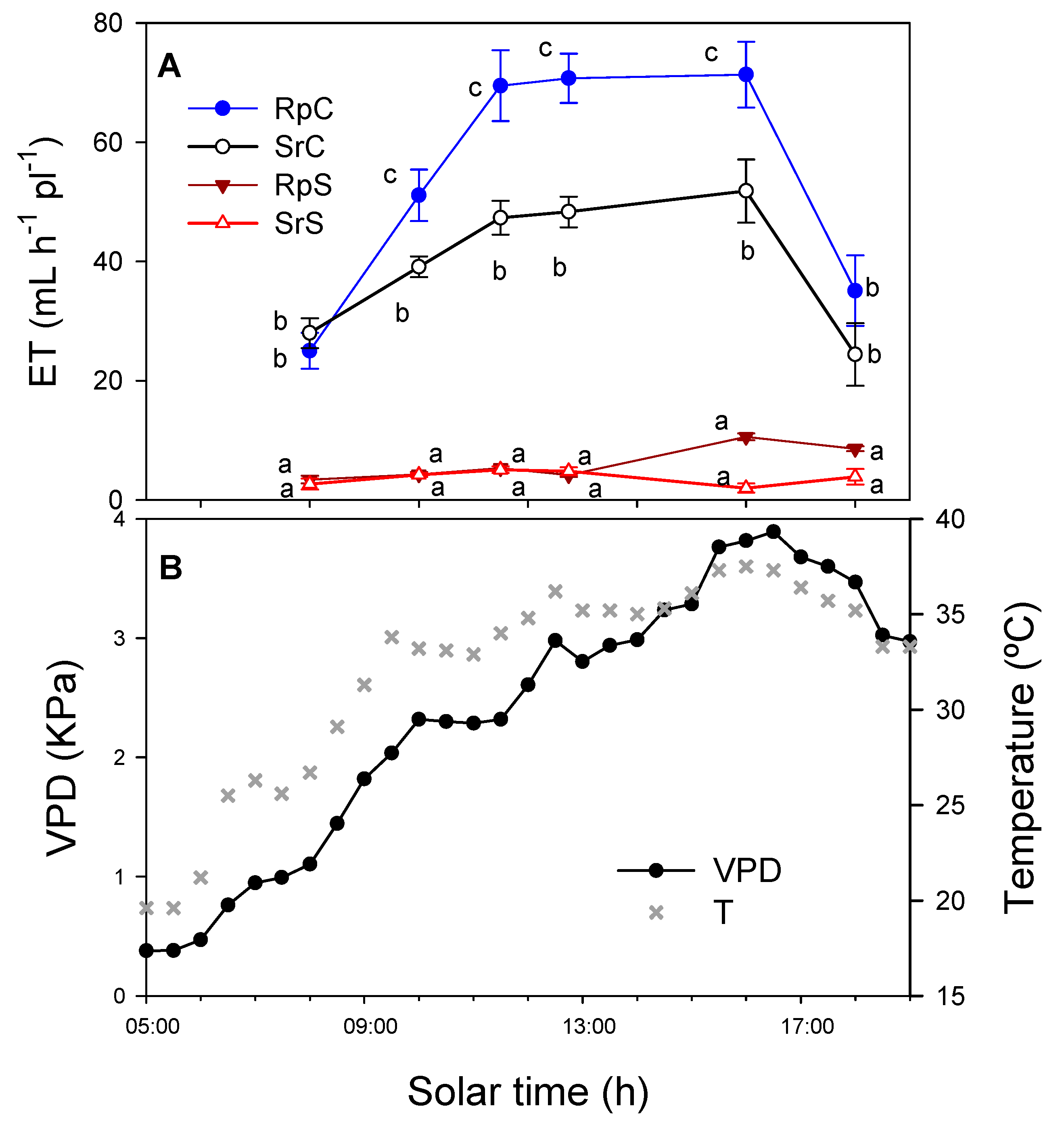
3.1. Substrate Water Content (SWC) and Evapotranspiration (ET)
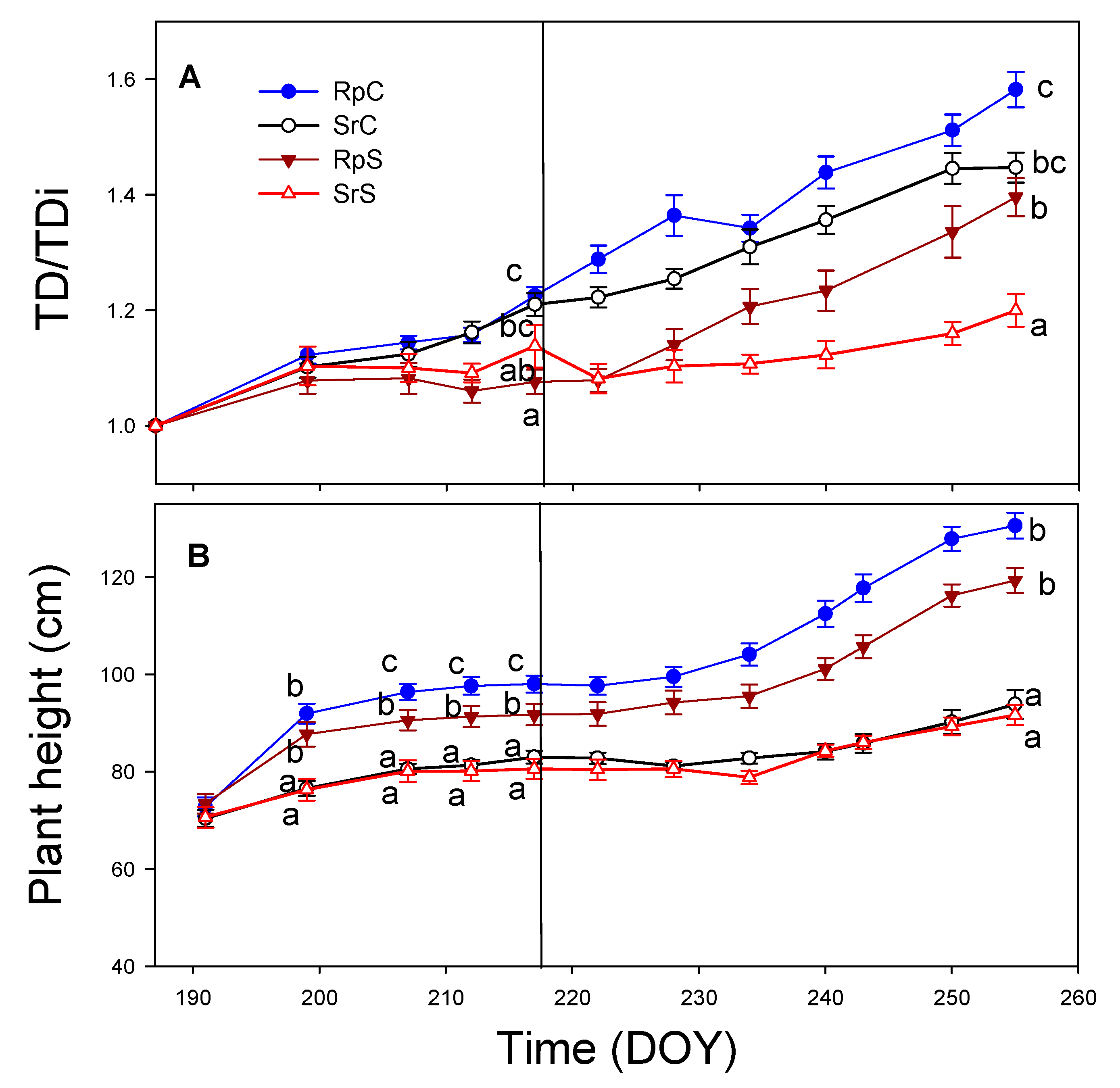
3.2. Plant Growth
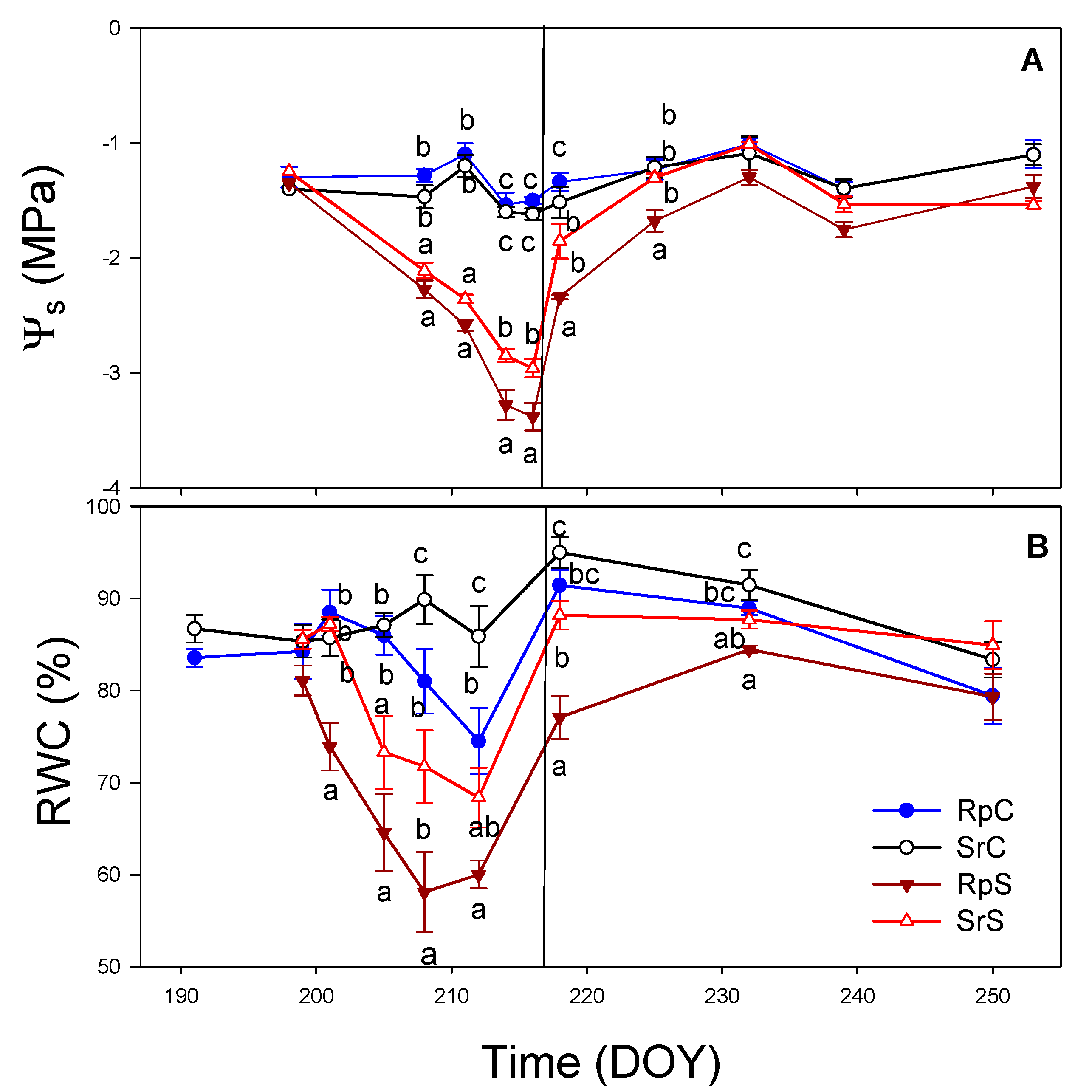
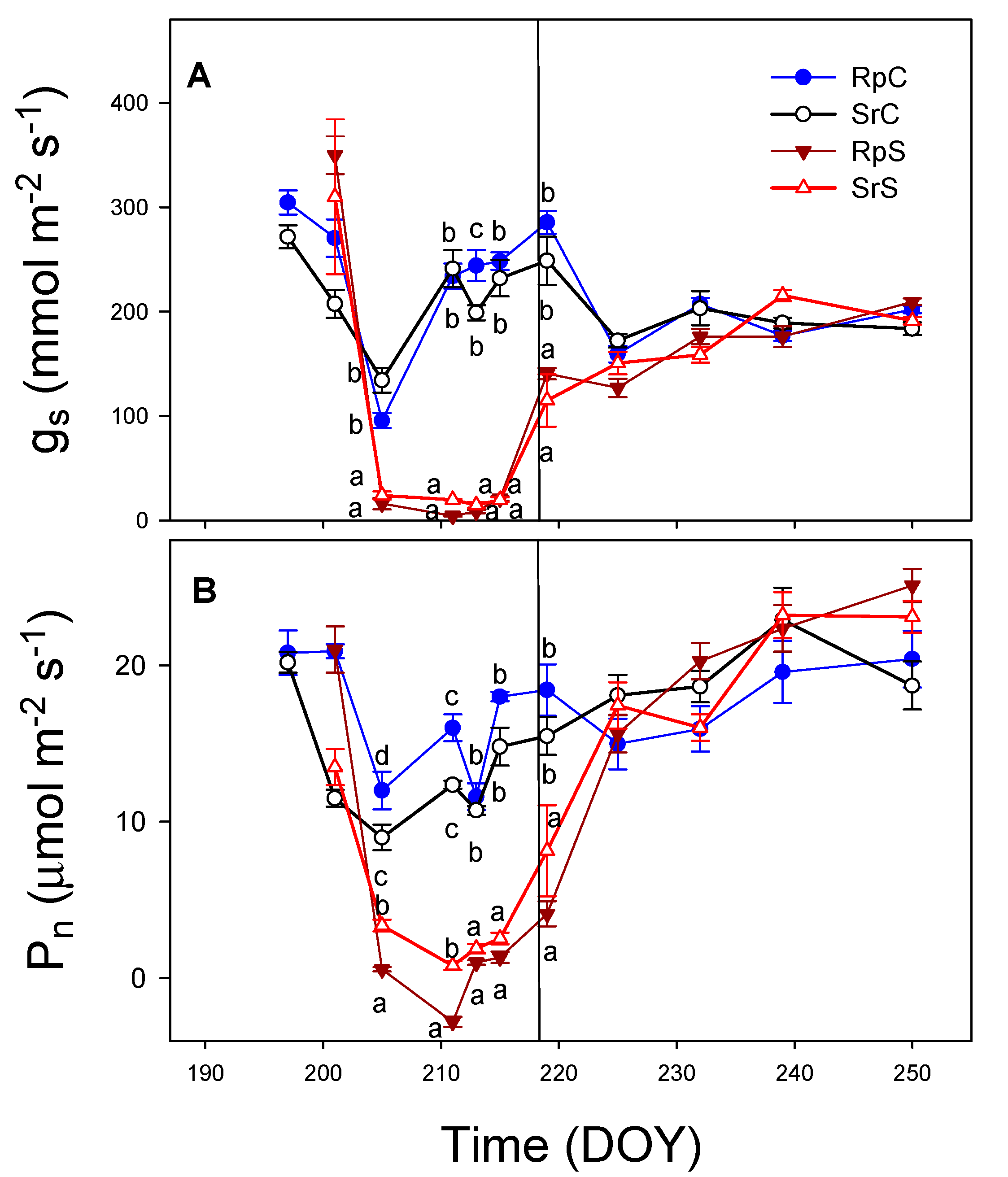
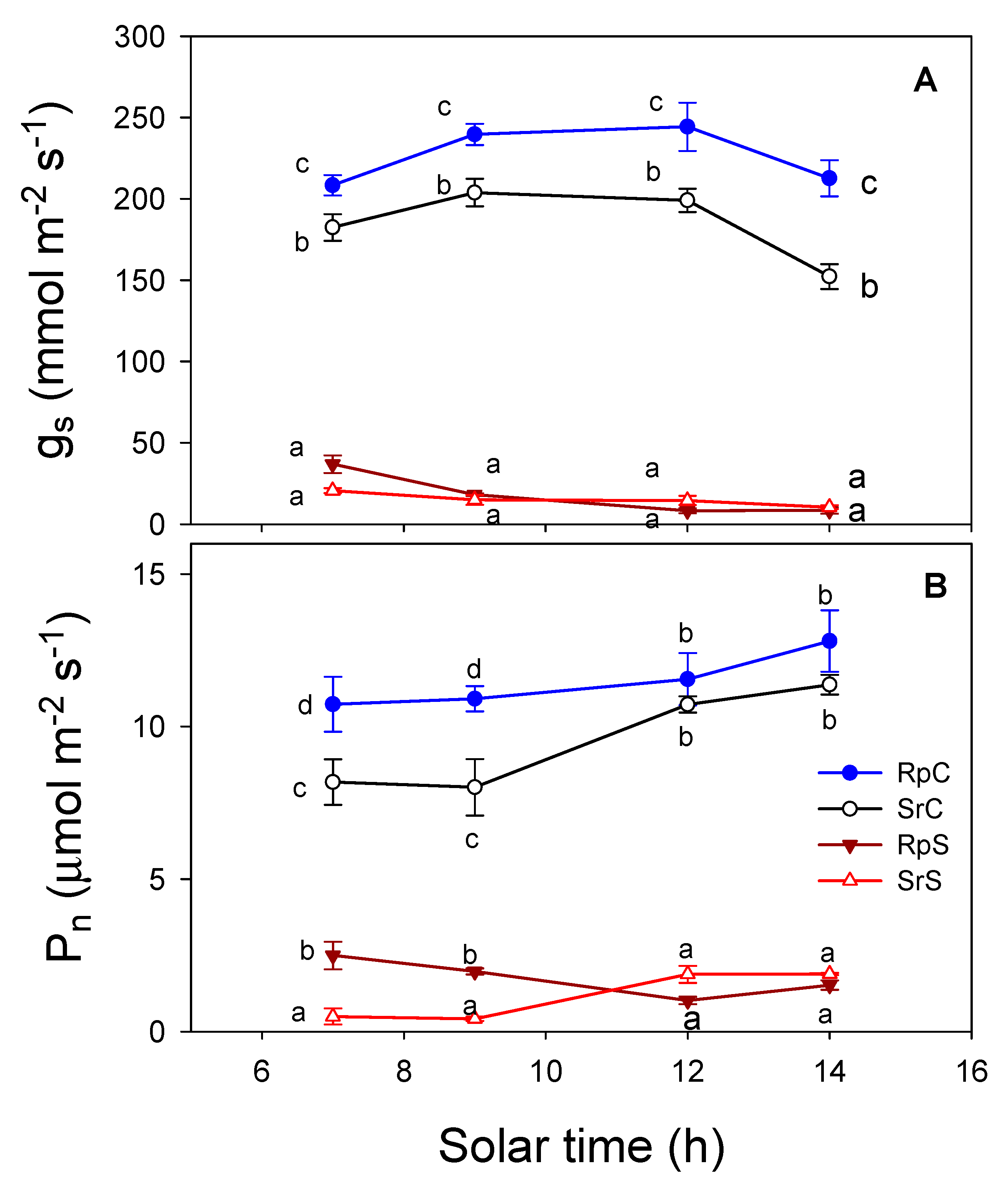
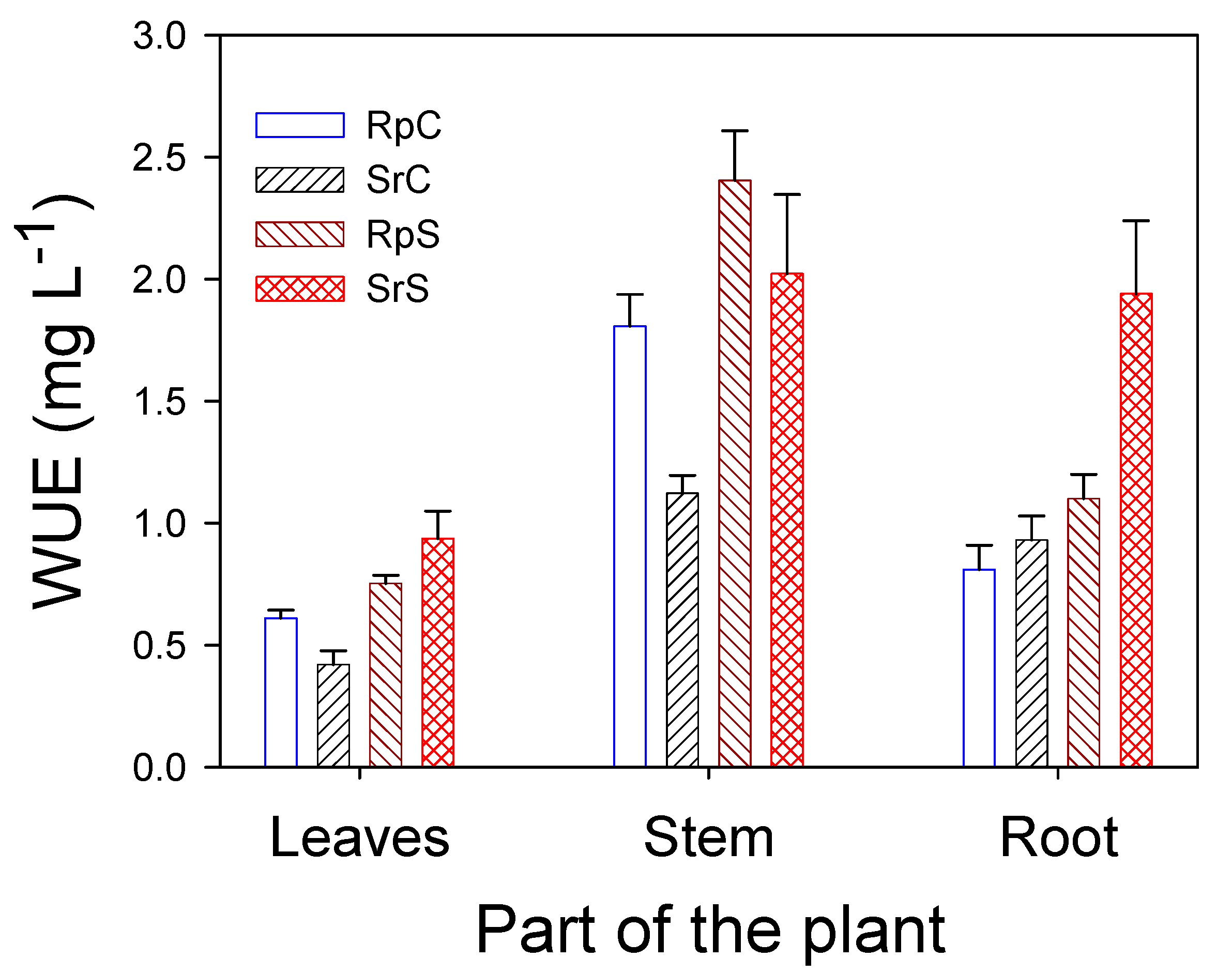
3.3. Plant Water Relations and Gas Exchange Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- MAPA. 2019 Encuesta sobre Superficies y Rendimientos de Cultivos en España (ESYRCE). Available online: https://www.mapa.gob.es/es/estadistica/temas/estadisticas-agrarias/agricultura/superficies-producciones-anuales-cultivos/ (accessed on 30 October 2020).
- Casanova-Gascón, J.; Figueras-Panillo, M.; Iglesias-Castellarnau, I.; Martín-Ramos, P. Comparison of SHD and Open-Center Training Systems in Almond Tree Orchards cv. ‘Soleta’. Agronomy 2019, 9, 874. [Google Scholar] [CrossRef] [Green Version]
- Espadador, M.; Orgaz, F.; Testi, L.; Lorite, I.J.; González-Dugo, V.; Fereres, E. Responses of transpiration and transpiration efficiency of almond trees to moderate water deficit. Sci. Hortic. 2017, 225, 6–14. [Google Scholar] [CrossRef]
- Girona, J.; Mata, M.; Marsal, J. Regulated deficit irrigation during the kernel-filling period and optimal irrigation rates in almond. Agric. Water Manag. 2005, 75, 152–167. [Google Scholar] [CrossRef]
- López-López, M.; Espadador, M.; Testi, L.; Lorite, J.I.; Orgaz, F.; Fereres, E. Water use of irrigated almond trees when subjected to water deficits. Agric. Water Manag. 2018, 195, 84–93. [Google Scholar] [CrossRef]
- Egea, G.; Nortes, P.A.; González-Real, M.M.; Baille, A.; Domingo, R. Agronomic response and water productivity of almond trees under contrasted deficit irrigation regimes. Agric. Water Manag. 2010, 97, 171–181. [Google Scholar] [CrossRef]
- Espadafor, M.; Orgaz, F.; Testi, L.; Lorite, I.J.; Villalobos, F.J. Transpiration of young almond trees in relation to intercepted radiation. Irrig. Sci. 2015, 33, 265–275. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, I.; Meyer, A.; Alfonso, S.; Gonçalves, B. Compared leaf anatomy and water relations of commercial and traditional Prunus dulcis (Mill.) cultivars under rain-fed conditions. Sci. Hortic. 2018, 229, 226–232. [Google Scholar] [CrossRef]
- Moriana, A.; Memmi, H.; Centeno, A.; Martín-Palomo, M.J.; Corell, M.; Torrecillas, A.; Pérez-López, D. Influence of rootstock on pistachio (Pistacia vera L. cv Kerman) water relations. Agric. Water Manag. 2018, 202, 263–270. [Google Scholar] [CrossRef]
- Álvarez, S.; Gómez-Bellot, M.J.; Acosta-Motos, J.R.; Sánchez-Blanco, M.J. Application of deficit irrigation in Phillyrea angustifolia for landscaping purposes. Agric. Water Manag. 2019, 218, 193–202. [Google Scholar] [CrossRef]
- Castel, J.R.; Fereres, E. Responses of Young Almond Trees to Two Drought Periods in the Field. J. Hortic. Sci. Biotechol. 1982, 57, 175–187. [Google Scholar] [CrossRef]
- Romero, P.; Botía, P. Daily and seasonal patterns of leaf water relations and gas exchange of regulated deficit-irrigated almond trees under semiarid conditions. Environ. Exp. Bot. 2006, 56, 158–173. [Google Scholar] [CrossRef]
- Goldhamer, D.; Viveros, M. Effects of preharvest irrigation cutoff durations and postharvest water deprivation on almond tree performance. Irrig. Sci. 2000, 19, 125–131. [Google Scholar] [CrossRef]
- Gutiérrez-Gordillo, S.; Durán Zuazo, V.H.; Hernández-Santana, V.; Ferrera Gil, F.; García Escalera, A.; Amores-Agüera, J.J.; García-Tejero, I.F. Cultivar Dependent Impact on Yield and Its Components of Young Almond Trees under Sustained-Deficit Irrigation in Semi-Arid Environments. Agronomy 2020, 10, 733. [Google Scholar] [CrossRef]
- Trifilò, P.; Lo Gullo, M.A.; Nardini, A.; Pernice, F.; Salleo, S. Rootstock effect on xylem conduit dimensions and vulnerability to cavitation of Olea europaea L. Trees 2007, 21, 549–556. [Google Scholar] [CrossRef]
- Ben Yahmed, J.; Ghrab, M.; Ben Mimoun, M.B. Eco-physiological evaluation of different scion-rootstock combination of almond grown in Mediterranean conditions. Fruits 2016, 71, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Balal, R.M.; Khan, M.M.; Shahid, M.A.; Mattson, N.S.; Abbas, T.; Ashfaq, M.; Garcia-Sanchez, F.; Ghazanfer, U.; Gimeno, V.; Iqbal, Z. Comparative studies on the physiobiochemical, enzymatic, and ionic modifications in salt-tolerant and salt-sensitive Citrus rootstocks under NaCl stress. J. Am. Soc. Hortic. Sci. 2012, 137, 86–95. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Imtiaz, M.; Kong, Q.; Cheng, F.; Ahmed, W.; Huang, Y.; Bie, Z. Grafting: A Technique to Modify Ion Accumulation in Horticultural Crops. Front. Plant Sci. 2016, 7, 1457. [Google Scholar] [CrossRef] [Green Version]
- Galindo, A.; Collado-González, J.; Griñán, I.; Corell, M.; Centeno, A.; Martín-Palomo, M.J.; Girón, I.J.; Rodríguez, P.; Cruz, Z.N.; Memmi, H.; et al. Deficit irrigation and emerging fruit crops as a strategy to save water in Mediterranean semiarid agrosystems. Agric. Water Manag. 2018, 202, 311–324. [Google Scholar] [CrossRef]
- Masson, A.; Monteuuis, O. Rubber tree clonal plantations: Grafted vs self-rooted plant material. Bois Forêts Des Trop. 2017, 332, 57–68. [Google Scholar] [CrossRef]
- Huang, W.; Liai, S.; Lv, H.; Khaldun, A.B.M.; Wang, Y. Characterization of the growth and fruit quality of tomato grafted on a woody medicinal plant, Lycium chinense. Sci. Hortic. 2015, 197, 447–453. [Google Scholar] [CrossRef]
- Nardini, A.; Gascó, A.; Raimondo, F.; Gortan, E.; Lo Gullo, M.A.; Caruso, T.; Salleo, S. Is rootstock-induced dwarfing in olive an effect of reduced plant hydraulic efficiency? Tree Physiol. 2006, 26, 1137–1144. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, S.; Pinochet, J.; Romero, J.; Gogorcena, Y.; Moreno, M.A.; Espada, J.L. Performance of peach and plum based rootstocks of different vigour on a late peach cultivar in replant and calcareous conditions. Sci. Hortic. 2011, 129, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, S.; Dridi, J.; Gutiérrez, D.; Moret, D.; Irigoyen, J.J.; Moreno, M.A.; Gogorcena, Y. Physiological, biochemical and molecular response in four Prunus rootstocks submitted to drought stress. Tree Physiol. 2013, 33, 1061–1075. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Blanco, M.J.; Álvarez, S.; Navarro, A.; Bañón, S. Changes in leaf water relations, gas exchange, growth and flowering quality in potted geranium plants irrigated with different water regimes. J. Plant Physiol. 2009, 166, 467–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acosta-Motos, J.R.; Hernández, J.A.; Álvarez, S.; Barba-Espín, G.; Sánchez-Blanco, M.J. The long-term resistance mechanisms, critical irrigation threshold and relief capacity shown by Eugenia myrtifolia plants in response to saline reclaimed water. Plant Physiol. Biochem. 2017, 111, 244–256. [Google Scholar] [CrossRef]
- Álvarez, S.; Bañón, S.; Sánchez-Blanco, M.J. Regulated deficit irrigation in different phenological stages of potted geranium plants: Water consumption, water relations and ornamental quality. Acta Physiol. Plant 2013, 35, 1257–1267. [Google Scholar] [CrossRef] [Green Version]
- Scholander, P.F.; Hammel, H.T.; Bradstreet, E.D.; Hemingsen, E.A. Sap pressure in vascular plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Turner, N.C. Measurement of plant water status by the pressure chamber technique. Irrig. Sci. 1988, 9, 289–308. [Google Scholar] [CrossRef]
- Barrs, H.D. Determination of water deficits in plants tissue. In Water Deficits and Plant Growth; Kozlowski, T.T., Ed.; Academic Press: New York, NY, USA, 1968; pp. 235–368. [Google Scholar]
- Begg, J.E.; Turner, N.C. Water potential gradients in field tobacco. Plant Physiol. 1970, 46, 343–346. [Google Scholar] [CrossRef] [Green Version]
- Gijón, M.C.; Gimenez, C.; Perez-López, D.; Guerrero, J.; Couceiro, J.F.; Moriana, A. Rootstock influences the response of pistachio (Pistacia vera L. cv. Kerman) to water stress and rehydration. Sci. Hortic. 2010, 125, 666–671. [Google Scholar] [CrossRef]
- Hsiao, T.C. Plant Responses to Water Stress. Ann. Rev. Plant Physiol. 1973, 24, 519–570. [Google Scholar] [CrossRef]
- Ruiz Sánchez, M.C.; Domingo, R.; Torrecillas, A.; Pérez-Pastor, A. Water stress preconditioning to improve drought resistance in young apricot plants. Plant Sci. 2000, 156, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Álvarez, S.; Rodríguez, P.; Broetto, F.; Sánchez-Blanco, M.J. Long term responses and adaptive strategies of Pistacia lentiscus under moderate and severe deficit irrigation and salinity: Osmotic and elastic adjustment, growth, ion uptake and photosynthetic activity. Agric. Water Manag. 2018, 202, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Franco, J.A.; Bañón, S.; Vicente, M.J.; Miralles, J.; Martínez-Sánchez, J.J. Root development in horticultural plants grown under abiotic stress conditions—A review. J. Hortic. Sci. Biotechnol. 2011, 86, 543–556. [Google Scholar] [CrossRef]
- Sánchez-Blanco, M.J.; Ortuño, M.F.; Bañón, S.; Álvarez, S. Deficit irrigation as a strategy to control growth in ornamental plants and enhance their ability to adapt to drought conditions. J. Hortic. Sci. Biotechnol. 2019, 94, 137–150. [Google Scholar] [CrossRef]
- Yadollahi, A.; Arzani, K.; Ebadi, A.; Winthersohn, M.; Karimi, S. The response of different almond genotypes to moderate and severe water stress in order to screen for drought tolerance. Sci. Hortic. 2011, 129, 403–413. [Google Scholar] [CrossRef]
- Bañón, S.; Fernández, J.A.; Franco, J.A.; Torrecillas, A.; Alarcón, J.J.; Sánchez-Blanco, M.J. Effects of water stress and night temperature pre-conditioning on water relations and morphological and anatomical changes of Lotus creticus plants. Sci. Hort. 2004, 101, 333–342. [Google Scholar] [CrossRef]
- Abrisqueta, J.M.; Mounzer, O.; Álvarez, S.; Conejero, W.; García-Orellana, Y.; Tapia, L.M.; Vera, J.; Abrisqueta, I.; Ruiz-Sánchez, M.C. Root dynamics of peach submitted to partial rootzone drying and continuos deficit irrigation. Agric. Water Manag. 2008, 95, 959–967. [Google Scholar] [CrossRef]
- Rubio-Cabetas, M.J. Almond Rootstocks: Overview. In XVI GREMPA Meeting on Almonds and Pistachios; Kodad, O., López-Francos, A., Rovira, M., Socias i Company R, Eds.; Options Méditerranéennes: Série A, Séminaires Méditerranéens; CIHEAM: Zaragoza, Spain, 2016; Volume 119, pp. 133–143. [Google Scholar]
- Tous, J.; Romero, A.; Hermoso, J. New trends in olive orchard design for continuous mechanical harvesting. Adv. Hortic. Sci. 2010, 24, 43–52. [Google Scholar]
- Spann, T.M.; Beede, R.H.; Dejong, T.M. Preformation in vegetative buds of pistachio (Pistacia vera): Relationship to shoot morphology, crown structure and rootstock vigor. Tree Physiol. 2007, 27, 1189–1196. [Google Scholar] [CrossRef] [Green Version]
- Basile, B.; Marsal, J.; DeJong, T.M. Daily shoot extension growth of peach trees growing on rootstocks that reduce scion growth is related to daily dynamics of stem water potential. Tree Physiol. 2003, 23, 695–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorce, C.; Massai, R.; Picciarelli, P.; Lorenzi, R. Hormonal relationships in xylem sap of grafted and ungrafted Prunus rootstocks. Sci. Hortic. 2002, 93, 333–342. [Google Scholar] [CrossRef]
- Lliso, I.; Forner, J.B.; Talón, M. The dwarfing mechanism of citrus rootstocks F&A 418 and #23 is related to competition between vegetative and reproductive growth. Tree Physiol. 2004, 24, 225–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motisi, A.; Pernice, F.; Sottile, F.; Caruso, T. Rootstock effect on the stem water potential gradients in cv. ‘Armking’ nectarine trees. Acta Hortic. 2004, 658, 75–79. [Google Scholar] [CrossRef]
- Karimi, S.; Yadollahi, A.; Nazari-Moghadam, R.; Imani, A.; Arzani, K. In vitro Screening of Almond (Prunus dulcis (Mill.)) Genotypes for Drought Tolerance. J. Biolog. Environ. Sci. 2012, 6, 263–270. [Google Scholar]
- Navarro, A.; Álvarez, S.; Castillo, M.; Bañón, S.; Sánchez-Blanco, M.J. Changes in tissue-water relations, photosynthetic activity, and growth of Myrtus communis plants in response to different conditions of water availability. J. Hortic. Sci. Biotechnol. 2009, 84, 541–547. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Dugo, V.; Zarco-Tejada, P.; Berni, J.A.J.; Suárez, L.; Goldhamer, D.; Fereres, E. Almond tree canopy temperature reveals intra-crown variability that is water stress-dependent. Agric. For. Meteorol. 2012, 154, 156–165. [Google Scholar] [CrossRef]
- Lenton, R. Irrigation in the twenty-first century: Reflections on science, policy and society. Irrig. Drain. 2014, 63, 154–157. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Wei, Z.; Liu, F. ABA-mediated modulation of elevated CO2 on stomatal response to drought. Curr. Opin. Plant Biol. 2020, 56, 174–180. [Google Scholar] [CrossRef]
- Shackel, K.A. Water relations of woody perennial plant species. OENO One 2007, 41, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torrecillas, A.; Alarcón, J.J.; Domingo, R.; Planes, J.; Sánchez-Blanco, M.J. Strategies for drought resistance in leaves of two almond cultivars. Plant Sci. 1996, 118, 135–143. [Google Scholar] [CrossRef]
- Rouhi, V.; Samson, R.; Lemeur, R.; VanDame, P. Photosynthetic gas exchange characteristics in three different almond species during drought stress and subsequent recovery. Environ. Exp. Bot. 2007, 59, 117–129. [Google Scholar] [CrossRef]
- Villalobos, F.J.; Perez-Priego, O.; Testi, L.; Morales, A.; Orgaz, F. Effects of water supply on carbon and water exchange of olive trees. Eur. J. Agron. 2012, 40, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Pérez, J.G.; Syvertsen, J.P.; Botía, P.; García-Sánchez, F. Leaf water relations and net gas exchange responses of salinized carrizo citrange seedlings during drought stress and recovery. Ann. Bot. 2007, 100, 335–345. [Google Scholar] [CrossRef] [Green Version]
- Álvarez, S.; Sánchez-Blanco, M.J. Comparison of individual and combined effects of salinity and deficit irrigation on physiological, nutritional and ornamental aspects of tolerance in Callistemon laevis plants. J. Plant Physiol. 2015, 185, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Peñuelas, J.; Munné-Bosch, S.; Llusia, J.; Filella, I. Leaf reflectance and photo- and antioxidant protection in field-grown summer-stressed Phillyrea angustifolia. Optical signals of oxidative stress? New Phytol. 2004, 162, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Franco, J.A.; Martínez-Sánchez, J.J.; Fernández, J.A.; Bañón, S. Selection and nursery production of ornamental plants for landscaping and xerogardening in semi-arid environments. J. Hortic. Sci. Biotechnol. 2006, 81, 3–17. [Google Scholar] [CrossRef]
- Álvarez, S.; Sánchez-Blanco, M.J. Changes in growth rate, root morphology and water use efficiency of potted Callistemon citrinus plants in response to different levels of water deficit. Sci. Hortic. 2013, 156, 54–62. [Google Scholar] [CrossRef] [Green Version]
| Period | Parameters | RpC | SrC | RpS | SrS |
|---|---|---|---|---|---|
| Stress | Leaf DW (g pl−1) | 14.9 ± 0.9 b | 12.6 ± 0.9 b | 3.8 ± 0.7 a | 3.6 ± 0.7 a |
| Stem DW (g pl−1) | 48.6 ± 4.5 c | 25.4 ± 2.9 b | 28.5 ± 1.9 b | 16.1 ± 0.8 a | |
| Root DW (g pl−1) | 21.6 + 2.5 bc | 26.9 + 1.2 c | 10.6 + 1.0 a | 20.3 + 0.7 b | |
| Root to shoot ratio | 1.21 + 0.07 a | 2.36 + 0.08 b | 2.16 + 0.05 ab | 6.06 + 0.39 c | |
| Total leaf area (cm2) | 1306 ± 66 c | 970 ± 57 b | 297 ± 55 a | 316 ± 67 a | |
| Number of leaves | 405.0 ± 14.0 c | 336.7 ± 19.9 b | 129.2 ± 22.5 a | 129.3 ± 20.7 a | |
| Leaf blade area (cm2) | 3.2 ± 0.2 b | 3.0 ± 0.2 b | 1.9 ± 0.2 a | 2.0 ± 0.3 a | |
| Recovery | Leaf DW (g pl−1) | 26.3 ± 1.6 c | 14.3 ± 1.4 ab | 16.0 ± 16.0 b | 11.6 ± 0.8 a |
| Stem DW (g pl−1) | 83.9 ± 5.4 d | 33.8 ± 2.5 b | 33.8 ± 33.8 c | 24.2 ± 1.4 a | |
| Root DW (g p−1) | 32.9 + 2.6 b | 35.5 + 1.9 b | 18.7 + 0.5 a | 24.2 + 0.3 a | |
| Root to shoot ratio | 1.32 + 0.05 a | 2.42 + 0.05 b | 1.13 + 0.05 a | 2.22 + 0.04 b | |
| Total leaf area (cm2) | 2278 ± 197 c | 1120 ± 146.4 a | 1594 ± 89 b | 917 ± 55 a | |
| Number of leaves | 491.3 ± 51.9 c | 345.8 ± 48.3 b | 403.8 ± 21.7 b | 215.0 ± 26.4 a | |
| Leaf blade area (cm2) | 4.2 ± 0.3 | 3.6 ± 0.3 | 3.9 ± 0.3 | 4.4 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez, S.; Martín, H.; Barajas, E.; Rubio, J.A.; Vivaldi, G.A. Rootstock Effects on Water Relations of Young Almond Trees (cv. Soleta) When Subjected to Water Stress and Rehydration. Water 2020, 12, 3319. https://doi.org/10.3390/w12123319
Álvarez S, Martín H, Barajas E, Rubio JA, Vivaldi GA. Rootstock Effects on Water Relations of Young Almond Trees (cv. Soleta) When Subjected to Water Stress and Rehydration. Water. 2020; 12(12):3319. https://doi.org/10.3390/w12123319
Chicago/Turabian StyleÁlvarez, Sara, Hugo Martín, Enrique Barajas, José Antonio Rubio, and Gaetano Alessandro Vivaldi. 2020. "Rootstock Effects on Water Relations of Young Almond Trees (cv. Soleta) When Subjected to Water Stress and Rehydration" Water 12, no. 12: 3319. https://doi.org/10.3390/w12123319





